Submitted:
01 April 2024
Posted:
03 April 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
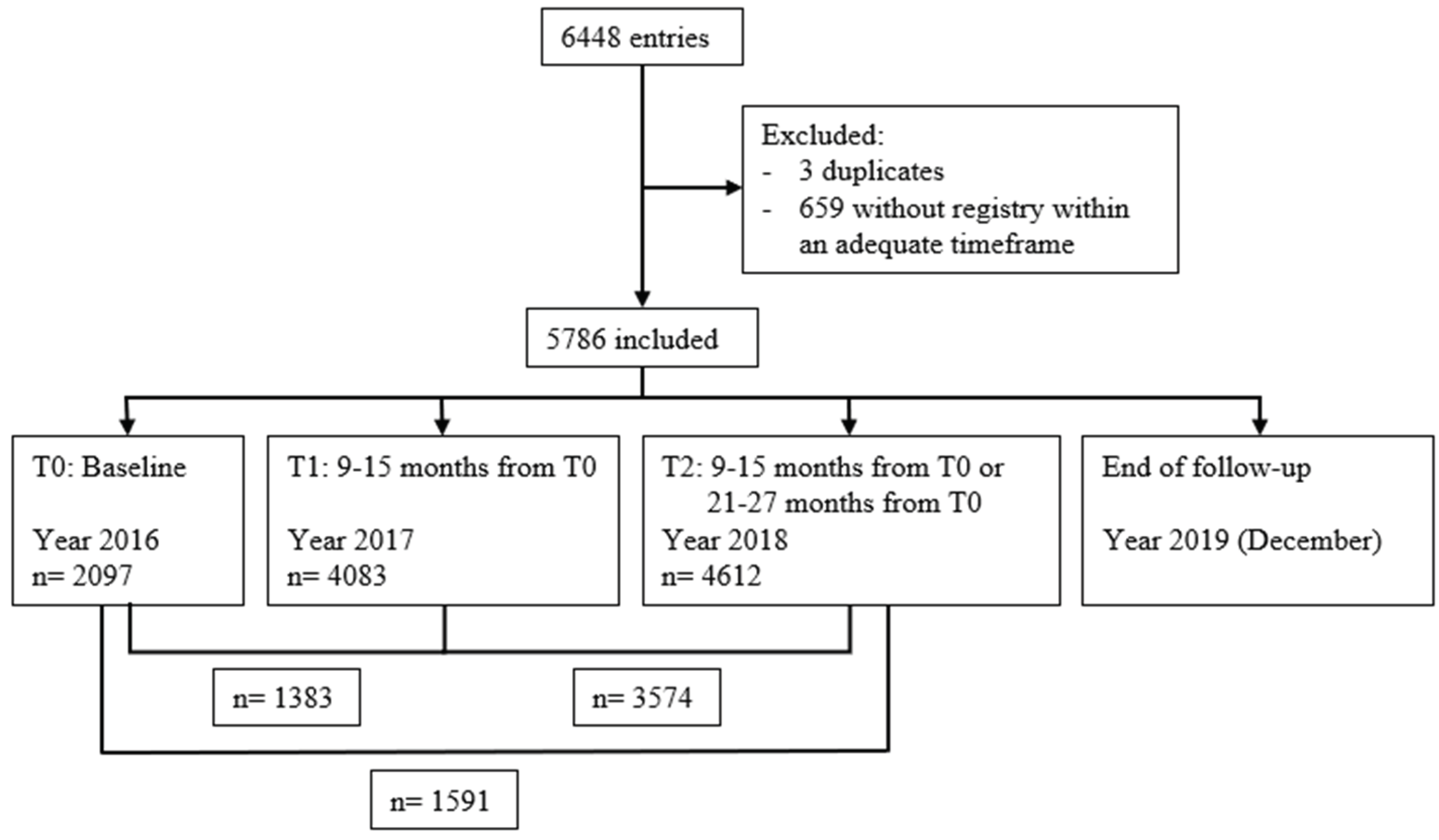
2.1. Type of Study and Selection of Participants
2.2. Setting Characterization
2.3. Data Collection Methods
2.4. Statistical Analysis
3. Results
3.1. Sample Characterisation
3.2. IWGDF Risk Factors and Degree Progression at One and Two Years
3.3. IWGDF Classification Accuracy in Predicting DFU Development at One, Two, and Three Years
4. Discussion
4.1. Main Findings
4.2. Strengths and Weaknesses of the Study
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Magliano, D.J. and E.J. Boyko, IDF diabetes atlas. 2022.
- Armstrong, D.G.; Boulton, A.J.; Bus, S.A. Diabetic foot ulcers and their recurrence. New England Journal of Medicine 2017, 376, 2367–2375. [Google Scholar] [CrossRef] [PubMed]
- Wukich, D.K.; Raspovic, K.M.; Suder, N.C. Patients with diabetic foot disease fear major lower-extremity amputation more than death. Foot & ankle specialist 2018, 11, 17–21. [Google Scholar] [CrossRef]
- Lazzarini, P.A.; et al. Diabetes-related lower-extremity complications are a leading cause of the global burden of disability. Diabetic Medicine 2018, 35, 1297–1299. [Google Scholar] [CrossRef] [PubMed]
- Lazzarini, P.A.; et al. A new declaration for feet's sake: Halving the global diabetic foot disease burden from 2% to 1% with next generation care. Diabetes/metabolism research and reviews 2023, e3747. [Google Scholar] [CrossRef] [PubMed]
- Barshes, N.R.; et al. The system of care for the diabetic foot: objectives, outcomes, and opportunities. Diabetic foot & ankle 2013, 4, 21847. [Google Scholar] [CrossRef]
- Jeffcoate, W. Stratification of foot risk predicts the incidence of new foot disease, but do we yet know that the adoption of routine screening reduces it? Diabetologia 2011, 54, 991–993. [Google Scholar] [CrossRef] [PubMed]
- Bus, S.; et al. IWGDF guidance on the prevention of foot ulcers in at-risk patients with diabetes. Diabetes/metabolism research and reviews 2016, 32, 16–24. [Google Scholar] [CrossRef] [PubMed]
- Bus, S.A.; et al. Guidelines on the prevention of foot ulcers in persons with diabetes (IWGDF 2023 update). Diabetes/Metabolism Research and Reviews 2024, 40, e3651. [Google Scholar] [CrossRef] [PubMed]
- Vandenbroucke, J.P.; et al. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE): explanation and elaboration. Annals of internal medicine 2007, 147, W-163–W-194. [Google Scholar] [CrossRef]
- Instituto Nacional de Estatistica. Censos 2021. 2024 [cited 2024 21/03/2024].
- (DGS), D.-G.o.H., Norma nº 005/2011: Diagnóstico Sistemático do Pé Diabético 2011: https://normas.dgs.min-saude.pt/2011/01/21/diagnostico-sistematico-do-pe-diabetico/#:~:text=Norma%20n%C2%BA%20005%2F2011&text=O%20exame%20cl%C3%ADnico%20dos%20p%C3%A9s,alto%20risco.
- (SPMS), H.M.S.S. SClínico | Cuidados de Saúde Primários (CSP). 2020 [cited 2024 21/03/2024].
- Schaper, N.C.; et al. Practical guidelines on the prevention and management of diabetes-related foot disease (IWGDF 2023 update). Diabetes/Metabolism Research and Reviews, 2023: p. e3657. [CrossRef]
- van Netten, J.J.; et al. Definitions and criteria for diabetes-related foot disease (IWGDF 2023 update). Diabetes/Metabolism Research and Reviews 2023, e3654. [CrossRef]
- Team, R.C. R: A language and environment for statistical computing, Vienna, Austria. Published online 2021.
- Cheng, H.T.; et al. Worldwide Epidemiology of Diabetes-Related End-Stage Renal Disease, 2000-2015. Diabetes Care 2021, 44, 89–97. [Google Scholar] [CrossRef]
- Directorate-General of Health (DGS), National Program for Diabetes – Challenges and Strategies. 2023: Lisbon: NPD.
- Martins-Mendes, D.; et al. The independent contribution of diabetic foot ulcer on lower extremity amputation and mortality risk. Journal of Diabetes and its Complications 2014, 28, 632–638. [Google Scholar] [CrossRef]
- Stoekenbroek, R.M.; et al. How common are foot problems among individuals with diabetes? Diabetic foot ulcers in the Dutch population. Diabetologia 2017, 60, 1271–1275. [Google Scholar] [CrossRef]
- Bubun, J.; et al. Validity and Reliability Diabetic Foot Check-up as a Simple Screening Test of Diabetic Foot Ulcers in a Community. The International Journal of Lower Extremity Wounds 2023, 15347346231178181. [Google Scholar] [CrossRef] [PubMed]
- Edelman, D.; Sanders, L.J.; Pogach, L. Reproducibility and accuracy among primary care providers of a screening examination for foot ulcer risk among diabetic patients. Preventive medicine 1998, 27, 274–278. [Google Scholar] [CrossRef] [PubMed]
- Crawford, F.; et al. The risk of foot ulceration in people with diabetes screened in community settings: findings from a cohort study. QJM: An International Journal of Medicine 2011, 104, 403–410. [Google Scholar] [CrossRef] [PubMed]
- Pallin, J.A; et al. Do we screen, examine or assess to identify the “at-risk” foot in diabetes—time for agreed terms and definitions? Diabetic Medicine 2023, 40. [Google Scholar] [CrossRef] [PubMed]
| Baseline (T0) |
1 year (T1) |
Change from baseline to 1 year (T0 to T1) |
2 years (T2) |
Change from 1 to 2 years (T1 to T2) |
Change from baseline to 2 years (T0 to T2) |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Present | Sample | Present | Sample | Progression | Regression | Sample | Present | Sample | Progression | Regression | Sample | Progression | Regression | Sample | |
| Risk factors | |||||||||||||||
|
LOPS [n (%)] |
202 (9.2) |
2190 | 465 (10.8) |
4308 | 36 (2.5) |
34 (2.3) |
1451 | 429 (8.8) |
4863 | 73 (1.9) |
54 (1.4) |
3801 | 48 (2.8) |
42 (2.5) |
1692 |
|
PAD [n (%)] |
60 (2.7) |
2259 | 97 (2.2) |
4323 | 8 (0.5) |
2 (0.1) |
1537 | 115 (2.4) |
4865 | 16 (0.4) |
13 (0.3) |
3814 | 9 (0.5) |
5 (0.3) |
1756 |
| Foot deformity [n (%)] | 1104 (46.8) | 2357 | 1953 (42.5) |
4597 | 55 (3.4) |
24 (1.5) |
1621 | 2184 (42.4) |
5148 | 94 (2.3) |
52 (1.3) |
4100 | 89 (4.8) |
49 (2.6) |
1865 |
| Previous DFU [n (%)] | 133 (5.6) |
2380 | 247 (5.4) |
4590 | 30 (1.8) |
4 (0.2) |
1655 | 312 (6.1) |
5131 | 79 (1.9) |
16 (0.4) |
4091 | 89 (4.7) |
8 (0.4) |
1890 |
| Previous LEA [n (%)] | 48 (2.1) |
2340 | 78 (1.7) |
4542 | 4 (0.2) |
1 (<0.1) |
1602 | 85 (1.7) |
5096 | 5 (0.1) |
2 (<0.1) |
4037 | 6 (0.3) |
2 (0.1) |
1844 |
| Risk group | |||||||||||||||
|
Group 0 (Very low risk) [n (%)] |
1890 (90.1) |
2097 | 3706 (90.8) |
4083 | To 1: 11 (0.8) To 2: 18 (1.3) To 3: 9 (0.7) |
NA | 1383 | 4172 (90.5) |
4612 | To 1: 32 (0.9) To 2: 23 (0.6) To 3: 20 (0.6) |
NA | 3574 | To 1: 13 (0.8) To 2: 17 (1.1) To 3: 16 (1.0) |
NA | 1591 |
|
Group 1 (Low risk) [n (%)] |
72 (3.4) |
143 (3.5) |
To 2: 1 (0.1) To 3: 0 (0.0) |
To 0: 13 (0.9) | 174 (3.8) |
To 2: 2 (0.1) To 3: 2 (0.1) |
To 0: 19 (0.5) |
To 2: 4 (0.3) To 3: 0 (0.0) |
To 0: 14 (0.9) | ||||||
|
Group 2 (Moderate risk) [n (%)] |
82 (3.9) |
137 (3.3) |
To 3: 1 (0.1) | To 0: 16 (1.2) To 1: 0 (0.0) |
136 (2.9) |
To 3: 12 (0.3) | To 0: 25 (0.7) To 1: 1 (<0.1) |
To 3: 6 (0.4) | To 0: 21 (1.3) To 1: 2 (0.1) |
||||||
|
Group 3 (High risk) [n (%)] |
53 (2.5) |
97 (2.4) |
NA | To 0: 4 (0.3) To 1: 0 (0.0) To 2: 0 (0.0) |
130 (2.8) |
NA | To 0: 9 (0.3) To 1: 1 (<0.1) To 2: 2 (<0.1) |
NA | To 0: 4 (0.3) To 1: 0 (0.0) To 2: 0 (0.0) |
||||||
| Total | 40 (3) | 33 (2.4) | 91 (2.6) | 57 (1.6) | 56 (3.6) | 41 (2.6) | |||||||||
|
IWGDF risk group (based on the baseline assessment) |
Diabetes-related foot ulcer development | |||||
| 1 year | 2 years | 3 years | ||||
| Present | Sample | Present | Sample | Present | Sample | |
| Group 0 (Very low risk) [n (%)] | 3 (0.2) | 1890 | 5 (0.3) | 1890 | 13 (0.7) | 1888* |
| Group 1 (Low risk) [n (%)] | 0 (0.0) | 72 | 0 (0.0) | 72 | 1 (1.4) | 72 |
| Group 2 (Moderate risk) [n (%)] | 0 (0.0) | 82 | 0 (0.0) | 82 | 6 (7.4) | 81 # |
| Group 3 (High risk) [n (%)] | 4 (7.5) | 53 | 4 (7.5) | 53 | 6 (11.3) | 53 |
| Total | 7 (0.3) | 2097 | 9 (0.4) | 2097 | 26 (1.2) | 2097 |
| Sensitivity [% (95% CI)] |
Specificity [% (95% CI)] |
PPV [% (95% CI)] |
NPV [% (95% CI)] |
LR+ (95% CI) |
LR- (95% CI) |
|
|---|---|---|---|---|---|---|
| At 1 year | ||||||
| Group 1 to 3 | 57.1 (20.5-93.8) |
90.5 (89.2-91.7) |
2.0 (0.0-0.4) |
99.8 (99.7-100.0) |
6.0 (3.1-11.5) |
0.5 (0.2-1.1) |
| Group 2 to 3 | 93.9 (92.9-94.9) |
3.1 (1.1-6.0) |
99.9 (99.7-100.0) |
9.4 (4.8-18.2) |
||
| Group 3 | 97.8 (97.2-98.5) |
8.2 (0.5-15.8) |
26.5 (13.1-53.5) |
0.4 (0.2-1.0) |
||
| At 2 years | ||||||
| Group 1 to 3 | 44.4 (12.0-76.9) |
90.3 (89.0-91.6) |
1.9 (0.0-0.4) |
99.7 (99.5-100.0) |
4.6 (2.2-9.6) |
0.6 (0.3-1.1) |
| Group 2 to 3 | 93.7 (92.7-94.8) |
3.0 (0.0-0.6) |
99.8 (99.5-100.0) |
7.1 (3.3-15.0) |
||
| Group 3 | 97.7 (97.0-98.3) |
7.6 (0.0-14.7) |
18.9 (8.7-41.4) |
0.6 (0.3-1.0) |
||
| At 3 years | ||||||
| Group 1 to 3 | 50.0 (30.8-69.2) |
90.7 (89.4-91.9) |
6.3 (0.3-9.6) |
99.3 (98.9-99.7) |
5.4 (3.6-8.1) |
0.6 (0.4-0.8) |
| Group 2 to 3 | 46.2 (27.0-65.3) |
94.1 (93.1-95.1) |
9.0 (4.1-13.8) |
7.8 (5.0-12.3) |
||
| Group 3 | 23.1 (0.7-39.3) |
97.7 (97.1-98.4) |
11.3 (2.8-19.9) |
99.0 (98.6-99.5) |
10.2 (4.7-21.6) |
0.8 (0.6-1.0) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




