Submitted:
03 April 2024
Posted:
03 April 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Core-Only CaCO3 Microparticles
2.1. Loading Methods
2.2. Demonstration and Limitations
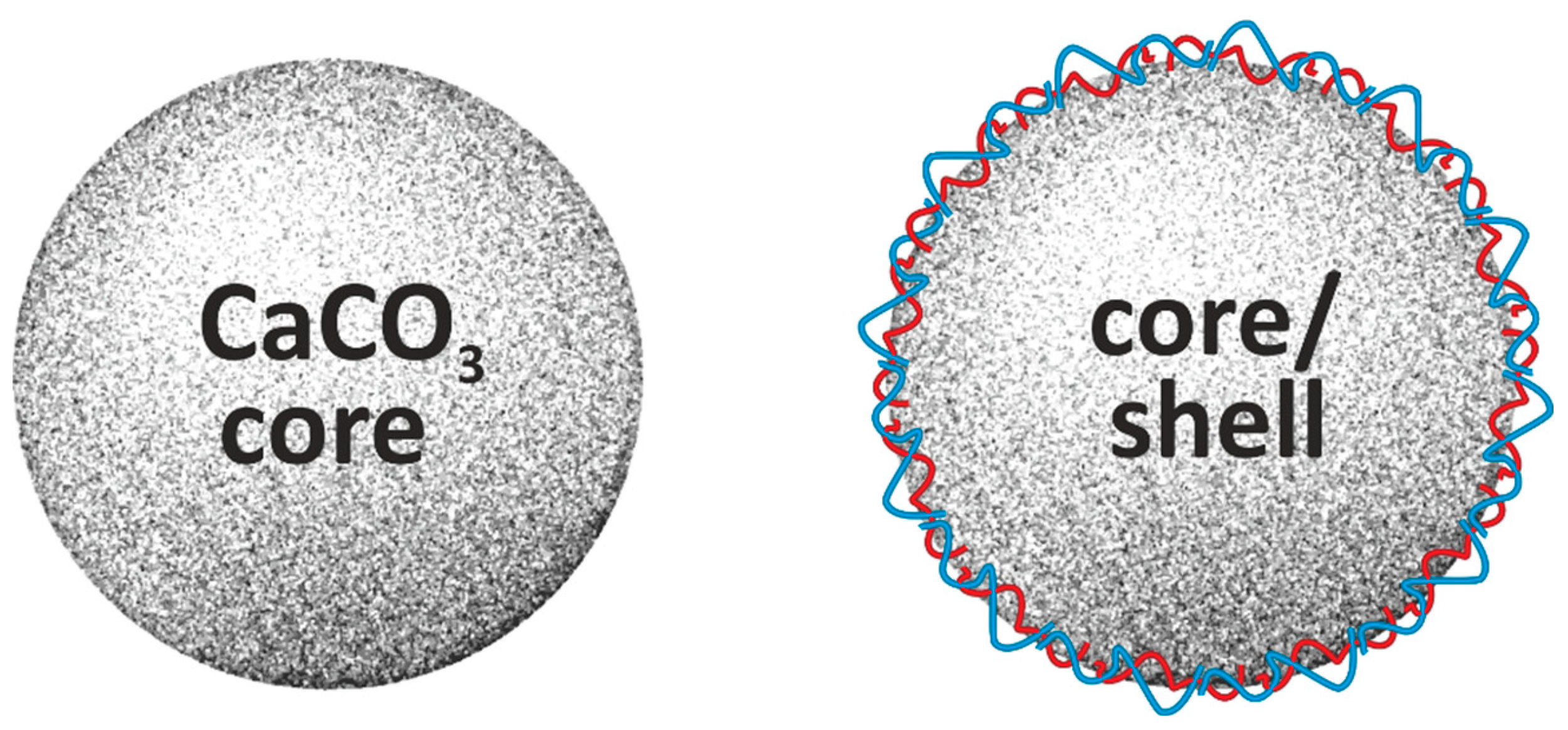
3. CaCO3-Based Core/Shell Systems
3.1. Methods of Fabrication
3.2. Delivery of Small Molecules
3.3. Delivery of Proteins
3.4. Delivery of Nucleic Acids
4. CaCO3-Based Hollow Microcapsules
4.1. Methods of Fabrication
4.2. Delivery of Small Molecules
4.3. Delivery of Proteins
4.4. Delivery of Nucleic Acids
5. Conclusion
6. Outlook: In Vivo Studies
6.1. Modulation of the pH of Tumor Environment
6.2. Biodistribution and Biocompatibility
6.3. Retention, Stability, and Toxicity
6.4. Vaccinal Applications
| Particle type | Size | Cargo type | Encapsulated molecule | Shell composition | Ref. |
|---|---|---|---|---|---|
| Core | 1 μm | Small molecule | Doxorubicin | Hyaluronate/glutamate | [52] |
| Core | 0.6–3.2 µm | - | - | - | [6] |
| Core | 0.43 µm | Fluorescent dye | Rhodamine 6G | - | [40] |
| Core | 0.52 µm | - | - | - | [1] |
| Core | 0.4–2.7 µm | - | - | - | [7] |
| Core | n/a | Protein | BSA | - | [41] |
| Core | 17.9 μm | Small molecule | Ibuprofen, nifedipine, losartan potassium, and metronidazole benzoate | - | [48] |
| Core | 3.1–23.5 µm | Small molecule | Aspirin, vanillin | - | [42] |
| Core | 17.9 μm | Protein | Lysozyme, BSA | - | [49] |
| Core | 3.4 μm | Protein | Superoxide dismutase | - | [43] |
| Core | 10 μm | Protein | Catalase, insulin, aprotinin | - | [44] |
| Core | 0.8-1.6 μm | Small molecule | Doxorubicin | - | [21] |
| Core | 4–5 μm | Protein | Catalase | - | [51] |
| Core | 1 μm | Protein | Ovalbumin, pneumolysin | - | [113] |
| Core | 5.45 μm | Protein | β-lactamase | - | [50] |
| Core | 1.3 μm | - | - | - | [114] |
| Core | 4–7 μm | Radionuclide | 224Ra | - | [45] |
| Core | 1–3, 3–15 μm | Radionuclide | 224Ra | - | [46] |
| Core | 0.2-1.1 μm | Nucleic acid | DNA | - | [34] |
| Core/shell | 2 μm | Small molecule | Doxorubicin | Poly-L-ornithine/ fucoidan | [115] |
| Core/shell | 0.2 μm | Small molecule | Doxorubicin | Oleic acid/PEG | [116] |
| Core/shell | 3 μm | Small molecule | Doxorubicin | PDDA/PSS | [63] |
| Core/shell | ∼10 μm | Protein | Ovalbumin, cancer cell lysate | Poly(HPMA-APMA) with TLR7/8-agonists | [64] |
| Core/shell | 0.65, 3.2 μm | Radionuclide | 225Ac | HSA/TA | [111] |
| Core/shell | ∼2 μm | Protein | BSA | PLA | [65] |
| Core/shell | 2–4 μm | Fluorescent dye | Nile Red, rhodamine 110 | CaCO3 | [66] |
| Core/shell, shell | 2–2.5 μm | Small molecule | Doxorubicin | PAH/PSS/QD | [18] |
| Shell | 4.75 μm | Protein | Lactalbumine, lysozyme, horseradish peroxidase, chymotrypsin | - | [39] |
| Shell | 5.4 μm | - | - | PAH/PSS | [117] |
| Shell | 9 μm | - | - | PLL, PR, DA, COL/HA, CS, DS, HS | [15] |
| Shell | 3–6 μm | Protein | Insulin | PAH/PSS, PVS, DS | [20] |
| Shell | 5 μm | Fluorescent dye | FITC-dextran | HA/PAH, PLL | [70] |
| Shell | 3 μm | Fluorescent dye | FITC-dextran | pARG/DS, p(HPMA-DMAE)/PSS, PAH/PSS | [71] |
| Shell | ∼1 μm | Fluorescent dye, protein | Rhodamine B, methylene blue, insulin | Phenylboronic –modified alginate/PVPON | [94] |
| Shell | 1.8–3.8 μm | - | - | pArg/DS | [118] |
| Shell | 0.5 μm | Small molecule | Doxorubicin | pArg/DS | [32] |
| Shell | 3–5 μm | Extract | Gratiola officinalis extract | PAH/PSS/DS | [97] |
| Shell | 4 μm | Small molecule | Apigenin, ascorbic acid | PAH/DS | [96] |
| Shell | 0.25–0.5 μm | Small molecule | Gemcitabine, clodronate | pArg/DS | [95] |
| Shell | 3.3–4.8 μm | Protein | BSA, chymotrypsin, lysozyme | PAH/PSS | [103] |
| Shell | 5.0–8.3 μm | Small molecule | Tetracycline hydrochloride | PAH/pectin | [99] |
| Shell | 5.0 μm | Cells | Escherichia coli | PAH/PSS | [98] |
| Shell | 4.5 μm | Small molecule | Doxorubicin, nimbin | PAH/PMA/NR | [119] |
| Shell | 5.0 μm | Small molecule | Vitamin B12 | PAH/PSS | [100] |
| Shell | 5.0 μm | Protein | BSA | PAH/PSS | [105] |
| Shell | 4.2–6.3 μm | - | - | PAH/PSS/QD | [36] |
| Shell | 3–4 μm | Protein | Ovalbumin, horseradish peroxidase | pArg/DS | [104] |
| Shell | 3 μm | Nucleic acid | G-quadruplex DNA, double stranded DNA | PMA/PVPON | [29] |
| Shell | 0.65, 3.3 μm | Nucleic acid | mRNA, siRNA | pArg/DS | [107] |
| Shell | ∼3 μm | Nucleic acid | mRNA, pDNA, plasmid | pArg/DS/SiO2 | [106] |
| Shell | 1–4 μm | Fluorescent dye | Tetramethylrhodamine dextran | PAH/DNA | [120] |
| Shell | 2.84 μm | Labeled protein | BSA-Cy7 | pArg/DS | [110] |
| Shell | 3–5 μm | Small molecule | Doxorubicin | Chitosan/alginate | [69] |
| Shell | 3–6 μm | Labeled protein | FITC-BSA | PLL/CS | [121] |
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bahrom H, Goncharenko AA, Fatkhutdinova LI, Peltek OO, Muslimov AR, Koval OY, et al. Controllable Synthesis of Calcium Carbonate with Different Geometry: Comprehensive Analysis of Particle Formation, Cellular Uptake, and Biocompatibility. ACS Sustainable Chemistry and Engineering. 2019;7(23):19142-56. [CrossRef]
- Trushina DB, Bukreeva T V., Antipina MN. Size-Controlled Synthesis of Vaterite Calcium Carbonate by the Mixing Method: Aiming for Nanosized Particles. Crystal Growth and Design. 2016;16(3):1311-9. [CrossRef]
- Vostrikova A V., Prikhozhdenko ES, Mayorova OA, Goryacheva IY, Tarakina N V., Sukhorukov GB, et al. Thermal carbonization in nanoscale reactors: Controlled formation of carbon nanodots inside porous CaCO3 microparticles. Scientific Reports. 2018;8(1):1-7. [CrossRef]
- Trofimov AD, Ivanova AA, Zyuzin M V., Timin AS. Porous inorganic carriers based on silica, calcium carbonate and calcium phosphate for controlled/modulated drug delivery: Fresh outlook and future perspectives. Vol. 10, Pharmaceutics. 2018. [CrossRef]
- Lam SF, Bishop KW, Mintz R, Fang L, Achilefu S. Calcium carbonate nanoparticles stimulate cancer cell reprogramming to suppress tumor growth and invasion in an organ-on-a-chip system. Scientific Reports. 2021;11(1):1-12. [CrossRef]
- Ševčík R, Šašek P, Viani A. Physical and nanomechanical properties of the synthetic anhydrous crystalline CaCO3 polymorphs: vaterite, aragonite and calcite. Journal of Materials Science. 2018;53(6):4022-33. [CrossRef]
- Svenskaya YI, Fattah H, Inozemtseva OA, Ivanova AG, Shtykov SN, Gorin DA, et al. Key Parameters for Size- and Shape-Controlled Synthesis of Vaterite Particles. Crystal Growth and Design. 2018;18(1):331-7. [CrossRef]
- Han C, Hu Y, Wang K, Luo G. Preparation and in-situ surface modification of CaCO3 nanoparticles with calcium stearate in a microreaction system. Powder Technology. 2019;356:414-22. [CrossRef]
- Niu YQ, Liu JH, Aymonier C, Fermani S, Kralj D, Falini G, et al. Calcium carbonate: controlled synthesis, surface functionalization, and nanostructured materials. Chemical Society Reviews. 2022;51(18):7883-943. [CrossRef]
- Fadia P, Tyagi S, Bhagat S, Nair A, Panchal P, Dave H, et al. Calcium carbonate nano- and microparticles: synthesis methods and biological applications. 3 Biotech. 2021;11(11):1-30. [CrossRef]
- Byrappa K, Ohara S, Adschiri T. Nanoparticles synthesis using supercritical fluid technology - towards biomedical applications. Advanced Drug Delivery Reviews. 2008;60(3):299-327. [CrossRef]
- Pai RK, Pillai S. Nanoparticles of amorphous calcium carbonate by miniemulsion: Synthesis and mechanism. CrystEngComm. 2008;10(7):865-72. [CrossRef]
- Anton N, Benoit JP, Saulnier P. Design and production of nanoparticles formulated from nano-emulsion templates-A review. Journal of Controlled Release. 2008;128(3):185-99. [CrossRef]
- Babou-Kammoe R, Hamoudi S, Larachi F, Belkacemi K. Synthesis of CaCO 3 nanoparticles by controlled precipitation of saturated carbonate and calcium nitrate aqueous solutions. The Canadian Journal of Chemical Engineering. 15 févr 2012;90(1):26-33. [CrossRef]
- Campbell J, Abnett J, Kastania G, Volodkin D, Vikulina AS. Which biopolymers are better for the fabrication of multilayer capsules? A comparative study using vaterite CaCO3as templates. ACS Applied Materials and Interfaces. 2021;13(2):3259-69. [CrossRef]
- Chesneau C, Larue L, Belbekhouche S. Design of Tailor-Made Biopolymer-Based Capsules for Biological Application by Combining Porous Particles and Polysaccharide Assembly. Pharmaceutics. 2023;15(6). [CrossRef]
- Tan C, Dima C, Huang M, Assadpour E, Wang J, Sun B, et al. Advanced CaCO3-derived delivery systems for bioactive compounds. Advances in Colloid and Interface Science. 2022;309(September):102791. [CrossRef]
- Kalenichenko D, Nifontova G, Karaulov A, Sukhanova A, Nabiev I. Designing functionalized polyelectrolyte microcapsules for cancer treatment. Nanomaterials. 2021;11(11):3055. [CrossRef]
- Li G, Zhao Y, Zhang J, Hao J, Xu D, Cao Y. CaCO3 loaded lipid microspheres prepared by the solid-in-oil-in-water emulsions technique with propylene glycol alginate and xanthan gum. Frontiers in Nutrition. 2022;9(1). [CrossRef]
- Yoshida K, Ono T, Kashiwagi Y, Takahashi S, Sato K, Anzai JI. pH-dependent release of insulin from layer-by-layer-deposited polyelectrolyte microcapsules. Polymers. 2015;7(7):1269-78.
- Dou J, Zhao F, Fan W, Chen Z, Guo X. Preparation of non-spherical vaterite CaCO3 particles by flash nano precipitation technique for targeted and extended drug delivery. Journal of Drug Delivery Science and Technology. 2020;57(April):101768. [CrossRef]
- Gundogdu D, Alemdar C, Turan C, Hazal Husnugil H, Banerjee S, Erel-Goktepe I. Tuning stimuli-responsive properties of alginate hydrogels through layer-by-layer functionalization for dual-responsive dual drug release. Colloids and Surfaces A: Physicochemical and Engineering Aspects. 2023;676(PA):132213. [CrossRef]
- Decher G, Hong JD, Schmitt J. Buildup of ultrathin multilayer films by a self-assembly process: III. Consecutively alternating adsorption of anionic and cationic polyelectrolytes on charged surfaces. Thin Solid Films. 1992;210-211(PART 2):831-5. [CrossRef]
- Decher G, Hong JD. Buildup of ultrathin multilayer films by a self-assembly process, 1 consecutive adsorption of anionic and cationic bipolar amphiphiles on charged surfaces. Makromolekulare Chemie Macromolecular Symposia. 2 juin 1991;46(1):321-7.
- Vikulina AS, Campbell J. Biopolymer-Based Multilayer Capsules and Beads Made via Templating : Advantages , Hurdles and Perspectives. Nanomaterials. 2021;11:2502.
- Volodkin DV, Madaboosi N, Blacklock J, Skirtach AG, Möhwald H. Surface-Supported Multilayers Decorated with Bio-active Material Aimed at Light-Triggered Drug Delivery. Langmuir. 15 déc 2009;25(24):14037-43. [CrossRef]
- De Geest BG, Skirtach AG, Mamedov AA, Antipov AA, Kotov NA, De Smedt SC, et al. Ultrasound-Triggered Release from Multilayered Capsules. Small. 2007;3(5):804-8. [CrossRef]
- Déjugnat C, Sukhorukov GB. pH-Responsive Properties of Hollow Polyelectrolyte Microcapsules Templated on Various Cores. Langmuir. 1 août 2004;20(17):7265-9.
- Alford A, Tucker B, Kozlovskaya V, Chen J, Gupta N, Caviedes R, et al. Encapsulation and ultrasound-triggered release of G-Quadruplex DNA in multilayer hydrogel microcapsules. Polymers. 2018;10(12). [CrossRef]
- Campbell J, Kastania G, Volodkin D. Encapsulation of Low-Molecular-Weight Drugs into Polymer Multilayer Capsules Templated on Vaterite CaCO 3 Crystals. micromachines. 2020;11(717). [CrossRef]
- Li S, Lian B. Application of Calcium Carbonate as a Controlled Release Carrier for Therapeutic Drugs. Minerals. 2023;13(9):1-11. [CrossRef]
- Gileva A, Trushina D, Yagolovich A, Gasparian M, Kurbanova L, Smirnov I, et al. Doxorubicin-Loaded Polyelectrolyte Multilayer Capsules Modified with Antitumor DR5-Specific TRAIL Variant for Targeted Drug Delivery to Tumor Cells. Nanomaterials. 2023;13(5). [CrossRef]
- Fujiwara M, Shiokawa K, Araki M, Ashitaka N, Morigaki K, Kubota T, et al. Encapsulation of proteins into CaCO3 by phase transition from vaterite to calcite. Crystal Growth and Design. 2010;10(9):4030-7. [CrossRef]
- Zhao D, Wang CQ, Zhuo RX, Cheng SX. Modification of nanostructured calcium carbonate for efficient gene delivery. Colloids and Surfaces B: Biointerfaces. 2014;118:111-6. [CrossRef]
- Popova V, Poletaeva Y, Chubarov A, Dmitrienko E. pH-Responsible Doxorubicin-Loaded Fe3O4@CaCO3 Nanocomposites for Cancer Treatment. Pharmaceutics. 26 févr 2023;15(3):771.
- Nifontova G, Ramos-Gomes F, Baryshnikova M, Alves F, Nabiev I, Sukhanova A. Cancer cell targeting with functionalized quantum dot-encoded polyelectrolyte microcapsules. Frontiers in Chemistry. 2019;7(JAN):1-11. [CrossRef]
- Trushina DB, Borodina TN, Belyakov S, Antipina MN. Calcium carbonate vaterite particles for drug delivery: Advances and challenges. Materials Today Advances. 2022;14(2022):100214. [CrossRef]
- Ishikawa F, Murano M, Hiraishi M, Yamaguchi T, Tamai I, Tsuji A. Insoluble powder formulation as an effective nasal drug delivery system. Pharmaceutical Research. 2002;19(8):1097-104. [CrossRef]
- Volodkin D V., Larionova NI, Sukhorukov GB. Protein encapsulation via porous CaCO3 microparticles templating. Biomacromolecules. 2004;5(5):1962-72. [CrossRef]
- Parakhonskiy B V., Haase A, Antolini R. Sub-micrometer vaterite containers: Synthesis, substance loading, and release. Angewandte Chemie - International Edition. 2012;51(5):1195-7. [CrossRef]
- Farzan M, Roth R, Québatte G, Schoelkopf J, Huwyler J, Puchkov M. Loading of porous functionalized calcium carbonate microparticles: Distribution analysis with focused ion beam electron microscopy and mercury porosimetry. Pharmaceutics. 2019;11(1):1-14. [CrossRef]
- Levy CL, Matthews GP, Laudone GM, Beckett S, Turner A, Schoelkopf J, et al. Mechanism of adsorption of actives onto microporous functionalised calcium carbonate (FCC). Adsorption. 2017;23(4):603-12. [CrossRef]
- Binevski P V., Balabushevich NG, Uvarova VI, Vikulina AS, Volodkin D. Bio-friendly encapsulation of superoxide dismutase into vaterite CaCO3 crystals. Enzyme activity, release mechanism, and perspectives for ophthalmology. Colloids and Surfaces B: Biointerfaces. 2019;181(May):437-49.
- Feoktistova NA, Balabushevich NG, Skirtach AG, Volodkin D, Vikulina AS. Inter-protein interactions govern protein loading into porous vaterite CaCO3crystals. Physical Chemistry Chemical Physics. 2020;22(17):9713-22. [CrossRef]
- Li RG, Napoli E, Jorstad IS, Bønsdorff TB, Juzeniene A, Bruland ØS, et al. Calcium Carbonate Microparticles as Carriers of 224 Ra: Impact of Specific Activity in Mice with Intraperitoneal Ovarian Cancer. Current Radiopharmaceuticals. 2020;14(2):145-53. [CrossRef]
- Westrøm S, Malenge M, Jorstad IS, Napoli E, Bruland ØS, Bønsdorff TB, et al. Ra-224 labeling of calcium carbonate microparticles for internal α-therapy: Preparation, stability, and biodistribution in mice. Journal of Labelled Compounds and Radiopharmaceuticals. 30 mai 2018;61(6):472-86.
- Feoktistova NA, Vikulina AS, Balabushevich NG, Skirtach AG, Volodkin D. Bioactivity of catalase loaded into vaterite CaCO3 crystals via adsorption and co-synthesis. Materials & Design. 5 janv 2020;185:108223. [CrossRef]
- Preisig D, Haid D, Varum FJO, Bravo R, Alles R, Huwyler J, et al. Drug loading into porous calcium carbonate microparticles by solvent evaporation. European Journal of Pharmaceutics and Biopharmaceutics. 2014;87(3):548-58. [CrossRef]
- Roth R, Schoelkopf J, Huwyler J, Puchkov M. Functionalized calcium carbonate microparticles for the delivery of proteins. European Journal of Pharmaceutics and Biopharmaceutics. 2018;122(October 2017):96-103. [CrossRef]
- Ramalapa B, Crasson O, Vandevenne M, Gibaud A, Garcion E, Cordonnier T, et al. Protein-polysaccharide complexes for enhanced protein delivery in hyaluronic acid templated calcium carbonate microparticles. Journal of Materials Chemistry B. 2017;5(35):7360-8. [CrossRef]
- Vikulina AS, Feoktistova NA, Balabushevich NG, Skirtach AG, Volodkin D. The mechanism of catalase loading into porous vaterite CaCO3 crystals by co-synthesis. Physical Chemistry Chemical Physics. 2018;20(13):8822-31. [CrossRef]
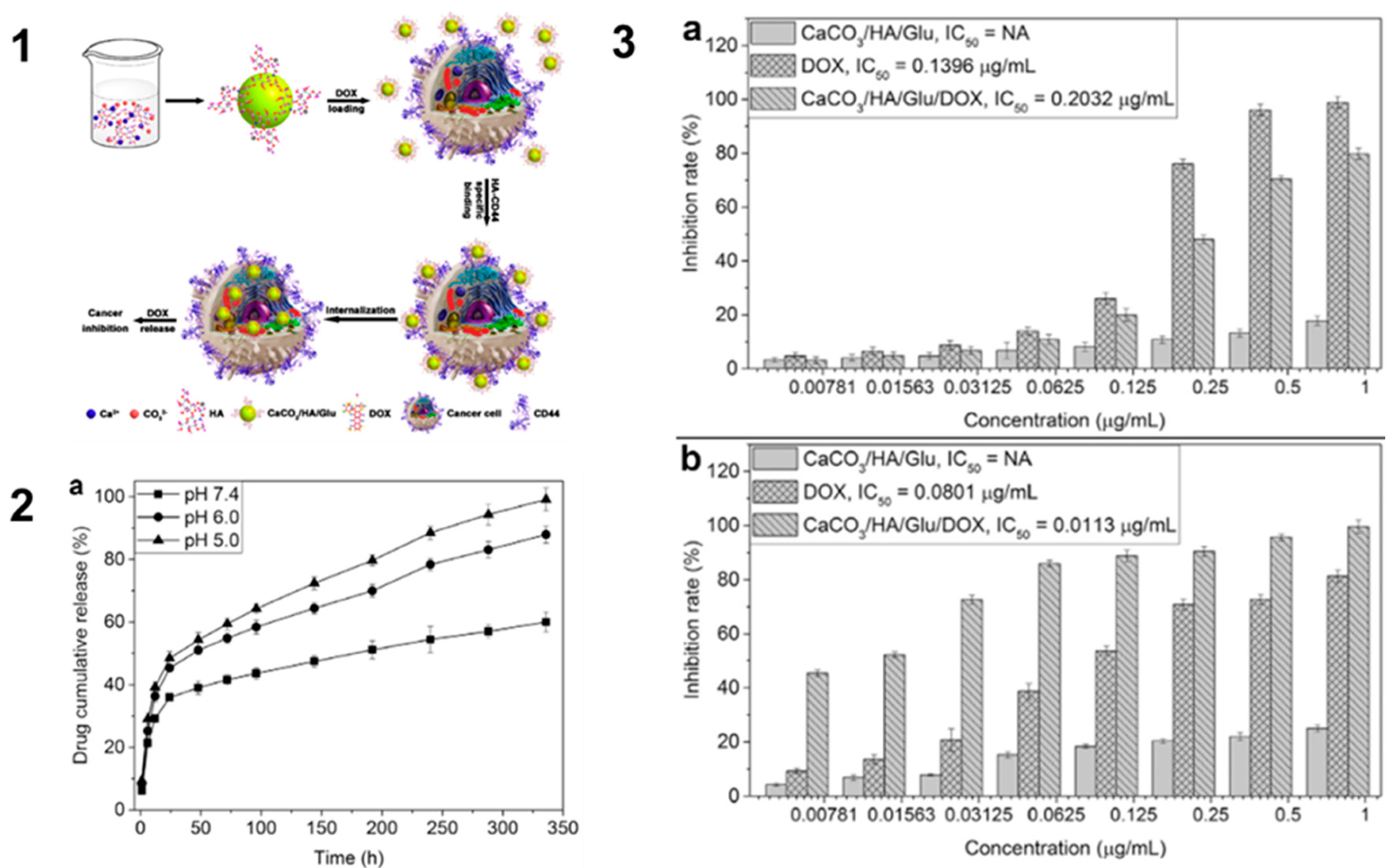
- Guo Y, Li H, Shi W, Zhang J, Feng J, Yang X, et al. Targeted delivery and pH-responsive release of doxorubicin to cancer cells using calcium carbonate/hyaluronate/glutamate mesoporous hollow spheres. Journal of Colloid and Interface Science. 2017;502:59-66.
- Feoktistova NA, Balabushevich NG, Skirtach AG, Volodkin D, Vikulina AS. Inter-protein interactions govern protein loading into porous vaterite CaCO3 crystals. Phys Chem Chem Phys. 6 mai 2020;22(17):9713-22. [CrossRef]
- Wang P, Kankala RK, Fan J, Long R, Liu Y, Wang S. Poly-L-ornithine/fucoidan-coated calcium carbonate microparticles by layer-by-layer self-assembly technique for cancer theranostics. J Mater Sci: Mater Med. 10 mai 2018;29(5):68. [CrossRef]
- Wang C, Chen S, Yu Q, Hu F, Yuan H. Taking advantage of the disadvantage: employing the high aqueous instability of amorphous calcium carbonate to realize burst drug release within cancer cells. J Mater Chem B. 15 mars 2017;5(11):2068-73. [CrossRef]
- Guo Y, Li H, Shi W, Zhang J, Feng J, Yang X, et al. Targeted delivery and pH-responsive release of doxorubicin to cancer cells using calcium carbonate/hyaluronate/glutamate mesoporous hollow spheres. Journal of Colloid and Interface Science. 15 sept 2017;502:59-66.
- Muslimov AR, Antuganov D, Tarakanchikova YV, Karpov TE, Zhukov MV, Zyuzin MV, et al. An investigation of calcium carbonate core-shell particles for incorporation of 225Ac and sequester of daughter radionuclides: in vitro and in vivo studies. Journal of Controlled Release. 10 févr 2021;330:726-37. [CrossRef]
- Lu J, Jiao Y, Cao G, Liu Z. Multimode CaCO3/pneumolysin antigen delivery systems for inducing efficient cellular immunity for anti-tumor immunotherapy. Chemical Engineering Journal. 15 sept 2021;420:129746.
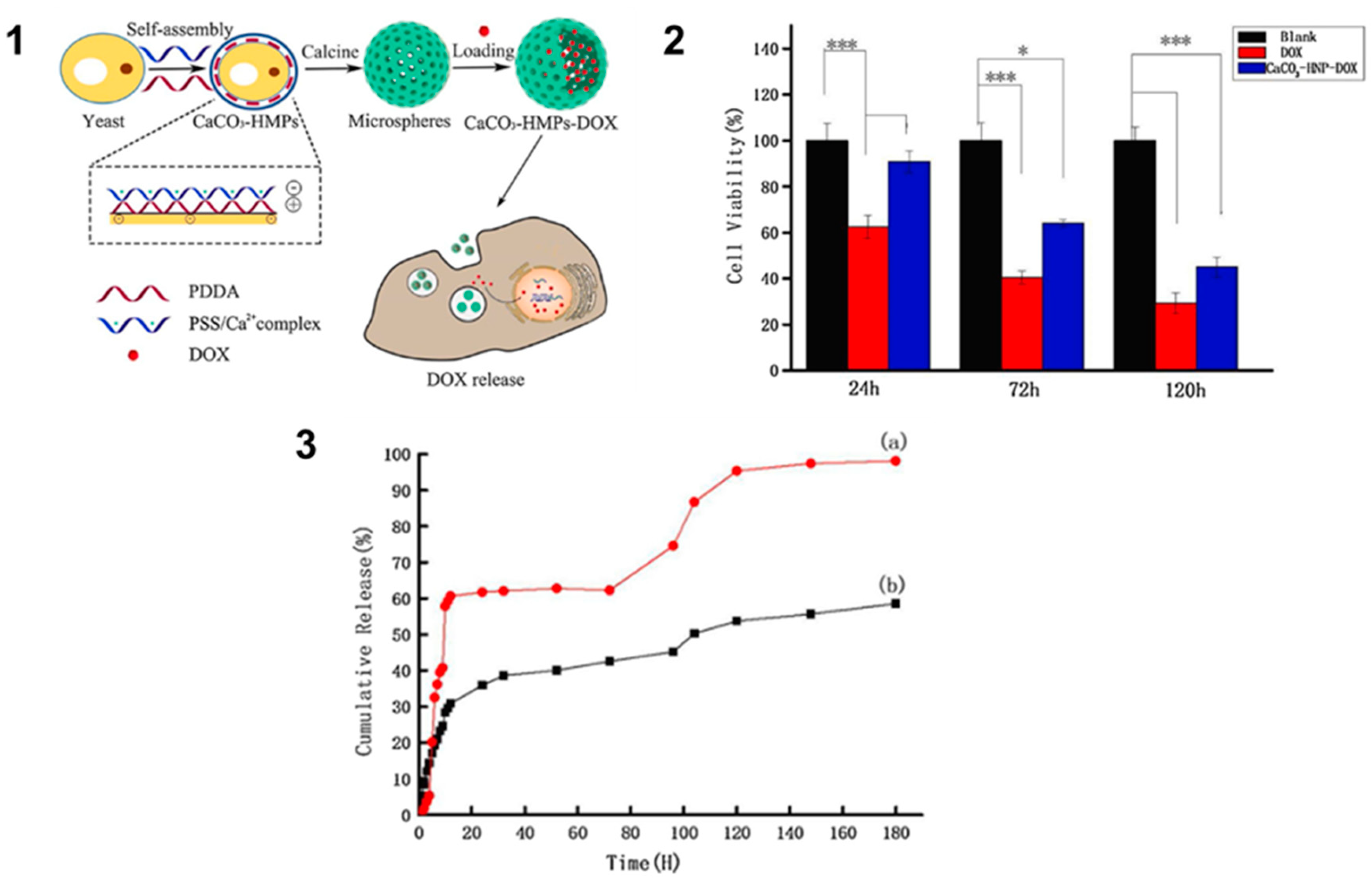
- Wei Y, Sun R, Su H, Xu H, Zhang L, Huang D, et al. Synthesis and characterization of porous CaCO3 microspheres templated by yeast cells and the application as pH value-sensitive anticancer drug carrier. Colloids and Surfaces B: Biointerfaces. 1 mars 2021;199:111545. [CrossRef]
- Ramalapa B, Crasson O, Vandevenne M, Gibaud A, Garcion E, Cordonnier T, et al. Protein–polysaccharide complexes for enhanced protein delivery in hyaluronic acid templated calcium carbonate microparticles. J Mater Chem B. 13 sept 2017;5(35):7360-8. [CrossRef]
- Kudryavtseva VL, Zhao L, Tverdokhlebov SI, Sukhorukov GB. Fabrication of PLA/CaCO3 hybrid micro-particles as carriers for water-soluble bioactive molecules. Colloids and Surfaces B: Biointerfaces. 1 sept 2017;157:481-9. [CrossRef]
- Bewernitz MA, Lovett AC, Gower LB. Liquid–Solid Core-Shell Microcapsules of Calcium Carbonate Coated Emulsions and Liposomes. Applied Sciences. janv 2020;10(23):8551. [CrossRef]
- Wei Y, Sun R, Su H, Xu H, Zhang L, Huang D, et al. Synthesis and characterization of porous CaCO3 microspheres templated by yeast cells and the application as pH value-sensitive anticancer drug carrier. Colloids and Surfaces B: Biointerfaces. 2021;199(September 2020):111545. [CrossRef]
- Lybaert L, Ryu KA, Nuhn L, De Rycke R, De Wever O, Chon AC, et al. Cancer Cell Lysate Entrapment in CaCO3 Engineered with Polymeric TLR-Agonists: Immune-Modulating Microparticles in View of Personalized Antitumor Vaccination. Chemistry of Materials. 2017;29(10):4209-17. [CrossRef]
- Kudryavtseva VL, Zhao L, Tverdokhlebov SI, Sukhorukov GB. Fabrication of PLA/CaCO3 hybrid micro-particles as carriers for water-soluble bioactive molecules. Colloids and Surfaces B: Biointerfaces. 2017;157:481-9. [CrossRef]
- Bewernitz MA, Lovett AC, Gower LB. Liquid–solid core-shell microcapsules of calcium carbonate coated emulsions and liposomes. Applied Sciences (Switzerland). 2020;10(23):1-18. [CrossRef]
- Nifontova G, Tsoi T, Karaulov A, Nabiev I, Sukhanova A. Structure-function relationships in polymeric multilayer capsules designed for cancer drug delivery. Biomaterials Science. 2022;10(18):5092-115. [CrossRef]
- Nifontova G, Zvaigzne M, Baryshnikova M, Korostylev E, Ramos-Gomes F, Alves F, et al. Next-Generation Theranostic Agents Based on Polyelectrolyte Microcapsules Encoded with Semiconductor Nanocrystals: Development and Functional Characterization. Nanoscale Research Letters. 2018;13. [CrossRef]
- Zhao Q, Han B, Wang Z, Gao C, Peng C, Shen J. Hollow chitosan-alginate multilayer microcapsules as drug delivery vehicle: doxorubicin loading and in vitro and in vivo studies. Nanomedicine: Nanotechnology, Biology, and Medicine. 2007;3(1):63-74. [CrossRef]
- Szarpak A, Cui D, Dubreuil F, De Geest BG, De Cock LJ, Picart C, et al. Designing hyaluronic acid-based layer-by-layer capsules as a carrier for intracellular drug delivery. Biomacromolecules. 2010;11(3):713-20. [CrossRef]
- De Geest BG, Vandenbroucke RE, Guenther AM, Sukhorukov GB, Hennink WE, Sanders NN, et al. Intracellularly Degradable Polyelectrolyte Microcapsules. Advanced Materials. 18 avr 2006;18(8):1005-9. [CrossRef]
- Ariga K, Lvov YM, Kawakami K, Ji Q, Hill JP. Layer-by-layer self-assembled shells for drug delivery. Advanced Drug Delivery Reviews. 2011;63(9):762-71. [CrossRef]
- Prikhozhdenko ES, Gusliakova OI, Kulikov OA, Mayorova OA, Shushunova NA, Abdurashitov AS, et al. Target delivery of drug carriers in mice kidney glomeruli via renal artery. Balance between efficiency and safety. Journal of Controlled Release. 10 janv 2021;329:175-90. [CrossRef]
- Zheng P, Ding B, Shi R, Jiang Z, Xu W, Li G, et al. A Multichannel Ca2+ Nanomodulator for Multilevel Mitochondrial Destruction-Mediated Cancer Therapy. Advanced Materials. 2021;33(15):2007426. [CrossRef]
- Nifontova G, Ramos-Gomes F, Baryshnikova M, Alves F, Nabiev I, Sukhanova A. Cancer Cell Targeting With Functionalized Quantum Dot-Encoded Polyelectrolyte Microcapsules. Front Chem. 30 janv 2019;7:34. [CrossRef]
- Flemke J, Maywald M, Sieber V. Encapsulation of Living E. coli Cells in Hollow Polymer Microspheres of Highly Defined Size. Biomacromolecules. 14 janv 2013;14(1):207-14. [CrossRef]
- Gileva A, Trushina D, Yagolovich A, Gasparian M, Kurbanova L, Smirnov I, et al. Doxorubicin-Loaded Polyelectrolyte Multilayer Capsules Modified with Antitumor DR5-Specific TRAIL Variant for Targeted Drug Delivery to Tumor Cells. Nanomaterials. janv 2023;13(5):902. [CrossRef]
- Gundogdu D, Alemdar C, Turan C, Husnugil HH, Banerjee S, Erel-Goktepe I. Tuning Stimuli-responsive Properties of Alginate Hydrogels through Layer-by-layer Functionalization for Dual-responsive Dual Drug Release. Colloids and Surfaces A: Physicochemical and Engineering Aspects. 2023;132213. [CrossRef]
- Kalenichenko D, Nifontova G, Karaulov A, Sukhanova A, Nabiev I. Designing Functionalized Polyelectrolyte Microcapsules for Cancer Treatment. Nanomaterials. nov 2021;11(11):3055. [CrossRef]
- Mihai M, Racovita S, Vasiliu AL, Doroftei F, Barbu-Mic C, Schwarz S, et al. Autotemplate Microcapsules of CaCO3/Pectin and Nonstoichiometric Complexes as Sustained Tetracycline Hydrochloride Delivery Carriers. ACS Appl Mater Interfaces. 25 oct 2017;9(42):37264-78.
- Navolokin N, Lomova M, Bucharskaya A, Godage O, Polukonova N, Shirokov A, et al. Antitumor Effects of Microencapsulated Gratiola officinalis Extract on Breast Carcinoma and Human Cervical Cancer Cells In Vitro. Materials. janv 2023;16(4):1470. [CrossRef]
- Nehru S, Guru A, Pachaiappan R, Hatamleh AA, Al-Dosary MA, Arokiyaraj S, et al. Co-encapsulation and release of apigenin and ascorbic acid in polyelectrolyte multilayer capsules for targeted polycystic ovary syndrome. Int J Pharm. 28 déc 2023;651:123749. [CrossRef]
- Novoselova MV, Loh HM, Trushina DB, Ketkar A, Abakumova TO, Zatsepin TS, et al. Biodegradable Polymeric Multilayer Capsules for Therapy of Lung Cancer. ACS Appl Mater Interfaces. 5 févr 2020;12(5):5610-23. [CrossRef]
- Trushina DB, Bukreeva TV, Borodina TN, Belova DD, Belyakov S, Antipina MN. Heat-driven size reduction of biodegradable polyelectrolyte multilayer hollow capsules assembled on CaCO3 template. Colloids and Surfaces B: Biointerfaces. 2018;170:312-21. [CrossRef]
- De Temmerman ML, Rejman J, Grooten J, De Beer T, Vervaet C, Demeester J, et al. Lyophilization of Protein-Loaded Polyelectrolyte Microcapsules. Pharm Res. 1 juill 2011;28(7):1765-73. [CrossRef]
- Maiorova LA, Erokhina SI, Pisani M, Barucca G, Marcaccio M, Koifman OI, et al. Encapsulation of vitamin B12 into nanoengineered capsules and soft matter nanosystems for targeted delivery. Colloids and Surfaces B: Biointerfaces. 1 oct 2019;182:110366. [CrossRef]
- Musin EV, Kim AL, Tikhonenko SA. Destruction of Polyelectrolyte Microcapsules Formed on CaCO3 Microparticles and the Release of a Protein Included by the Adsorption Method. Polymers (Basel). 1 mars 2020;12(3):520. [CrossRef]
- Alford A, Tucker B, Kozlovskaya V, Chen J, Gupta N, Caviedes R, et al. Encapsulation and Ultrasound-Triggered Release of G-Quadruplex DNA in Multilayer Hydrogel Microcapsules. Polymers (Basel). 5 déc 2018;10(12):1342. [CrossRef]
- Tarakanchikova YV, Muslimov AR, Zyuzin MV, Nazarenko I, Timin AS, Sukhorukov GB, et al. Layer-by-Layer-Assembled Capsule Size Affects the Efficiency of Packaging and Delivery of Different Genetic Cargo. Particle & Particle Systems Characterization. 2021;38(2):2000228. [CrossRef]
- Timin AS, Muslimov AR, Lepik KV, Epifanovskaya OS, Shakirova AI, Mock U, et al. Efficient gene editing via non-viral delivery of CRISPR–Cas9 system using polymeric and hybrid microcarriers. Nanomedicine: Nanotechnology, Biology and Medicine. 1 janv 2018;14(1):97-108. [CrossRef]
- De Geest BG, Vandenbroucke RE, Guenther AM, Sukhorukov GB, Hennink WE, Sanders NN, et al. Intracellularly Degradable Polyelectrolyte Microcapsules. Advanced Materials. 2006;18(8):1005-9. [CrossRef]
- Yoshida K, Ono T, Kashiwagi Y, Takahashi S, Sato K, Anzai J ichi. pH-Dependent Release of Insulin from Layer-by-Layer-Deposited Polyelectrolyte Microcapsules. Polymers. juill 2015;7(7):1269-78.
- Szarpak A, Cui D, Dubreuil F, De Geest BG, De Cock LJ, Picart C, et al. Designing Hyaluronic Acid-Based Layer-by-Layer Capsules as a Carrier for Intracellular Drug Delivery. Biomacromolecules. 8 mars 2010;11(3):713-20. [CrossRef]
- Belbekhouche S, Charaabi S, Carbonnier B. Glucose-sensitive capsules based on hydrogen-bonded (polyvinylpyrrolidone / phenylboronic –modified alginate) system. Colloids and Surfaces B: Biointerfaces. 2019;177(January):416-24.
- Novoselova M V., Loh HM, Trushina DB, Ketkar A, Abakumova TO, Zatsepin TS, et al. Biodegradable Polymeric Multilayer Capsules for Therapy of Lung Cancer. ACS Applied Materials and Interfaces. 2020;12(5):5610-23. [CrossRef]
- Nehru S, Guru A, Pachaiappan R, Hatamleh AA, Al-Dosary MA, Arokiyaraj S, et al. Co-encapsulation and release of apigenin and ascorbic acid in polyelectrolyte multilayer capsules for targeted polycystic ovary syndrome. International Journal of Pharmaceutics. 2024;651(December 2023):123749. [CrossRef]
- Navolokin N, Lomova M, Bucharskaya A, Godage O, Polukonova N, Shirokov A, et al. Antitumor Effects of Microencapsulated Gratiola officinalis Extract on Breast Carcinoma and Human Cervical Cancer Cells In Vitro. Materials. 2023;16(4). [CrossRef]
- Flemke J, Maywald M, Sieber V. Encapsulation of living E. coli cells in hollow polymer microspheres of highly defined size. Biomacromolecules. 2013;14(1):207-14. [CrossRef]
- Mihai M, Racovita S, Vasiliu AL, Doroftei F, Barbu-Mic C, Schwarz S, et al. Autotemplate Microcapsules of CaCO3/Pectin and Nonstoichiometric Complexes as Sustained Tetracycline Hydrochloride Delivery Carriers. ACS Applied Materials and Interfaces. 2017;9(42):37264-78.
- Maiorova LA, Erokhina SI, Pisani M, Barucca G, Marcaccio M, Koifman OI, et al. Encapsulation of vitamin B12 into nanoengineered capsules and soft matter nanosystems for targeted delivery. Colloids and Surfaces B: Biointerfaces. 2019;182(May). [CrossRef]
- Svenskaya Y, Garello F, Lengert E, Kozlova A, Verkhovskii R, Bitonto V, et al. Biodegradable polyelectrolyte/magnetite capsules for MR imaging and magnetic targeting of tumors. Nanotheranostics. 2021;5(3):362-77. [CrossRef]
- Borbora A, Manna U. Impact of chemistry on the preparation and post-modification of multilayered hollow microcapsules. Chemical Communications. 2021;57(17):2110-23. [CrossRef]
- Petrov AI, Volodkin D V., Sukhorukov GB. Protein-calcium carbonate coprecipitation: A tool for protein encapsulation. Biotechnology Progress. 2005;21(3):918-25. [CrossRef]
- De Temmerman ML, Rejman J, Grooten J, De Beer T, Vervaet C, Demeester J, et al. Lyophilization of protein-loaded polyelectrolyte microcapsules. Pharmaceutical Research. 2011;28(7):1765-73. [CrossRef]
- Musin E V., Kim AL, Tikhonenko SA. Destruction of polyelectrolyte microcapsules formed on CaCO3 microparticles and the release of a protein included by the adsorption method. Polymers. 2020;12(3):1-8. [CrossRef]
- Timin AS, Muslimov AR, Lepik K V., Epifanovskaya OS, Shakirova AI, Mock U, et al. Efficient gene editing via non-viral delivery of CRISPR–Cas9 system using polymeric and hybrid microcarriers. Nanomedicine: Nanotechnology, Biology, and Medicine. 2018;14(1):97-108. [CrossRef]
- Tarakanchikova Y V., Muslimov AR, Zyuzin M V., Nazarenko I, Timin AS, Sukhorukov GB, et al. Layer-by-Layer-Assembled Capsule Size Affects the Efficiency of Packaging and Delivery of Different Genetic Cargo. Particle and Particle Systems Characterization. 2021;38(2):1-10. [CrossRef]
- Machtakova M, Thérien-Aubin H, Landfester K. Polymer nano-systems for the encapsulation and delivery of active biomacromolecular therapeutic agents. Chemical Society Reviews. 2022;51(1):128-52. [CrossRef]
- Som A, Raliya R, Tian L, Akers W, Ippolito JE, Singamaneni S, et al. Monodispersed calcium carbonate nanoparticles modulate local pH and inhibit tumor growth in vivo. Nanoscale. 2016;8(25):12639-47. [CrossRef]
- Prikhozhdenko ES, Gusliakova OI, Kulikov OA, Mayorova OA, Shushunova NA, Abdurashitov AS, et al. Target delivery of drug carriers in mice kidney glomeruli via renal artery. Balance between efficiency and safety. Journal of Controlled Release. 2021;329(November 2020):175-90. [CrossRef]
- Muslimov AR, Antuganov D, Tarakanchikova Y V., Karpov TE, Zhukov M V., Zyuzin M V., et al. An investigation of calcium carbonate core-shell particles for incorporation of 225Ac and sequester of daughter radionuclides: in vitro and in vivo studies. Journal of Controlled Release. 2021;330(September 2020):726-37. [CrossRef]
- d’Amora M, Liendo F, Deorsola FA, Bensaid S, Giordani S. Toxicological profile of calcium carbonate nanoparticles for industrial applications. Colloids and Surfaces B: Biointerfaces. 2020;190(March):110947. [CrossRef]
- Lu J, Jiao Y, Cao G, Liu Z. Multimode CaCO3/pneumolysin antigen delivery systems for inducing efficient cellular immunity for anti-tumor immunotherapy. Chemical Engineering Journal. 2021;420(P1):129746.
- Lin J, Huang L, Xiang R, Ou H, Li X, Chen A, et al. Blood compatibility evaluations of CaCO3 particles. Biomedical Materials (Bristol). 2021;16(5). [CrossRef]
- Wang P, Kankala RK, Fan J, Long R, Liu Y, Wang S. Poly-L-ornithine/fucoidan-coated calcium carbonate microparticles by layer-by-layer self-assembly technique for cancer theranostics. Journal of Materials Science: Materials in Medicine. 2018;29(5). [CrossRef]
- Wang C, Chen S, Yu Q, Hu F, Yuan H. Taking advantage of the disadvantage: employing the high aqueous instability of amorphous calcium carbonate to realize burst drug release within cancer cells. Journal of Materials Chemistry B. 2017;5(11):2068-73. [CrossRef]
- Antipov AA, Shchukin D, Fedutik Y, Petrov AI, Sukhorukov GB, Möhwald H. Carbonate microparticles for hollow polyelectrolyte capsules fabrication. Colloids and Surfaces A: Physicochemical and Engineering Aspects. 2003;224(1-3):175-83. [CrossRef]
- Trushina DB, Bukreeva T V., Borodina TN, Belova DD, Belyakov S, Antipina MN. Heat-driven size reduction of biodegradable polyelectrolyte multilayer hollow capsules assembled on CaCO3 template. Colloids and Surfaces B: Biointerfaces. 2018;170(March):312-21. [CrossRef]
- Sharma V, Vijay J, Ganesh MR, Sundaramurthy A. Multilayer capsules encapsulating nimbin and doxorubicin for cancer chemo-photothermal therapy. International Journal of Pharmaceutics. 2020;582(April):119350. [CrossRef]
- Lin YH, Singuru MMR, Marpaung DSS, Liao WC, Chuang MC. Ethylene Glycol-Manipulated Syntheses of Calcium Carbonate Particles and DNA Capsules toward Efficient ATP-Responsive Cargo Release. ACS Applied Bio Materials. 2023;6(8):3351-60. [CrossRef]
- Zhao Q, Li B. pH-controlled drug loading and release from biodegradable microcapsules. Nanomedicine: Nanotechnology, Biology, and Medicine. 2008;4(4):302-10.
 PEGylation : [55,73,74].
PEGylation : [55,73,74].  Vectorization : [75]. Encapsulated Molecules:
Vectorization : [75]. Encapsulated Molecules:  Drug : [76,77,78,79,80,81,82,83,84]
Drug : [76,77,78,79,80,81,82,83,84]  Protein : [85,86,87]
Protein : [85,86,87]  Nucleic acid : [88,89,90].
Nucleic acid : [88,89,90].  Shell composition : poly(allylamine hydrochloride)/poly(sodium 4-styrenesulfonate) [76,79,81,86,87,91,92] ; hyaluronic acid/poly(allylamine hydrochloride), hyaluronic acid/poly-L-lysine [93] ; poly(arginine)/dextran sulfate [77,84,85,90,91] ; polylactic acid/dextran sulfate [83,89] ; poly(allylamine hydrochloride)/dextran sulfate [82,92] ; poly(isopropyl oxazoline)/alginate [78] ; pectin/poly(allylamine hydrochloride) [80] ; poly(methacrylic acid)/poly(N-vinyl-2-pyrrolidone) [88].
Shell composition : poly(allylamine hydrochloride)/poly(sodium 4-styrenesulfonate) [76,79,81,86,87,91,92] ; hyaluronic acid/poly(allylamine hydrochloride), hyaluronic acid/poly-L-lysine [93] ; poly(arginine)/dextran sulfate [77,84,85,90,91] ; polylactic acid/dextran sulfate [83,89] ; poly(allylamine hydrochloride)/dextran sulfate [82,92] ; poly(isopropyl oxazoline)/alginate [78] ; pectin/poly(allylamine hydrochloride) [80] ; poly(methacrylic acid)/poly(N-vinyl-2-pyrrolidone) [88].
 PEGylation : [55,73,74].
PEGylation : [55,73,74].  Vectorization : [75]. Encapsulated Molecules:
Vectorization : [75]. Encapsulated Molecules:  Drug : [76,77,78,79,80,81,82,83,84]
Drug : [76,77,78,79,80,81,82,83,84]  Protein : [85,86,87]
Protein : [85,86,87]  Nucleic acid : [88,89,90].
Nucleic acid : [88,89,90].  Shell composition : poly(allylamine hydrochloride)/poly(sodium 4-styrenesulfonate) [76,79,81,86,87,91,92] ; hyaluronic acid/poly(allylamine hydrochloride), hyaluronic acid/poly-L-lysine [93] ; poly(arginine)/dextran sulfate [77,84,85,90,91] ; polylactic acid/dextran sulfate [83,89] ; poly(allylamine hydrochloride)/dextran sulfate [82,92] ; poly(isopropyl oxazoline)/alginate [78] ; pectin/poly(allylamine hydrochloride) [80] ; poly(methacrylic acid)/poly(N-vinyl-2-pyrrolidone) [88].
Shell composition : poly(allylamine hydrochloride)/poly(sodium 4-styrenesulfonate) [76,79,81,86,87,91,92] ; hyaluronic acid/poly(allylamine hydrochloride), hyaluronic acid/poly-L-lysine [93] ; poly(arginine)/dextran sulfate [77,84,85,90,91] ; polylactic acid/dextran sulfate [83,89] ; poly(allylamine hydrochloride)/dextran sulfate [82,92] ; poly(isopropyl oxazoline)/alginate [78] ; pectin/poly(allylamine hydrochloride) [80] ; poly(methacrylic acid)/poly(N-vinyl-2-pyrrolidone) [88].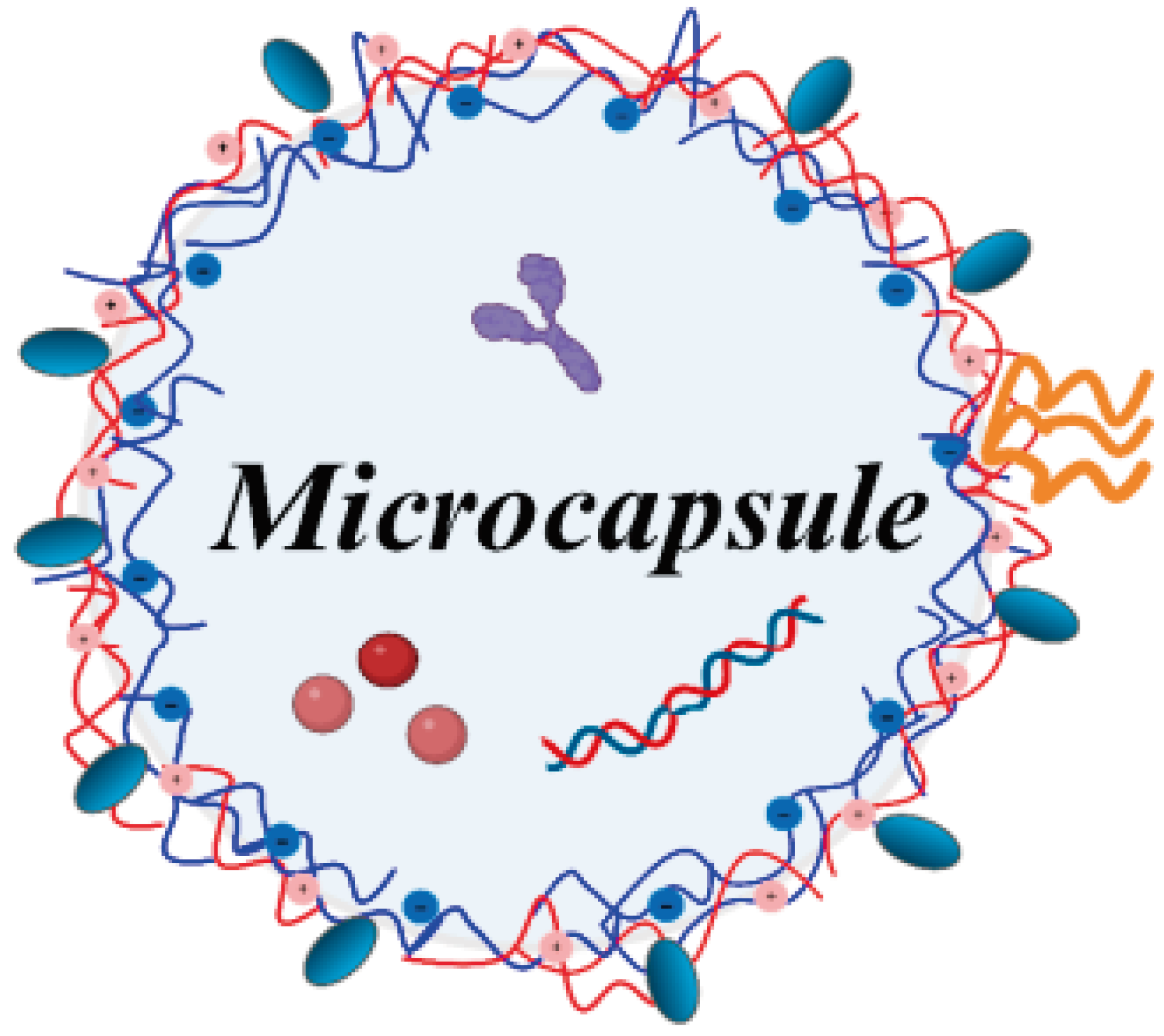

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





