Submitted:
02 April 2024
Posted:
03 April 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Results
2.1. Thermodynamics of Fusion
2.2. Condensed phase heat capacities
2.3. Vapor Pressures and Vaporization Enthalpies
3. Discussion
3.1. Discrepancy of the Fusion Data
3.1. Fusion and Solution Thermochemistry at 298.15 K
3.2. Vaporization and Sublimation Thermochemistry
4. Materials and Methods
4.1. Materials
4.2. Solution calorimetry
4.3. Differential scanning calorimetry
4.4. Fast Scanning Calorimetry
4.5. Computations
4.6. Molecular Refractivity
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tsukushi, I.; Yamamuro, O.; Matsuo, T. Solid state amorphization of organic molecular crystals using a vibrating mill. Solid State Commun. 1995, 94, 1013–1018. [Google Scholar] [CrossRef]
- Tsukushi, I.; Yamamuro, O.; Ohta, T.; Matsuo, T.; Nakano, H.; Shirota, Y. A calorimetric study on the configurational enthalpy and low-energy excitation of ground amorphous solid and liquid-quenched glass of 1, 3, 5-tri--naphthylbenzene. J. Phys. Cond. Matt. 1996, 8, 245. [Google Scholar] [CrossRef]
- Magill, J.H.; Plazek, D.J. Crystallization Kinetics of 1 : 3 :5 Tri-α-naphthyl Benzene. Nature 1966, 209, 70–71. [Google Scholar] [CrossRef]
- Magill, J. Physical Properties of Aromatic Hydrocarbons. III. A Test of the Adam—Gibbs Relaxation Model for Glass Formers Based on the Heat-Capacity Data of 1, 3, 5-tri-α-Naphthylbenzene. J. Chem. Phys. 1967, 47, 2802–2807. [Google Scholar] [CrossRef]
- Ngai, K.; Magill, J.; Plazek, D. Flow, diffusion and crystallization of supercooled liquids: Revisited. J. Chem. Phys. 2000, 112, 1887–1892. [Google Scholar] [CrossRef]
- Swallen, S.F.; Bonvallet, P.A.; McMahon, R.J.; Ediger, M. Self-diffusion of tris-naphthylbenzene near the glass transition temperature. Phys. Rev. Lett. 2003, 90, 015901. [Google Scholar] [CrossRef] [PubMed]
- Hofer, K.; Perez, J.; Johari, G.P. Detecting enthalpy ‘cross-over’ in vitrified solids by differential scanning calorimetry. Philos. Mag. Lett. 1991, 64, 37–43. [Google Scholar] [CrossRef]
- Whitaker, C.M.; McMahon, R.J. Synthesis and characterization of organic materials with conveniently accessible supercooled liquid and glassy phases: Isomeric 1, 3, 5-tris (naphthyl) benzenes. J. Phys. Chem. 1996, 100, 1081–1090. [Google Scholar] [CrossRef]
- Magill, J.; Plazek, D. Physical Properties of Aromatic Hydrocarbons. II. Solidification Behavior of 1, 3, 5-Tri-α-Naphthylbenzene. J. Chem. Phys. 1967, 46, 3757–3769. [Google Scholar] [CrossRef]
- Dawson, K.; Zhu, L.; Kopff, L.A.; McMahon, R.J.; Yu, L.; Ediger, M. Highly stable vapor-deposited glasses of four tris-naphthylbenzene isomers. J. Phys. Chem. Lett. 2011, 2, 2683–2687. [Google Scholar] [CrossRef]
- Whitaker, K.R.; Ahrenberg, M.; Schick, C.; Ediger, M. Vapor-deposited α, α, β-tris-naphthylbenzene glasses with low heat capacity and high kinetic stability. J. Chem. Phys. 2012, 137, 154502. [Google Scholar] [CrossRef]
- Liu, T.; Cheng, K.; Salami-Ranjbaran, E.; Gao, F.; Li, C.; Tong, X.; Lin, Y.-C.; Zhang, Y.; Zhang, W.; Klinge, L.; et al. The effect of chemical structure on the stability of physical vapor deposited glasses of 1,3,5-triarylbenzene. J. Chem. Phys. 2015, 143, 084506. [Google Scholar] [CrossRef]
- Ediger, M.D.; Swallen, S.; Kearns, K.; Yu, L.; Wu, T. Unusually stable glasses and methods for forming same. US Patent 2012. [Google Scholar]
- Gutzow, I.; Schmelzer, J. The vitreous state; Springer: 2015; 576 p.
- Costa, J.C.S.; Rocha, R.M.; Vaz, I.C.M.; Torres, M.C.; Mendes, A.; Santos, L.M.N.B.F. Description and Test of a New Multilayer Thin Film Vapor Deposition Apparatus for Organic Semiconductor Materials. J. Chem. Eng. Data 2015, 60, 3776–3791. [Google Scholar] [CrossRef]
- Lima, C.F.; Costa, J.C.; Silva, A.M.; Mendes, A.; Santos, L.M. Solid–Liquid–Gas Phase Equilibria for Small Phenylene-Thiophene Co-Oligomers. J. Chem. Eng. Data 2022, 67, 3033–3045. [Google Scholar] [CrossRef]
- Magill, J.; Ubbelohde, A. Interlocking of polyphenyl molecules in the pre-freezing region. Trans. Faraday Soc. 1958, 54, 1811–1821. [Google Scholar] [CrossRef]
- Elseiver. https://www.reaxys.com/ Reaxys. 2024.
- Yagofarov, M.I.; Nagrimanov, R.N.; Solomonov, B.N. New aspects in the thermochemistry of solid-liquid phase transitions of organic non-electrolytes. J. Mol. Liq. 2018, 256, 58–66. [Google Scholar] [CrossRef]
- Bolmatenkov, D.N.; Yagofarov, M.I.; Sokolov, A.A.; Ziganshin, M.A.; Solomonov, B.N. The heat capacities and fusion thermochemistry of sugar alcohols between 298.15 K and Tm: The study of D-sorbitol, D-mannitol and myo-inositol. J. Mol. Liq. 2021; 115545. [Google Scholar]
- Sokolov, A.A.; Yagofarov, M.I.; Balakhontsev, I.S.; Nizamov, I.I.; Mukhametzyanov, T.A.; Solomonov, B.N.; Yurkshtovich, Y.N.; Stepurko, E.N. Thermodynamic Properties of 3-and 4-Ethoxyacetanilides between 80 and 480 K. Molecules 2023, 28, 7027. [Google Scholar] [CrossRef]
- Solomonov, B.N.; Yagofarov, M.I. An approach for the calculation of vaporization enthalpies of aromatic and heteroaromatic compounds at 298.15 K applicable to supercooled liquids. J. Mol. Liq. 2020, 319, 114330. [Google Scholar] [CrossRef]
- Yagofarov, M.I.; Solomonov, B.N. Interpolation of the temperature dependence of the fusion enthalpy of aromatic compounds between 298.15 K and the melting temperature. Int. J. Thermophys. 2022, 43, 90. [Google Scholar] [CrossRef]
- Cebe, P.; Thomas, D.; Merfeld, J.; Partlow, B.P.; Kaplan, D.L.; Alamo, R.G.; Wurm, A.; Zhuravlev, E.; Schick, C. Heat of fusion of polymer crystals by fast scanning calorimetry. Polymer 2017, 126, 240–247. [Google Scholar] [CrossRef]
- Bolmatenkov, D.N.; Yagofarov, M.I.; Valiakhmetov, T.F.; Rodionov, N.O.; Solomonov, B.N. Vaporization enthalpies of benzanthrone, 1-nitropyrene, and 4-methoxy-1-naphthonitrile: prediction and experiment. J. Chem. Thermodyn. 2021, 168, 106744. [Google Scholar] [CrossRef]
- Solomonov, B.N.; Yagofarov, M.I.; Nagrimanov, R.N. Additivity of vaporization enthalpy: group and molecular contributions exemplified by alkylaromatic compounds and their derivatives. J. Mol. Liq. 2021, 342, 117472. [Google Scholar] [CrossRef]
- Solomonov, B.N.; Varfolomeev, M.A.; Nagrimanov, R.N.; Novikov, V.B.; Zaitsau, D.H.; Verevkin, S.P. Solution calorimetry as a complementary tool for the determination of enthalpies of vaporization and sublimation of low volatile compounds at 298.15 K. Thermochim. Acta 2014, 589, 164–173. [Google Scholar] [CrossRef]
- Biswas, N.; Dey, G.; Das, L.; Das, S.; Ruidas, N.; Chakraborty, A. Geometry, vibrations and torsional potential of 1-phenyl naphthalene: A combined ab-initio and experimental study. J. Mol. Struct. 2024, 1296, 136844. [Google Scholar] [CrossRef]
- Le Fevre, R.; Sundaram, A.; Sundaram, K. 593. Molecular polarisability. The molar Kerr constants and conformations of eight polyaryls as solutes. J. Chem. Soc. 1963, 3180–3188. [Google Scholar] [CrossRef]
- Armarego, W.L. Purification of laboratory chemicals; Butterworth-Heinemann: 2017.
- Yagofarov, M.I.; Sokolov, A.A.; Balakhontsev, I.S.; Nizamov, I.I.; Solomonov, B.N. Thermochemistry of fusion, solution and hydrogen bonding in benzamide, N-methylbenzamide, and acetanilide. Thermochim. Acta 2023, 728, 179579. [Google Scholar] [CrossRef]
- Buzyurov, A.V.; Nagrimanov, R.N.; Zaitsau, D.H.; Mukhametzyanov, T.A.; Abdelaziz, A.; Solomonov, B.N.; Schick, C. Application of the Flash DSC 1 and 2+ for vapor pressure determination above solids and liquids. Thermochim. Acta 2021, 706, 179067. [Google Scholar] [CrossRef]
- Bolmatenkov, D.N.; Yagofarov, M.I.; Valiakhmetov, T.F.; Rodionov, N.O.; Solomonov, B.N. Vaporization enthalpies of benzanthrone, 1-nitropyrene, and 4-methoxy-1-naphthonitrile: Prediction and experiment. J. Chem. Thermodyn. 2022, 168, 106744. [Google Scholar] [CrossRef]
- Yagofarov, M.I.; Lapuk, S.E.; Mukhametzyanov, T.A.; Ziganshin, M.A.; Schick, C.; Solomonov, B.N. Application of fast scanning calorimetry to the fusion thermochemistry of low-molecular-weight organic compounds: Fast-crystallizing m-terphenyl heat capacities in a deeply supercooled liquid state. Thermochim. Acta 2018, 668, 96–102. [Google Scholar] [CrossRef]
- Fuller, E.N.; Schettler, P.D.; Giddings, J.C. New Method for Prediction of Binary Gas-Phase Diffusion Coefficients. Ind. Eng. Chem. 1966, 58, 18–27. [Google Scholar] [CrossRef]
- Reid, R.C.; Prausnitz, J.M.; Poling, B.E. The properties of gases and liquids; McGraw-Hill, Inc.: New York:, 1987. [Google Scholar]
- Buzyurov, A.V.; Nagrimanov, R.N.; Zaitsau, D.H.; Mukhametzyanov, T.A.; Solomonov, B.N.; Abdelaziz, A.; Schick, C. Application of the Flash DSC 1 and 2+ for vapor pressure determination above solids and liquids. Thermochim. Acta 2021, 706, 179067. [Google Scholar] [CrossRef]
- Ferro-Costas, D.; Fernández-Ramos, A. A combined systematic-stochastic algorithm for the conformational search in flexible acyclic molecules. Frontiers Chem. 2020, 8, 16. [Google Scholar] [CrossRef] [PubMed]
- Ayala, P.Y.; Schlegel, H.B. Identification and treatment of internal rotation in normal mode vibrational analysis. J. Chem. Phys. 1998, 108, 2314–2325. [Google Scholar] [CrossRef]
- Cervinka, C.; Fulem, M.; Ruzicka, K. Evaluation of accuracy of ideal-gas heat capacity and entropy calculations by density functional theory (DFT) for rigid molecules. J. Chem. Eng. Data 2012, 57, 227–232. [Google Scholar] [CrossRef]
- Pfaendtner, J.; Yu, X.; Broadbelt, L.J. The 1-D hindered rotor approximation. Theor. Chem. Acc. 2007, 118, 881–898. [Google Scholar] [CrossRef]
- Kilpatrick, J.E.; Pitzer, K.S. Energy levels and thermodynamic functions for molecules with internal rotation. III. Compound rotation. J. Chem. Phys. 1949, 17, 1064–1075. [Google Scholar] [CrossRef]
- Marston, C.C.; Balint-Kurti, G.G. The Fourier grid Hamiltonian method for bound state eigenvalues and eigenfunctions. J. Chem. Phys. 1989, 91, 3571–3576. [Google Scholar] [CrossRef]
- Balint-Kurti, G.G.; Ward, C.L.; Marston, C.C. Two computer programs for solving the Schrödinger equation for bound-state eigenvalues and eigenfunctions using the Fourier grid Hamiltonian method. Comput. Phys. Commun. 1991, 67, 285–292. [Google Scholar] [CrossRef]
- Balint-Kurti, G.G.; Dixon, R.N.; Marston, C.C. Grid methods for solving the Schrödinger equation and time dependent quantum dynamics of molecular photofragmentation and reactive scattering processes. Int. Rev. Phys. Chem. 1992, 11, 317–344. [Google Scholar] [CrossRef]
- Cervinka, C.; Fulem, M.; Růžička, K.t. Evaluation of uncertainty of ideal-gas entropy and heat capacity calculations by density functional theory (DFT) for molecules containing symmetrical internal rotors. J. Chem. Eng. Data 2013, 58, 1382–1390. [Google Scholar] [CrossRef]
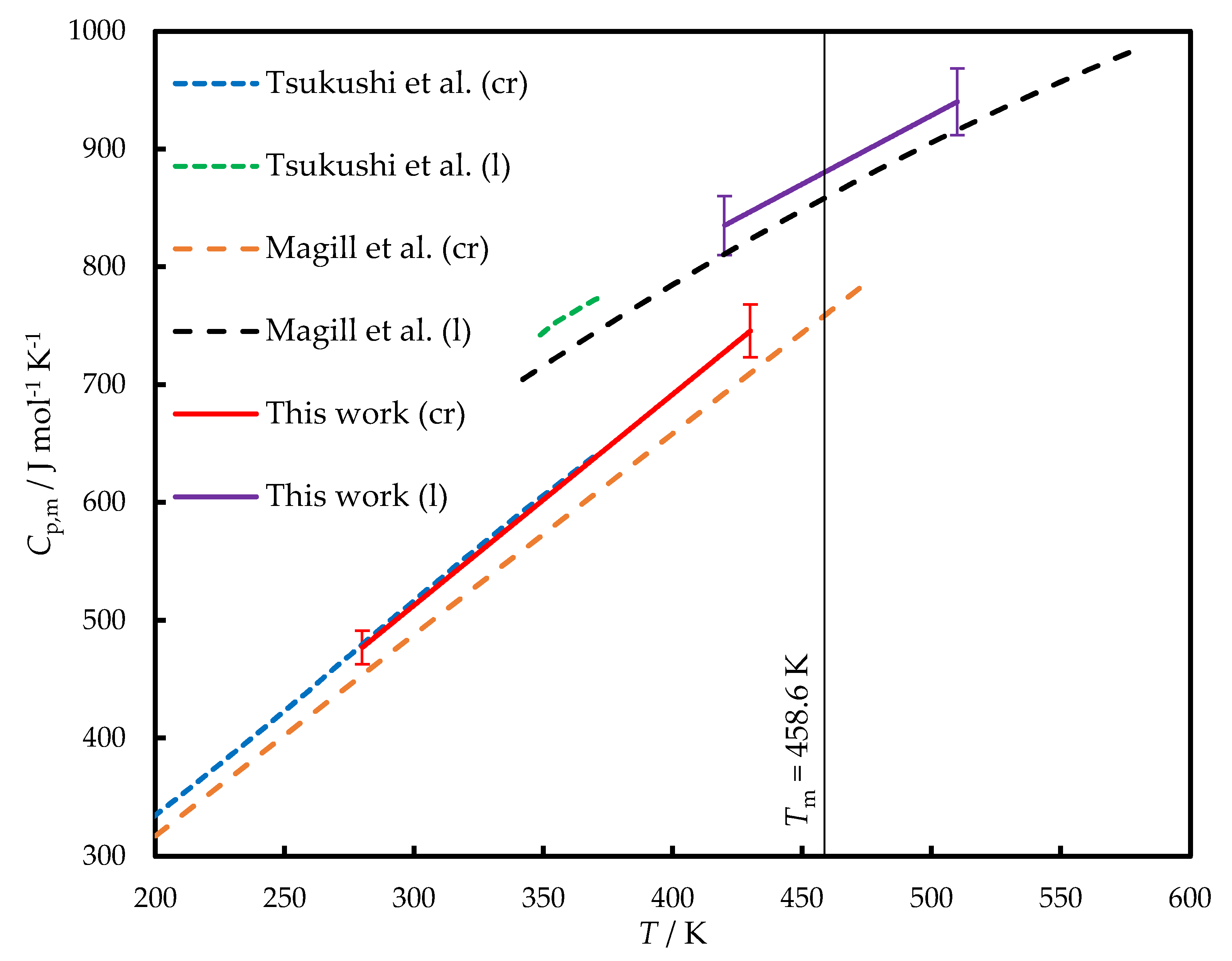
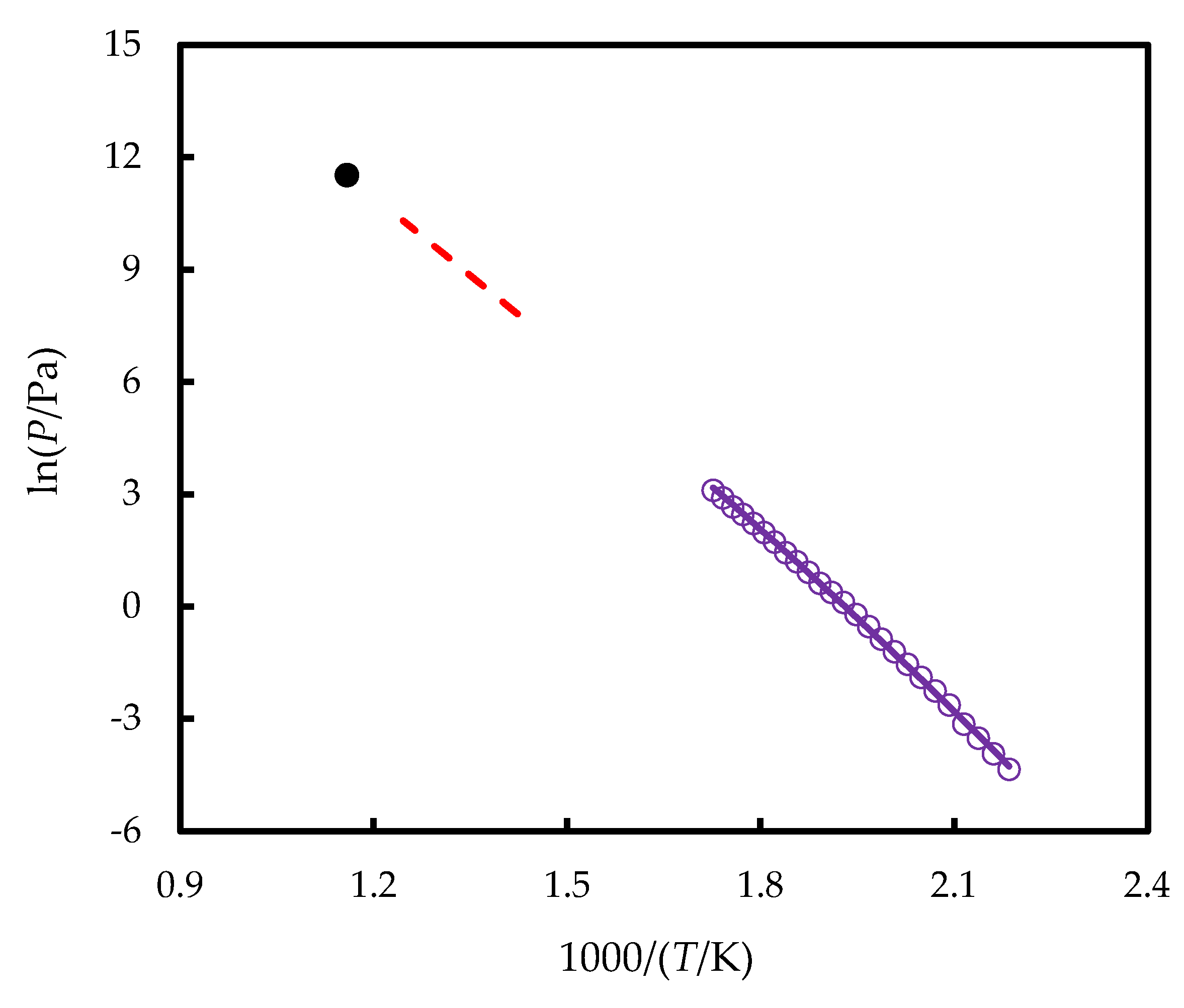
| Tm / K | (Tm)/ kJ mol-1 | Ref. |
| 458.6±0.4 a | 32.9±0.5 a | This work |
| 456.3 | 33.3 | [1] |
| 472 | 42.4±0.6 | [4] |
| 472.2 | 41.8 | [9] |
| T / K | pa / Pa | T / K | pa / Pa | T / K | pa / Pa |
| 457.6 | 1.29·10-2 | 503.3 | 4.19·10-1 | 548.9 | 5.59 |
| 462.7 | 1.95·10-2 | 508.3 | 5.85·10-1 | 554.0 | 7.22 |
| 467.8 | 2.95·10-2 | 513.4 | 8.03·10-1 | 559.0 | 9.15 |
| 472.8 | 4.33·10-2 | 518.5 | 1.11 | 564.1 | 11.6 |
| 477.9 | 7.24·10-2 | 523.5 | 1.45 | 569.2 | 14.3 |
| 483.0 | 1.05·10-1 | 528.6 | 1.86 | 574.3 | 18.3 |
| 488.1 | 1.50·10-1 | 533.7 | 2.49 | 579.3 | 22.2 |
| 493.1 | 2.14·10-1 | 538.8 | 3.29 | ||
| 498.2 | 2.99·10-1 | 543.8 | 4.19 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





