1. Introduction
Currently, the problem of new and returning infections is attracting increasing attention of researchers in terms of global conservation of biosecurity throughout the world. In Russia, foci of tick-borne encephalitis (TBE) and Lyme disease are widespread and have the greatest epidemiological significance. Along with them, pathogens of tick-borne rickettsiosis, human granulocytic anaplasmosis, and human monocytic ehrlichiosis are found in vectors [
1,
2,
3,
4]. The causative agents of these infections (Tick-borne encephalitis virus
, Borrelia spp., Anaplasma spp. Ehrlichia spp., Rickettsia) are also actively detected in natural foci of Primorsky Territory (southern Far East) [
5,
6]. The attention of researchers is aimed at identifying cases of mixed infection of people, as well as ticks, with pathogens of natural focal infections [
7,
8].
Contact between people and arthropods occurs mainly in the natural habitats of ixodid ticks, as well as in anthropurgic foci, including urban areas, suburban areas, summer cottages, places of public recreation and gathering of wild plants [
9]. Every year, about 7 thousand cases of tick bites are registered in the Primorsky Territory, the geography of referrals for bites and detection of pathogens covers most of the regions of Primorye. According to 2018 data, we registered cases of human bites by ticks
Ixodes persulcatus (
I. persulcatus) in 87.6%, ticks of the genera
Haemaphysalis and
Dermacentor - in 8.2% and 4.2% [
9]. The constant increase in the number of people in contact with natural foci makes the problem of tick-borne infections one of the most pressing for public health. Solving this problem requires an individual approach in laboratory verification of pathogens in ticks and in diagnosing tick-borne infections in people affected by a tick bite.
This is also due to the fact that targeted studies of ixodid ticks and people after their bites provide a lot of unexpected information. Thus, a new Haseki tick virus (HSTV) was detected in ixodid ticks and sick people in the Primorsky Territory and in the Asian part of Russia [
10]. Previously, a similar virus, BLTV 4, was first described in China [
11]. The complete HSTV polyprotein has been deciphered, and the genome structure suggests that the viral pathogens HSTV and BLTV 4 can be classified as new genera within
Flaviviridae [
10]. In addition, in the territories adjacent to the Primorsky Territory in China, and then in South Korea and Japan, severe fever with thrombocytopenia syndrome (SFTS), which occurs after a tick bite, was discovered [
12,
13,
14,
15]. The SFTS virus was found to belong to the
Phlebovirus genus group of the
Bunyaviridae family. These findings indicate the prospect of further discoveries of other new viruses in Ixodid ticks.
An example of this was our comprehensive studies of the half-fed tick
I. persulcatus, removed from a person in 2016, when, against the background of co-infection with the murine ectromelia virus (ECTV), we were able to verify the new virus Kiparis-144, later KiparisV [
16]. It was shown that when these two viruses are co-cultivated in the body of warm-blooded animals, the new KiparisV virus acts as an amplifier or “accelerator” of the replication of the mousepox virus, dramatically increasing its virulence. This identified feature of the relationship between these two viruses is reminiscent of the recently described symbiosis of two new satellite phage systems (Mulch and Flayer), which infect Streptomyces species, performing the functions of helpers [
17].
This study is aimed at solving the issues of indicating a new virus, called Kiparis-144 (KiparisV), in ticks and in humans, and establishing the features of its interaction during co-infection with other pathogens.
2. Materials and Methods
2.1. Human and Tick Samples
We isolated a new pathogen from an underfed tick, Ixodes persulcatus, taken from a patient on May 21, 2016 in the south of the Russian Far East (Nadezhdinsky district, Kiparisovo village, Primorsky Territory).
We analyzed 439 serum samples obtained from patients who reported tick bites in the Primorsky Territory (southern Far East, Russia). Samples were collected in 2018–2022. The study was conducted in accordance with the Declaration of Helsinki, written informed consent was obtained from all patients for medical intervention (Appendix No. 2 to the order of the Ministry of Health of Russia dated November 12, 2021 No. 1051n). Local rules for coding human blood samples were also followed. To conduct a study of patient blood samples to verify tick-borne infections, permission was obtained from the local ethics committee G.P. Somov Research Institute of Epidemiology and Microbiology Rospotrebnadzor (protocol No. 2 of November 16, 2021).
2.2. Enzyme Immunoassay (ELISA)
Determination of specific antibodies of class M and G against the causative agents of TBE and Lyme disease in blood serum was detected using ELISA kits - “VectoTBEV-IgM”, “VectoTBEV-IgG”, “LymeBest-IgM”, “LymeBest-IgG” (JSC «Vector – Best», Novosibirsk).
2.3. Real-Time PCR (qPCR)
The tick was examined for the presence of genetic markers, such as tick-borne encephalitis virus (TBE), Borrelia burgdorferi sensu lato, Anaplasma phagocytophilum, and Ehrlichia muris/Ehrlichia chaffeensis, by real-time polymerase chain reaction (qPCR) using the kit“AmpliSense TBEV, B. burgdorferi s.l., A. phagocytophilum, E. chaffeensis/E. muris-FL” (Central Research Institute of Epidemiology, Moscow, Russia), according to the manufacturer’s instructions on a cycler with fluorescent detection “ROTOR-GENE Q” (QIAGEN, Hilden, Germany).
2.4. Indirect Immunofluorescent Antibody (IFA) Method
The indirect IFA method was used to detect the antigen in Pig embryo kidney (PEK) culture cells infected with test samples in 3 tubes. Cells from these tubes were collected at certain times of the experiments, and then slides were prepared from the mixture of cells on objective glasses. The antigen of the virus was detected in the cells by applying specific immune serum to the slides and, subsequently, fluorescent immunoglobulins (FITS) in the working dilution specified in the manufacturer’s instructions (Branch “MEDGAMAL”, N.F. Gamaleya NIIEM). Slides were viewed in 3 fields on a fluorescent microscope (MC-200 TF A-1120 Vienna, Austria). The average percentage of cells with the content of the fluorescent virus antigen in relation to the total number of cells in the field of view was considered.
2.5. Electron Microscopy (EM)
Electron microscopic examination of viral samples was carried out using the method negative staining. The PEK cell line was infected with experimental samples and the supernatant was collected after 1 day. Viral particles were visualized in the supernatants. Supernatants from each sample (1 mL) were centrifuged at 3000× g for 20 min at 4◦C to remove cell debris. The clarified supernatants were then centrifuged at 13,000× g for 40 min at 4◦C. The pellets were resuspended in 10 µL PBS. Formvar carbon-coated copper grids were floated in droplets of virus suspension for 10 min and stained with 2% phosphotungstic acid for 1 min at room temperature. Subsequently, the grids were examined by transmission electronic microscopy [
18]. The samples were viewed using a JEM-100S transmission electron microscope (JEOL, Tokyo, Japan) at an accelerating voltage of 80 kV.
3. Results
3.1. Detection of Specific Antibodies to the KiparisV in Humans after Tick Bites
Initially, we were faced with the task of determining the threshold for test positivity in IFA. For this purpose, we examined 101 blood sera from people who reported a tick bite. We obtained comparative data on the number of individuals with antibodies in the short term (up to 5 days) and long term (more than 6 days) of blood sampling after tick bites (
Table 1).
The percentage of positive results in groups of people with an antibody titer of 1:40 ranged from 7.6 to 8.0, with an average of 7.9%. An antibody titer of 1:40 was conditionally adopted as a stable threshold for positivity in the IFA assay. Subsequently, we used this indicator to study the specific immune response to the new virus in people affected by a tick bite in the Primorsky Territory (the southern territory of the Russian Far East).
A total of 439 blood samples were examined.
Table 2 shows that the percentage of positive results in people with tick bites in the territories of Vladivostok, its suburbs, the adjacent Nadezhdinsky district, and the village of Kiparisovo ranged from 11.3 ± 4.3% to 14.5 ± 2.8%, geometric mean antibody titer (GMAT) - from 1:45 to 1:64. The immune response rate in other territories of the Primorsky Territory was higher - 23.6 ± 3.7%, GMAT - 1:74. The intensity of the immune response to the new virus in people of the Primorsky Territory averaged 16.4±1.7% (n=72), GMAT - 1:69.
The results of a serological study in IFA against KiparisV in the blood of persons affected by a tick bite showed that a significant part of them were attributable to antibody titers of 1:40 and 1:80 (45.5% and 41.6%). However, there are samples with high titers of 1:160 (8.3%; n=6) and even 1:320 - (4.1%; n=3). This means that cases of clinical manifestations of infection caused by the KiparisV cannot be excluded in humans.
3.2. Human Case
Mono-KiparisV human infections. The patient was hospitalized on June 6, 2018 in the infectious diseases department of the hospital with complaints of weakness and malaise, fever up to 38.8°C, chills, fever, body pain. From the anamnesis it is known that on 06/05/2018 he became acutely ill. From the epidemiological history it became known that there was no contact with infectious patients and no hypothermia; 2 weeks ago a tick was sucked on near the village of Kiparisovo, the tick was not examined.
On examination the condition is of moderate severity. Consciousness is clear. There are no meningeal symptoms. The skin is of normal color, there are no rashes, and no changes were detected at the site of the bite. There is no peripheral edema, the lymph nodes are not enlarged. The mucous membrane of the oropharynx is clearly hyperemic, the posterior wall of the pharynx is tuberous, the tonsils are not enlarged, there is no plaque on them, the tongue is moist and not coated. The chest is evenly involved in the act of breathing; the respiratory rate is 17 per minute. Pulse 85 beats per minute, blood pressure 110/80 mm. A clinical blood test revealed leukocytosis, neutrophilia, lymphocytopenia, and mild thrombocytopenia. When examining blood in Elisa, IgM and IgG antibodies to tick-borne pathogens (TBEV and Borrelia spp.) were not detected. A clinical diagnosis was established: Adenoviral infection, pharyngitis of moderate severity. After 6 days, the patient was discharged after recovery in satisfactory condition.
Taking into account the face of a tick bite near the village of Kiparisovo two weeks before the acute onset of the disease, the blood of this patient was additionally examined. Using the IFA method, antibodies to the antigen of the KiparisV virus were detected at a titer of 1:160, which allowed us to consider this case as an example of a mono-infection caused by this virus, which we had previously isolated from the I. persulcatus tick in the specified territory.
A case of co-infection. We give an example of another case of long-term observation of a patient after a tick bite on June 13, 2021 on the island. Russian (Vladivostok). The patient was observed for a long time by an infectious disease specialist for Lyme disease. Serological studies were carried out using ELISA to detect antibodies to tick-borne pathogens (TBEV and
Borrelia spp.) 11, 44, 97, 106, 222 and 358 days after the tick bite. In addition, these blood serums were examined at the IFA for the detection of antibodies to the KiparisV antigen (
Table 3).
Based on these results, there was a long-term, sustained detection of antibodies to B. burgdorfery spp. only IgM class, but not IgG, i.e. there was no switching of the immune response to the production of later IgG antibodies. Clinical symptoms of Lyme disease accompanied the patient during the observation period, i.e. there was no improvement in the patient's condition. In addition, during all periods of observation, we detected antibodies to the KiparisV in titers up to 1:80. The detection of these antibodies and antibodies to B. burgdorferi indicated a case of co-infection caused by Borrelia spp. and the KiparisV virus.
3.3. Electron Microscopy of Viral Co-Isolates
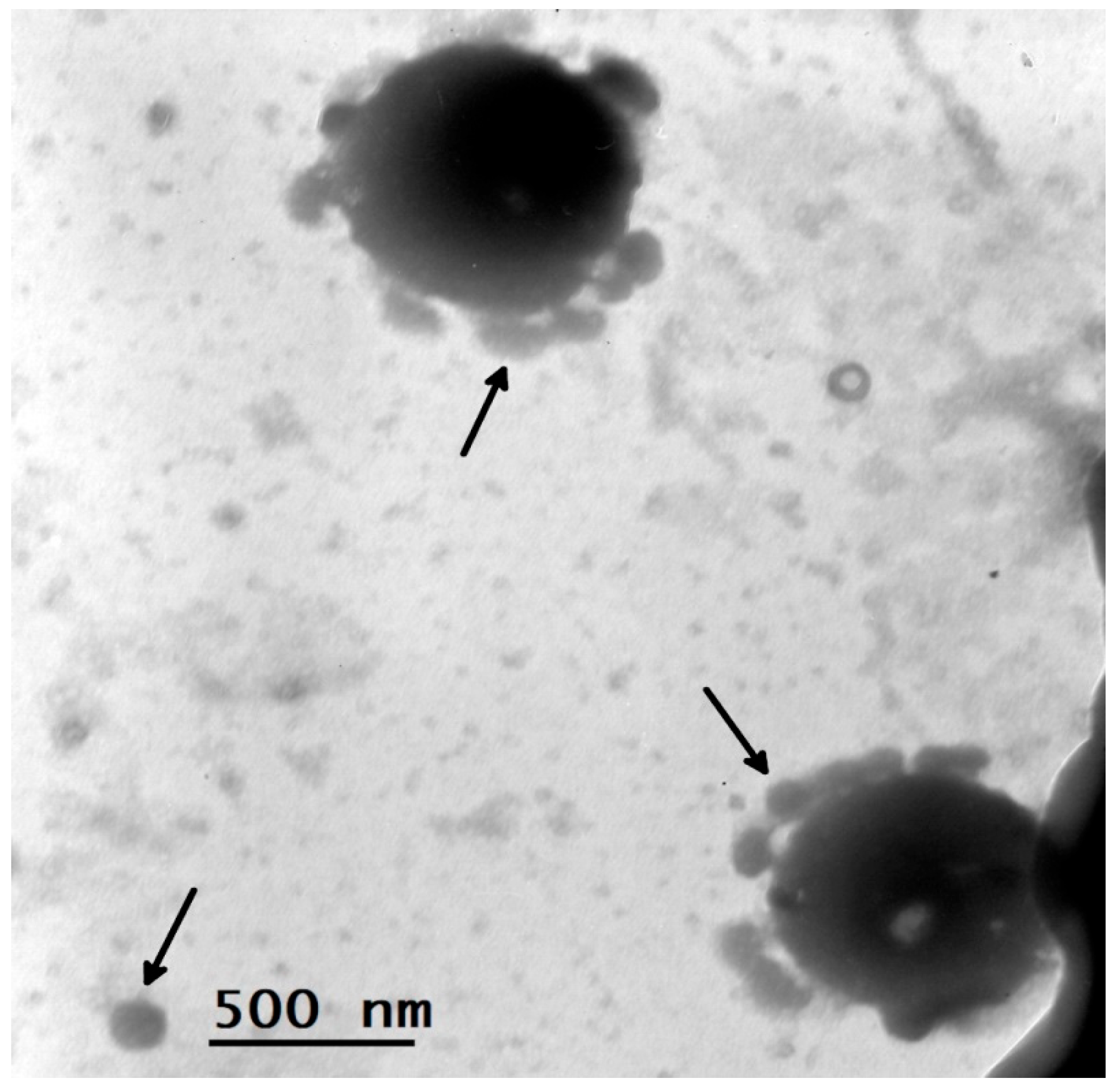
Additional evidence was obtained of the prevalence of the new virus in ixodid ticks, in which qPCR studies did not reveal genetic markers of infectious agents (Tick-borne encephalitis virus, Borrelia spp., Anaplasma spp. Ehrlichia spp., Rickettsia). We performed immunostaining (IFA) studies on KiparisV viral protein expression in PEK cells. 6 specimens of I. persulcatus attached to humans were studied, and an antigen to this virus was detected in two cases. A suspension of a tick with a high antigenic load (bright immunofluorescent glow at +4) was taken for electron microscopic examination.
Viral particles were visualized in cell culture supernatants using EM analysis.
Figure 1 shows large particles of different sizes from 1000 nm to 500 nm, around which the process of adhesion of viral particles about 100 nm in size is observed. Single or merged several copies of freely lying particles of the same small virus, up to 100 nm in size, were found.
We described a similar relationship between the new KiparisV virus and ectromelia virus (ECTV) in a previous article [
16]. To compare the electron microscopic picture of KiparisV and ECTV we present
Figure 2.
Shown here are dense conglomerates consisting of fused pox-viral particles with small virus particles up to 100 nm in size adhered to them. The electron microscopic picture demonstrated the phenomenon of co-cultivation of two viruses – the ECTV virus and the KiparisV virus, which we described previously [
16].
4. Discussion
The wide distribution of natural foci of combined infections transmitted by ixodid ticks of various species, which have common carriers of various pathogens, often causes the identification of several pathogens in one vector and the manifestation of mixed pathology in humans [
3,
4,
5,
7]. Cases of co-infection of the new Haseki tick virus (HSTV) with other tick-borne infections, such as double or triple infection with tick-borne encephalitis virus, bacteria
Borrelia spp.,
Anaplasma spp., have also been described in humans. [
10]. The identification of a new virus KiparisV [
16] in a half-fed
I persulcatus tick removed from a human dictated the need to study, first of all, its role in human pathology, as well as conducting research to determine the characteristics of its behavior in the tick microbiome.
According to the literature, mixed infection of ticks is a fairly common event and is recorded in 6.3–25.8% of cases [
8,
9]. The detected activation of the ECTV virus during co-cultivation with the KiparisV virus revealed an important feature - a sharp increase in the virulence and pathogenicity of the mouse virus [
16]. Previously, we drew attention to this feature of increased pathogenicity of individual pathogens in humans with the simultaneous detection of several pathogens of tick-borne infections after tick bites. Thus, back in 2005, during co-infection of people with the TBE virus and rickettsia, we noted an increase in clinical symptoms and an increase in laboratory indicators for rickettsiosis [
19]. In 2022, we described a case of triple co-infection (TBEV +
B. burgdorfery + SARS-Cov2), where we showed in the dynamics of observation for up to 2 years the predominance of not only the clinical symptoms of Lyme disease, but also the dominance of serological indicators for
Borrelia spp. [
20]. Due to the identification of high rates of infection of ticks with
Borrelia spp. compared to other pathogens, some authors consider the natural dominance of Lyme disease in combination with very mild forms of tick-borne encephalitis [
21]. It is probably the clinical manifestations of Lyme disease that force such patients to seek medical help, which contributes to the detection of inapparant forms of tick-borne encephalitis [
8].
The dominance of
Borrelia in the body of a tick simultaneously infected with the TBE virus and
B. burgdorferi was experimentally proven [
22]. According to this author [
22], in nature there is a gradual replacement of the tick-borne encephalitis virus by pathogens of a bacterial nature
(Borrelia spp.). It has been shown that in the body of a tick both pathogens are in an antagonistic relationship: ticks infected with
Borrelia spp. are either not susceptible to a highly virulent strain of TBEV administered parenterally, or the replication of the virus in their body is inhibited.
In the present study, we described a case of dual infection with KiparisV virus and
Borrelia spp., which we observed over the course of a year. In our opinion, under the influence of the KiparisV virus, there was also an increase in the activity of
Borrelia spp. with the constant production of antibodies to them only of the IgM class and clinical symptoms of Lyme disease. This manifestation is possible during competition between infectious agents, when one of the pathogens can also change the metabolism of the infected organism in the direction of enhancing immunity against another pathogen [
23].
Using the example of a mono-infection, it was shown that after a tick bite near the village of Kiparisovo, a patient developed clinical symptoms characteristic of acute respiratory diseases after 2 weeks. Antibodies to TBEV and Borrelia spp were not detected in ELISA, but antibodies to the KiparisV virus were detected in IFA at a titer of 1:160. This allowed us to interpret the described case as a mono-infection caused by a new virus. A study of blood sera from people affected by tick bites indicated a fairly frequent (up to 16.4±1.7%) encounter of people with the KiparisV virus in the territory of natural foci of the Primorsky Territory.
Thus, we have shown the widespread distribution of the new KiparisV virus in the Primorsky Territory, which is capable of causing clinical symptoms of acute respiratory infections in mono-infection, as well as long-term activation of Borrelia spp. in co-infection.
We were able to obtain a picture of such relationships between the KiparisV and other pathogens through electron microscopic studies (
Figure 1 and
Figure 2). The behavior of microorganisms in the tick microbiome was carried out at the early stages of virus reproduction, i.e. on the first day of its accumulation in the supernatant of infected PEK cells. Previously, we showed the features of the interaction of KiparisV with a well-known viral pathogen - ECTV, which has lipoprotein villi covering the surface of the outer shell of the virion [
16]. Apparently, the KiparisV, upon coinfection, can bind to the surface glycoproteins of the ECTV virus, contributing to a multiple increase in the virulence of ECTV (up to 10,000 times on the 5th passage). Moreover, here the dominant mouse ectromelia virus performed the function of an indicator for verification of the second virus - KiparisV. At the same time, researchers conducting molecular genetic studies of the tick virome are often unable to isolate new pathogens, probably because they do not have an indicator such as ECTV and permissive models for their isolation [
24,
25,
26].
Figure 1 shows a similar picture of the interaction of the KiparisV virus with unknown representatives of the tick microbiome - pathogens of a viral or bacterial nature. But the main thing here is that KiparisV probably uses other pathogens of the tick microbiome for its reproduction and at the same time can change their virulence.
Understanding the microbiome requires multifaceted research to describe the species, numbers, functions, and relationships of microorganisms in specific environments and their effects on the environment and hosts. Currently, in microbiology, the virosphere is considered as many copies of virus-like mobile genetic elements (MGE), and satellites are virus-like MGE, the replication of which depends on another virus [
17]. Thus, there are reports of viruses infecting bacterial hosts; several families of satellite nucleic acids, which are usually called phage satellites, have been described [
27]. This phenomenon of the formation of a pair of viruses, when many small viruses bind to the surface of a large pathogen, is not limited to the ECTV+KiparisV system shown by us. Co-cultivation of various pathogens is widespread, at least in the isolated tick ecosystem or in its microbiome.
The results of our study, although preliminary, emphasize the urgent need for a comprehensive study of the entire spectrum of potential symbiotic interactions of microorganisms in ticks. Based on these new data in microbiology and on the results we obtained, it can be assumed that the new KiparisV virus, when co-cultivated with other pathogens, performs the functions of a satellite, often increasing the pathogenicity of the main pathogen. Moreover, we can say that a unique relationship develops in the tick microbiome with the deliberate and non-random nature of the symbiotic relationships of different pathogens. Tagile de Carvalho et al. [
28] also believe that co-cultivation in a satellite-helper system can significantly increase the virulence of some infectious agents. The phenomenon we describe, where many small viruses bind to the surface of a large pathogen, differs from known virus-like mobile genetic elements (MGEs) and satellites, the replication of which depends on another virus [
17]. It can be assumed that this phenomenon of the formation of host-helper virus pairs is not limited to the ECTV+KiparisV system shown by us. Co-cultivation of various pathogens is widespread, at least in the isolated tick ecosystem or in its microbiome. Apparently, this is why in natural ecosystems, as a result of changes in the population gene pool, there is a threat of the constant emergence of new genetic clusters. These processes underlie the emergence of new and recurring infections, the solution of which represents current fundamental and applied problems [
29].