1. Introduction
1.1. Escherichia coli
E. coli is a gram negative bacterium, facultative anaerobe, not sporogenous, belonging to the family of
Enterobacteriaceae [
1]. The majority of animal species' intestinal flora have
E. coli as the primary facultative anaerobe, which is often free of pathogenicity. However, many strains have evolved pathogenetic processes that enable them to cause a variety of illnesses in both humans and animals, including some extremely serious ones [
2].
E. coli can be classified into pathotypes based on their pathogenetic profile, which takes into account the virulence factors, the diseases caused and the phylogenetic profile [
3]. Among
E. coli causing enteric diseases, several pathotypes have been identified, namely intestinal pathogenic
E. coli (IPEC) which includes enteropathogenic
E. coli (EPEC), enterohemorrhagic
E. coli (EHEC), enterotoxigenic
E. coli (ETEC), enteroaggregative
E. coli (EAEC), diffusely adherent
E. coli (DAEC), enteroinvasive
E. coli (EIEC); and extraintestinal pathogenic
E. coli (ExPEC) which includes uropathogenic
E. coli (UPEC), neonatal meningitis
E. coli (NMEC), sepsis-associated
E. coli (SEPEC), avian pathogenic
E. coli (APEC), and mammary pathogenic
E. coli (MPEC) [
4].
Among the different pathotypes the group represented by Shiga toxin-producing
E. coli (STEC) is of particular interest. This group includes strains that produce at least one member of a class of potent cytotoxins called Shiga toxins. The STECs, also called Verotoxin producing
E. coli (VTEC), are named after the Shiga toxin (Stx), which is very similar to a cytotoxin produced by
Shigella dysenteriae serotype 1 [
5].
Among STEC strains the ones having particular pathogenicity for humans are often also referred as enterohemorragic
E. coli (EHEC). This pathotype is a zoonotic agent which causes a potentially fatal human illness whose clinical spectrum includes bloody diarrhea, hemorrhagic colitis (HC), and the hemolytic uremic syndrome (HUS) [
6]. Since 1982, among STEC strains, EHEC has been a major source of food safety concern. The first strain included in this group is
E. coli serotype O157: H7, and this strain is still the most widespread EHEC serotype in the United States of America and Europe [
7].
E. coli serotype O157:H7 is mostly associated with outbreaks and sporadic cases of HC and HUS in many countries; however, non-O157 STEC have been implicated in outbreaks around the world, and the number of reported cases has steadily increased every year. Centers for Disease Control and Prevention (CDC) has identified other six O groups, besides O157, to be of growing concern for public health and responsible for 71% of all illnesses caused by STEC: O26, O45, O103, O111, O121 and O145 [
8]. The European Food and Safety Authority (EFSA) has identified five serogroups O26, O103, O111, O145, O157 (“Big five”) [
7], as those of major concern to human health in Europe. Nowadays considerable attention is drawn to non-O157 STEC strains particularly after the occurrence of a severe foodborne outbreak happened in 2011 in Germany caused by consumption of sprouts contaminated by STEC O104:H4 [
9,
10,
11,
12,
13,
14].
1.2. Reservoirs
Ruminants, especially cattle, are a major reservoir of a diverse group of STEC despite they are not a source of diseases for these animals. Indeed, cattle are asymptomatic excretors of STECs which are permanent or transient members of their normal intestinal flora [
15].
Only the gastrointestinal tract of ruminants can be considered with certainty as a reservoir for these bacteria. The outbreaks investigated from 1982 up to now have highlighted how ruminants, and the bovine species in particular, are almost always involved in the transmission of these bacteria to humans [
15].
The persistence of STEC in the individual animal is due to the ability these bacteria have in colonizing specific portions of the gastrointestinal tract. The different possible interactions between the microorganism and its host influence the fecal elimination pattern: a low level (<10³ CFU/g of feces) and short duration (<10 days) elimination occurs when colonization it is limited to the rumen; low levels of elimination are also observed when colonization is extended to the cecum and colon but for longer periods (> 30 days) [
16].
1.3. Zoonotic spillover
Cattle farming is undoubtedly the major source of environmental contamination from STEC [
15], but the pathogens have also been recovered from goats, deers, horses, dogs, and birds [
5,
8,
13]. Works from various researchers have demonstrated that STEC infections in humans should not be associated only with cattle spillover, since there are several proofs of other sources of contamination like the outbreak of STEC happened in Norfolk (UK) which was related to wild rabbits. The high genetic similarity between STEC strains isolated from domestic pets (dogs and cats) and cattle, and the presence of STEC in wildlife animals like red deers and psitaccine birds highlight the possibility that new reservoirs can enhance human exposition and risk of infection [
18,
19,
20,
21].
Nonetheless, outbreaks of STEC are generally ascribed to the consumption of contaminated foods of bovine origin, especially undercooked ground beef patties and unpasteurized milk. For example, in studies of retail ground beef in North America, the prevalence of STEC ranged from 9% to 36.4%, with
E. coli O157 isolated from 0% to 3.7% of the samples tested [
22].Raw milk and raw milk-products are among the main food sources of STEC infection in humans; therefore, identification of pathogens at herd level is of primary importance for public health [
11]. Fecal contamination can be considered the only relevant route to explain the presence of STEC in raw milk. Therefore, the key point in the control of these pathogens is the reduction of fecal contamination of milk [
23].
The control of the circulation of STECs on the farm is complex and involves the herd management as a whole. Currently, only the managerial practices aimed at limiting the presence of STEC in milk are proposed. These measures include: the limitation of the circulation of STEC within the individual farm by hygiene measures (e.g. bedding hygiene, water supplies, alleys cleaning) and the minimization of fecal contamination of milk during milking [
24,
25].
1.4. STEC Detection Methods
In order to obtain laboratory confirmation of STEC infection, one of the following requirements needs to be fulfilled, according to the European Centre for Disease Prevention and Control (ECDC): direct detection of the nucleic acid of
stx1 or
stx2 gene(s) without strain isolation; isolation of nonsorbitol fermenting (NSF)
E. coli O157 (without testing for Stx or
stx genes); and isolation/cultivation of an
E. coli strain that produces Stx or harbors the relative gene(s) [
26].
As demonstrated by Dastmalchi et al. 2012 [
27] and Renter et al. 2004 [
28], molecular approaches for STEC identification in feces have only been used after bacterial colonies isolation on specific plates (e.g., MacConkey agar) from the aforementioned matrix or after an enrichment step of the matrix, no reports of direct molecular analysis on feces samples have been discovered in the literature. Indeed, after bacterial isolation, two sets of endpoint PCR or Real Time PCR are needed to confirm serotype identification and to evaluate the presence of virulence factors that identify
E. coli serotypes as
E. coli STEC; the entire procedure is time consuming (55/60 h), even though really precise in STEC identification [
29].
Serological methods are commonly used for STEC infection diagnosis, but even in this case most of the analysis cannot be performed directly on the sample but needs a prior step of bacterial isolation on agar plates or at least an enrichment step [
1]. To this day in literature there are several examples of different immunological assays (e.g., traditional ELISA, lateral flow immunoassay, monoclonal antibodies…) with common limitations like cross-reaction with other pathogens (i.e.,
Brucella abortus,
Yersinia enterocolitica,
Vibrio cholera,
Escherichia hermanni,
Citrobacter freundii,
Citrobacter sedlakii and
Salmonella) or even viruses like the two cases of norovirus outbreaks in the United States which yielded false positives for STEC infections [
26,
30,
31,
32].
The DNA-based methods for researching STECs have the advantage of being rapid, and not requiring special reagents, such as the specific Shiga anti-toxin antisera, nor the essential equipment for the use of cell cultures. There are numerous PCR methods for the search of
stx1 and
stx2 genes, capable of detecting the presence of all known Shiga toxin subtypes. These tests can be performed both on single bacterial colonies and on mixed cultures, such as enrichment media, or samples as such [
33].
1.5. Gap Analysis
For what concerns milk and milk products, the World Health Organization in 2018 summarized the main critical points to be considered when establishing surveillance and control programs related to STEC infections and food contaminations. The report, and consequently most of the national surveillance programs, focuses on raw milk and raw milk cheeses ready for retail completely neglecting the possibility to evaluate the source of contamination directly at the start of the dairy chain [
34], and more important, to reduce the risks for a spread of these pathogens within the herd.
This approach is the result of a lack of knowledge (gaps) in preventing the disease from spreading in dairy and beef herds at the beginning of the food chain, although the problems associated with STEC/EHEC foodborne disease have been recognized for several years. A recent and useful gap analysis made through the Discontools project [
35] highlighted the main critical points and gaps that need to be filled regarding STEC surveillance and control. A lot has been made through the years to improve the epidemiological landscape of this pathogen; the analysis, which can be examined in the Discontools website, goes deeply into illustrating all the issues and the relative possible solutions like diagnostic methods, vaccines development, alternative antibiotic therapy and epidemiology of the pathogen. Among the several issues reported, in our opinion, one of the most important is related to the epidemiologic analysis. In fact, the Discontools analysis [
35] identifies two major gaps in the understanding of STEC epidemiology: the mechanisms of spreading of the disease among herds and how animals are exposed within a farm. Strictly related to the epidemiological analysis, there are gaps in the diagnostic approach. Indeed, new diagnostic approaches and methods are needed to identify mainly non-O157 serotypes in carrier animals, and to assess the spread of these serotypes among animals, the contamination of food for human consumption, and human risk related to these foods [
35].
1.6. Aims of the Pilot Study
The presence of STEC in animals, the severity of the disease for human beings, and the role of the environment in maintain these pathogens, support the importance of this group of pathogens in a One Health framework. Moreover,the increasing number of reports on the presence of contaminated foods with STEC serotypes [
35], the probable underestimation of the presence of these pathogens in dairy herds [
11], and the gaps identified related to the epidemiology and detection of these pathogens in dairy herds supported the development of a project trying to fil these gaps, and to develop a new, effective surveillance program to be applied to dairy herds. Within this framework, a pilot study was designed:
to assess the viability of new molecular methodologies applied to raw milk filters enabling the identification of the presence of STEC serotypes in milk production, as a way to have an estimation of the prevalence of these pathogens in the herds;
to apply the same methods to identify the presence of these pathogens in calf feces, as a way to identify a potential way of spreading, but also a critical point for a potential prevention of the spread.
For what concerns milk production, we already have shown that milk filters may be a more useful matrix compared to raw milk, as a way to identify herds harboring STEC serotypes. Indeed, in-line filters or raw milk filters or (RMF) made by non-woven fabric are components of milking machines aimed to catch debris as well as feces particles. The filters are usually changed before milking. In the RMF, the dimension of the pores, usually 100–150 μm, are too big to prevent pathogenic bacteria to be retained by the filter, but previous studies showed their usefulness to identify pathogens [
36,
37,
38].
The presence of STEC in calf has been reported in few studies [
39,
40,
41], but at best of our knowledge, this approach was never thought as a way to identify potential vector animals within and between herds, and a potential critical point for control measures. The availability of new commercial molecular assays allows to identify non-O157 serotypes in milk and milk products which simplify and make more efficient the detection process. However, these methods were not assessed and validated for other biological matrix such as RMF and feces. These validations are pivotal to apply them to a surveillance program based on these matrices.
2. Materials and Methods
2.1. Herds and Animals
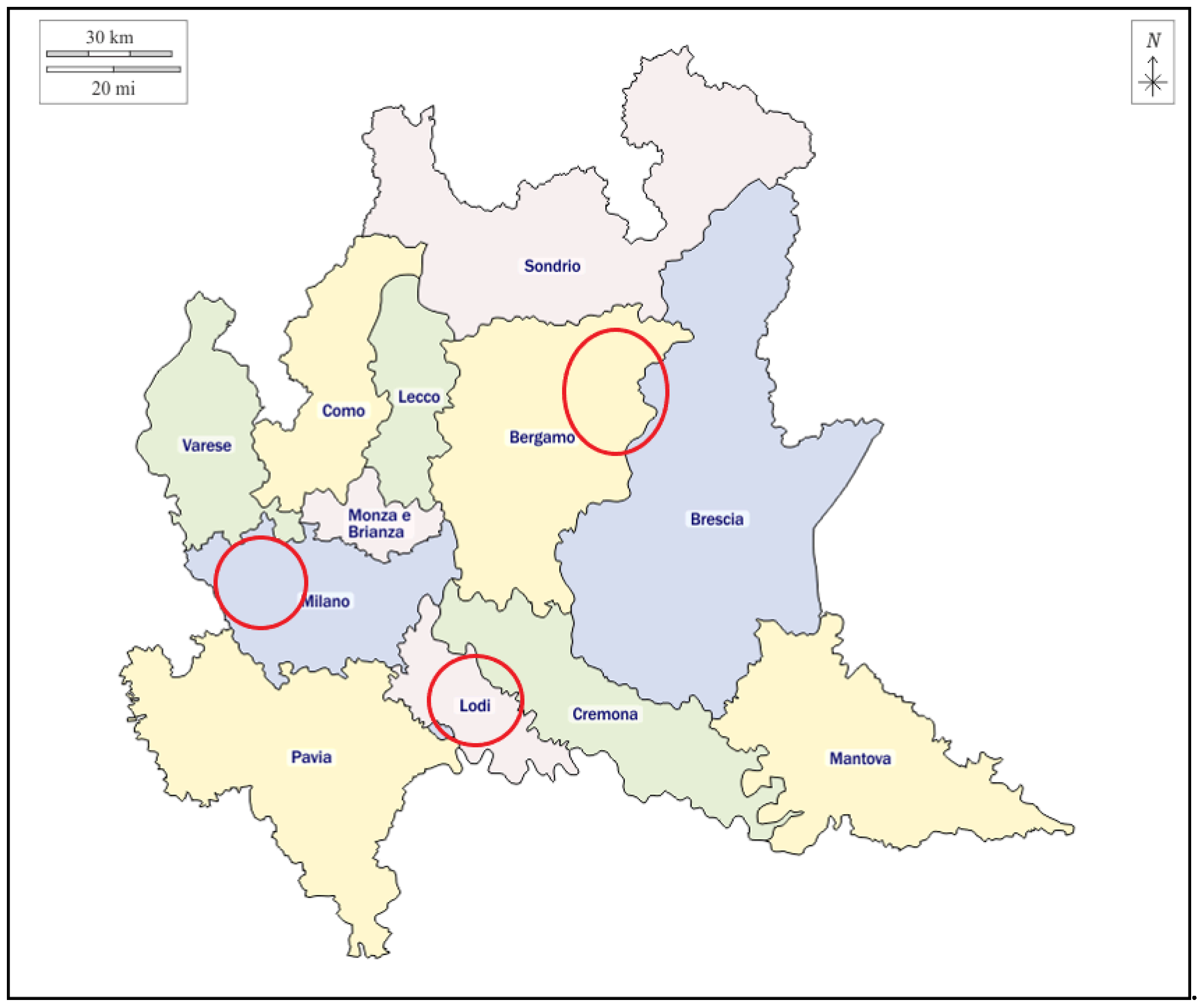
Bulk tank milk (BTM) and RMF samples were collected from 15 different dairy herds of the Lombardy region, while fecal samples were collected from calves belonging to 3 different dairy herds of the Milano province. Samples were divided by location and time of sampling.
2.2. Samples Collection
Milk and filter samples were collected by technicians of the Regional Breeding Association (ARAL) in different areas of Lombardy within the routine sampling for milk quality assessment. For raw milk analysis, about 25 ml of BTM were sampled, whereas the whole milk filter was taken after milking. For each sampling time both BTM and RMF were sampled in each herd. Calf feces were collected by herd veterinarians during routine protocols for enteritis prevention. Ten to fifteen g of feces were sampled directly from the rectal ampoule of the animal. Samples were collected in sterile tubes (milk and feces) and in disposable sterile bag for milk filters. All the samples were immediately frozen (-20°C), delivered to our laboratories and kept frozen (-20°C) until processed.
2.3. Samples Preparation
Prior to the enrichment step every sample has been thawed at room temperature (23±5°C) inside a laminar flow hood avoiding sample contamination. All the samples (raw milk, milk filters and bovine feces) have been prepared for the DNA extraction process following strict sterility procedures in order to protect the operator from the pathogen and to avoid contaminations that could lead to incorrect results. After thawing each sample has been put in a Falcon tube (50 ml falcon tube for milk filters and 15 ml falcon tubes for milk and bovine feces) and enriched with buffered peptone water (BPW) (Biomérieux, Marcy-l’Étoile, France) with a 1:10 ratio, as suggested by food sample enrichment protocol of Real Time PCR producer. The enriched samples have been incubated at 37ºC and 5% CO2 for 24 hours and then 1 mL of enriched sample has been transferred into a 1,5 mL tube to proceed with the extraction step or to be stored in a -20°C freezer.
2.4. DNA Extraction
The DNA extraction process has been carried out with the commercial SureTect™ STEC extraction kit (ThermoFisher Scientific, Waltham, Massachussets, USA). Briefly 10 µL of proteinase K (ThermoFisher Scientific, Waltham, Massachussets, USA) have been added to the side of the SureTect Lysis Tube, then 10 µL of diluted sample have been added to the bottom of the tube. The tubes have been capped and incubated in a thermoblock at 37°C for 10 minutes and then at 95°C for 5 minutes. After the incubation the supernatant, containing the sample’s DNA, has been used to proceed with the Real-Time PCR assay.
2.5. Real Time PCR Assay
2.5.1. Escherichia Coli O157:H7 and STEC Virulence Factors Identification
This commercial kit is based on TaqMan™ PCR technology. Dye-labeled probes target unique DNA sequences specific to E. coli STEC. This assay detects the presence of STEC stx, eae genes and E. coli O157:H7 serotype from food and environmental safety samples. The molecular designs of the primers and probes of this assay are proprietary and for this reason cannot be shown. To perform the assay 20 µL of the sample processed with the SureTect™ STEC extraction kit (ThermoFisher Scientific, Waltham, Massachussets, USA) have been loaded in the PCR tube in order to resuspend the lyophilized master mix already present in the tube. The tubes have been capped with optical cap strips and loaded on the Applied Biosystems™ QuantStudio™ 5 Food Safety System (ThermoFisher Scientific, Waltham, Massachussets, USA) to start the Real Time PCR run. The results have been analyzed with ThermoFisher Scientific RapidFinder™ Analysis Software v1.1. The PCR running conditions consisted of an initial denaturation at 95°C for 7 min followed by 50 cycles of denaturation at 95°C for 5 s, annealing and extension at 60°C for 45 s. The samples which resulted positive for at least the stx gene have been processed further with the ThermoFisher Scientific SureTect™ E. coli STEC Identification kit.
2.5.2. STEC Serotype Identification
The ThermoFisher Scientific SureTect™ E. coli STEC Identification commercial kit is used for the rapid qualitative detection of E. coli STEC serotypes (O26, O45, O103, O111, O121, O145) from food and environmental safety samples. To perform the assay 20 µL of the sample processed with the SureTect™ STEC extraction kit (ThermoFisher Scientific, Waltham, Massachussets, USA) have been loaded in the PCR tube in order to resuspend the lyophilized master mix already present in the tube. The tubes have been capped with optical cap strips and loaded on the Applied Biosystems™ QuantStudio™ 5 Food Safety System (ThermoFisher Scientific, Waltham, Massachussets, USA) to start the Real Time PCR run. The results have been analyzed with ThermoFisher Scientific RapidFinder™ Analysis Software v1.1. The PCR running conditions consisted of an initial denaturation at 95°C for 7 min followed by 50 cycles of denaturation at 95°C for 5 s, annealing and extension at 60°C for 45 s.
2.6. Protocol Validation
The diagnostic procedure previously described are aimed to process food and environmental samples. Therefore, we preliminary assessed the accuracy of these procedure applied to the different matrices we want to investigate (raw milk, raw milk filters and feces). Following the study of Albonico et al. [
11], we artificially contaminated negative samples of raw milk filters and calf feces with specific
E. coli STEC serotypes (O157, O26, O45, O103, O111, O121, O145), provided from European Union Reference Laboratory VTEC (ISS Rome, Italy), in order to assess the assay sensibility on samples different from food matrices. Each sample has been inoculated with 10 or 10
2 CFU of a single
E. coli serotype and then treated as described above. The negative samples were also tested without artificial contamination to make sure that these kind of matrices do not yield false positives with this specific assay.
2.7. Statistical Analysis
All the data were analyzed on SPSS 28.0.1.1 (IBM Corp., Armonk, NY, USA, 2022) and on XLSTAT 2023.1.1 (Lumivero, New York, USA). Statistical association between variables has been determined through χ2 test and Fisher’s exact test.
3. Results
3.1. Protocol Validation
The negative controls tested negative for all the target genes in the assay and all the contaminated controls tested positive for the expected virulence and serotype genes at both 10 and 102 CFU inoculum concentrations, enabling us to further proceed with the unknown BTM, RMF and feces.
3.2. Data Description
A total of 290 samples coming from 18 different dairy herds were collected and analyzed from January to December 2022 (
Figure 1). 88 were BTM, 104 RMF and 98 calves’ feces samples. Samples have been considered as positive results following a principle of maximum precaution and the criterion of direct detection of the nucleic acid of
stx1 or
stx2 gene(s) without strain isolation [
26]. All raw results of Real-Time PCR analysis are provided in supplementary table S1.
Regarding virulence genes identification, we found three BTM samples positive for the
stx gene, 10 for
eae gene and no sample positive for both genes. When we considered RMF a total of 6 samples tested positive for
stx presence, 25 for
eae presence and 37 for the presence of both genes (
Table 1).
When fecal samples were considered, 72 samples were positive for the
stx gene, 84 for the
eae gene and 71 samples for both genes. For pre-weaning samples one sample was positive for the
stx gene, 13 for the
eae gene and 71 for both genes; for post-weaning instead no samples were positive for
stx or
eae gene and 35 were positive for both genes. These results are summarized in
Table 2.
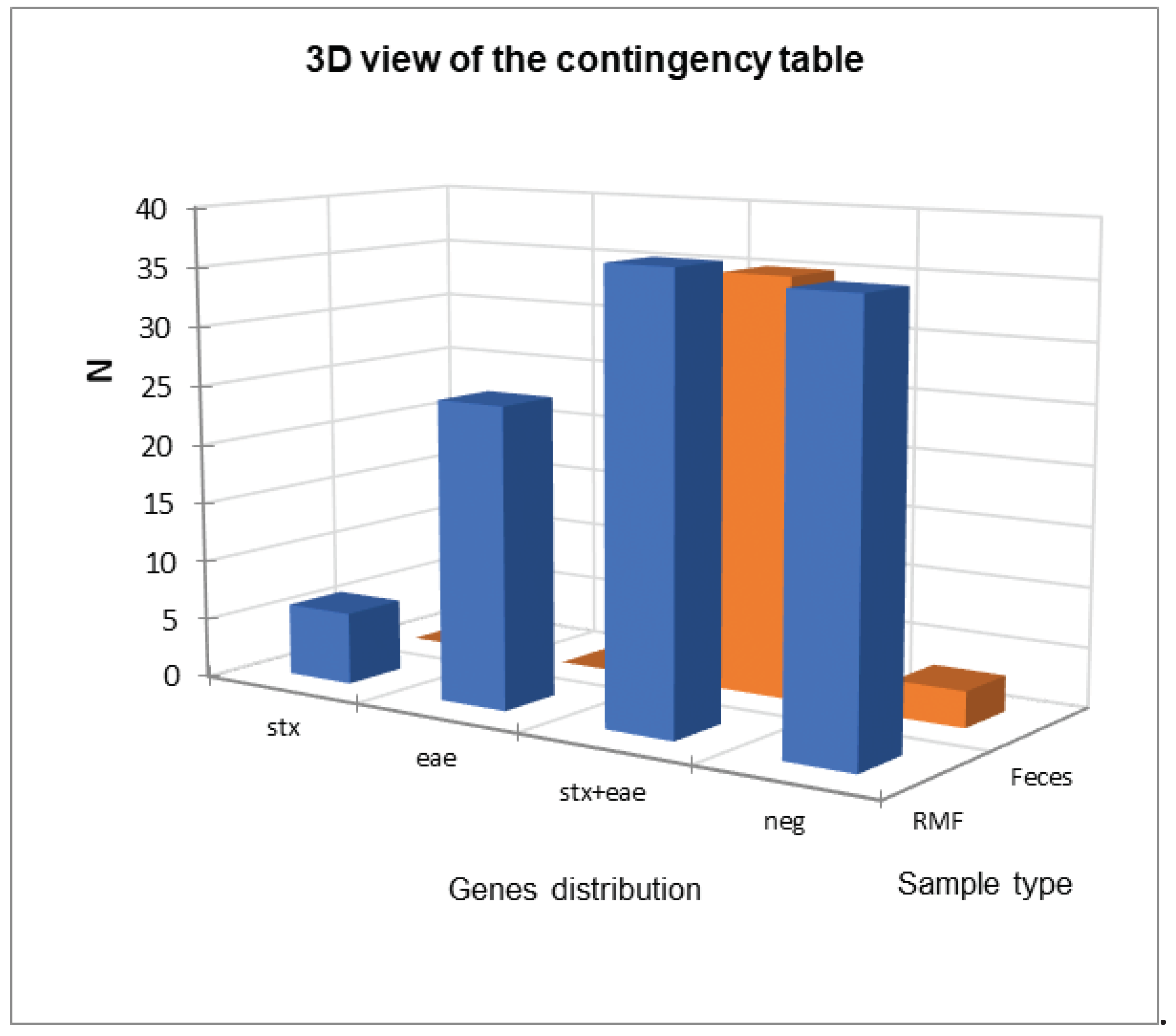
The comparison between the distributions of virulence genes in RMF and fecal samples was reported in
Figure 2, and the statistical analysis reported in
Table 3 showed a statistical difference (α=0.05) between them mainly due to a frequency higher-than-expected in
stx+eae positive fecal samples and, conversely a lower-than-expected frequency in RMF samples.
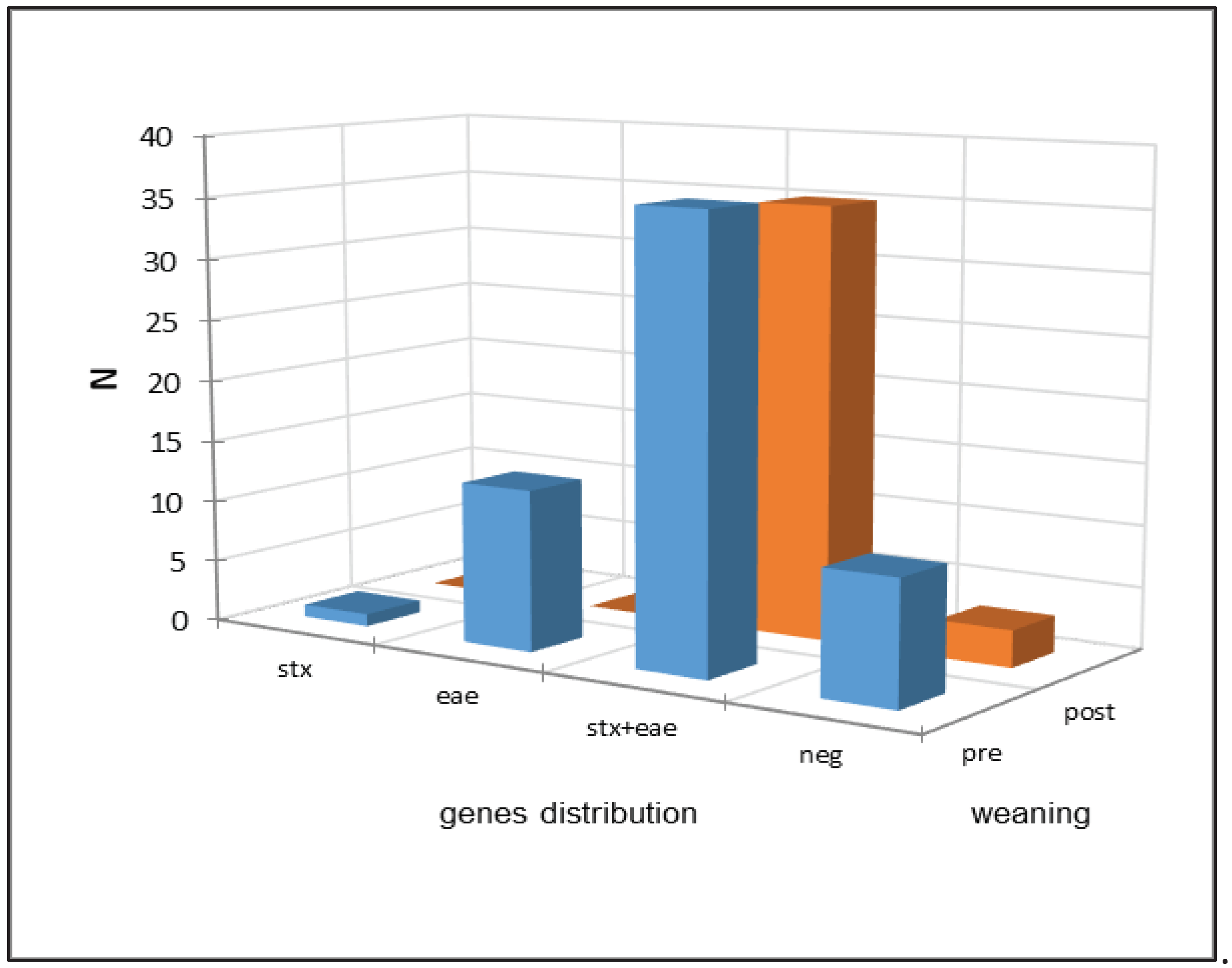
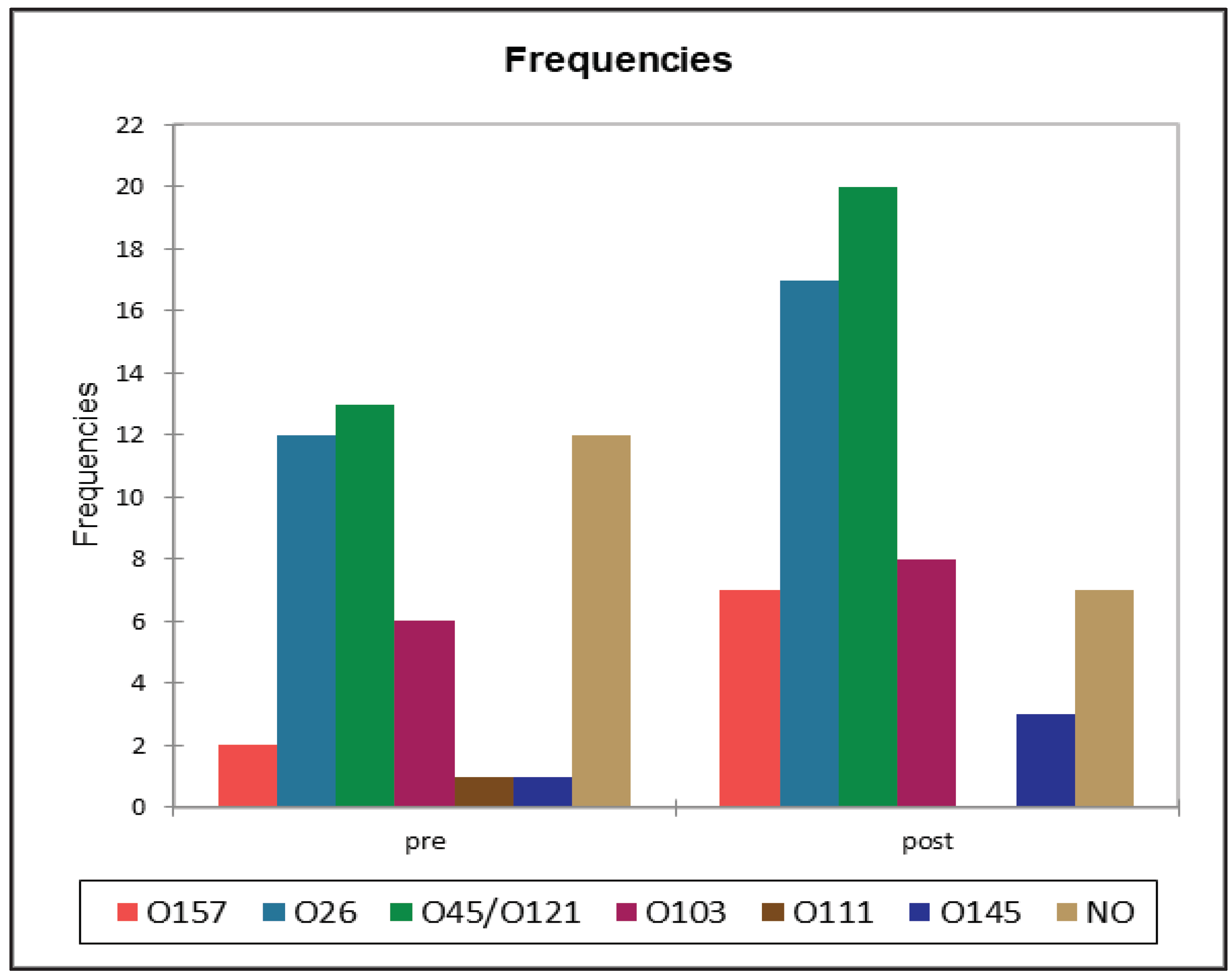
Figure 3 reports the comparison between the distributions of virulence genes in fecal samples taken before and after weaning and
Table 4 reports the results of the statistical analysis. The comparison between the distributions of virulence genes in pre- and post-weaning fecal samples (
Table 4) showed a statistical difference (α=0.05) between them mainly due to a frequency higher-than-expected in
stx+eae positives in post-weaning fecal samples as well as a lower-than-expected frequency of
eae positive samples. As expected, the pattern was reversed in pre-weaning samples.
3.3. Serotypes Distribution
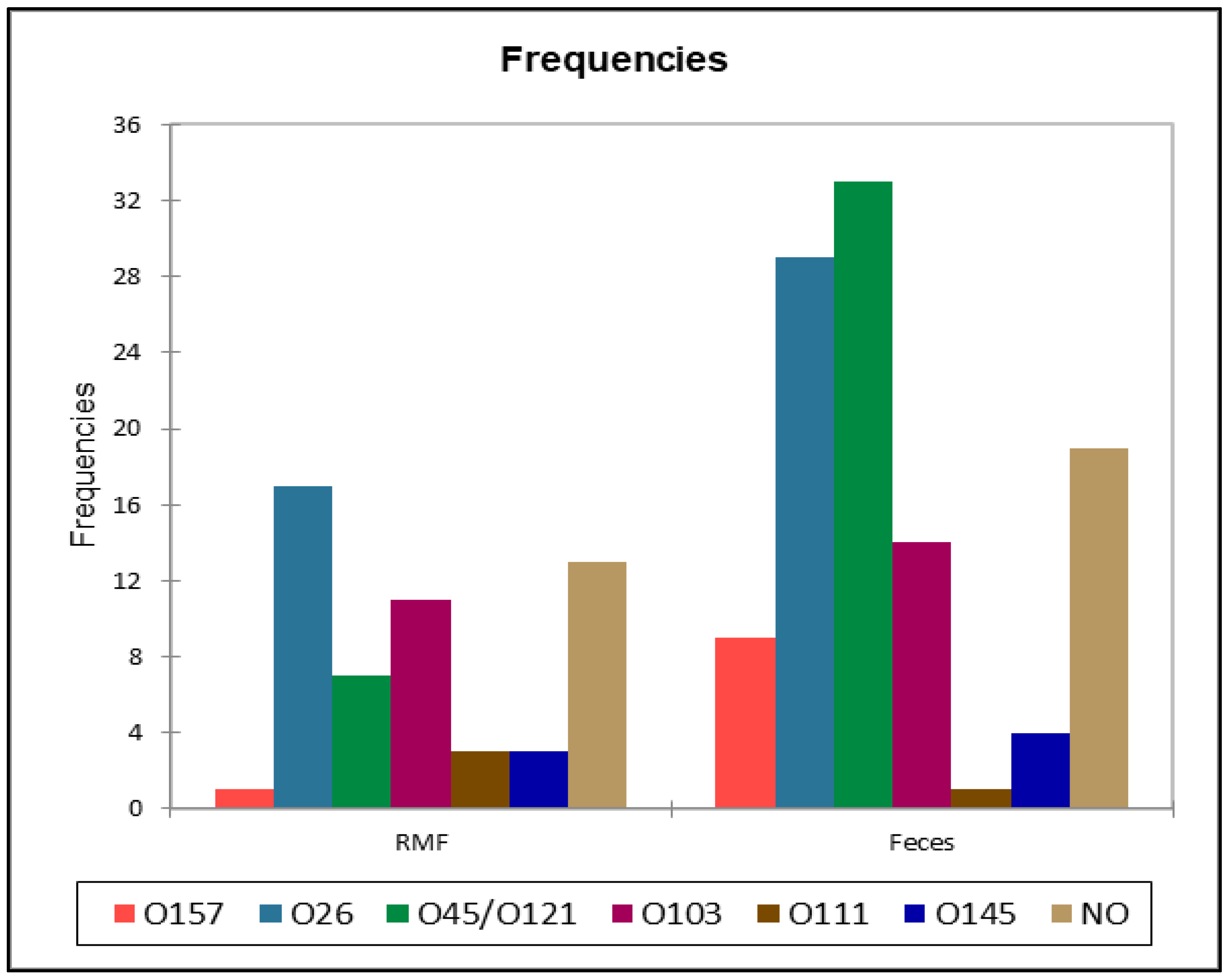
A total of 83 (70.3%) samples out of 118 samples positive for
stx gene resulted positive for at least one STEC serotype. None of serotypes included in the “big seven” panel were found in BTM samples, while in RMF O157 (
n=1), O26 (
n=17), O45/ O121 (
n=7), O103 (
n=11), O111 (
n=3), O145 (
n=3) were identified, and 13
stx positive samples from RMF were negative for serotype identification. These results are summarized in
Figure 4 and
Table 5. The serotype identification of fecal samples led to the identification of O157 (
n=9), O26 (
n=29), O45/ O121 (
n=32), O103 (
n=14), O111 (
n=1), O145 (
n=4) serotypes, while for 19
stx positive samples, the serotype was not identified (
Table 5).
All the serotypes identified and classified by type of matrix were reported in
Table 5 to visualize the relative abundance. The comparison of the serotypes distributions (
Figure 5 and
Table 6) between RMF and feces showed a single significant results with a lower-than-expected frequency of O45/O121 in RMF samples, and the opposite in faces, when pre- and post-weaning distribution were analyzed, we did not find any statistically significant difference at the Fisher’s exact test(α=0.05).
4. Discussion
Escherichia coli STEC represent a serious threat to public health and need an efficient surveillance program in order to prevent outbreaks in humans. Nowadays the only existent surveillance program in Italy, as well as in other Countries (e.g. France), involves the analyses performed on food (i.e. raw milk, dairy products, raw beef, vegetables) without considering the epidemiologic situation at herd level.
The evidence of several gaps in the current knowledge on the epidemiology of the disease, particularly concerning the spread of the infection within the herd, and, therefore, in the surveillance approach at herd level, support studies aiming to try to increase our knowledge on this problem.
The pilot study described in this paper aimed to apply current available diagnostic kit in matrices different from milk and milk products; and to verify if their application may be helpful in the diagnosis of STEC at herd level (calf feces and RMF). The results confirmed that the commercial kit may be applied to RMF and feces, as well as to the target matrices (milk and milk products).
4.1 STEC Prevalence in the Different Matrices
The results of the analysis with these diagnostic kits on BTM, RMF and calf feces showed a different epidemiological pattern related to the matrix. Indeed, the proportion of positive samples in BTM was very low (3.4% of
stx-positive samples), while the proportion on the RMF of the same herds were significantly higher (41.3% of
stx-positive samples), as well as in calf feces. These differences were not unexpected [
11,
27,
38,
39,
42], and may be explained in the following way:
Milk: 25 ml of raw milk are sampled from the bulk tank, capable of holding 150 to 10000 liters of milk at 4°C, which result in a poor detection level, particularly when the prevalence of STEC positive cows is very low and/or when milking practice are optimal.
Milk Filters: with this type of sample is easier to find a positivity since the main task of the filter is to block and retain any type of fecal or litter debris coming from the milking routine, and all the milk pass through the filter; therefore, there is no dilution effect.
Bovine feces: Since E. coli STEC is part of the intestinal microflora of bovines the higher prevalence of positive samples with this type of matrix is expected.
The large differences observed in the prevalence of STEC in BTM and RMF confirmed previous studies comparing these two matrices [
11] that showed as RMF gave a higher frequency of positive results. These results support the importance of selecting a proper matrix to monitor the presence of the pathogen at herd level, like the RMF. Indeed, sampling BTM could lead to an underestimation of the prevalence of this pathogen, and for this reason we think that this matrix is not the most appropriate to monitor the presence of STEC at herd level.
The presence of positive STEC feces in both pre- and post-weaning calves suggest that the infection can be transmitted from positive cows to calves either during calving or by contaminated colostrum and milk. The observed significant higher-than- expected frequency of
stx +
eae genes compared to RMF frequency supports this hypothesis. The evidence of these ways of transmission also suggests a potential preventive measure based on the use of stored STEC negative milk and colostrum as applied to prevent paratuberculosis transmission [
43]. However, how the pathogens enter the herd is still to be elucidated. The most probable ways are the purchase of infected animals (calf, heifer, or cow), and the presence of the pathogens in the environment such as in water (pools, wells) of fresh forage.
4.2. Distribution of Serotypes
The distribution of serotypes among the different samples showed only one significant difference represented by O45/O121 higher-than- expected frequency of these serotypes in feces when compared to RMF. Another results worth mentioning is the low prevalence of O157 isolates that represented, overall, less than 20% of the serotypes. Moreover, the most important information arisen from these analysis, in our opinion, is represented by the fact that 33 out of 113 (29,2%) samples tested positive for the stx virulence gene but negative for the identification of the “top seven” serotypes.
The current monitoring programs usually consider only the “big five” or the “big seven” without considering other STEC serotypes that nowadays may represent a growing threat to the public health [
29,
44].
Following these results, a plausible idea would be to include other common STEC serotypes in the monitoring program, similarly to what has been done in the work of Capps et al. 2021, in order to screen the samples for other six most common STEC non top seven serotypes (O2, O74, O109, O131, O168, and O171) and evaluate the prevalence and the resulting burden on public health of these serotypes [
44].
5. Conclusions
The results obtained so far support our hypothesis that STEC prevalence at herd level is highly underestimated and that the surveillance program needs critical and extensive improvements in order to be more efficient in detecting and preventing STEC infections. The results of this pilot study also suggest that prevention at calves’ level may be considered to reduce the risk of spreading the infection within the herd and will support further research projects investigating this aspect of STEC transmission chain. The epidemiology of these infections and the characteristics of the pathogens clearly show how a One Health approach will be pivotal to improve our capabilities to control the spread of these infections.
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on Preprints.org. Table S1: raw binary results of Real-Time PCR analysis
Author Contributions
Conceptualization V.S. and A.Z.; methodology, V.S. and F.Z.; validation, V.S., A.Z. and F.Z.; formal analysis, V.S. and A.Z.; investigation, V.S., A.Z. and F.Z.; resources, V.S. and F.Z.; data curation, V.S. and F.Z.; writing—original draft preparation, V.S. and F.Z.; writing—review and editing, V.S. and A.Z.; funding acquisition, A.Z.All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
According to national legislation, the Ethics Committee approval was not required. Furthermore, this study was conducted in accordance with EU Directive 2010/63/EU on the protection of animals used for scientific purposes.
Data Availability Statement
No data available.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Gyles, C.L. Escherichia coli in Domestic Animals and Humans; Gyles, C.L. (Eds.) ; CABI, 1994; ISBN 0851989217.
- Gyles, C.L.; Prescott, J.F.; Songer, J.G.; Thoen, C.O. Pathogenesis of Bacterial Infections in Animals: Fourth Edition; Wiley-Blackwell, 2010; ISBN 9780813812373.
- Nataro, J.P.; Kaper, J.B. Diarrheagenic Escherichia coli. Clin. Microbiol. Rev. 1998, 11, 142–201. [Google Scholar] [CrossRef]
- Sora, V.M.; Meroni, G.; Martino, P.A.; Soggiu, A.; Bonizzi, L.; Zecconi, A. Extraintestinal pathogenic escherichia coli: Virulence factors and antibiotic resistance. Pathogens 2021, 10. [Google Scholar] [CrossRef]
- Gyles, C.L. Shiga toxin-producing Escherichia coli: an overview. J. Anim. Sci. 2007, 85. [Google Scholar] [CrossRef]
- Karmali, M.A.; Gannon, V.; Sargeant, J.M. Verocytotoxin-producing Escherichia coli (VTEC). Vet. Microbiol. 2010, 140, 360–370. [Google Scholar] [CrossRef]
- EFSA Panel on Biological Hazards Scientific Opinion on VTEC-seropathotype and scientific criteria regarding pathogenicity assessment. EFSA J. 2013, 11, 3138. [CrossRef]
- USDA Working Group usda. 2011.
- Costa, M.; Sucari, A.; Epszteyn, S.; Oteiza, J.; Gentiluomo, J.; Melamed, C.; Figueroa, Y.; Mingorance, S.; Grisaro, A.; Spioussas, S.; et al. Comparison of six commercial systems for the detection of non-O157 STEC in meat and vegetables. Food Microbiol. 2019, 84. [Google Scholar] [CrossRef]
- Li, B.; Liu, H.; Wang, W. Multiplex real-time PCR assay for detection of Escherichia coli O157:H7 and screening for non-O157 Shiga toxin-producing E. coli. BMC Microbiol. 2017, 17, 1–13. [Google Scholar] [CrossRef]
- Albonico, F.; Gusmara, C.; Gugliotta, T.; Loiacono, M.; Mortarino, M.; Zecconi, A. A new integrated approach to analyze bulk tank milk and raw milk filters for the presence of the E. coli serogroups frequently associated with VTEC status. Res. Vet. Sci. 2017, 115, 401–406. [Google Scholar] [CrossRef]
- Varcasia, B.; Tomassetti, F.; De Santis, L.; Di Giamberardino, F.; Lovari, S.; Bilei, S.; De Santis, P. Presence of Shiga Toxin-Producing Escherichia coli (STEC) in Fresh Beef Marketed in 13 Regions of ITALY (2017). Microorganisms 2018, 6, 126. [Google Scholar] [CrossRef]
- Vélez, M. V.; Colello, R.; Etcheverría, A.I.; Padola, N.L. Shiga toxin producing Escherichia coli: the challenge of adherence to survive. Rev. Argent. Microbiol. 2023, 55, 100–107. [Google Scholar] [CrossRef]
- Montero, D.A.; Canto, F. Del; Velasco, J.; Colello, R.; Padola, N.L.; Salazar, J.C.; Martin, C.S.; Oñate, A.; Blanco, J.; Rasko, D.A.; et al. Cumulative acquisition of pathogenicity islands has shaped virulence potential and contributed to the emergence of LEE-negative Shiga toxin-producing Escherichia coli strains. Emerg. Microbes Infect. 2019, 8, 486–502. [Google Scholar] [CrossRef]
- Caprioli, A.; Morabito, S.; Brugère, H.; Oswald, E. Enterohaemorrhagic Escherichia coli: Emerging issues on virulence and modes of transmission. Vet. Res. 2005, 36, 289–311. [Google Scholar] [CrossRef]
- Duffy, G.; Burgess, C.M.; Bolton, D.J. A review of factors that affect transmission and survival of verocytotoxigenic Escherichia coli in the European farm to fork beef chain. Meat Sci. 2014, 97, 375–383. [Google Scholar] [CrossRef]
- Peng, Z.; Liang, W.; Hu, Z.; Li, X.; Guo, R.; Hua, L.; Tang, X.; Tan, C.; Chen, H.; Wang, X.; et al. O-serogroups, virulence genes, antimicrobial susceptibility, and MLST genotypes of Shiga toxin-producing Escherichia coli from swine and cattle in Central China. BMC Vet. Res. 2019, 15, 1–13. [Google Scholar] [CrossRef]
- Scaife, H.R.; Cowan, D.; Finney, J.; Kinghorn-Perry, S.F.; Crook, B. Wild rabbits (Oryctolagus cuniculus) as potential carriers of verocytotoxin-producing Escherichia coli. Vet. Rec. 2006, 159, 175–178. [Google Scholar] [CrossRef]
- Szczerba-Turek, A.; Chierchia, F.; Socha, P.; Szweda, W. Shiga Toxin-Producing Escherichia coli in Faecal Samples from Wild Ruminants. animals 2023, 13. [Google Scholar] [CrossRef]
- Sanches, L.A.; Gomes, M. da S.; Teixeira, R.H.F.; Cunha, M.P.V.; Oliveira, M.G.X. de; Vieira, M.A.M.; Gomes, T.A.T.; Knobl, T. Captive wild birds as reservoirs of enteropathogenic E. coli (EPEC) and Shiga-toxin producing E. coli (STEC). Brazilian J. Microbiol. 2017, 48, 760–763. [Google Scholar] [CrossRef]
- Bentancor, A.; Rumi, M. V.; Carbonari, C.; Gerhardt, E.; Larzábal, M.; Vilte, D.A.; Pistone-Creydt, V.; Chinen, I.; Ibarra, C.; Cataldi, A.; et al. Profile of Shiga toxin-producing Escherichia coli strains isolated from dogs and cats and genetic relationships with isolates from cattle, meat and humans. Vet. Microbiol. 2012, 156, 336–342. [Google Scholar] [CrossRef]
- Karama, M.; Mainga, A.O.; Cenci-Goga, B.T.; Malahlela, M.; El-Ashram, S.; Kalake, A. Molecular profiling and antimicrobial resistance of Shiga toxin-producing Escherichia coli O26, O45, O103, O121, O145 and O157 isolates from cattle on cow-calf operations in South Africa. Sci. Rep. 2019, 9, 1–15. [Google Scholar] [CrossRef]
- Hussein, H.S.; Sakuma, T. Shiga toxin-producing Escherichia coli: Pre- and postharvest control measures to ensure safety of dairy cattle products. J. Food Prot. 2005, 68, 199–207. [Google Scholar] [CrossRef]
- Etcheverría, A.I.; Padola, N.L. Shiga toxin-producing Escherichia coli: Factors involved in virulence and cattle colonization. Virulence 2013, 4, 366–372. [Google Scholar] [CrossRef]
- Farrokh, C.; Jordan, K.; Auvray, F.; Glass, K.; Oppegaard, H.; Raynaud, S.; Thevenot, D.; Condron, R.; De Reu, K.; Govaris, A.; et al. Review of Shiga-toxin-producing Escherichia coli (STEC) and their significance in dairy production. Int. J. Food Microbiol. 2013, 162, 190–212. [Google Scholar] [CrossRef]
- Rastawicki, W.; Śmietańska, K.; Rokosz-Chudziak, N.; Wołkowicz, T. Antibody response to lipopolysaccharides and recombinant proteins of Shiga toxin (STX)-producing Escherichia coli (STEC) in children with haemolytic uraemic syndrome in Poland. Lett. Appl. Microbiol. 2020, 70, 440–446. [Google Scholar] [CrossRef]
- Dastmalchi Saei, H.; Ayremlou, N. Characterization of Shiga toxin-producing Escherichia coli (STEC) in feces of healthy and diarrheic calves in Urmia region, Iran. Iran. J. Microbiol. 2012, 4, 63–69. [Google Scholar]
- Renter, D.G.; Sargeant, J.M.; Hungerford, L.L. Distribution of Escherichia coli O157:H7 within and among cattle operations in pasture-based agricultural areas. Am. J. Vet. Res. 2004, 65, 1367–1376. [Google Scholar] [CrossRef]
- ISO/TS 13136:2012 Microbiology of food and animal feed Real-time polymerase chain reaction (PCR)-based method for the detection of food-borne pathogens Horizontal method for the detection of Shiga toxin-producing Escherichia coli (STEC) and the determination of O157, O111,. 2012.
- Parsons, B.D.; Zelyas, N.; Berenger, B.M.; Chui, L. Detection, characterization, and typing of shiga toxin-producing Escherichia coli. Front. Microbiol. 2016, 7, 1–12. [Google Scholar] [CrossRef]
- Wang, J.; Katani, R.; Li, L.; Hegde, N.; Roberts, E.L.; Kapur, V.; DebRoy, C. Rapid detection of Escherichia coli O157 and shiga toxins by lateral flow immunoassays. Toxins (Basel). 2016, 8. [Google Scholar] [CrossRef]
- Wijnsma, K.L.; van Bommel, S.A.M.; van der Velden, T.; Volokhina, E.; Schreuder, M.F.; van den Heuvel, L.P.; van de Kar, N.C.A.J. Fecal diagnostics in combination with serology: best test to establish STEC-HUS. Pediatr. Nephrol. 2016, 31, 2163–2170. [Google Scholar] [CrossRef]
- Beutin, L.; Fach, P. Detection of Shiga Toxin-Producing Escherichia coli from Nonhuman Sources and Strain Typing. Microbiol. Spectr. 2014, 2, 1–23. [Google Scholar] [CrossRef]
- World Health Organization Shiga toxin-producing Escherichia coli (STEC) and food: attribution, characterization, and monitoring MICROBIOLOGICAL RISK ASSESSMENT SERIES 31 REPORT Shiga toxin-producing Escherichia coli (STEC) and food: attribution, characterisation, and monitoring; 2019; ISBN 978-92-5-130682-6.
- DISCONTOOLS. Available online: https://www.discontools.eu/database.html?rid=9839&v=html (accessed on Dec 5, 2023).
- Murphy, B.P.; Murphy, M.; Buckley, J.F.; Gilroy, D.; Rowe, M.T.; McCleery, D.; Fanning, S. In-line milk filter analysis: Escherichia coli O157 surveillance of milk production holdings. Int. J. Hyg. Environ. Health 2005, 208, 407–413. [Google Scholar] [CrossRef]
- Giacometti, F.; Serraino, A.; Finazzi, G.; Daminelli, P.; Losio, M.N.; Bonilauri, P.; Arrigoni, N.; Garigliani, A.; Mattioli, R.; Alonso, S.; et al. Foodborne pathogens in in-line milk filters and associated on-farm risk factors in dairy farms authorized to produce and sell raw milk in northern Italy. J. Food Prot. 2012, 75, 1263–1269. [Google Scholar] [CrossRef]
- Van Kessel, J.A.S.; Karns, J.S.; Lombard, J.E.; Kopral, C.A. Prevalence of Salmonella enterica, Listeria monocytogenes, and Escherichia coli virulence factors in bulk tank milk and in-line filters from U.S. dairies. J. Food Prot. 2011, 74, 759–768. [Google Scholar] [CrossRef]
- Salaheen, S.; Kim, S.W.; Springer, H.R.; Hovingh, E.P.; Van Kessel, J.A.S.; Haley, B.J. Genomic diversity of antimicrobial-resistant and Shiga toxin gene-harboring non-O157 Escherichia coli from dairy calves. J. Glob. Antimicrob. Resist. 2023, 33, 164–170. [Google Scholar] [CrossRef]
- Fernández, M.; Casaux, M.L.; Fraga, M.; Vignoli, R.; Bado, I.; Zunino, P.; Umpiérrez, A. Shiga Toxin-Producing Escherichia coli (STEC) Associated with Calf Mortality in Uruguay. Microorganisms 2023, 11, 1–12. [Google Scholar] [CrossRef]
- Auvray, F.; Bièche-Terrier, C.; Um, M.M.; Dupouy, V.; Nzuzi, N.; David, L.; Allais, L.; Drouet, M.; Oswald, E.; Bibbal, D.; et al. Prevalence and characterization of the seven major serotypes of Shiga toxin-producing Escherichia coli (STEC) in veal calves slaughtered in France. Vet. Microbiol. 2023, 282. [Google Scholar] [CrossRef]
- Dell’Orco, F.; Gusmara, C.; Loiacono, M.; Gugliotta, T.; Albonico, F.; Mortarino, M.; Zecconi, A. Evaluation of virulence factors profiles and antimicrobials resistance of Escherichia coli isolated from bulk tank milk and raw milk filters. Res. Vet. Sci. 2019, 123, 77–83. [Google Scholar] [CrossRef]
- Geraghty, T.; Graham, D.A.; Mullowney, P.; More, S.J. A review of bovine Johne’s disease control activities in 6 endemically infected countries. Prev. Vet. Med. 2014, 116, 1–11. [Google Scholar] [CrossRef]
- Capps, K.M.; Ludwig, J.B.; Shridhar, P.B.; Shi, X.; Roberts, E.; DebRoy, C.; Cernicchiaro, N.; Phebus, R.K.; Bai, J.; Nagaraja, T.G. Identification, Shiga toxin subtypes and prevalence of minor serogroups of Shiga toxin-producing Escherichia coli in feedlot cattle feces. Sci. Rep. 2021, 11, 1–12. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).