Submitted:
03 April 2024
Posted:
03 April 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Study 1: Establishing Expert Consensus on Key Indicators of QoL in Colorectal Cancer Survivors
2.1. Materials and Methods
2.1.1. Identification of the Initial Pool of Quality of Life Domains and Subdomains
2.1.2. Evaluation of the Importance of (Sub)Domains
Participants
Questionnaire
Consensus Criterion and Analyses
2.2. Results
2.2.1. Round 1
2.1.2. Round 2
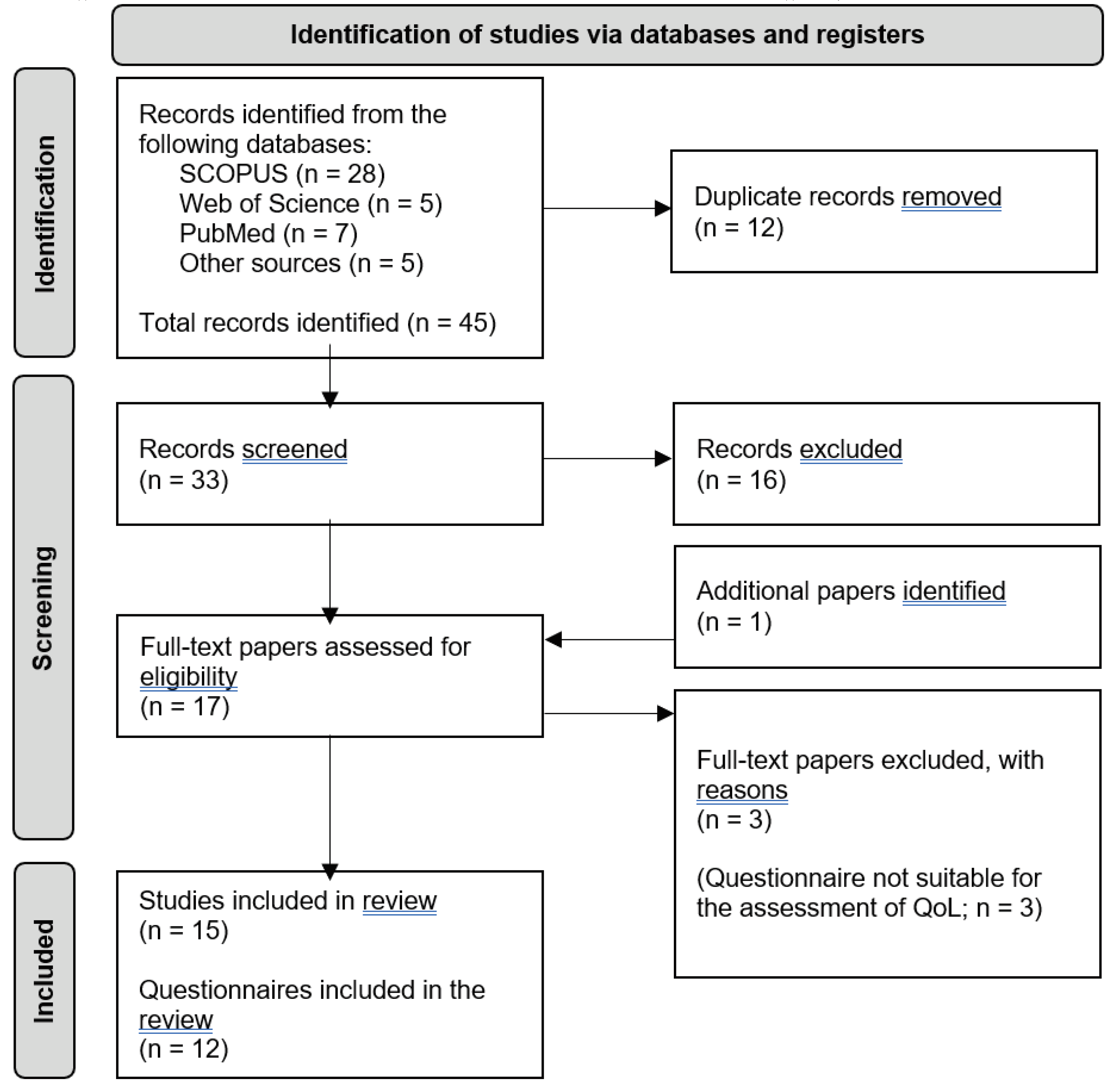
3. Study 2: Scoping Review of Quality of Life Questionnaires for Colorectal Cancer Survivors
3.1. Materials and Methods
3.1.1. Overview
3.1.2. Identifying the Research Questions
- -
- RQ2a: Which quality of life questionnaires for colorectal cancer survivors exist?
- -
- RQ2b: What domains of quality of life do they assess?
- -
- RQ2c: How do the identified domains of reviewed quality of life questionnaires overlap with the identified key indicators of quality of life by expert consensus?
3.1.3. Identifying Relevant Studies
3.1.4. Identifying Relevant Studies
3.1.5. Charting the Data
3.1.6. Collating, Summarizing, and Reporting Results
3.1.7. Consultation Exercises
3.2. Results
3.2.1. Characteristics of the Reviewed Questionnaires
3.2.2. Overview of the QoL Domains Assessed by Reviewed Questionnaires
4. Discussion
4.1. Limitations of the Study
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A: Supplemental Tables
| QoL domain | Consensus reached in Study 1 | ABCRC | EORTC QLQ-C30 | EORTC QLQ-CR38 | EORTC QLQ-CR29 | EORTC QLQ-ELD14 | FACT-C | mCOH-QOL-O | QLACS | QWLQ-CS | SF-36v2 | SF-12v2 | WHOQOL-BREF | ||
| a | b | a | b | ||||||||||||
| Physical Health and Well-being | * | X | X | X | X | X | X | X | |||||||
| Psychological Health and Well-being | * | X | X | X | |||||||||||
| Social Health and Well-being | X | X | X | ||||||||||||
| Spiritual Health and Well-being | X | X | |||||||||||||
| General QoL | X | X | X | ||||||||||||
| QoL areas assessed | |||||||||||||||
| … of all domain areas of Study 1 (4 + 1 general QoL)) | 2 | 1 | 2 | 3 | 5 | 1 | 1 | 3 | |||||||
| … of areas of consensus reached in Study 1 (2) | 1 | 1 | 2 | 2 | 1 | 1 | 2 | ||||||||
| QoL domain | Consensus reached in Study 1 | ABCRC | EORTC QLQ-C30 | EORTC QLQ-CR38 | EORTC QLQ-CR29 | EORTC QLQ-ELD14 | FACT-C | mCOH-QOL-O | QLACS | QWLQ-CS | SF-36v2 | SF-12v2 | WHOQOL-BREF | ||
| a | b | a | b | ||||||||||||
| Functional ability and Mobility | * | X | X | ||||||||||||
| Activities of daily living | * | X | |||||||||||||
| Fatigue/Vitality | * | X | X | X | X | X | |||||||||
| Sleep and rest | X | ||||||||||||||
| Pain and discomfort | * | X | X | X | X | X | |||||||||
| Abdominal pain | X | ||||||||||||||
| Buttock pain | X | ||||||||||||||
| Health perceptions | X | X | |||||||||||||
| Physical symptoms | * | X | |||||||||||||
| General gastrointestinal | X | X | |||||||||||||
| Hair loss | X | X | |||||||||||||
| Dry mouth | X | X | |||||||||||||
| Dyspnea | X | ||||||||||||||
| Taste problems | X | X | |||||||||||||
| Nausea, vomiting | X | ||||||||||||||
| Skin problems | X | X | |||||||||||||
| Urinary problems | X | ||||||||||||||
| Urinary frequency | X | ||||||||||||||
| Urinary incontinence | X | ||||||||||||||
| Painful urination | X | ||||||||||||||
| Stool frequency | X | ||||||||||||||
| Defecation problems | X | X | |||||||||||||
| Stool irregularities | X | ||||||||||||||
| Constipation | X | ||||||||||||||
| Diarrhea | X | ||||||||||||||
| Faecal incontinence | X | X | |||||||||||||
| Intoxications | X | ||||||||||||||
| Bloated abdomen | X | X | |||||||||||||
| Flatulence | X | ||||||||||||||
| Health distress | |||||||||||||||
| Weight loss/gain | X | X | |||||||||||||
| Physical Health and comorbidities | * | ||||||||||||||
| Other in physical domain | |||||||||||||||
| Appetite, nutrition | X | X | |||||||||||||
| Sexual enjoyment | X | ||||||||||||||
| Sexual functioning | X | ||||||||||||||
| Sexual problems | X | ||||||||||||||
| Sexual dysfunction, impotence | X | X | X | ||||||||||||
| Sexual interest | X | X | |||||||||||||
| Dysapreunia | X | X | |||||||||||||
| Disease-specific effects on physical well-being | X | ||||||||||||||
| Change in health | X | ||||||||||||||
| QoL areas assessed | |||||||||||||||
| … of all domain areas of Study 1 (10) | 4 | 3 | 1 | 2 | 2 | 1 | 2 | 3 | 3 | ||||||
| … of areas of consensus reached in Study 1 (6) | 4 | 2 | 1 | 1 | 1 | 2 | 2 | 2 | |||||||
| … of ‘Other’ domain areas (9) | 1 | 1 | 3 | 3 | 3 | 1 | 1 | 1 | |||||||
| QoL domain | Consensus reached in Study 1 | ABCRC | EORTC QLQ-C30 | EORTC QLQ-CR38 | EORTC QLQ-CR29 | EORTC QLQ-ELD14 | FACT-C | mCOH-QOL-O | QLACS | QWLQ-CS | SF-36v2 | SF-12v2 | WHOQOL-BREF | ||
| a | b | a | b | ||||||||||||
| Anxiety | X | X | |||||||||||||
| Depression | * | ||||||||||||||
| Psychological distress | * | X | |||||||||||||
| Cognitive functioning, concentration and attention | X | X | X | ||||||||||||
| Uncertainty | X | ||||||||||||||
| Fear of Recurrence | X | ||||||||||||||
| Isolation/Abandonment and feelings of belonging | |||||||||||||||
| Positive feelings and affect | X | ||||||||||||||
| Negative feelings and affect | X | ||||||||||||||
| Loss of interest in usual activities | * | ||||||||||||||
| Other in psychological domain | |||||||||||||||
| General mental health | X | X | |||||||||||||
| Emotional functioning and well-being | X | X | X | ||||||||||||
| Disease-specific effects on psychological well-being | X | ||||||||||||||
| QoL areas assessed | |||||||||||||||
| … of all domain areas of Study 1 (10) | 1 | 1 | 1 | 1 | 1 | 1 | 4 | ||||||||
| … of areas of consensus reached in Study 1 (3) | 1 | ||||||||||||||
| … of ‘Other’ domain areas (3) | 1 | 1 | 1 | 1 | 1 | 1 | |||||||||
| QoL domain | Consensus reached in Study 1 | ABCRC | EORTC QLQ-C30 | EORTC QLQ-CR38 | EORTC QLQ-CR29 | EORTC QLQ-ELD14 | FACT-C | mCOH-QOL-O | QLACS | QWLQ-CS | SF-36v2 | SF-12v2 | WHOQOL-BREF | ||
| a | b | a | b | ||||||||||||
| Family functioning | X | X | |||||||||||||
| Martial functioning | X | ||||||||||||||
| Affection/Sexuality | |||||||||||||||
| Self Concept/Appearance | X | X | X | X | |||||||||||
| Enjoyment/Leisure (participation and opportunities) | |||||||||||||||
| Social activity and limitations | X | ||||||||||||||
| Financial Concerns | X | X | X | ||||||||||||
| Social Support | |||||||||||||||
| Employment | X | X | |||||||||||||
| … general feelings about working life | X | ||||||||||||||
| … job characteristics | X | ||||||||||||||
| … social structure and culture | X | ||||||||||||||
| … contact with supervisor | X | ||||||||||||||
| … contact with other actors at work | X | ||||||||||||||
| … organisational characteristics | X | ||||||||||||||
| … work perceptions | X | ||||||||||||||
| … effects of the disease and treatment (on work) | X | ||||||||||||||
| Role limitations due to health or psychical problems | X | ||||||||||||||
| … due to physical problems | X | X | |||||||||||||
| … due to emotional problems | X | X | |||||||||||||
| Other in social domain | |||||||||||||||
| Social adjustment to ostomy | X | ||||||||||||||
| Social functioning | X | X | X | ||||||||||||
| QoL areas assessed | |||||||||||||||
| … of domain areas of Study 1 (10) | 4 | 2 | 1 | 1 | 1 | 4 | 1 | 1 | 1 | ||||||
| … of ‘Other’ domain areas (2) | 1 | 1 | 8 | 1 | 1 | ||||||||||
| QoL domain | Consensus reached in Study 1 | ABCRC | EORTC QLQ-C30 | EORTC QLQ-CR38 | EORTC QLQ-CR29 | EORTC QLQ-ELD14 | FACT-C | mCOH-QOL-O | QLACS | QWLQ-CS | SF-36v2 | SF-12v2 | WHOQOL-BREF | ||
| a | b | a | b | ||||||||||||
| SPIRITUAL QOL | |||||||||||||||
| Meaning of Illness | |||||||||||||||
| Religiosity | |||||||||||||||
| Hope | |||||||||||||||
| Transcendence | |||||||||||||||
| Inner Strength | |||||||||||||||
| QoL areas assessed | |||||||||||||||
| … of domain areas of Study 1 (5) | |||||||||||||||
| QoL domain | Consensus reached in Study 1 | ABCRC | EORTC QLQ-C30 | EORTC QLQ-CR38 | EORTC QLQ-CR29 | EORTC QLQ-ELD14 | FACT-C | mCOH-QOL-O | QLACS | QWLQ-CS | SF-36v2 | SF-12v2 | WHOQOL-BREF | ||
| a | b | a | b | ||||||||||||
| Cancer-specific scale | X | ||||||||||||||
| Treatment-related symptoms | X | ||||||||||||||
| Stoma-related symptoms etc. | X | X | X | X | |||||||||||
| Embarrassment (by stoma, bowel movement, ...) | X | X | |||||||||||||
| Environment domain | X | ||||||||||||||
| Disease burden | X | ||||||||||||||
| Benefits of cancer | X | ||||||||||||||
| Functional well-being | X | ||||||||||||||
| Maintenance purposes | X | ||||||||||||||
| Private life | X | ||||||||||||||
| Other QoL areas assessed | 1 | 2 | 2 | 2 | 2 | 2 | 1 | 1 | 1 | ||||||
References
- Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021 May 4;71(3):209–49. [CrossRef]
- WCFR. https://www.wcrf.org/cancer-trends/worldwide-cancer-data/. 2022.
- Siegel RL, Wagle NS, Cercek A, Smith RA, Jemal A. Colorectal cancer statistics, 2023. CA Cancer J Clin. 2023 May;73(3):233–54.
- Allemani C, Matsuda T, Di Carlo V, Harewood R, Matz M, Nikšić M, et al. Global surveillance of trends in cancer survival 2000–14 (CONCORD-3): analysis of individual records for 37 513 025 patients diagnosed with one of 18 cancers from 322 population-based registries in 71 countries. The Lancet. 2018 Mar;391(10125):1023–75. [CrossRef]
- Cheng V, Oveisi N, McTaggart-Cowan H, Loree JM, Murphy RA, De Vera MA. Colorectal Cancer and Onset of Anxiety and Depression: A Systematic Review and Meta-Analysis. Current Oncology. 2022 Nov 15;29(11):8751–66. [CrossRef]
- Sihvola S, Kuosmanen L, Kvist T. Resilience and related factors in colorectal cancer patients: A systematic review. European Journal of Oncology Nursing. 2022 Feb;56:102079. [CrossRef]
- Lim CYS, Laidsaar-Powell RC, Young JM, Kao SC, Zhang Y, Butow P. Colorectal cancer survivorship: A systematic review and thematic synthesis of qualitative research. Eur J Cancer Care (Engl). 2021 Jul 18;30(4). [CrossRef]
- Révész D, van Kuijk SMJ, Mols F, van Duijnhoven FJB, Winkels RM, Kant Ij, et al. External validation and updating of prediction models for estimating the 1-year risk of low health-related quality of life in colorectal cancer survivors. J Clin Epidemiol. 2022 Dec;152:127–39.
- Chan RJ, Chan A, Yates P, Molassiotis A. A step forward in addressing cancer survivorship in the Asia-Pacific region. BMC Med. 2017 Dec 26;15(1):17. [CrossRef]
- von Blanckenburg P, Seifart U, Conrad N, Exner C, Rief W, Nestoriuc Y. Quality of life in cancer rehabilitation: the role of life goal adjustment. Psychooncology. 2014 Oct;23(10):1149–56. [CrossRef]
- Joshy G, Thandrayen J, Koczwara B, Butow P, Laidsaar-Powell R, Rankin N, et al. Disability, psychological distress and quality of life in relation to cancer diagnosis and cancer type: population-based Australian study of 22,505 cancer survivors and 244,000 people without cancer. BMC Med. 2020 Dec 1;18(1):372. [CrossRef]
- Briggs LG, Sentana-Lledo D, Lage DE, Trinh QD, Morgans AK. Optimal assessment of quality of life for patients with prostate cancer. Ther Adv Med Oncol. 2022 Jan 10;14:175883592211413. [CrossRef]
- Miller KA, Stal J, Gallagher P, Weng Z, Freyer DR, Kaslander JN, et al. Time from Diagnosis and Correlates of Health-Related Quality of Life among Young Adult Colorectal Cancer Survivors. Cancers (Basel). 2021 Aug 11;13(16):4045. [CrossRef]
- The ACTION Study Group. Health-related quality of life and psychological distress among cancer survivors in Southeast Asia: results from a longitudinal study in eight low- and middle-income countries. BMC Med. 2017 Dec 13;15(1):10.
- Tay MRJ, Wong CJ, Aw HZ. Assessment of Health-Related Quality of Life and Distress in an Asian Community-Based Cancer Rehabilitation Program. Current Oncology. 2022 Sep 27;29(10):7012–20. [CrossRef]
- European Commission (EC). Research and Innovation. Conquering Cancer: Mission Possible Report of the Mission Board for Cancer. Luxembourg; 2020.
- European Commission (EC). Europe’s Beating Cancer Plan Communication from the Commission to the European Parliament and the Council. Brussels; 2021.
- Bianchi V, Spitale A, Ortelli L, Mazzucchelli L, Bordoni A. Quality indicators of clinical cancer care (QC 3 ) in colorectal cancer. BMJ Open. 2013 Jul;3(7):e002818. [CrossRef]
- Harrison M, Milbers K, Hudson M, Bansback N. Do patients and health care providers have discordant preferences about which aspects of treatments matter most? Evidence from a systematic review of discrete choice experiments. BMJ Open. 2017 May 17;7(5):e014719. [CrossRef]
- Aldaz BE, Treharne GJ, Knight RG, Conner TS, Perez D. Oncology healthcare professionals’ perspectives on the psychosocial support needs of cancer patients during oncology treatment. J Health Psychol. 2017 Sep 1;22(10):1332–44. [CrossRef]
- Ware JE, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care. 1992 Jun;30(6):473–83.
- Lin CY, Hwang JS, Wang WC, Lai WW, Su WC, Wu TY, et al. Psychometric evaluation of the WHOQOL-BREF, Taiwan version, across five kinds of Taiwanese cancer survivors: Rasch analysis and confirmatory factor analysis. Journal of the Formosan Medical Association. 2019 Jan;118(1):215–22. [CrossRef]
- de Jong M, Tamminga SJ, de Boer AGEM, Frings-Dresen MHW. The Quality of Working Life Questionnaire for Cancer Survivors (QWLQ-CS): a Pre-test Study. BMC Health Serv Res. 2016 Dec 2;16(1):194.
- Rotonda C, Conroy T, Mercier M, Bonnetain F, Uwer L, Miny J, et al. Validation of the French version of the colorectal-specific quality-of-life questionnaires EORTC QLQ-CR38 and FACT-C. Quality of Life Research. 2008 Apr 13;17(3):437–45. [CrossRef]
- Grant M, Ferrell B, Dean G, Uman G, Chu D, Krouse R. Revision and Psychometric Testing of the City of Hope Quality of Life–Ostomy Questionnaire. Quality of Life Research. 2004 Oct;13(8):1445–57. [CrossRef]
- Muzzatti B, Annunziata MA. Assessing quality of life in long-term cancer survivors: a review of available tools. Supportive Care in Cancer. 2013 Nov 1;21(11):3143–52. [CrossRef]
- Nguyen H, Butow P, Dhillon H, Sundaresan P. A review of the barriers to using Patient-Reported Outcomes (PROs) and Patient-Reported Outcome Measures (PROMs) in routine cancer care. J Med Radiat Sci. 2021 Jun 19;68(2):186–95. [CrossRef]
- Mlakar I, Lin S, Nateqi J, Gruarin S, Diéguez L, Piairo P, et al. Establishing an Expert Consensus on Key Indicators of the Quality of Life among Breast Cancer Survivors: A Modified Delphi Study. J Clin Med. 2022 Apr 5;11(7):2041. [CrossRef]
- Pietersma S, de Vries M, van den Akker-van Marle ME. Domains of quality of life: results of a three-stage Delphi consensus procedure among patients, family of patients, clinicians, scientists and the general public. Quality of Life Research. 2013 Nov 17. [CrossRef]
- Tung J, Speechley KN, Gofton T, Gonzalez-Lara LE, Graham M, Naci L, et al. Towards the assessment of quality of life in patients with disorders of consciousness. Quality of Life Research. 2020 May 14;29(5):1217–27. [CrossRef]
- Hasson F, Keeney S, McKenna H. Research guidelines for the Delphi survey technique. J Adv Nurs. 2000 Oct 28;32(4):1008–15. [CrossRef]
- Powell C. The Delphi technique: myths and realities. J Adv Nurs. 2003 Feb 10;41(4):376–82. [CrossRef]
- Beiderbeck D, Frevel N, von der Gracht HA, Schmidt SL, Schweitzer VM. Preparing, conducting, and analyzing Delphi surveys: Cross-disciplinary practices, new directions, and advancements. MethodsX. 2021;8:101401. [CrossRef]
- Ferrell BR, Hassey Dow K, Grant M. Measurement of the quality of life in cancer survivors. Quality of Life Research. 1995 Dec;4(6):523–31. [CrossRef]
- Lavdantini M, Tsitsis N. Definitions and conceptual models of quality of life in cancer patients. Health Science Journal. 2015;9:1–5.
- Muñoz C, Juarez G, Muñoz ML, Portnow J, Fineman I, Badie B, et al. The Quality of Life of Patients with Malignant Gliomas and Their Caregivers. Soc Work Health Care. 2008 Oct 22;47(4):455–78. [CrossRef]
- Mollica M, Nemeth L, Newman SD, Mueller M. Breast Cancer in African Americans: From Patient to Survivor. Journal of Transcultural Nursing. 2014;25(4):334–40.
- Mlakar I, Lin S, Aleksandraviča I, Arcimoviča K, Eglītis J, Leja M, et al. Patients-centered SurvivorShIp care plan after Cancer treatments based on Big Data and Artificial Intelligence technologies (PERSIST): a multicenter study protocol to evaluate efficacy of digital tools supporting cancer survivors. BMC Med Inform Decis Mak. 2021 Dec 14;21(1):243. [CrossRef]
- Borgiel AEM, Dunn E V., Lamont CT, Macdonald PJ, Evensen MK, Bass MJ, et al. Recruiting family physicians as participants in research. Fam Pract. 1989;6(3):168–72. [CrossRef]
- Vanmeerbeek M, Govers P, Schippers N, Rieppi S, Mortelmans K, Mairiaux P. Searching for consensus among physicians involved in the management of sick-listed workers in the Belgian health care sector: a qualitative study among practitioners and stakeholders. BMC Public Health. 2016 Dec 17;16(1):164. [CrossRef]
- Freitas Â, Santana P, Oliveira MD, Almendra R, Bana e Costa JC, Bana e Costa CA. Indicators for evaluating European population health: a Delphi selection process. BMC Public Health. 2018 Dec 27;18(1):557. [CrossRef]
- Koo TK, Li MY. A Guideline of Selecting and Reporting Intraclass Correlation Coefficients for Reliability Research. J Chiropr Med. 2016 Jun;15(2):155–63. [CrossRef]
- R Core Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria; 2020.
- Revelle W. Psych: Procedures for Psychological, Psychometric, and Personality Research . Evanston: Northwestern University; 2019.
- Kassambara A. Rstatix: Pipe-Friendly Framework for Basic Statistical Tests. San Francisco: GitHub, Inc.; 2021.
- Benjamini Y, Hochberg Y. Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. Journal of the Royal Statistical Society: Series B (Methodological). 1995 Jan 5;57(1):289–300. [CrossRef]
- Arksey H, O’Malley L. Scoping studies: towards a methodological framework. Int J Soc Res Methodol. 2005 Feb;8(1):19–32. [CrossRef]
- Levac D, Colquhoun H, O’Brien KK. Scoping studies: advancing the methodology. Implementation Science. 2010 Dec 20;5(1):69. [CrossRef]
- Tricco AC, Lillie E, Zarin W, O’Brien KK, Colquhoun H, Levac D, et al. PRISMA Extension for Scoping Reviews (PRISMA-ScR): Checklist and Explanation. Ann Intern Med. 2018 Oct 2;169(7):467–73.
- Martín-Martín A, Orduna-Malea E, Thelwall M, Delgado López-Cózar E. Google Scholar, Web of Science, and Scopus: A systematic comparison of citations in 252 subject categories. J Informetr. 2018 Nov;12(4):1160–77. [CrossRef]
- Calderon C, Ferrando PJ, Lorenzo-Seva U, Ferreira E, Lee EM, Oporto-Alonso M, et al. Psychometric properties of the Spanish version of the European Organization for Research and Treatment of Cancer Quality of Life Questionnaire (EORTC QLQ-C30). Quality of Life Research. 2022 Jun 20;31(6):1859–69. [CrossRef]
- El Alami Y, Essangri H, Majbar MA, Boutayeb S, Benamr S, El Malki HO, et al. Psychometric validation of the Moroccan version of the EORTC QLQ-C30 in colorectal Cancer patients: cross-sectional study and systematic literature review. BMC Cancer. 2021 Dec 27;21(1):99. [CrossRef]
- Gujral S, Conroy T, Fleissner C, Sezer O, King PM, Avery KNL, et al. Assessing quality of life in patients with colorectal cancer: An update of the EORTC quality of life questionnaire. Eur J Cancer. 2007 Jul;43(10):1564–73. [CrossRef]
- Al-Shandudi M, Al-Mandhari M, Chan MF, Al-Hajri T, Al-Balushi M, Al-Azri M. Health-Related Quality of Life of Omani Colorectal Cancer Survivors. Cancer Control. 2022 Jan 11;29:107327482210841. [CrossRef]
- Whistance RN, Conroy T, Chie W, Costantini A, Sezer O, Koller M, et al. Clinical and psychometric validation of the EORTC QLQ-CR29 questionnaire module to assess health-related quality of life in patients with colorectal cancer. Eur J Cancer. 2009 Nov;45(17):3017–26. [CrossRef]
- Lorca LA, Sacomori C, Fasce Pineda G, Vidal Labra R, Cavieres Faundes N, Plasser Troncoso J, et al. Validation of the EORTC QLQ-ELD 14 questionnaire to assess the health-related quality of life of older cancer survivors in Chile. J Geriatr Oncol. 2021 Jun;12(5):844–7. [CrossRef]
- Wendel CS, Grant M, Herrinton L, Temple LKF, Hornbrook MC, McMullen CK, et al. Reliability and validity of a survey to measure bowel function and quality of life in long-term rectal cancer survivors. Quality of Life Research. 2014 Dec 3;23(10):2831–40. [CrossRef]
- Jane Mohler M, Joel Coons S, Hornbrook MC, Herrinton LJ, Wendel CS, Grant M, et al. The Health-Related Quality of Life in Long-Term Colorectal Cancer Survivors Study: objectives, methods and patient sample. Curr Med Res Opin. 2008 Jul 1;24(7):2059–70. [CrossRef]
- Avis NE, Smith KW, McGraw S, Smith RG, Petronis VM, Carver CS. Assessing Quality of Life in Adult Cancer Survivors (QLACS). Quality of Life Research. 2005 May;14(4):1007–23. [CrossRef]
- Ashley L, Smith AB, Jones H, Velikova G, Wright P. Traditional and Rasch psychometric analyses of the Quality of Life in Adult Cancer Survivors (QLACS) questionnaire in shorter-term cancer survivors 15months post-diagnosis. J Psychosom Res. 2014 Oct;77(4):322–9. [CrossRef]
- Escobar A, Trujillo-Martín M del M, Rueda A, Pérez-Ruiz E, Avis NE, Bilbao A. Cross-cultural adaptation, reliability and validity of the Spanish version of the Quality of Life in Adult Cancer Survivors (QLACS) questionnaire: application in a sample of short-term survivors. Health Qual Life Outcomes. 2015 Dec 16;13(1):182. [CrossRef]
- Boome I te, Somers AMJ, Graupner C, Kimman ML, Gidding- Slok AHM, Breukink SO. Development and content validation of the Assessment of Burden of ColoRectal Cancer (ABCRC)-tool. European Journal of Surgical Oncology. 2022 Aug;48(8):1807–14.
- Siafaka V, Mavridis D, Tsonis O, Tzamakou E, Christogiannis C, Tefa L, et al. The WHOQOL-BREF instrument: Psychometric evaluation of the Greek version in patients with advanced cancer and pain and associations with psychological distress. Palliat Support Care. 2022 Aug 19;1–11. [CrossRef]
- Oliveira SEH, Carvalho H, Esteves F. Toward an understanding of the quality of life construct: Validity and reliability of the WHOQOL-Bref in a psychiatric sample. Psychiatry Res. 2016 Oct;244:37–44. [CrossRef]
- Leung KF, Wong WW, Tay MSM, Chu MML, Ng SSW. Development and validation of the interview version of the Hong Kong Chinese WHOQOL-BREF. Quality of Life Research. 2005 Jun 1;14(5):1413–9. [CrossRef]
- McNair AGK, Whistance RN, Forsythe RO, Macefield R, Rees J, Pullyblank AM, et al. Core Outcomes for Colorectal Cancer Surgery: A Consensus Study. PLoS Med. 2016 Aug 9;13(8):e1002071.
- Dirven L, Petersen MAa, Aaronson NK, Chie WC, Conroy T, Costantini A, et al. Development and Psychometric Evaluation of an Item Bank for Computerized Adaptive Testing of the EORTC Insomnia Dimension in Cancer Patients (EORTC CAT-SL). Appl Res Qual Life. 2021 Apr 6;16(2):827–44. [CrossRef]
- Saver BG, Martin SA, Adler RN, Candib LM, Deligiannidis KE, Golding J, et al. Care that Matters: Quality Measurement and Health Care. PLoS Med. 2015 Nov 17;12(11):e1001902.
- Bellizzi KM, Mustian KM, Palesh OG, Diefenbach M. Cancer survivorship and aging. Cancer. 2008 Dec 3;113(S12):3530–9. [CrossRef]
| Round 1 | Round 2 | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| All participants | Group 1 | Group 2 | |||||||
| f | % | f | % | f | % | f | % | ||
| N | 54 | 25 | 9 | 16 | |||||
| Gender | Female | 33 | 61.1 | 16 | 64.0 | 4 | 44.4 | 12 | 75.0 |
| Male | 21 | 38.9 | 9 | 36.0 | 5 | 55.6 | 4 | 25.0 | |
| Speciality* | Gastroenterology | 4 | 7.4 | 1 | 4.0 | - | - | 1 | 6.3 |
| Medical Oncology | 16 | 29.6 | 9 | 36.0 | 4 | 44.4 | 5 | 31.3 | |
| Nutrition | 2 | 3.7 | 2 | 8.0 | 1 | 11.1 | 1 | 6.3 | |
| Oncology Nursing | 5 | 9.3 | - | - | - | - | - | - | |
| Physiotherapy | 1 | 1.9 | 4 | 16.0 | - | - | 4 | 25.0 | |
| Psychology | 5 | 9.3 | - | - | - | - | - | - | |
| Psychotherapy | - | - | - | - | - | - | - | - | |
| Radiology | 1 | 1.9 | - | - | - | - | - | - | |
| Radiotherapy Oncology | 2 | 3.7 | 2 | 8.0 | 2 | 22.2 | - | - | |
| Surgery | 8 | 14.8 | 5 | 20.0 | 2 | 22.2 | 3 | 18.8 | |
| Other | 11 | 20.4 | 3 | 12.0 | 1 | 11.1 | 2 | 12.5 | |
| Country | Austria | - | - | 3 | 12.0 | - | - | 3 | 18.8 |
| Belgium | 10 | 18.5 | 10 | 40.0 | 3 | 33.3 | 7 | 43.8 | |
| Latvia | 6 | 11.1 | 5 | 20.0 | 3 | 33.3 | 2 | 12.5 | |
| Portugal | 22 | 40.7 | 2 | 8.0 | - | - | 2 | 12.5 | |
| Slovenia | 2 | 3.7 | 3 | 12.0 | 2 | 22.2 | 1 | 6.3 | |
| Spain | 13 | 24.1 | 1 | 4.0 | 1 | 11.1 | - | - | |
| Switzerland | 1 | 1.9 | 1 | 4.0 | - | - | 1 | 6.3 | |
| Years in practice | M | 15.7 | 10.2 | 12.2 | 9.1 | ||||
| SD | 10.3 | 8.3 | 9.7 | 7.6 | |||||
| Round 1 | Round 2 | ||||||||||||||
| All participants | Group 1c | Group 2d | t-teste | ||||||||||||
| M | SD | % Agreementa | CRb | M | SD | M | SD | M | SD | df | t | Adj. pf | % Agreementg | CRh | |
| General domains | |||||||||||||||
| Physical Health and Well-being | 6.3 | 1.0 | 94.4 | * | 6.3 | 0.5 | 6.1 | 0.3 | 6.4 | 0.5 | 23 | -1.41 | 0.87 | 100.0 | * |
| Psychological Health and Well-being | 6.0 | 1.0 | 94.4 | * | 6.3 | 0.8 | 6.1 | 0.8 | 6.4 | 0.8 | 23 | -0.79 | 0.87 | 80.0 | * |
| Social Health and Well-being | 5.6 | 1.0 | 83.3 | * | 5.8 | 0.9 | 5.7 | 0.5 | 5.9 | 1.0 | 23 | -0.57 | 0.88 | 68.0 | - |
| Spiritual Health and Well-being | 4.9 | 1.6 | 61.1 | - | - | - | - | - | - | - | - | - | - | - | - |
| Physical QoL | |||||||||||||||
| Functional ability and Mobility | 6.2 | 0.9 | 96.0 | * | 6.5 | 0.7 | 6.7 | 0.5 | 6.5 | 0.8 | 20 | 0.70 | 0.87 | 90.9 | * |
| Activities of daily living | 6.1 | 0.9 | 94.0 | * | 6.6 | 0.6 | 6.7 | 0.5 | 6.6 | 0.7 | 20 | 0.20 | 0.94 | 95.5 | * |
| Fatigue/Vitality | 5.9 | 0.8 | 96.0 | * | 6.0 | 0.6 | 6.2 | 0.4 | 5.9 | 0.6 | 20 | 1.21 | 0.87 | 95.5 | * |
| Sleep and rest | 5.7 | 1.1 | 90.0 | * | 5.7 | 0.8 | 5.4 | 0.7 | 5.9 | 0.9 | 20 | -1.36 | 0.87 | 68.2 | - |
| Pain and discomfort | 6.4 | 0.9 | 94.0 | * | 7.0 | 0.2 | 7.0 | 0.0 | 6.9 | 0.3 | 20 | 0.83 | 0.87 | 100.0 | * |
| Health perceptions | 5.6 | 0.9 | 90.0 | * | 5.3 | 0.8 | 5.2 | 0.4 | 5.4 | 1.0 | 20 | -0.47 | 0.91 | 31.8 | - |
| Physical symptoms | 6.2 | 0.8 | 98.0 | * | 6.4 | 0.5 | 6.4 | 0.5 | 6.3 | 0.5 | 20 | 0.63 | 0.87 | 100.0 | * |
| Health distress | 5.7 | 1.0 | 94.0 | * | 5.3 | 0.8 | 5.0 | 0.7 | 5.5 | 0.9 | 20 | -1.31 | 0.87 | 31.8 | - |
| Weight loss/gain | 6.0 | 0.9 | 92.0 | * | 5.8 | 1.0 | 6.3 | 0.7 | 5.5 | 1.0 | 20 | 2.30 | 0.50 | 68.2 | - |
| Physical Health and comorbidities | 6.0 | 0.9 | 94.0 | * | 5.9 | 0.8 | 5.8 | 0.8 | 5.9 | 0.9 | 20 | -0.39 | 0.92 | 77.3 | * |
| Psychological QoL | |||||||||||||||
| Anxiety | 5.8 | 1.1 | 88.0 | * | 5.7 | 1.0 | 5.7 | 1.1 | 5.8 | 0.9 | 19 | -0.19 | 0.94 | 66.7 | - |
| Depression | 5.9 | 1.0 | 90.0 | * | 6.0 | 0.9 | 6.1 | 1.1 | 5.8 | 0.8 | 19 | 0.67 | 0.87 | 76.2 | * |
| Psychological distress | 5.8 | 1.1 | 84.0 | * | 6.0 | 0.7 | 6.1 | 0.8 | 6.0 | 0.7 | 19 | 0.33 | 0.92 | 76.2 | * |
| Cognitive functioning, concentration and attention | 5.5 | 1.3 | 82.0 | * | 5.5 | 1.0 | 5.3 | 1.2 | 5.6 | 0.9 | 19 | -0.54 | 0.88 | 52.4 | - |
| Uncertainty | 5.2 | 1.1 | 76.0 | * | 5.0 | 0.9 | 5.0 | 0.7 | 5.1 | 1.0 | 19 | -0.21 | 0.94 | 28.6 | - |
| Fear of Recurrence | 5.6 | 1.0 | 86.0 | * | 5.8 | 0.8 | 5.8 | 1.0 | 5.8 | 0.7 | 19 | -0.15 | 0.94 | 66.7 | - |
| Isolation/Abandonment and feelings of belonging | 5.5 | 1.2 | 82.0 | * | 5.6 | 0.9 | 5.0 | 0.9 | 6.0 | 0.6 | 19 | -3.13 | 0.17 | 61.9 | - |
| Positive feelings and affect | 5.6 | 1.0 | 88.0 | * | 5.8 | 0.8 | 5.4 | 0.7 | 6.0 | 0.7 | 19 | -1.72 | 0.87 | 66.7 | - |
| Negative feelings and affect | 5.5 | 1.1 | 86.0 | * | 5.8 | 0.5 | 5.7 | 0.5 | 5.8 | 0.6 | 19 | -0.69 | 0.87 | 71.4 | - |
| Loss of interest in usual activities | 5.5 | 1.3 | 80.0 | * | 6.0 | 0.8 | 5.8 | 1.2 | 6.2 | 0.4 | 19 | -1.06 | 0.87 | 90.5 | * |
| Social QoL | |||||||||||||||
| Family functioning | 5.7 | 1.2 | 78.0 | * | 5.9 | 1.1 | 6.1 | 1.1 | 5.7 | 1.3 | 17 | 0.77 | 0.87 | 68.4 | - |
| Martial functioning | 5.2 | 1.4 | 72.0 | - | - | - | - | - | - | - | - | - | - | - | - |
| Affection/Sexuality | 5.2 | 1.3 | 68.0 | - | - | - | - | - | - | - | - | - | - | - | - |
| Self Concept/Appearance | 5.4 | 1.2 | 78.0 | * | 5.3 | 0.7 | 5.3 | 0.5 | 5.3 | 0.9 | 17 | 0.09 | 0.94 | 36.8 | - |
| Enjoyment/Leisure (participation and opportunities) | 5.4 | 1.3 | 78.0 | * | 5.7 | 0.9 | 5.7 | 0.9 | 5.7 | 1.1 | 17 | -0.07 | 0.94 | 57.9 | - |
| Social activity and limitations | 5.6 | 1.2 | 80.0 | * | 5.8 | 0.8 | 5.8 | 0.4 | 5.9 | 1.0 | 17 | -0.34 | 0.92 | 73.7 | - |
| Financial Concerns | 5.2 | 1.3 | 76.0 | * | 4.8 | 1.1 | 4.7 | 0.7 | 5.0 | 1.3 | 17 | -0.67 | 0.87 | 21.1 | - |
| Social Support | 5.5 | 1.1 | 84.0 | * | 5.5 | 0.7 | 5.3 | 0.7 | 5.7 | 0.7 | 17 | -1.16 | 0.87 | 63.2 | - |
| Employment | 5.5 | 1.2 | 82.0 | * | 5.7 | 0.7 | 5.6 | 0.5 | 5.8 | 0.8 | 17 | -0.78 | 0.87 | 57.9 | - |
| Role limitations due to health or psychical problems | 5.4 | 1.2 | 78.0 | * | 5.5 | 0.6 | 5.3 | 0.5 | 5.7 | 0.7 | 17 | -1.33 | 0.87 | 47.4 | - |
| Spiritual QoL | |||||||||||||||
| Meaning of Illness | 5.2 | 1.5 | 72.0 | - | - | - | - | - | - | - | - | - | - | - | - |
| Religiosity | 4.1 | 1.9 | 48.0 | - | - | - | - | - | - | - | - | - | - | - | - |
| Hope | 5.2 | 1.4 | 68.0 | - | - | - | - | - | - | - | - | - | - | - | - |
| Transcendence | 4.4 | 1.6 | 50.0 | - | - | - | - | - | - | - | - | - | - | - | - |
| Inner Strength | 5.0 | 1.5 | 64.0 | - | - | - | - | - | - | - | - | - | - | - | - |
| Questionnaire | Reference | Type of paper | Construct assessed | Target population | Number of items | Response scale | Language of the questionnaire |
|---|---|---|---|---|---|---|---|
|
ABCRC (Assessment of Burden of Colorectal Cancer-tool) |
Boome et al., 2022 (62) | Development, content validation | Experienced burden of colorectal cancer and lifestyle parameters | Adult patients with colon and rectal cancer, patients with anastomosis, and patients with stoma | 27 (version for patients with stoma), 28 (version for colon cancer), 29 (version for rectal cancer) | 3- and 4-point; one open question | Dutch |
|
EORTC QLQ-C30 (European Organisation for Research and Treatment of Cancer, Quality of Life of Cancer Patients, version 3) |
Calderon et al., 2022 (51) | Validation | Quality of life | Adult patients with cancer (general) | 30 | 4-point Likert type and 7-point | Spanish |
| El Alami et al., 2021 (52) | Validation (Moroccan Arabic Version) | Adult patients with cancer (general); in this study colorectal cancer patients | Moroccan Arabic | ||||
|
EORTC QLQ-CR38 (European Organisation for Research and Treatment of Cancer, Quality of Life Questionnaire for Colorectal Cancer-38)1 |
Rotonda et al., 2008 (24) | Validation (French version) | Quality of life | Adult patients with colorectal cancer | 38 | 4-point Likert type and 7-point | French |
|
EORTC QLQ-CR29 (European Organisation for Research and Treatment of Cancer, Quality of Life Questionnaire for Colorectal Cancer-29)1, 2 |
Gujral et al., 2007 (53) | Content validation study (adaptation of the EORTC QLQ-CR38) | Quality of life | Adult patients with colorectal cancer | 29 | [no information available in the paper] | English |
| Al-Shandudi et al., 2022 (54) | Empirical study | Arabic | |||||
| Whistance et al., 2009 (55) | Clinical and psychometric validation | 4-point Likert type and 7-point | English, French, Taiwanese, Italian, German, Spanish | ||||
|
EORTC QLQ-ELD14 (European Organisation for Research and Treatment of Cancer, Quality of Life Questionnaire - Elderly Cancer Patients Module) |
Lorca et al., 2021 (56) | Validation for ageing cancer survivors | Quality of life | Ageing patients (>60 years) with cancer (in this study colorectal cancer survivors) | 14 | 7-point Likert scale and 4-point scale | Spanish version |
|
FACT-C (Functional Assessment of Cancer Therapy – Colorectal) |
Rotonda et al., 2008 (24) | Validation | Well-being | Adult patients with colorectal cancer | 34 | 5-point scale | French |
|
mCOH-QOL-O (Modified City of Hope Quality of Life - Ostomy questionnaire) |
Grant et al., 2004 (25) | Validation | Quality of life | Ostomy patients (cancer and non-cancer) | 41 | 11-point scale | English |
| Wendel et al., 2014 (57) | Rectal cancer survivors | 43 | |||||
| Mohler et al., 20083 (58) | Empirical paper | Colorectal cancer (CRC) survivors with and withoutostomies | 34 | English | |||
|
QLACS (Quality of Life in Adult Cancer Survivors questionnaire) |
Avis et al., 2005 (59) | Questionnaire development | Quality of life | Long-term cancer survivors (> or equal to 5 years post-diagnosis) | 47 | 7-point scale | English |
| Ashley et al., 2014 (60) | Psychometric validation | Short-term cancer survivors | |||||
| Escobar et al., 2015 (61) | Cross-cultural adaptation, reliability andvalidity of the Spanish version | Spanish | |||||
|
QWLQ-CS (Quality of Working Life Questionnaire for Cancer Survivors) |
De Jong et al., 20164 (23) | Item-development study | Quality of life in the work domain | Cancer survivors | 104 | 4-point scale | English |
|
SF-36v2 (SF-36v2 Health Survey) |
Ware & Sherbourne, 1992 (21) | Item selection study | Functional health and well-being | General population | 36 | several types of response scales differing by items | English |
| Mohler et al., 2008 (58) | Empirical study | ||||||
|
SF-12v2 (SF-12v2 Health Survey) |
Wendel et al., 2014 (57) | Validation paper – for use with long-term rectal cancer survivors | Functional health and well-being | [no information] | 12 | [no information] | English |
|
WHOQOL-BREF (World Health Organization Quality-of-Life Scale) |
Lin et al., 2019 (22) | Psychometric validation of Taiwan version | Quality of life | General population (sample of this study: cancer survivors, also colorectal) | 28 (original 26 items + 2 added in this study) | 5-point scale | Taiwanese |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





