Submitted:
02 April 2024
Posted:
03 April 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Experimental
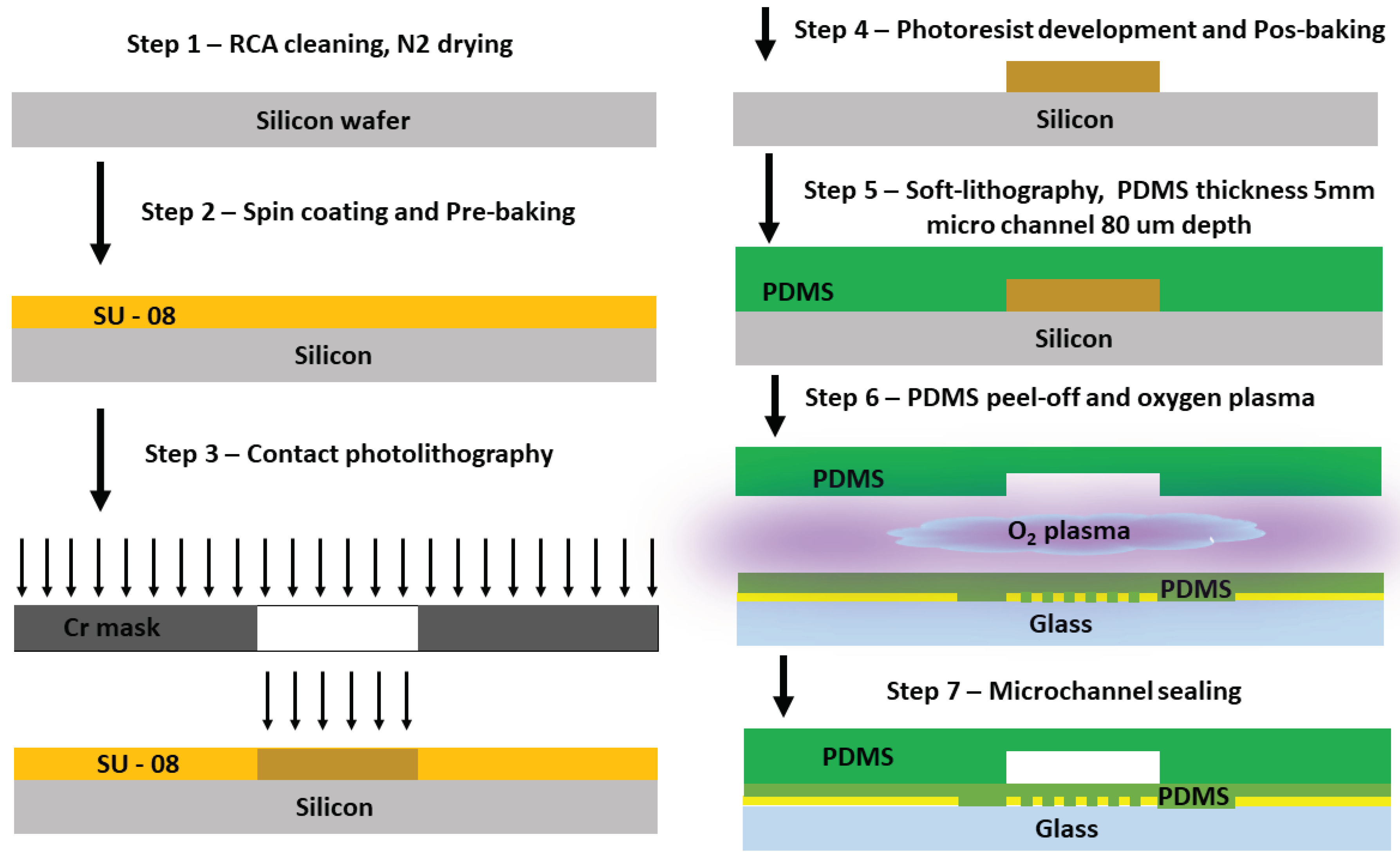
2.1. Microfluidic Channel Fabrication and Sensor Integration
2.2. Experimental Setup
3. Results and Discussion
Mechanism of Droplet Formation and Detection
4. Conclusion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Shembekar, N.; Hu, H.; Eustace, D.; Merten, C.A. Single-Cell Droplet Microfluidic Screening for Antibodies Specifically Binding to Target Cells. Cell Rep. 2018, 22, 2206–2215. [Google Scholar] [CrossRef]
- Ding, Y.; Howes, P.D.; deMello, A.J. Recent Advances in Droplet Microfluidics. Anal. Chem. 2020, 92, 132–149. [Google Scholar] [CrossRef]
- Shang, L.; Cheng, Y.; Zhao, Y. Emerging Droplet Microfluidics. Chem. Rev. 2017, 117, 7964–8040. [Google Scholar] [CrossRef]
- Huebner, A.; Sharma, S.; Srisa-Art, M.; Hollfelder, F.; Edel, J.B.; deMello, A.J. Microdroplets: A Sea of Applications? Lab. Chip 2008, 8, 1244. [Google Scholar] [CrossRef]
- Teh, S.-Y.; Lin, R.; Hung, L.-H.; Lee, A.P. Droplet Microfluidics. Lab. Chip 2008, 8, 198. [Google Scholar] [CrossRef]
- Atencia, J.; Beebe, D.J. Controlled Microfluidic Interfaces. Nature 2005, 437, 648–655. [Google Scholar] [CrossRef]
- Baroud, C.N.; Gallaire, F.; Dangla, R. Dynamics of Microfluidic Droplets. Lab. Chip 2010, 10, 2032. [Google Scholar] [CrossRef]
- Felton, H.; Hughes, R.; Diaz-Gaxiola, A. Negligible-Cost Microfluidic Device Fabrication Using 3D-Printed Interconnecting Channel Scaffolds. PLoS ONE 2021, 16, e0245206. [Google Scholar] [CrossRef]
- Zhang, J.; Xu, W.; Xu, F.; Lu, W.; Hu, L.; Zhou, J.; Zhang, C.; Jiang, Z. Microfluidic Droplet Formation in Co-Flow Devices Fabricated by Micro 3D Printing. J. Food Eng. 2021, 290, 110212. [Google Scholar] [CrossRef]
- Moiseeva, E.V.; Fletcher, A.A.; Harnett, C.K. Thin-Film Electrode Based Droplet Detection for Microfluidic Systems. Sens. Actuators B Chem. 2011, 155, 408–414. [Google Scholar] [CrossRef]
- Moraes Da Silva Junior, S.; Stiens, J.; Moshkalev, S.; Willibrordus Swart, J.; Lacerda De Orio, R.; Matvejev, V.; Zhang, Y.; Vandermeiren, W.; De Tandt, C. Microfluidic Devices on Glass for Liquid Mixtures Concentration with Coupled Thz Sensor. J. Integr. Circuits Syst. 2018, 13, 1–5. [Google Scholar] [CrossRef]
- Chen, C.; Zhao, Y.; Wang, J.; Zhu, P.; Tian, Y.; Xu, M.; Wang, L.; Huang, X. Passive Mixing inside Microdroplets. Micromachines 2018, 9, 160. [Google Scholar] [CrossRef]
- Whitesides, G.M. The Origins and the Future of Microfluidics. Nature 2006, 442, 368–373. [Google Scholar] [CrossRef]
- Nan, L.; Zhang, H.; Weitz, D.A.; Shum, H.C. Development and Future of Droplet Microfluidics. Lab. Chip, 2024. [Google Scholar] [CrossRef]
- Zhang, Z.; Kan, J.; Cheng, G.; Wang, H.; Jiang, Y. A Piezoelectric Micropump with an Integrated Sensor Based on Space-Division Multiplexing. Sens. Actuators Phys. 2013, 203, 29–36. [Google Scholar] [CrossRef]
- Liu, G.; Shen, C.; Yang, Z.; Cai, X.; Zhang, H. A Disposable Piezoelectric Micropump with High Performance for Closed-Loop Insulin Therapy System. Sens. Actuators Phys. 2010, 163, 291–296. [Google Scholar] [CrossRef]
- Li, L.; Ismagilov, R.F. Protein Crystallization Using Microfluidic Technologies Based on Valves, Droplets, and SlipChip. Annu. Rev. Biophys. 2010, 39, 139–158. [Google Scholar] [CrossRef]
- Belykh, S.S.; Yerin, C.V. Influence of the Microdroplets Sizes of Magnetic Emulsions on the Magneto-Optical Effect. Phys. Met. Metallogr. 2024. [Google Scholar] [CrossRef]
- Utharala, R.; Grab, A.; Vafaizadeh, V.; Peschke, N.; Ballinger, M.; Turei, D.; Tuechler, N.; Ma, W.; Ivanova, O.; Ortiz, A.G.; et al. A Microfluidic Braille Valve Platform for On-Demand Production, Combinatorial Screening and Sorting of Chemically Distinct Droplets. Nat. Protoc. 2022, 17, 2920–2965. [Google Scholar] [CrossRef]
- Xu, Z.; Wang, Y.; Sheng, K.; Rosenthal, R.; Liu, N.; Hua, X.; Zhang, T.; Chen, J.; Song, M.; Lv, Y.; et al. Droplet-Based High-Throughput Single Microbe RNA Sequencing by smRandom-Seq. Nat. Commun. 2023, 14, 5130. [Google Scholar] [CrossRef]
- Cerdeira, A.T.S.; Campos, J.B.L.M.; Miranda, J.M.; Araújo, J.D.P. Review on Microbubbles and Microdroplets Flowing through Microfluidic Geometrical Elements. Micromachines 2020, 11, 201. [Google Scholar] [CrossRef]
- Sonnen, K.F.; Merten, C.A. Microfluidics as an Emerging Precision Tool in Developmental Biology. Dev. Cell 2019, 48, 293–311. [Google Scholar] [CrossRef]
- Niu, X.; Zhang, M.; Peng, S.; Wen, W.; Sheng, P. Real-Time Detection, Control, and Sorting of Microfluidic Droplets. Biomicrofluidics 2007, 1, 044101. [Google Scholar] [CrossRef]
- Guo, M.T.; Rotem, A.; Heyman, J.A.; Weitz, D.A. Droplet Microfluidics for High-Throughput Biological Assays. Lab. Chip 2012, 12, 2146. [Google Scholar] [CrossRef]
- Xiao, Y.; Huang, Q.; Collins, J.W.; Brouchon, J.; Nelson, J.A.; Niziolek, Z.; O’Neil, A.; Ye, F.; Weitz, D.A.; Heyman, J.A. The Rapid Generation of Cell-Laden, FACS-Compatible Collagen Gels. Organoids 2023, 2, 204–217. [Google Scholar] [CrossRef]
- Chia, B.T.; Liao, H.-H.; Yang, Y.-J. A Novel Thermo-Pneumatic Peristaltic Micropump with Low Temperature Elevation on Working Fluid. Sens. Actuators Phys. 2011, 165, 86–93. [Google Scholar] [CrossRef]
- Scott, R.; Sethu, P.; Harnett, C.K. Three-Dimensional Hydrodynamic Focusing in a Microfluidic Coulter Counter. Rev. Sci. Instrum. 2008, 79, 046104. [Google Scholar] [CrossRef]
- Uhlen, M.; Quake, S.R. Sequential Sequencing by Synthesis and the Next-Generation Sequencing Revolution. Trends Biotechnol. 2023, 41, 1565–1572. [Google Scholar] [CrossRef]
- Vladisavljević, G.T.; Khalid, N.; Neves, M.A.; Kuroiwa, T.; Nakajima, M.; Uemura, K.; Ichikawa, S.; Kobayashi, I. Industrial Lab-on-a-Chip: Design, Applications and Scale-up for Drug Discovery and Delivery. Adv. Drug Deliv. Rev. 2013, 65, 1626–1663. [Google Scholar] [CrossRef]
- Nightingale, A.M.; Evans, G.W.H.; Xu, P.; Kim, B.J.; Hassan, S.; Niu, X. Phased Peristaltic Micropumping for Continuous Sampling and Hardcoded Droplet Generation. Lab. Chip 2017, 17, 1149–1157. [Google Scholar] [CrossRef]
- Nightingale, A.M.; Hassan, S.; Evans, G.W.H.; Coleman, S.M.; Niu, X. Nitrate Measurement in Droplet Flow: Gas-Mediated Crosstalk and Correction. Lab. Chip 2018, 18, 1903–1913. [Google Scholar] [CrossRef]
- Nightingale, A.M.; Hassan, S.; Makris, K.; Bhuiyan, W.T.; Harvey, T.J.; Niu, X. Easily Fabricated Monolithic Fluoropolymer Chips for Sensitive Long-Term Absorbance Measurement in Droplet Microfluidics. RSC Adv. 2020, 10, 30975–30981. [Google Scholar] [CrossRef]
- Da Silva Junior, S.M.; Stiens, J.; Moshkalev, S.; Swart, J.W.; Matvejev, V.; Zhang, Y.; De Tandt, C. Subterahertz Sensor in Microfluidic Devices for On-Line Determination and Control of Ethanol Concentration. J. Vac. Sci. Technol. B Nanotechnol. Microelectron. Mater. Process. Meas. Phenom. 2017, 35, 06GA02. [Google Scholar] [CrossRef]
- Nguyen, N.-T.; Lassemono, S.; Chollet, F.A. Optical Detection for Droplet Size Control in Microfluidic Droplet-Based Analysis Systems. Sens. Actuators B Chem. 2006, 117, 431–436. [Google Scholar] [CrossRef]
- Ribeiro, L.E.B.; de ALCÂNTARA, G.P.; Andrade, C.M.G.; Fruett, F. Analysis of the Planar Electrode Morphology Applied to Zeolite Based Chemical Sensors. Sens. Transducers 2015, 193, 80. [Google Scholar]
- Regiart, M.; Gimenez, A.M.; Lopes, A.T.; Carreño, M.N.P.; Bertotti, M. Ultrasensitive Microfluidic Electrochemical Immunosensor Based on Electrodeposited Nanoporous Gold for SOX-2 Determination. Anal. Chim. Acta 2020, 1127, 122–130. [Google Scholar] [CrossRef]
- Lee, G.; Lee, J.; Kim, J.; Choi, H.S.; Kim, J.; Lee, S.; Lee, H. Single Microfluidic Electrochemical Sensor System for Simultaneous Multi-Pulmonary Hypertension Biomarker Analyses. Sci. Rep. 2017, 7, 7545. [Google Scholar] [CrossRef]
- Ernst, A.; Streule, W.; Schmitt, N.; Zengerle, R.; Koltay, P. A Capacitive Sensor for Non-Contact Nanoliter Droplet Detection. Sens. Actuators Phys. 2009, 153, 57–63. [Google Scholar] [CrossRef]
- Kalantarifard, A.; Saateh, A.; Elbuken, C. Label-Free Sensing in Microdroplet-Based Microfluidic Systems. Chemosensors 2018, 6, 23. [Google Scholar] [CrossRef]
- Bento Ribeiro, L.E.; Piazzetta, M.H.; Gobbi, A.L.; Costa, J.S.; Fracassi Da Silva, J.A.; Fruett, F. Fabrication and Characterization of an Impedance Micro-Bridge for Lab-on-a-Chip. ECS Trans. 2010, 31, 155–163. [Google Scholar] [CrossRef]
- Micro Process Engineering: A Comprehensive Handbook; Wiley-VCH: Weinheim, 2009; ISBN 978-3-527-31550-5.






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




