Submitted:
03 April 2024
Posted:
03 April 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Glass Synthesis
2.2. Glass Characterization
3. Results
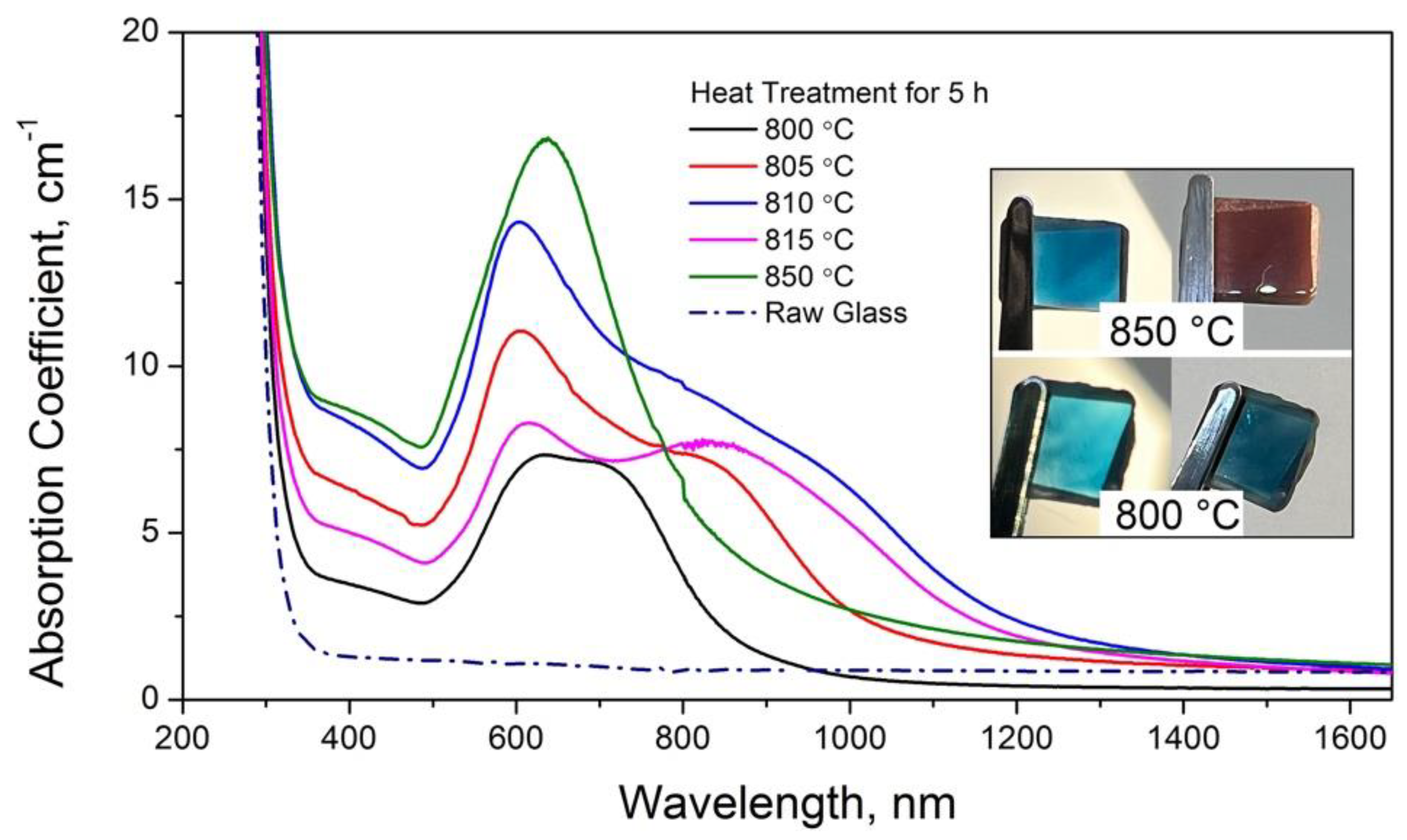
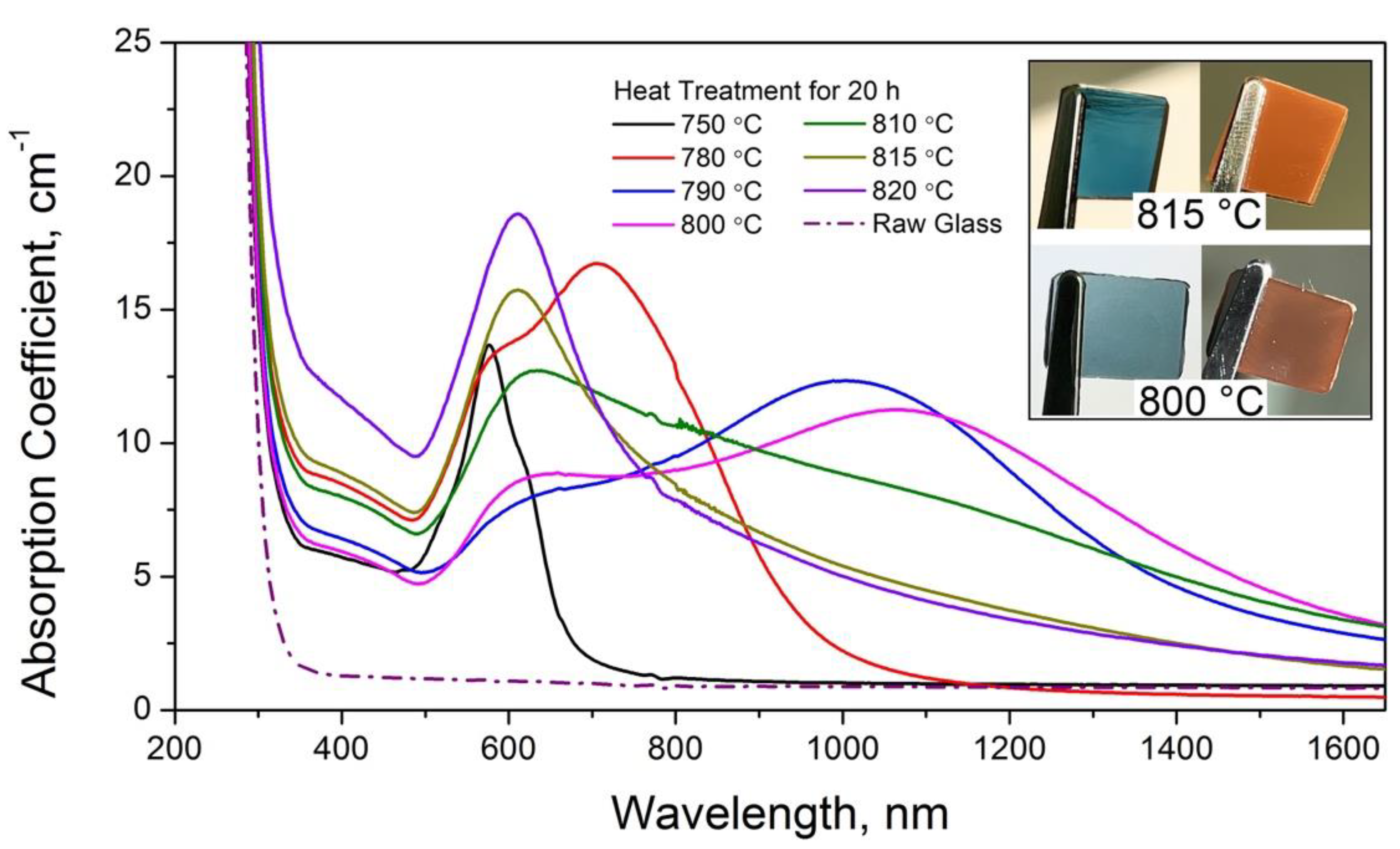
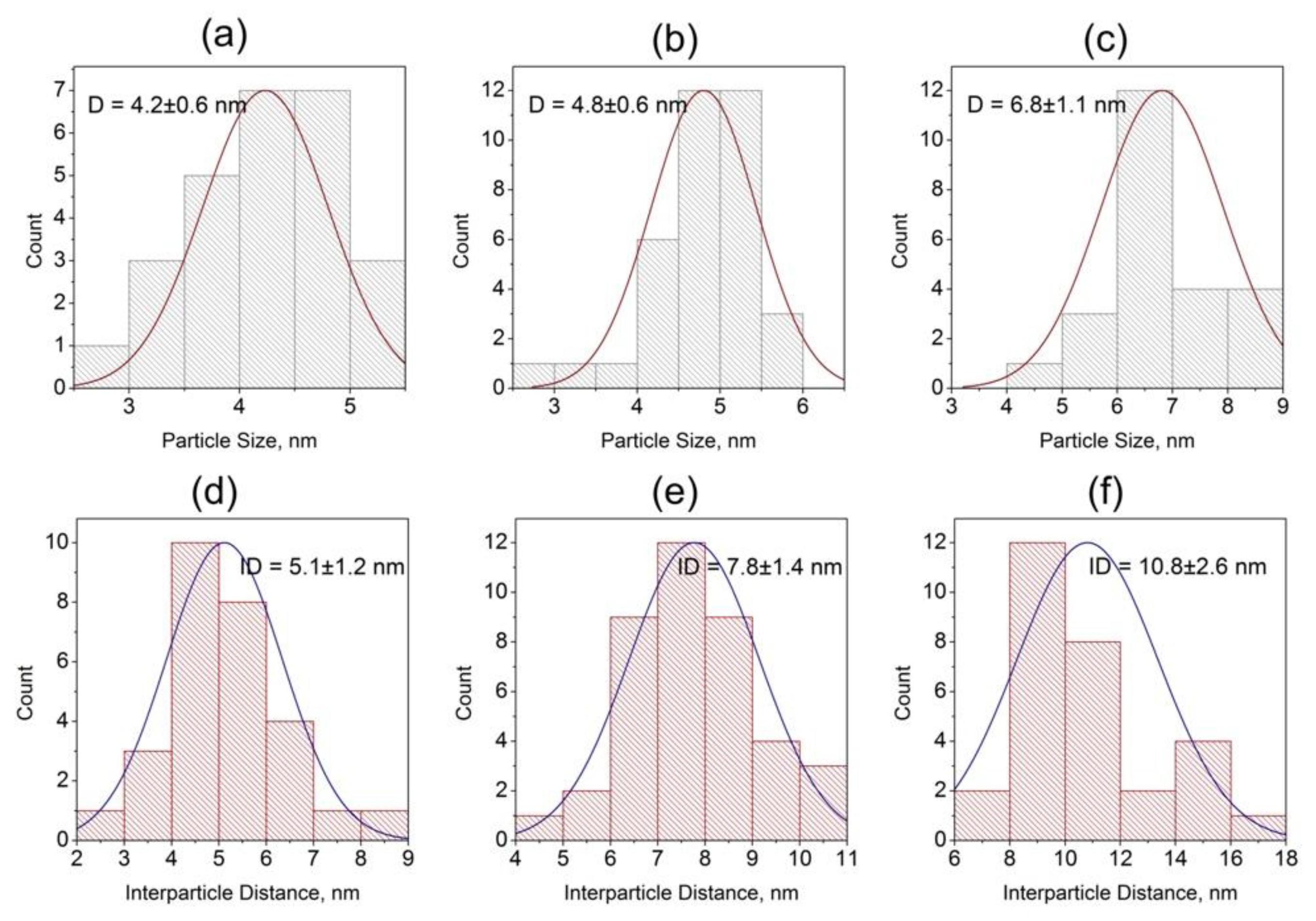
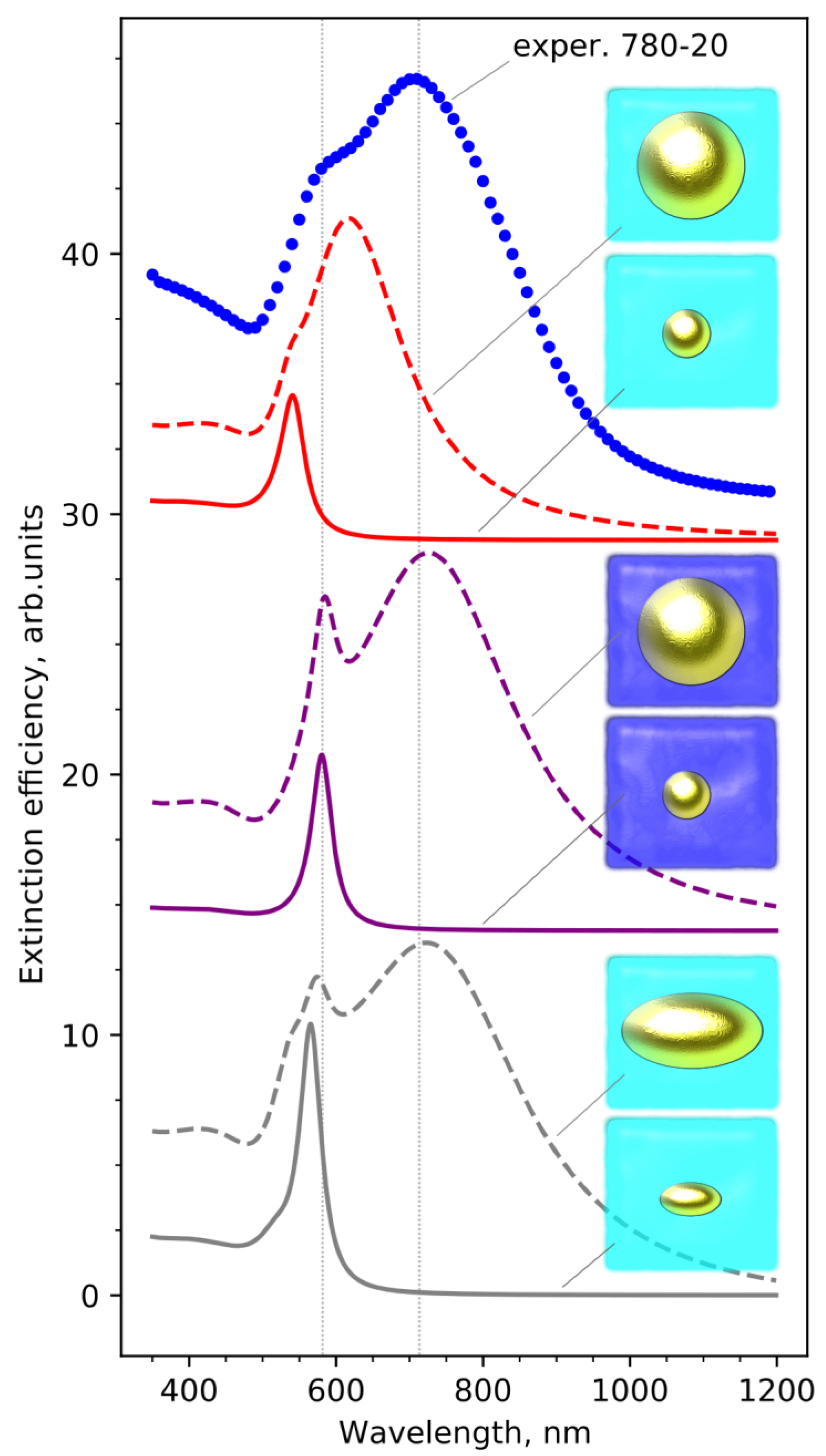
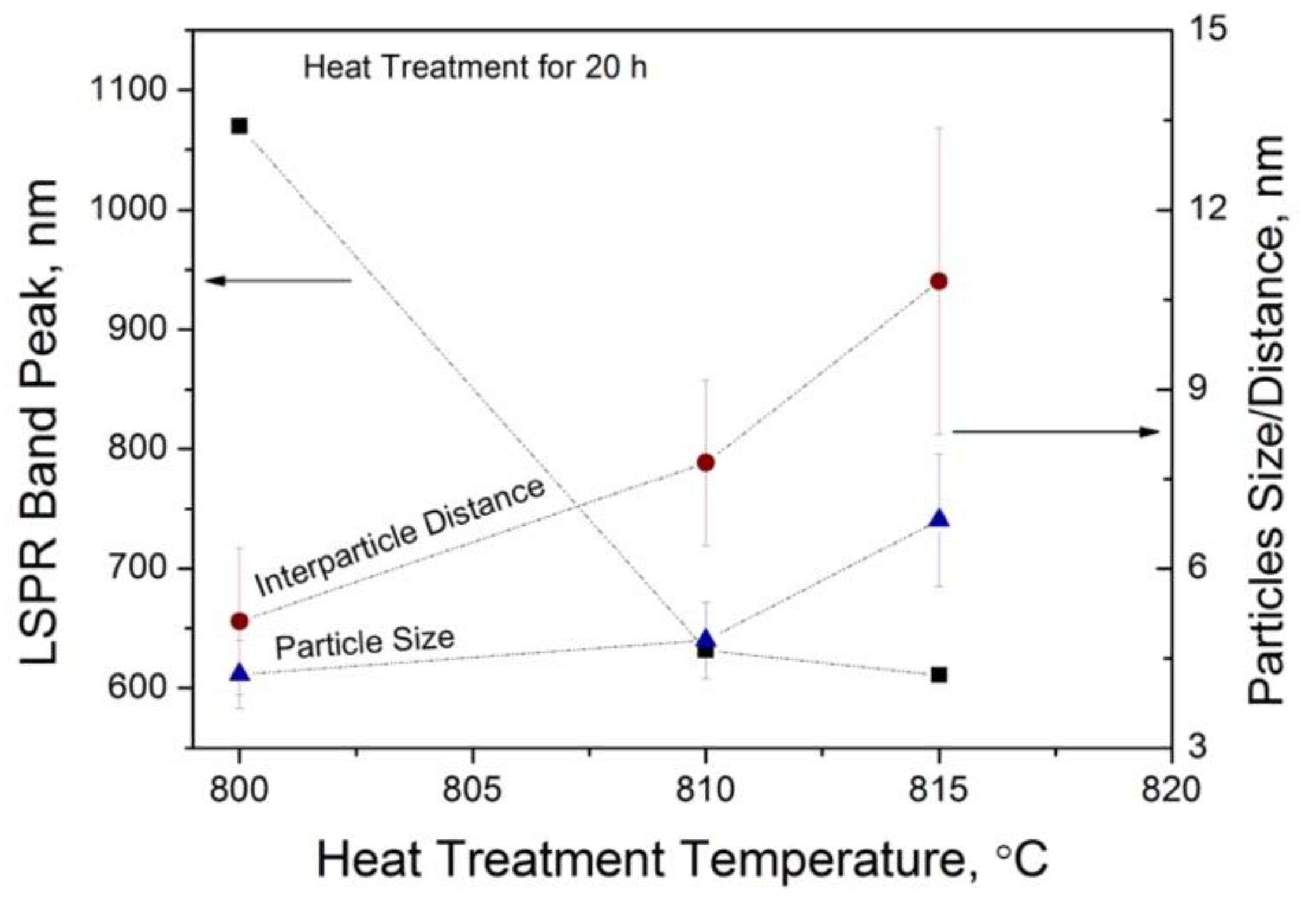
3.1. Optical Properties of Glasses upon Heat Treatment
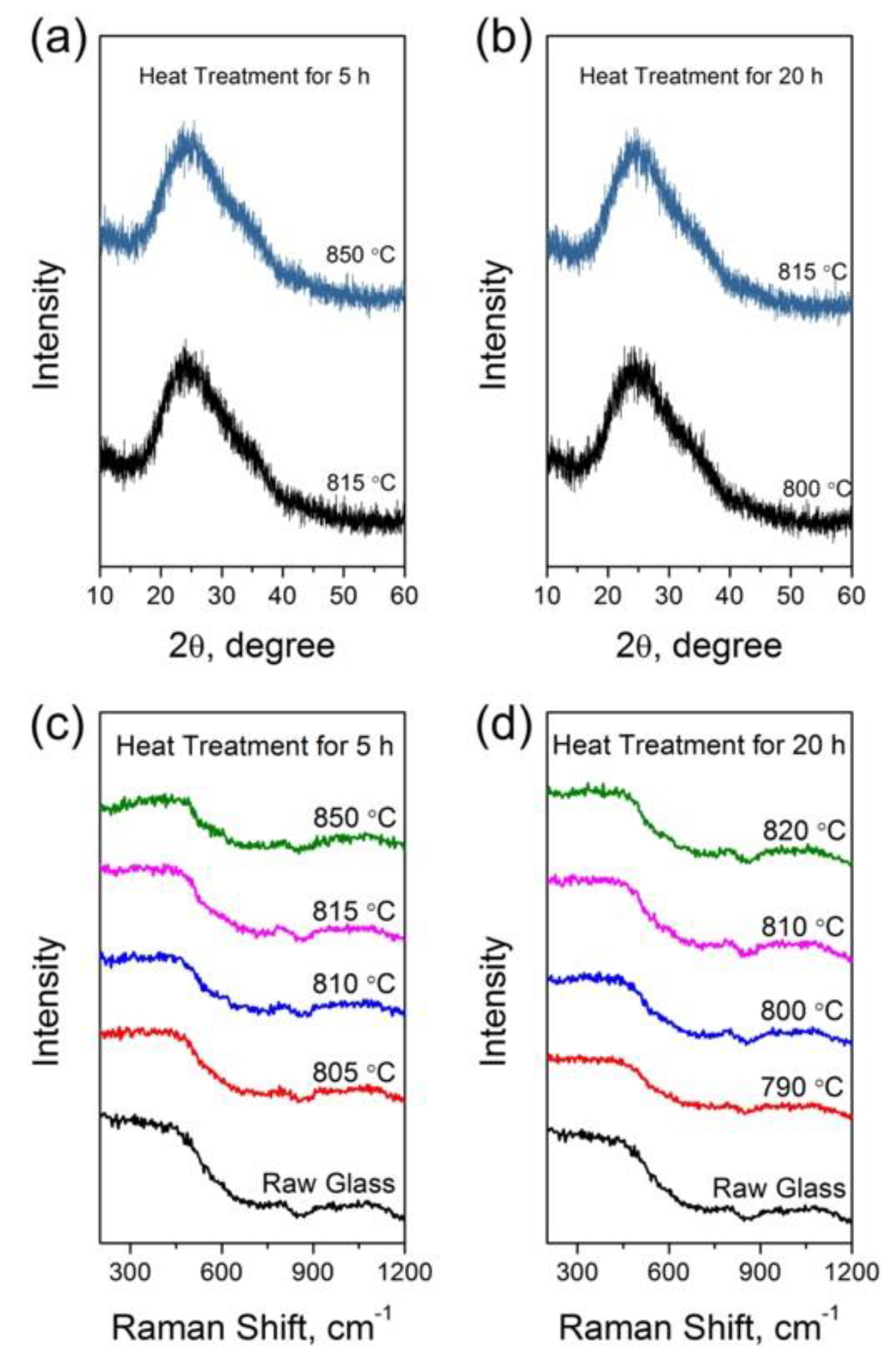
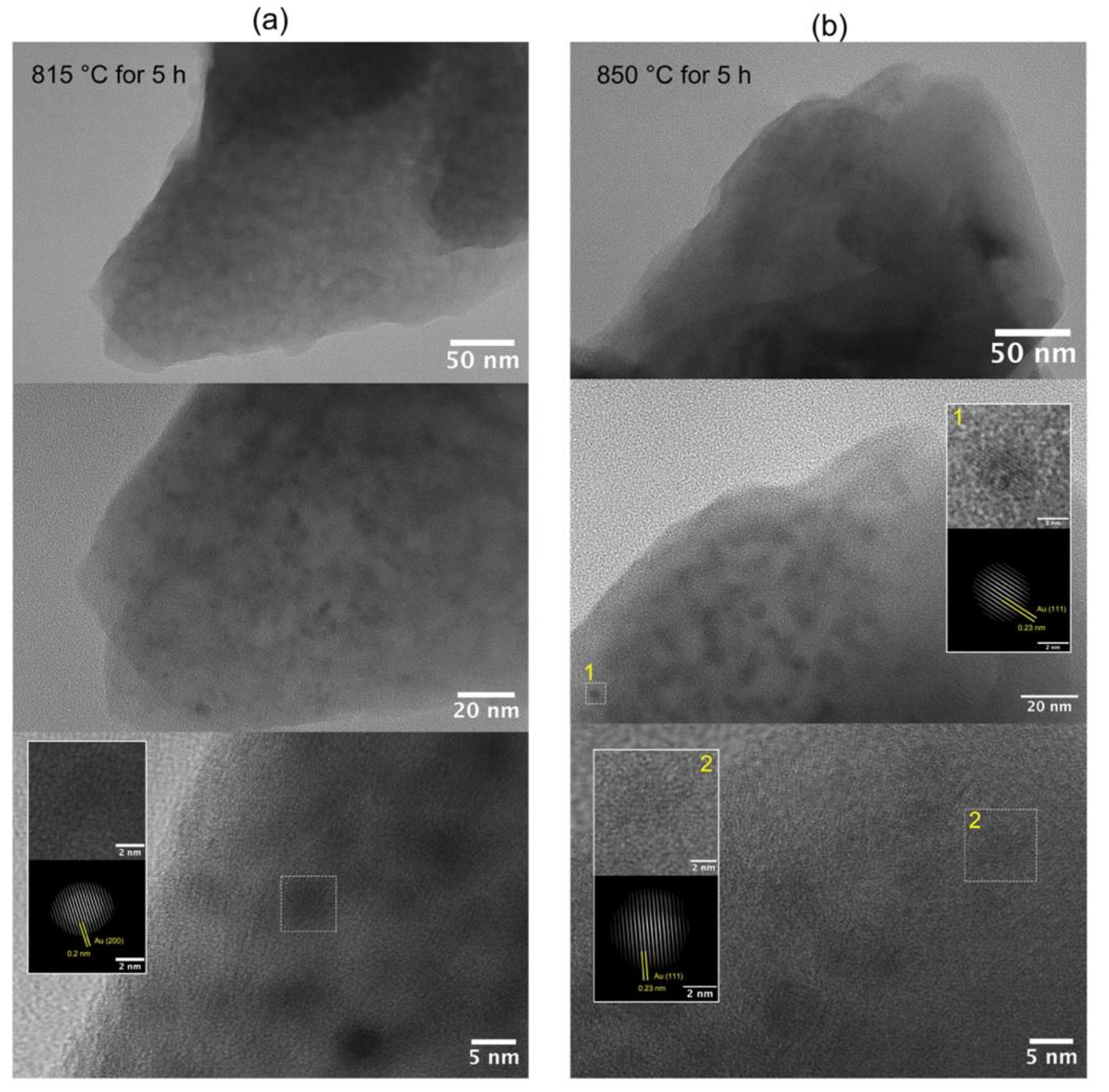
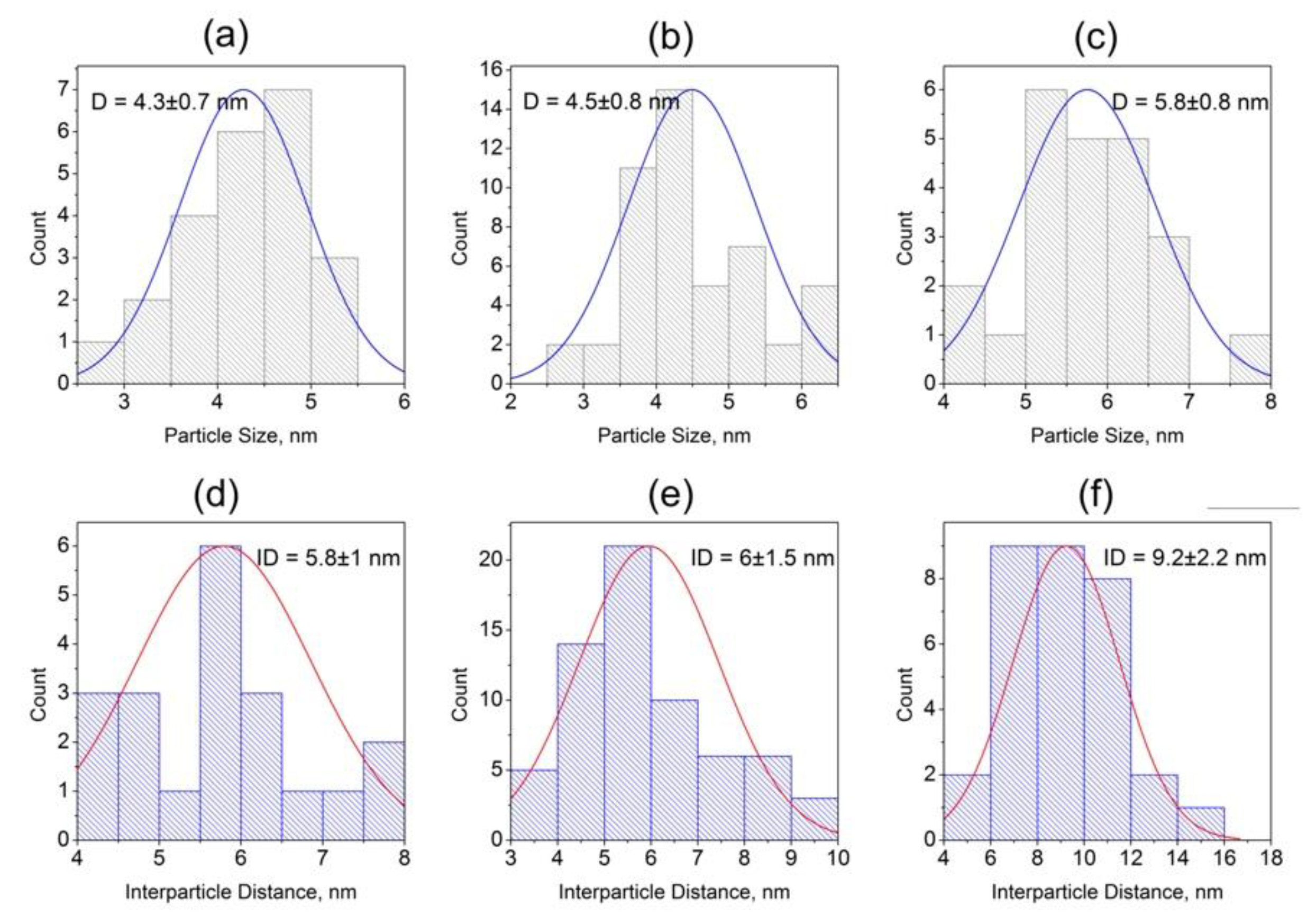
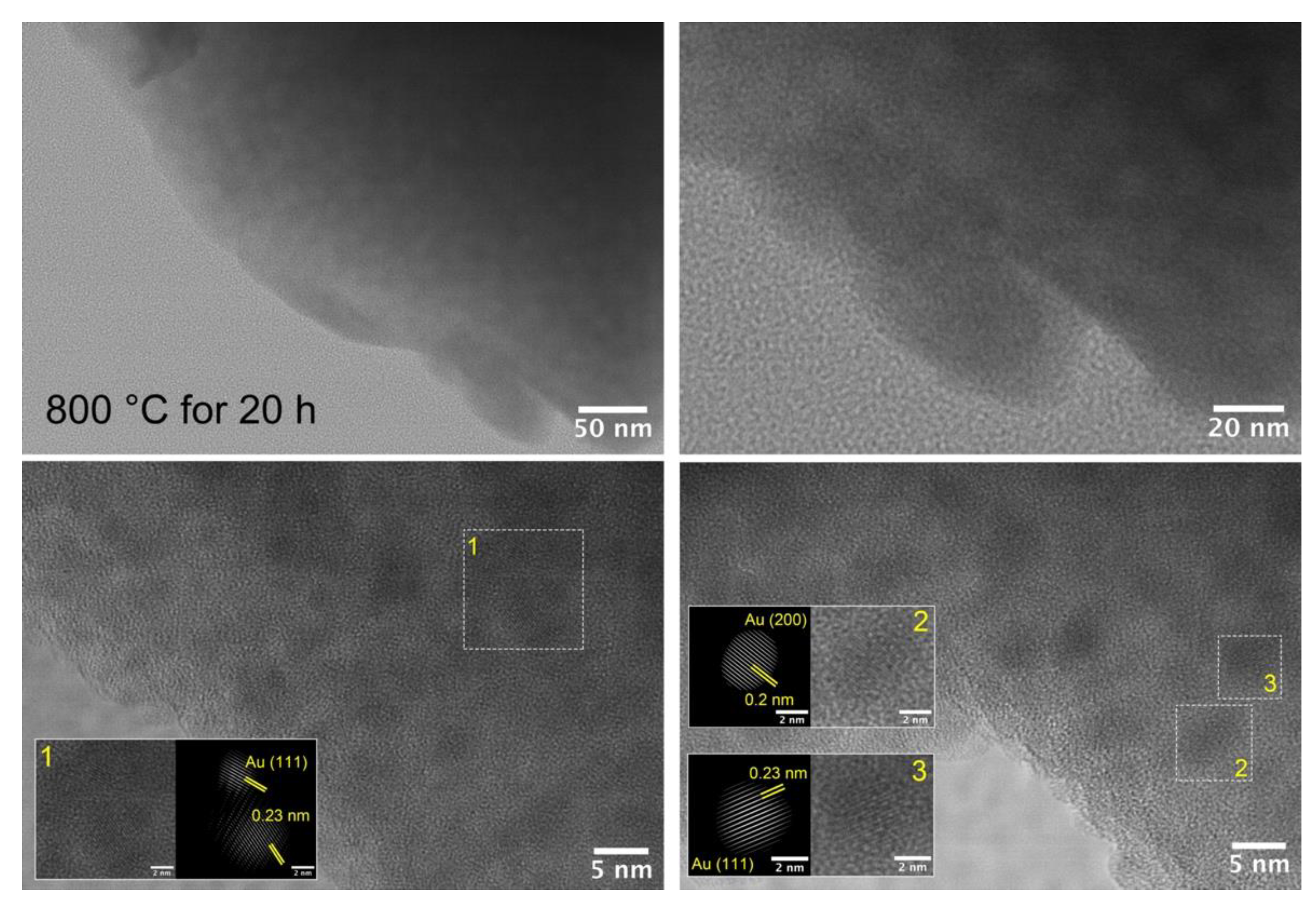
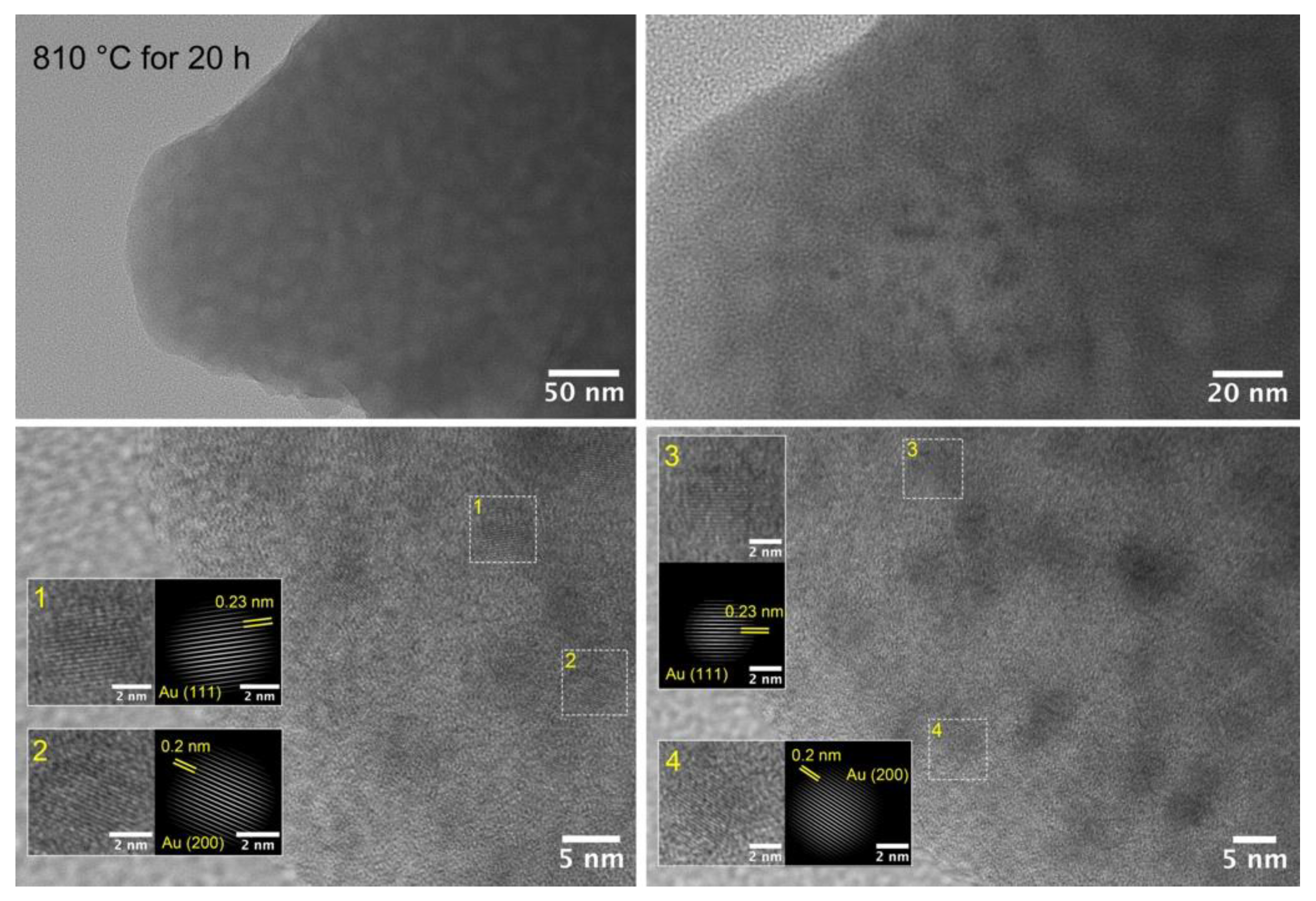
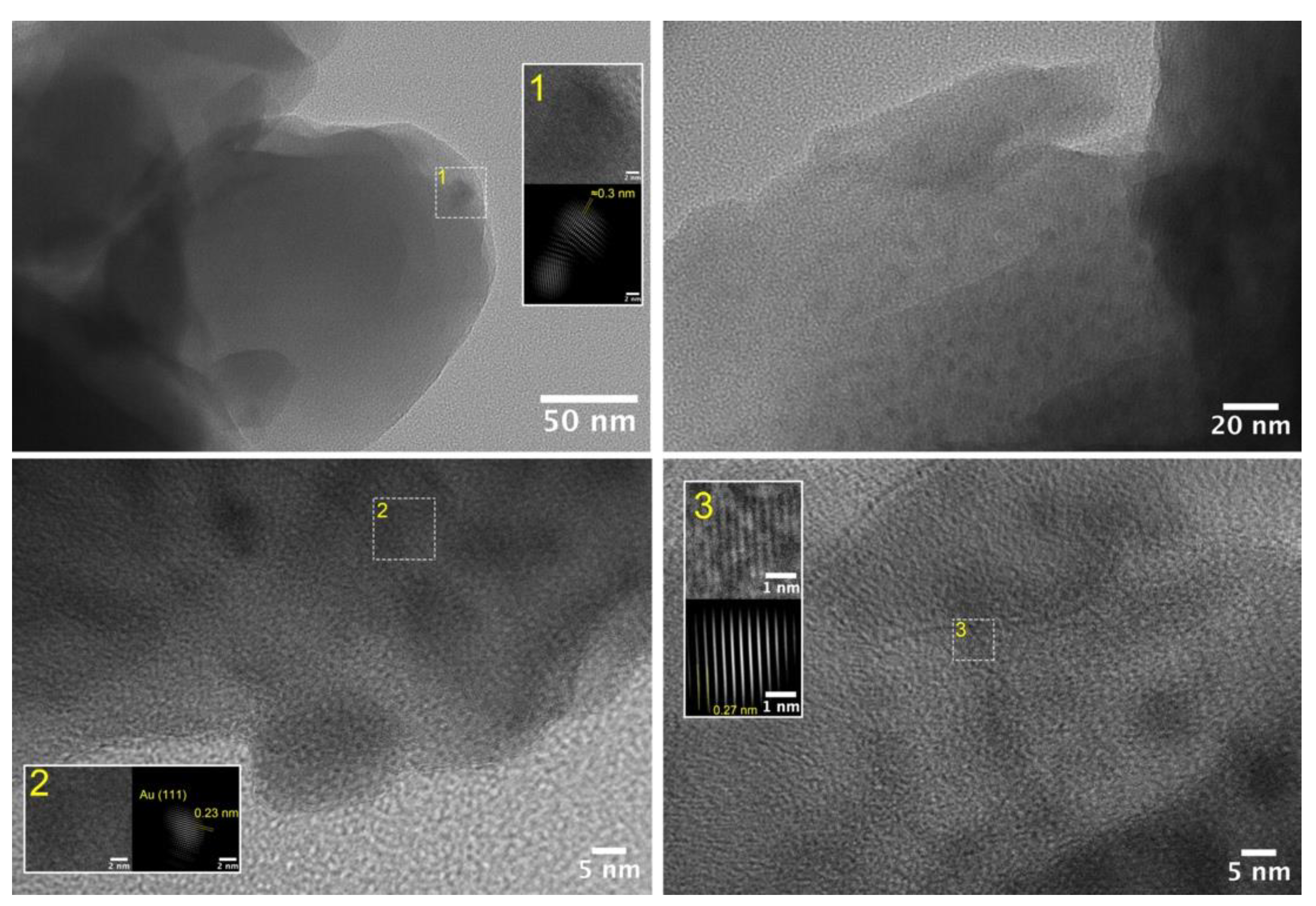
3.2. Structural Study of Glasses
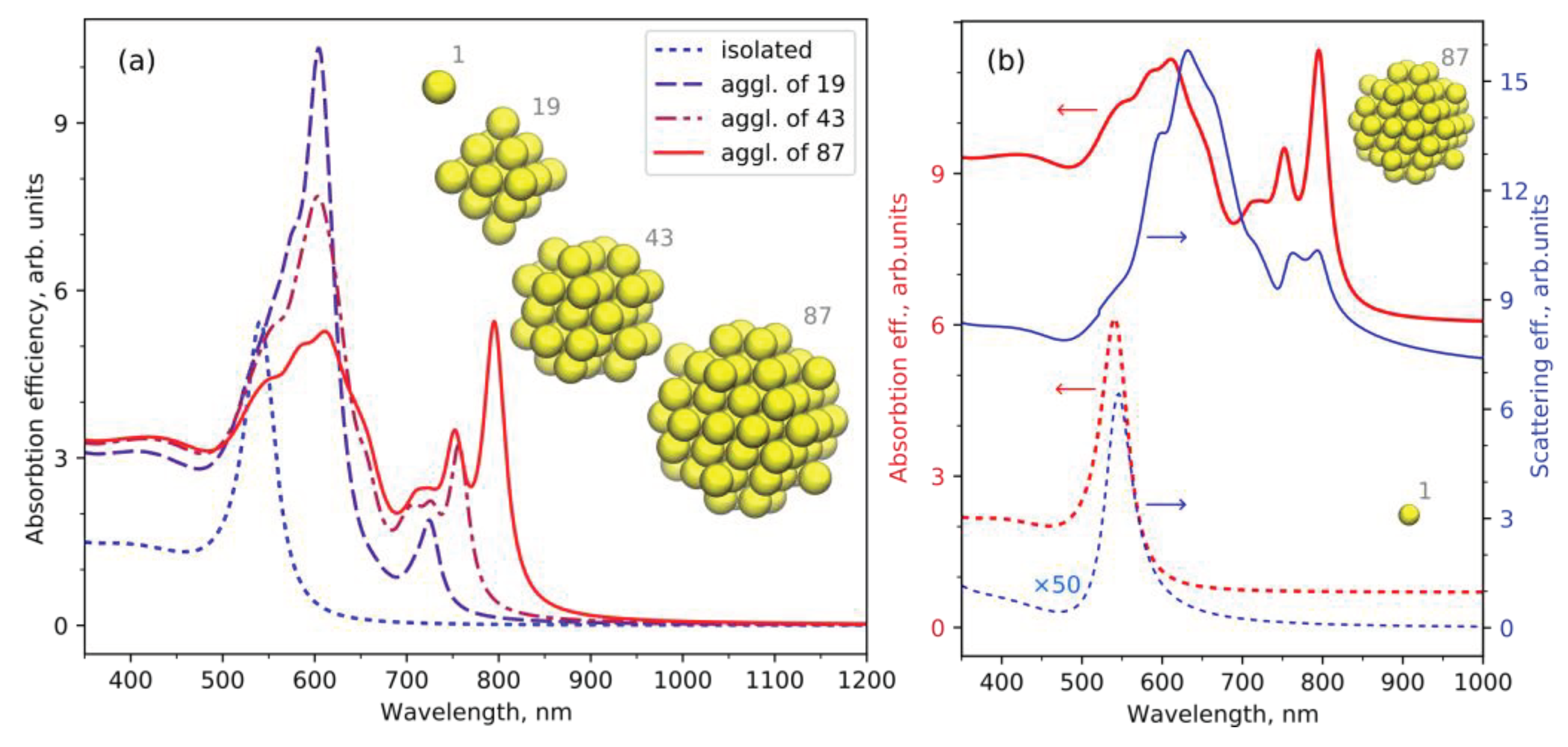
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Amendola, V. , Pilot, R., Frasconi, M., Maragò, O. and Iatì, M. Surface Plasmon Resonance in Gold Nanoparticles: A Review. Journal of Physics: Condensed Matter, 2017; 203002. [Google Scholar]
- Ferrari, E. Gold Nanoparticle-Based Plasmonic Biosensors. Biosensors (Basel) 2023, 13, 411. [Google Scholar] [CrossRef] [PubMed]
- Sarfraz, N.; Khan, I. Plasmonic Gold Nanoparticles (AuNPs): Properties, Synthesis and Their Advanced Energy, Environmental and Biomedical Applications. Chem Asian J 2021, 16, 720–742. [Google Scholar] [CrossRef] [PubMed]
- Shafiqa, A.R.; Abdul Aziz, A.; Mehrdel, B. Nanoparticle Optical Properties: Size Dependence of a Single Gold Spherical Nanoparticle. J Phys Conf Ser 2018, 1083, 012040. [Google Scholar] [CrossRef]
- Núñez-Leyva, J.M.; Kolosovas-Machuca, E.S.; Sánchez, J.; Guevara, E.; Cuadrado, A.; Alda, J.; González, F.J. Computational and Experimental Analysis of Gold Nanorods in Terms of Their Morphology: Spectral Absorption and Local Field Enhancement. Nanomaterials 2021, 11, 1696. [Google Scholar] [CrossRef] [PubMed]
- Djorović, A.; Oldenburg, S.J.; Grand, J.; Le Ru, E.C. Extinction-to-Absorption Ratio for Sensitive Determination of the Size and Dielectric Function of Gold Nanoparticles. ACS Nano 2020, 14, 17597–17605. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Peng, Y.; Yang, Y.; Li, Z.-Y. Plasmon-Enhanced Light–Matter Interactions and Applications. NPJ Comput Mater 2019, 5, 45. [Google Scholar] [CrossRef]
- Srabionyan, V.V.; Vetchinnikov, M.P.; Rubanik, D.S.; Durymanov, V.A.; Viklenko, I.A.; Avakyan, L.A.; Zinina, E.M.; Shakhgildyan, G.Yu.; Sigaev, V.N.; Bugaev, L.A. Local Electric Field Enhancement in the Vicinity of Aggregates of Ag, Au, Rb Containing Nanoparticles in Oxide Glasses. J Non Cryst Solids 2024, 631, 122927. [Google Scholar] [CrossRef]
- Shakhgildyan, G.; Lipatiev, A.; Lotarev, S.; Fedotov, S.; Sigaev, V. Glass: Home of the Periodic Table. Front Chem 2020, 8. [Google Scholar] [CrossRef] [PubMed]
- Shakhgil’dyan, G.Y.; Ziyatdinova, M.Z.; Kovgar, V.V.; Lotarev, S.V.; Sigaev, V.N.; Prusova, I.V. Effect of Gold Nanoparticles on the Spectral Luminescence Properties of Eu3+-Doped Phosphate Glass. Glass and Ceramics (English translation of Steklo i Keramika) 2019, 76. [Google Scholar] [CrossRef]
- Savinkov, V.I.; Shakhgil’Dyan, G.Y.; Paleari, A.; Sigaev, V.N. Synthesis of Optically Uniform Glasses Containing Gold Nanoparticles: Spectral and Nonlinear Optical Properties. Glass and Ceramics (English translation of Steklo i Keramika) 2013, 70, 143–148. [Google Scholar] [CrossRef]
- Som, T.; Karmakar, B. Plasmon Tuning of Nano-Au in Dichroic Devitrified Antimony Glass Nanocomposites by Refractive Index Control. Chem Phys Lett 2009, 479. [Google Scholar] [CrossRef]
- Chen, F.; Dai, S.; Xu, T.; Shen, X.; Lin, C.; Nie, Q.; Liu, C.; Heo, J. Surface-Plasmon Enhanced Ultrafast Third-Order Optical Nonlinearities in Ellipsoidal Gold Nanoparticles Embedded Bismuthate Glasses. Chem Phys Lett 2011, 514. [Google Scholar] [CrossRef]
- Wei, Y.; Zhao, J.; Fuhrmann, S.; Sajzew, R.; Wondraczek, L.; Ebendorff-Heidepriem, H. Controlled Formation of Gold Nanoparticles with Tunable Plasmonic Properties in Tellurite Glass. Light Sci Appl 2023, 12, 293. [Google Scholar] [CrossRef] [PubMed]
- Lipat’ev, G. Shakhgil’dyan, T. Lipat’eva, S. Lotarev, S. Fedotov, M. Vetchinnikov, E. Ignat’eva, N. Golubev, V.S. Formation of Luminescent and Birefringent Microregions in Phosphate Glass Containing Silver. Glass and Ceramics, 2016; 277–282. [Google Scholar]
- Srabionyan, V. V.; Heinz, M.; Kaptelinin, S.Y.; Avakyan, L.A.; Sukharina, G.B.; Skidanenko, A. V.; Pryadchenko, VasiliyV. ; Abdulvakhidov, K.G.; Mikheykin, AlexeyS.; Durymanov, VeniaminA.; et al. Effect of Thermal Post-Treatment on Surface Plasmon Resonance Characteristics of Gold Nanoparticles Formed in Glass by UV Laser Irradiation. J Alloys Compd 2019, 803, 354–363. [Google Scholar] [CrossRef]
- Heinz, M.; Srabionyan, V. V.; Avakyan, L.A.; Bugaev, A.L.; Skidanenko, A. V.; Pryadchenko, V. V.; Ihlemann, J.; Meinertz, J.; Patzig, C.; Dubiel, M.; et al. Formation and Implantation of Gold Nanoparticles by ArF-Excimer Laser Irradiation of Gold-Coated Float Glass. J Alloys Compd 2018, 736, 152–162. [Google Scholar] [CrossRef]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 Years of Image Analysis. Nat Methods 2012, 9, 671–675. [Google Scholar] [CrossRef] [PubMed]
- Martín-Yerga, D.; Yu, X.; Terekhina, I.; Henriksson, G.; Cornell, A. In Situ Catalyst Reactivation for Enhancing Alcohol Electro-Oxidation and Coupled Hydrogen Generation. Chemical Communications 2020, 56, 4011–4014. [Google Scholar] [CrossRef] [PubMed]
- Cormier, L.; Delbes, L.; Baptiste, B.; Montouillout, V. Vitrification, Crystallization Behavior and Structure of Zinc Aluminosilicate Glasses. J Non Cryst Solids 2021, 555, 120609. [Google Scholar] [CrossRef]
- Shakhgildyan, G.Yu.; Ziyatdinova, M.Z.; Vetchinnikov, M.P.; Lotarev, S.V.; Savinkov, V.I.; Presnyakova, N.N.; Lopatina, E.V.; Vilkovisky, G.A.; Sigaev, V.N. Thermally-Induced Precipitation of Gold Nanoparticles in Phosphate Glass: Effect on the Optical Properties of Er3+ Ions. J Non Cryst Solids 2020, 550, 120408. [Google Scholar] [CrossRef]
- Jiménez, J.A. Photoluminescence of Sm3+ Ions in Au-Doped Plasmonic and Dichroic Phosphate Glass. The European Physical Journal D 2023, 77, 124. [Google Scholar] [CrossRef]
- Jiménez, J.A. Dichroism in Plasmonic Cu Nanocomposite Glass: Selective Enhancement of the Orange-Red Emission from Sm3+. Optical Materials: X 2019, 1, 100002. [Google Scholar] [CrossRef]
- Singh, S.P.; Nath, M.; Karmakar, B. Quantum and Dielectric Confinements of Sub-10 Nm Gold in Dichroic Phosphate Glass Nanocomposites. Mater Chem Phys 2014, 146, 198–203. [Google Scholar] [CrossRef]
- Som, T.; Karmakar, B. Surface Plasmon Resonance and Enhanced Fluorescence Application of Single-Step Synthesized Elliptical Nano Gold-Embedded Antimony Glass Dichroic Nanocomposites. Plasmonics 2010, 5, 149–159. [Google Scholar] [CrossRef]
- Du, X.; Zhang, H.; Cheng, C.; Zhou, S.; Zhang, F.; Yu, Y.; Dong, G.; Qiu, J. Space-Selective Precipitation of ZnO Crystals in Glass by Using High Repetition Rate Femtosecond Laser Irradiation. Opt Express 2014, 22, 17908. [Google Scholar] [CrossRef]
- Montazerian, M.; Mancini, M.; Mauro, J.C. Advanced Tools for Unveiling Nucleation in Nanostructured Glass-Ceramics. Critical Reviews in Solid State and Materials Sciences 2023, 48, 411–439. [Google Scholar] [CrossRef]
- Jiazhi, L.; Ying, S.; Guanging, H. AN INVESTIGATION OF RELATIONSHIP BETWEEN PHASE SEPARATION AND CRYSTALLIZATION OF ZnO-Al2O3-SiO2 GLASSES. Le Journal de Physique Colloques 1982, 43, C9–231. [Google Scholar] [CrossRef]
- Pellerin, N.; Blondeau, J.-P.; Noui, S.; Allix, M.; Ory, S.; Veron, O.; De Sousa Meneses, D.; Massiot, D. Control of Selective Silicate Glass Coloration by Gold Metallic Nanoparticles: Structural Investigation, Growth Mechanisms, and Plasmon Resonance Modelization. Gold Bull 2013, 46. [Google Scholar] [CrossRef]
- Sigaev, V.N.; Savinkov, V.I.; Lotarev, S. V; Shakhgildyan, G.Y.; Lorenzi, R.; Paleari, A. Spatially Selective Au Nanoparticle Growth in Laser-Quality Glass Controlled by UV-Induced Phosphate-Chain Cross-Linkage. Nanotechnology 2013, 24, 225302–225302. [Google Scholar] [CrossRef] [PubMed]
- Simo, A.; Polte, J.; Pfänder, N.; Vainio, U.; Emmerling, F.; Rademann, K. Formation Mechanism of Silver Nanoparticles Stabilized in Glassy Matrices. J Am Chem Soc 2012, 134, 18824–18833. [Google Scholar] [CrossRef]
- Grand, J.; Adam, P.-M.; Grimault, A.-S.; Vial, A.; Lamy de la Chapelle, M.; Bijeon, J.-L.; Kostcheev, S.; Royer, P. Optical Extinction Spectroscopy of Oblate, Prolate and Ellipsoid Shaped Gold Nanoparticles: Experiments and Theory. Plasmonics 2006, 1, 135–140. [Google Scholar] [CrossRef]
- Pérez-Juste, J.; Pastoriza-Santos, I.; Liz-Marzán, L.M.; Mulvaney, P. Gold Nanorods: Synthesis, Characterization and Applications. Coord Chem Rev 2005, 249, 1870–1901. [Google Scholar] [CrossRef]
- Berger, A. Prolate Silver Particles in Glass Surfaces. J Non Cryst Solids 1993, 163, 185–194. [Google Scholar] [CrossRef]
- Mennig, M.; Berg, K.-J. Determination of Size Shape and Concentration of Spheroidal Silver Colloids Embedded in Glass by VIS-Spectroscopy. Materials Science and Engineering: B 1991, 9, 421–424. [Google Scholar] [CrossRef]
- Suszynska, M.; Krajczyk, L.; Capelletti, R.; Baraldi, A.; Berg, K.J. Microstructure and Silver Nanoparticles in Ion-Exchanged and Deformed Soda-Lime Silicate Glasses. J Non Cryst Solids 2003, 315, 114–123. [Google Scholar] [CrossRef]
- Stalmashonak, A.; Matyssek, C.; Kiriyenko, O.; Hergert, W.; Graener, H.; Seifert, G. Preparing Large-Aspect-Ratio Prolate Metal Nanoparticles in Glass by Simultaneous Femtosecond Multicolor Irradiation. Opt Lett 2010, 35, 1671. [Google Scholar] [CrossRef]
- Shakhgildyan, G.Yu.; Lipatiev, A.S.; Fedotov, S.S.; Vetchinnikov, M.P.; Lotarev, S.V.; Sigaev, V.N. Microstructure and Optical Properties of Tracks with Precipitated Silver Nanoparticles and Clusters Inscribed by the Laser Irradiation in Phosphate Glass. Ceram Int 2021, 47, 14320–14329. [Google Scholar] [CrossRef]
- Som, T.; Karmakar, B. Enhanced Frequency Upconversion of Sm3+ Ions by Elliptical Au Nanoparticles in Dichroic Sm3+: Au-Antimony Glass Nanocomposites. Spectrochim Acta A Mol Biomol Spectrosc 2010, 75, 640–646. [Google Scholar] [CrossRef]
- Mayer, K.M.; Hafner, J.H. Localized Surface Plasmon Resonance Sensors. Chem Rev 2011, 111, 3828–3857. [Google Scholar] [CrossRef]
- De, S.; Medda, S.K.; De, G. Refractive Index Controlled Plasmon Tuning of Au Nanoparticles in SiO 2 -ZrO 2 Film Matrices. J Nanosci Nanotechnol 2008, 8, 3868–3876. [Google Scholar] [CrossRef]
- De, G.; Medda, S.K.; De, S.; Pal, S. Metal Nanoparticle Doped Coloured Coatings on Glasses and Plastics through Tuning of Surface Plasmon Band Position. Bulletin of Materials Science 2008, 31, 479–485. [Google Scholar] [CrossRef]
- Shakhgildyan, G.; Durymanov, V.; Ziyatdinova, M.; Atroshchenko, G.; Golubev, N.; Trifonov, A.; Chereuta, O.; Avakyan, L.; Bugaev, L.; Sigaev, V. Effect of Gold Nanoparticles on the Crystallization and Optical Properties of Glass in ZnO-MgO-Al2O3-SiO2 System. Crystals (Basel) 2022, 12, 287. [Google Scholar] [CrossRef]
- Shakhgildyan, G.; Avakyan, L.; Ziyatdinova, M.; Atroshchenko, G.; Presnyakova, N.; Vetchinnikov, M.; Lipatiev, A.; Bugaev, L.; Sigaev, V. Tuning the Plasmon Resonance of Gold Nanoparticles in Phase-Separated Glass via the Local Refractive Index Change. J Non Cryst Solids 2021, 566, 120893. [Google Scholar] [CrossRef]
- Stelling, C.; Singh, C.R.; Karg, M.; König, T.A.F.; Thelakkat, M.; Retsch, M. Plasmonic Nanomeshes: Their Ambivalent Role as Transparent Electrodes in Organic Solar Cells. Sci Rep 2017, 7, 42530. [Google Scholar] [CrossRef]
- Aguilar, O.; de Castro, S.; Godoy, M.P.F.; Rebello Sousa Dias, M. Optoelectronic Characterization of Zn 1-x Cd x O Thin Films as an Alternative to Photonic Crystals in Organic Solar Cells. Opt Mater Express 2019, 9, 3638. [Google Scholar] [CrossRef]
- Ghosh, S.K.; Pal, T. Interparticle Coupling Effect on the Surface Plasmon Resonance of Gold Nanoparticles: From Theory to Applications. Chem Rev 2007, 107, 4797–4862. [Google Scholar] [CrossRef]
- Ung, T.; Liz-Marzán, L.M.; Mulvaney, P. Optical Properties of Thin Films of Au@SiO 2 Particles. J Phys Chem B 2001, 105, 3441–3452. [Google Scholar] [CrossRef]
- Nedyalkov, N.N.; Nakajima, Y.; Takami, A.; Koleva, M.; Karashanova, D.; Terakawa, M. Laser Induced Morphological and Optical Properties Changes in Au Doped Aluminum Oxide and Silicon Oxide Thin Films. Opt Laser Technol 2016, 79, 179–187. [Google Scholar] [CrossRef]
- Sendova, M.; Jiménez, J.A. Plasmonic Coupling in Silver Nanocomposite Glasses. The Journal of Physical Chemistry C 2012, 116, 17764–17772. [Google Scholar] [CrossRef]
- Mackowski, D.W.; Mishchenko, M.I. A Multiple Sphere T-Matrix Fortran Code for Use on Parallel Computer Clusters. J Quant Spectrosc Radiat Transf 2011, 112, 2182–2192. [Google Scholar] [CrossRef]
- Avakyan, L.A.; Heinz, M.; Skidanenko, A. V; Yablunovski, K.A.; Ihlemann, J.; Meinertz, J.; Patzig, C.; Dubiel, M.; Bugaev, L.A. Insight on Agglomerates of Gold Nanoparticles in Glass Based on Surface Plasmon Resonance Spectrum: Study by Multi-Spheres T-Matrix Method. Journal of Physics: Condensed Matter 2018, 30, 045901. [Google Scholar] [CrossRef]
- Rioux, D.; Vallières, S.; Besner, S.; Muñoz, P.; Mazur, E.; Meunier, M. An Analytic Model for the Dielectric Function of Au, Ag, and Their Alloys. Adv Opt Mater 2014, 2, 176–182. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




