1. Introduction
The effects of wild game feeding have already been widely investigated, but mostly the animal species have been in the focus and mainly the effects of supplementary winter feeding were examined in northern countries (Inslerman et al. 2006; Richardson 2006; Milner et al. 2014). However, in Central and Eastern Europe feeding for hunting purposes is more widespread (Apollonio et al. 2010). At these sites the goal is primarily to bait wild boar and thereby facilitate the hunt. A bait site is a small clearing established approx. 30–50 meters from hunting blinds, where feed generally scattered on the ground (Selva et al. 2014). Usually corn-cobs or seeds are used at the sites, although agricultural and food industry by-products (e.g. molasses, fresh and dried beet slices) are also applied (Selva et al. 2014). Given that agricultural products, especially cereals, contain weed seeds (Wilson et al., 2016; Gervilla et al., 2019), this practice can cause lead to the invasion of natural habitats by noxious weeds and exotic species. Due to the disturbance occasioned by extended anthropogenic activity, the increased game density, the bare and degraded soil produced by trampling, and the greater availability of nutrients, all bait sites may be considered important potential aids to invasion (Hobbs and Huenneke 1992; MacDougall and Turkington 2005).
However, only in recent years has more attention been paid to this problem. Significant degradation at bait sites was showed in different habitat types in Hungary (Rusvai et al. 2022 a,b) and in Slovakia (Kochjarová et al. 2023). It has been proved that the operation of bait sites over several years can significantly damage the physical and chemical parameters of the soil and degrade the soil seed bank as well (Rusvai et al 2022b). It has also been proved that in case of abandonment of these places, the abundance of weeds decreased significantly over time, but the number of weed species typically did not change, and the presence of segetal weed species was detectable even after almost a decade (Rusvai et al. 2021). Moreover, the density of weed seeds in the soil seed bank can also remain significant even after 10 years of abandonment (Rusvai et al. 2021). In Slovakia the number of non-native plant species in the national parks is increasing every year, and some researchers mention that these feeding grounds are one of the main sources of these plants (Kochjarová & Blanár 2018). Others found that some exotic species can be detectable even in the mountainous environment, at altitudes above 1000 m along these sites (Kochjarová et al. 2023). Therefore, it can be assumed that climate change can also affect the presence and the abundance of weed species at the feeding grounds and accordingly, it can affect the degree of degradation and possible regeneration processes at these places.
The aim of this study is to examine the impacts of baiting on herbaceous vegetation at bait sites in a typical Central European lower montane zone. We investigated the vegetation dynamics in two vegetation zones, oak and beech zones, in two areas with different moisture and shade conditions (forest and clearing). The most important background factor was changes in precipitation and temperature. The main questions of the study were the following:
1. Will the weed infestation be detectable at bait sites over a long period, and will the interannual changes be typical in every year, according to the fact that in August, more weed species are usually present with a greater cover at the bait sites?
2. Do the main meteorological factors play a role in the degree of weed infestation in each year, does precipitation or does higher or lower temperature affect the number and/or cover of weed species?
2. Materials and Methods
2.1. Description of the Study Area
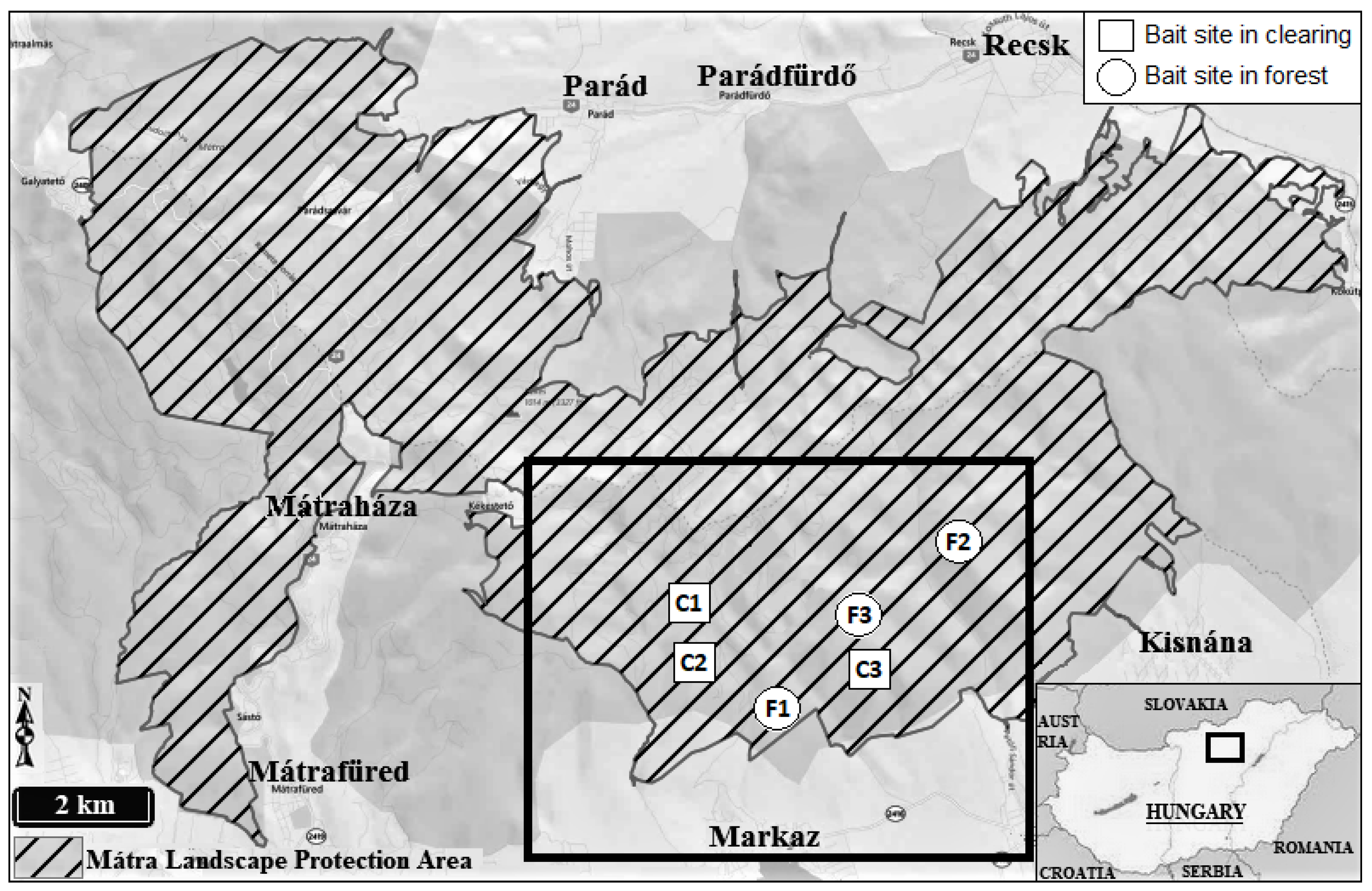
Altogether six bait sites were included in the assessment (
Figure 1). Three forest (F1, F2, F3) and three clearing sites (C1, C2, C3) were investigated in 2016, 2018, 2019 and 2020 in a Central European Lower Montane Zone, in Hungary, in the turkey oak–sessile oak zone. All bait sites have been in use for a long time (each for 5–10 years). Forest sites had at least 80% canopy cover, while clearing sites refer to old clearcuttings 50–100 m in diameter, characterized by grassland species. At all places vegetation was investigated using the transect method. The transects were set out from the center of the baits in 4 directions, closing an angle of 90° to each other. 22-22 1x1 meter tangential quadrats were placed on each of them, in which a percentage cover estimation was carried out in May and August of each year. In the present study, the coenological data of the individual units are evaluated together, in order to filter out spatial autocorrelation. To evaluate the role of the meteorological parameters’ average daily temperature and daily precipitation data between 2016 and 2020 was used. Data was collected from the nearest stations of the National Meteorological Network, using the Meteorological Database (HungaroMet 2020).
2.2. Coenosystematic Classification
The vegetation of the bait sites was evaluated according to type (forest vs. clearing, and examination period (May and August), based on their species list and abundance (cumulative cover of the taxons per bait site). To estimate the naturalness of communities, Borhidi Social Behaviour Type (SBT) classification (Borhidi, 1995) was used. According to this grouping, two main group was used: (1) Naturalness indicator species: stress tolerant specialists – S; competitors of natural habitats – C; stress tolerant generalists – G; natural pioneers – NP; disturbance tolerant plants – DT. (2) Degradation indicator species: native weed species – W; introduced crops running wild – I; adventitious weeds – A; ruderal competitors of the natural flora – RC; alien competitors, aggressive invaders – AC. To evaluate the role of meteorological factors, we calculated with monthly averages obtained from the basic data and compared it with the cumulative cover of weeds and natural species. The number and the abundance of alien weed species was also calculated to evaluate the role of bait sites in the maintenance of these plants. Species names were provided based on the nomenclature of Király (Király 2009)
2.3. Statistical Analysis
The analyses were performed with R statistical software (version 4.0.5, R Core Team 2021) using the packages “tidyverse” and ”rstatix”. The effects of different sites were tested by repeated–measures analysis of variance (ANOVA). The Tukey HSD test (post hoc test) was used to perform pair-wise multiple comparisons. For linear regression we used PAST program (Hammer et al. 2001).
3. Results
3.1. Species Pool and Abundance
Altogether 224 species were found at the six bait sites in the 4 years study period. A third of this (74 species, 33%) was weed species. The previously confirmed habitat differences (Rusvai et al. 2022) were clearly detectable in every year. At clearing sites more weed species were found in a higher abundance and the species pool was also different. 181 taxa were detected at the feeding sites located in clearing, of which 71 were degradation indicator species (39,2%), while only 134 species were present at the forest sites, of which 37 (27,6%) were weed.
In terms of the most abundant species, forest sites were dominated by natural weed species, like
Rumex crispus L. and
Fallopia convolvulus (L.) Á. Löve (
Table 1). However, the cover of these species was generally very low. The maximum coverage of
Rumex crispus L. per quadrat was only 65%, while the average cover of the other degradation indicator species was typically only 10-20% (
Chenopodium album L. – 20%;
Fallopia convolvulus (L.) Á. Löve – 15%;
Plantago major L. – 15%;
Galium aparine L. – 10%;
Ambrosia artemisiifolia L. – 10%;
Datura stramonium L. – 10%). The naturalness indicator species proved to be more abundant. At greater distances from the center of the bait sites, the coverage of these species was about 60-80%. Only
Urtica dioica L. was able to reach 100% in some quadrats, mainly close to the feeding places. However, the mass of disturbance-tolerant species (DT species) is still significant. Despite all of this, even within the group of natural species, disturbance-tolerant species (Borhidi indicator ‘DT’ category) dominated, which, in addition to the low plant cover values, clearly indicates the disturbance of these locations.
At clearing sites, the species pool and density were completely different (
Table 2). Ruderal and segetal weeds proved to be the most abundant and the average cover values of these species in the sampling units were also higher than in the forest sites.
Polygonum aviculare L.,
Xanthium spinosum L.,
Datura stramonium L. and
Tripleurospermum inodorum (L.) Sch.Bip often reached 100% cover in the quadrats, and other species could also very abundant (e.g. Bromus sterilis L. – 95%;
Capsella bursa-pastoris (L.) Medik – 90%;
Ballota nigra L. – 90%). Moreover, at these sites, alien species were also able to represent a significant mass. For example,
Ambrosia artemisiifolia L. and
Echinochloa crus-galli could reach 15-20%, while other non-indigenous plants, like
Galinsoga parviflora Cav. and
Erigeron annuus (L.) Pers. reached a maximum of 5%.
In terms of the spatial distribution of the above named species along the transects, the previously confirmed stress gradient (Rusvai et al. 2022) was also proved to be detectable in every year, every sampling period. The above mentioned degradation indicator species were the most abundant in the centre of the bait sites, especially in clearings, where the presence of weed species was dominant until 5-6 meters from the feeding sites. Meanwhile, the density of natural species increased with the distance. It should be emphasized, however, that although the continuous weed cover did not usually spread further than 5-10 meters, the presence of weed species could be detected even at greater distances in every year and every examination period, which clearly indicates the long-term degrading effect of these hunting facilities.
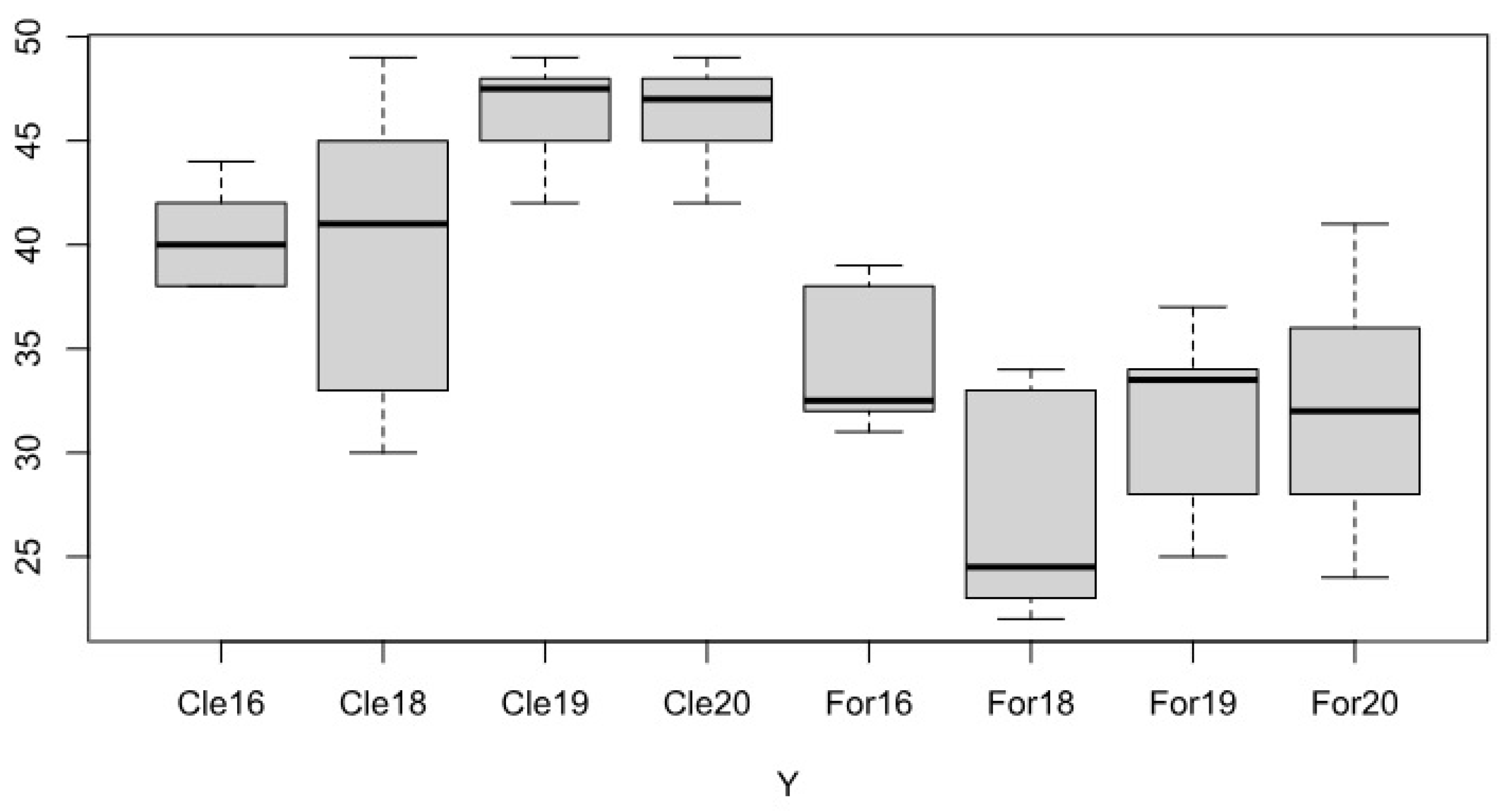
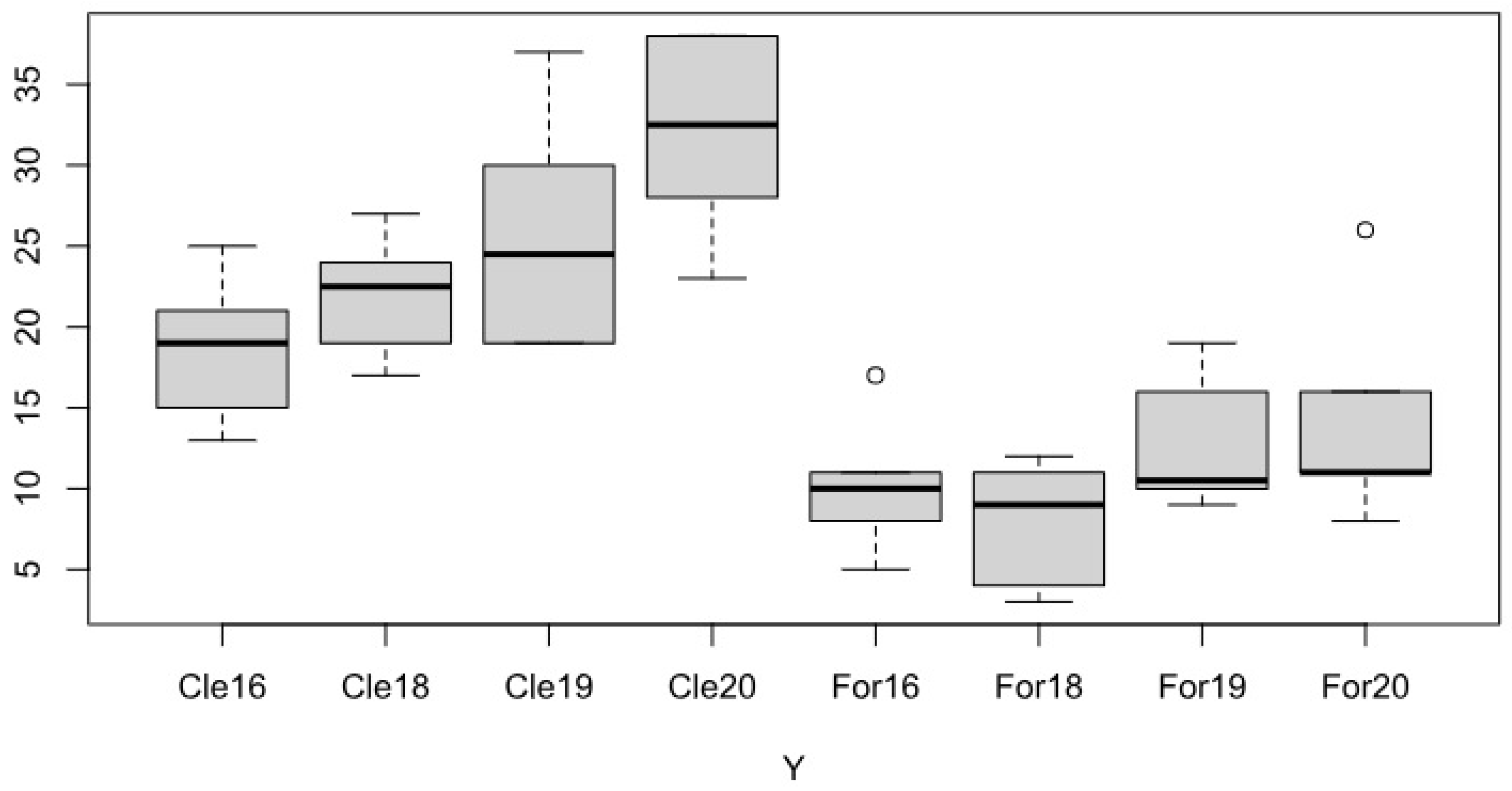
In general, it can be stated that there is a big difference between the samples in the forest and in the clearing in terms of cumulative coverage of naturalness (
Figure 2) and degradation (
Figure 3) indicator species. Accordign to ANOVA the number of native and also degradation indicator species was significantly higher (p<0.001) at clearing sites.
3.2. Intra- and Interannual Changes in Vegetation
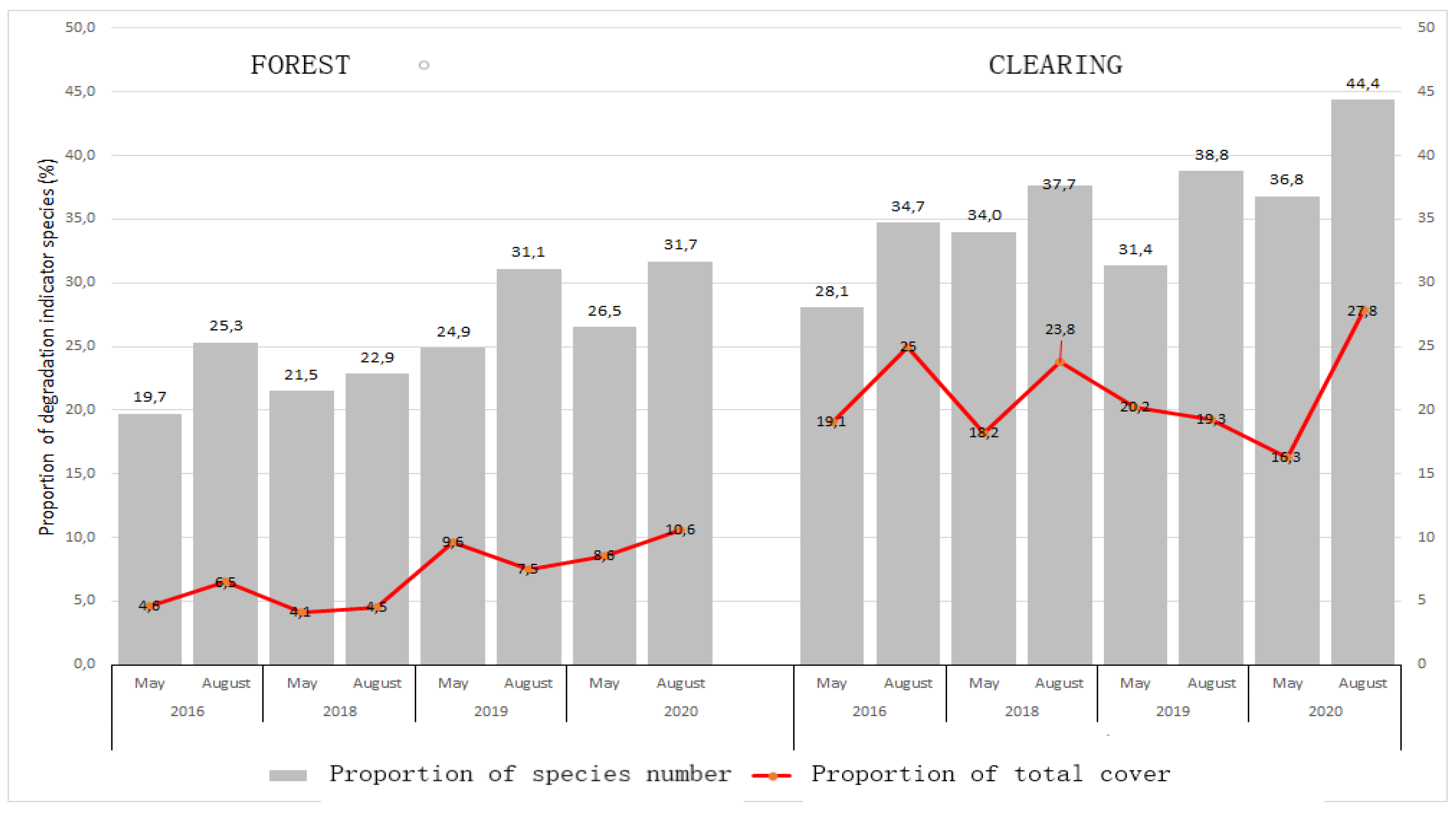
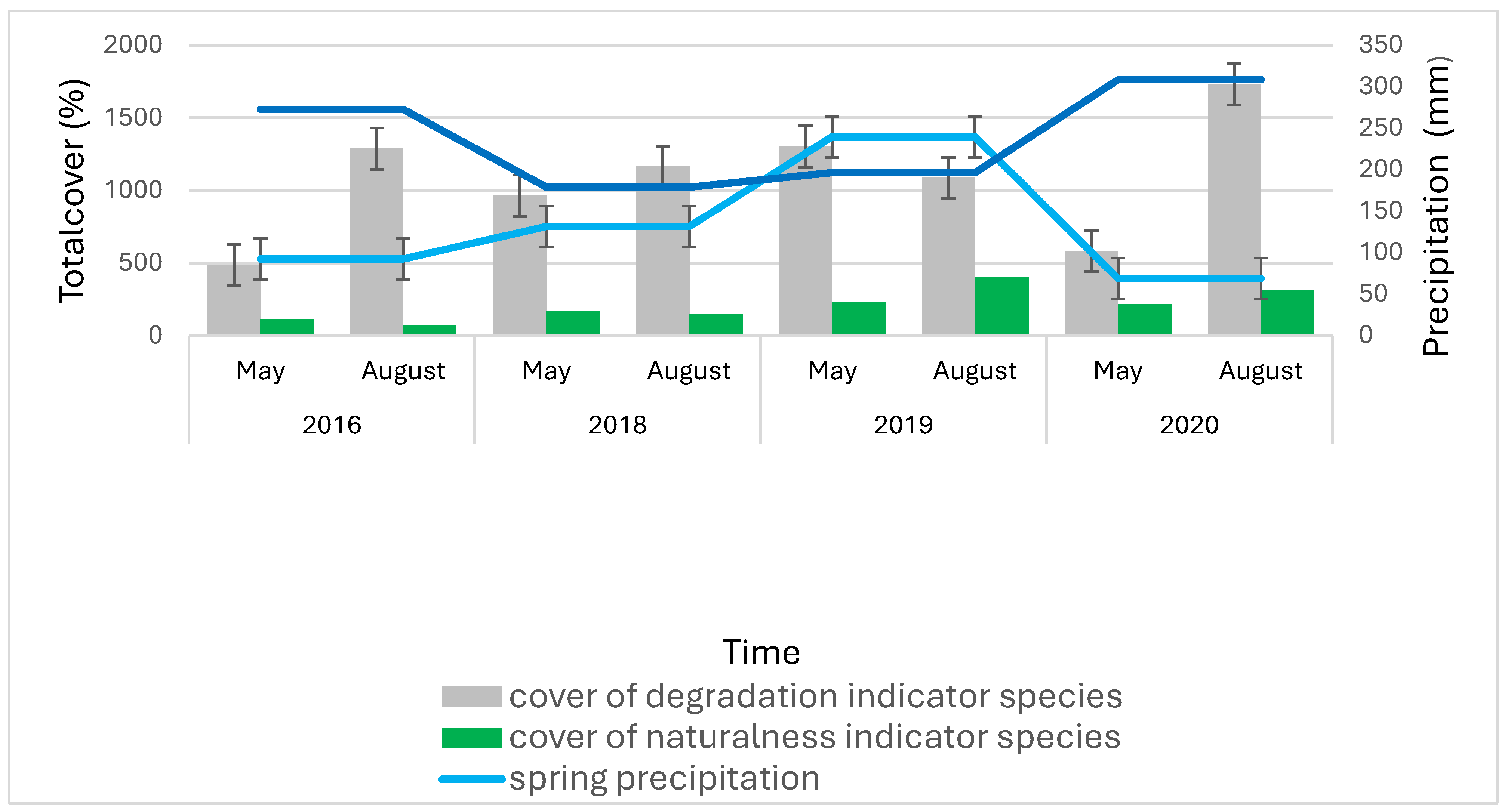
The well-known seasonal changes in field weed vegetation (Pinke et al. 2012) proved to be true in the case of bait sites: the increase in the number and coverage of weed species at the end of summer, resulting from their life-form, was generally detectable in all years and locations (
Figure 4).
The difference between the two habitat types is also clearly visible in the figure, according to which bait sites located in clearing turned out to be much more degraded. Based on the 4-year recording data, the total number of species, the number of degraded species and the number of natural species were significantly higher (p<0.001) at baits located in clearing, and the cumulative total coverage of each species was also much higher at these locations. However, the rate of growth over the years (and also within individual locations) was very variable, and an increasing trend was also observed in the mentioned parameters during the study, the reason for which can presumably be revealed by examining the meteorological and other parameters..
3.3. Influence of Meteorological Factors
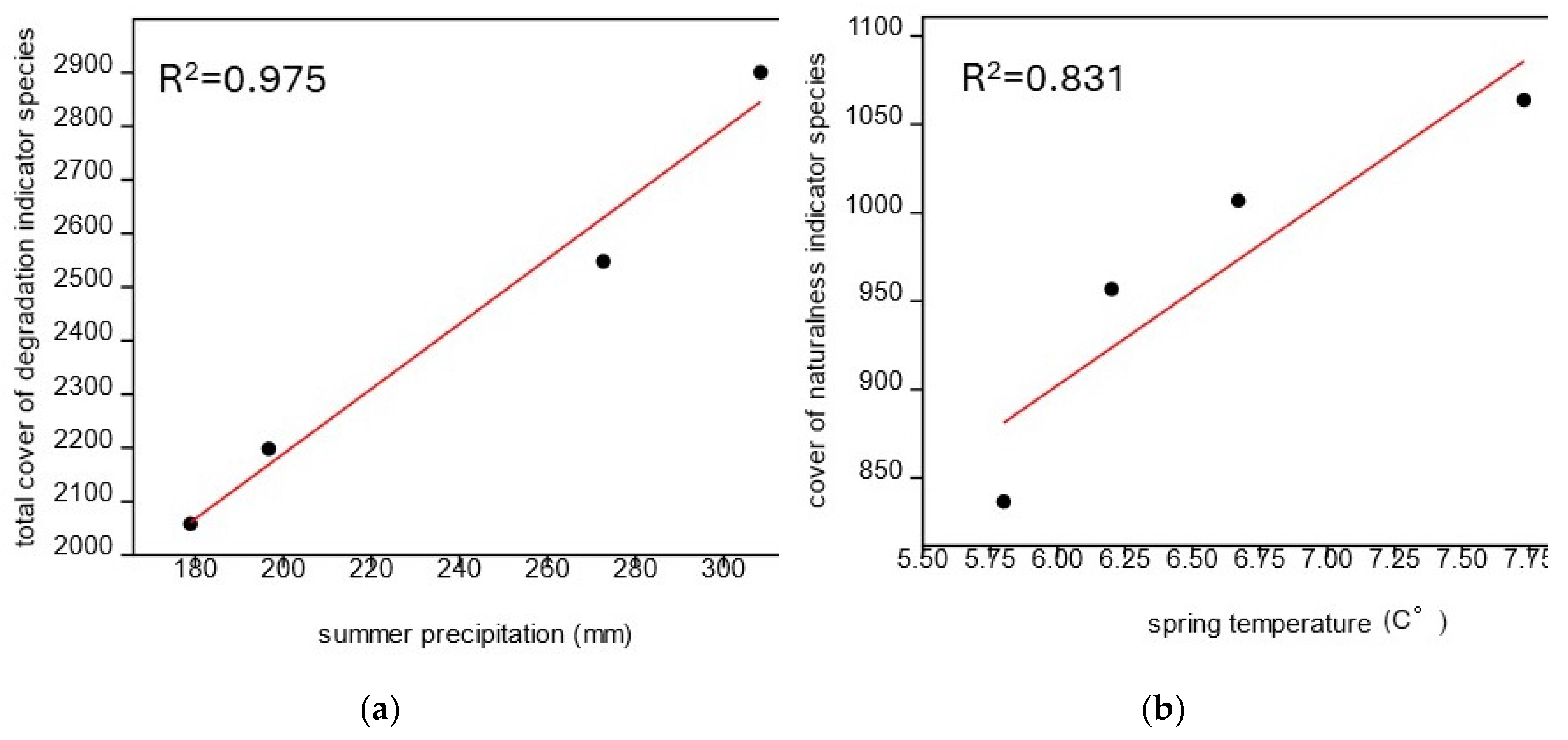
The extent of weed infestation was significantly influenced by the examined meteorological factors. At clearing sites, positive correlation (R
2=0.975) between the amount of summer precipitation and the total coverage of degradation indicator species was showed, as well as the average spring temperature and the coverage of naturalness indicator species (R
2=0.831) (
Figure 5).
Meanwhile, the distribution of precipitation also proved to be a decisive factor: in particular, warm, dry springs and the following rainy summers increased weed density (
Figure 6). In 2016 and 2020 a very dry spring followed by a relatively wet summer, as a result of which, after the sparse weeding in May, the mass of large weed species that require a significant amount of water for their growth (Lehoczky et al. 2016) increased significantly by August. In the years 2018 and 2019, with more spring precipitation, the change between the two aspects was generally not that significant. At that time, weed species already appeared in May, so the rate of growth in these years was not so significant. Moreover, in the year 2019, when both the spring and summer were especially rainy, the coverage of weed species even decreased slightly in terms of average values, while the abundance of natural species increased.
Presumably temperature has also played an important role in the characteristics of weed cover. In springs with a higher average temperature and a relatively large amount of precipitation (in years 2018 and 2019), significant weed growth could already develop in May. The most characteristic example is the case of Xanthium spinosum L. appearing on one of the sites. This species appeared in a high density already in May in 2018. This is due not only to the relatively large amount of spring precipitation, but also to the fact that this year was the warmest spring (average temperature: 7.7 °C). And since this weed requires a particularly high soil temperature for the initiation of germination (Vajdai 1996), in the colder springs of 2019 and 2020 (average temperature: 6.2 °C and 5.8 °C), this species could only appear in August, and typically not in too large density either.
Considering the abundance of alien species it can be said that the cover of non-native plant was very variable in each year and in each location. The proportion of these species from the total weed coverage varied between 2.2% to 46.5% during the study periods. However, if we examine the above mentioned meteorological factors, it can be seen, that the interannual changes within the year significantly affect the abundance of these species. The cover of alien weeds increased to a much greater extent in the years (in 2016 and 2020) when a dry spring was followed by a rainy summer (
Table 3).
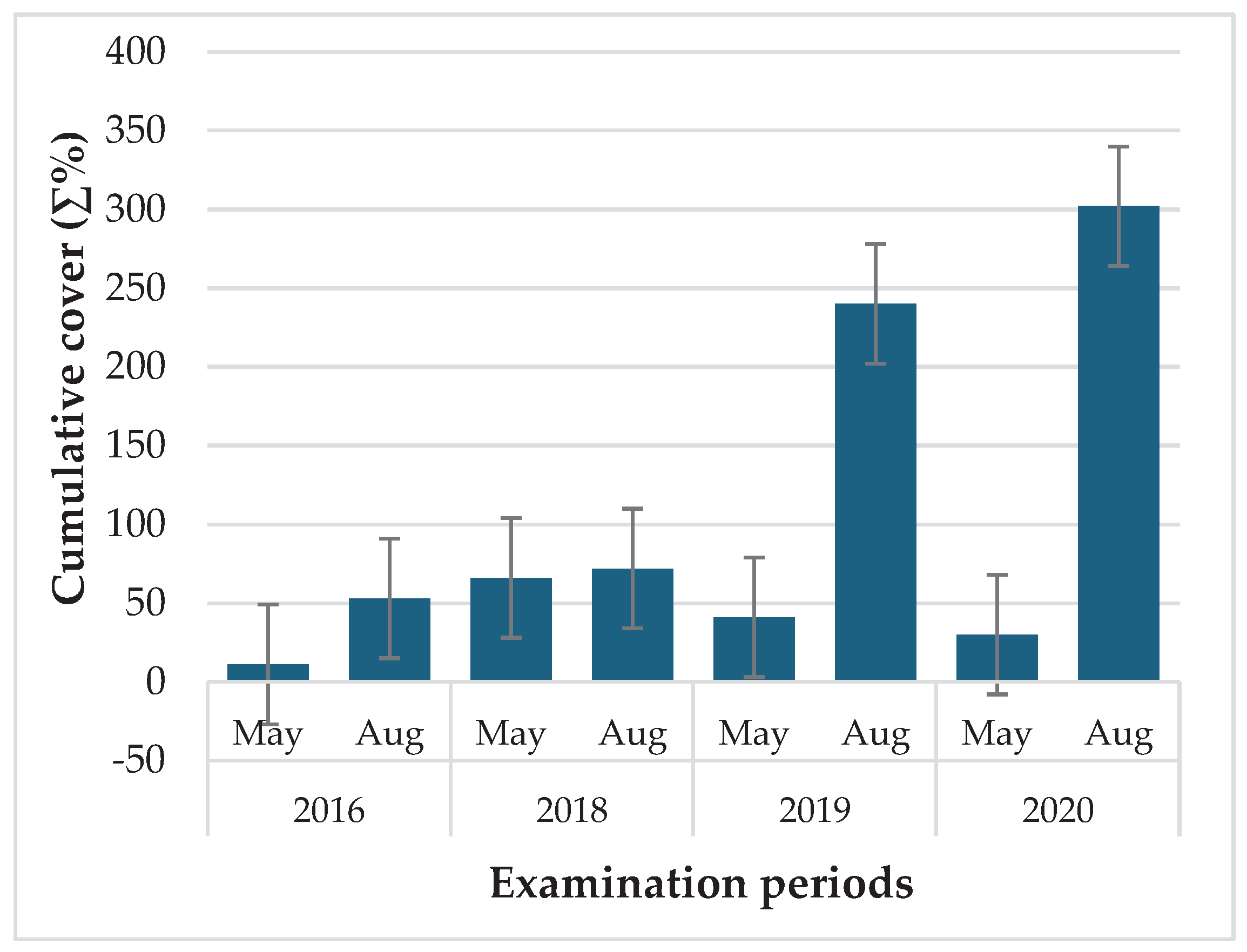
Although, invasive alien plant species (IAS) usually accounted for only a small proportion of the total weed coverage (0.2 to 3.6%), their presence is definitely important, especially because their coverage has increased over the years (
Figure 7). The most abundant species were
Ambrosia artemisiifolia L.,
Conyza canadensis L. and
Erigeron annuus (L.) Pers., which are all spreading alien species throughout Europe (Lambdon et al. 2008). Neither the temperature nor the precipitation data could support the trend, so it can presumably be the result of several other factors.
4. Discussion
4.1. Species Pool and Abundance
Based on the 4 years of investigation, it has been proven that feeding can cause significant and long-term degradation in natural habitats. Thereby, the assumption that was established investigating the impact of hunting establishments in Slovakia was confirmed here: thanks to the repeated supply of diaspores, the presence of species of external origin remains local, but constant (Kochjarová et al. 2023). However, the extent of this was different in the two habitat types. Thanks to the different moisture and shade conditions, it was shown that feeding places located in clearing are proved to be much more degraded. More weed species were found in a higher abundance, and mainly ruderal and segetal weeds dominated. Meanwhile, in forest sites the vegetation was sparse, and it characterized by natural species, appearing mostly in small patches. The previously proven stress gradient (Rusvai et al. 2022) was also consistent with this habitat difference in all year. The cover of degradation indicator species was the highest in the center of the baits, further their density and number of species decreased, while the number and cover of natural species increased. However, the spatial extent of this was different at each bait types. As expected, bait sites located in the clearing proved to be the most degraded. In this case, the area of the baits was typically characterized by continuous weed cover until 5-8 meters, while in the forest sites, the bare, litter-free soil surface was dominated with only a few weeds.
All these phenomena presumably due to the higher sensitivity of open habitats to invasions (Pauchard et al. 2009) and the specific environmental needs of weed species (Pinke & Pál 2005). In clearing sites many segetal and ruderal species was able to form homogeneous stands. As it has been also shown in Slovakia (Kochjarová et al. 2023), such species like Datura stramonium L., Xanthium spinosum L. and Polygonum aviculare L. was able to form high densities in the immediate vicinity of the feeding places. This result also clearly indicates that the most species-rich communities are not necessarily the most resistant to invasions (Von Holle 2013). And although weed invasion typically extends to the intermediate environment of the bait sites (Rusvai et al. 2022, Kochjarová et al. 2023), many examples are known when this led to the complete destruction of habitats (e.g. Bíró 1998; Molnár 2014). Thus, small mountain clearings are the most endangered, especially because bait sites are mainly established in these habitats.
4.2. Intra- and Interannual Changes in Vegetation
There was a significant difference between the vegetation of the examined periods: in August, more weed species were present at all sites, with a higher cover, however, climatic factors also played a significant role in the extent of this and in the species composition. The reason could be that most segetal weed species can typically germinate at higher soil temperatures (Berzsenyi 2000), so most of them could only reach a significant density by August. And although this is a clear phenomenon in field environments (Pinke et al. 2010), according to the results of this research, it proved to be also detectable in natural environment. Considering, that the rate of growth over the study was very variable, and an increasing trend was also observed during the 4 years examined, it is assumed that meteorological factors could play an important role in the intra- and interannual changes (see in detail in the next chapter).
Regardless of the weather components, the variation between the years may have been partially caused by several other factors, including unique habitat characteristics and other anthropogenic disturbances, like the decreasing wild boar population due to the spread of ASP (Sauter-Louis et al. 2021) and the slightly reduced hunting intensity due to restrictions caused by Covid-19 (Szabó 2021). These factors together could have resulted in the fact that, with decreasing disturbance, the seeds of the weed species hidden in the soil could more easily appear on the surface, resulting in greater coverage of them. In that way, the changes in the abundance of alien species can be partly explained by this. But since we only examined the average temperature and precipitation data here, it is not excluded that other weather factors may have played a role in this. For example, the uneven distribution of precipitation, the increase in frequency of extreme weather events, and also the unpredictable changes in the use of feeding sites (e.g. the quantity and quality of forage, as well as the method and frequency of their placement) can all play a role in the spread of alien species.
4.3. The Role of Meteorological Factors
The extent of weed infestation at bait sites was significantly influenced by the examined weather factors. The degree of degradation was more significant in drought years, while some regeneration processes were also observed in wetter periods. This result is consisted with the result of Jánoska (2006), who also experienced this phenomenon in a wild boar preserve, where the level of disturbance is similarly strong. The distribution of precipitation also proved to be a decisive factor. In particular, warm, dry springs and the following rainy summers increased weed density. As weed and alien plant species generally require higher temperatures to germinate than native plant, the increasingly frequent warmer springs will be more favorable for them (Wainwright et al. 2013).
As it has been proven, periods of drought also promoted the growth of weed species. These effects of climate change clearly promote the spread of weeds and alien species (Hulme 2016). For example the cover of Ambrosia artemisiifolia L., Conyza canadensis L. and Erigeron annuus (L.) Pers. has increased over the 4-year study, which can be examined by these facts. Among them, common ragweed was the most abundant, a species that a previous study has already indicated at bait sites across the country, sometimes in a significant density (Hirka and Csóka 2009). The authors highlight the role of bait sites and the road network connected to them in the spread of invasive species. The importance of this species was also emphasized by a study that examined the changes in vegetation at abandoned bait sites. It has been proven that common ragweed was able to appear even after nearly a decade of abandonment, and in this case too, it became especially abundant after warm, dry springs (Rusvai 2023).
Moreover, the above mentioned effects can be strengthened by other processes, such as the consequent weakening of forest health (Milad et al. 2011), which, combined with often inadequate forest management methods and other anthropogenic effects, can lead to the opening of forests (Dale et al. 2001). It can further promote the spread of weeds and invasive species (Kueffer et al. 2013) and also cause strong degradation in more closed forest areas (Laurence & Yensen 1991; Martin et al. 2009) and other valuable habitat patches as well (Rejmánek et al. 2013).
5. Conclusions
The degree of degradation is primarily determined by habitat characteristics (forest-clearing), but climatic factors (mainly precipitation and its distribution) has also played a significant role in the appearance and the abundance of naturalness and degradation indicator species species at bait sites. Because of climate change and increasing anthropogenic and other disturbances, these feeding places may pose an increasing threat in a several ways to the surrounding natural habitats in the future.
In the first instance, the contributing factors of the nutrient enrichment due to forage, the increased amount of urine and waste, the persistent weed seeds introduced with contaminated feed, digging and trampling due to a higher concentration of animals (which have been proven in previous research by Rusvai et al. 2022), the effects of seed dispersal mechanisms (Blossey & Gorchov 2017) and the above mentioned climatic factors, bait sites can even be the focal points of biological invasions (Spurrier & Drees 2000, Davis & Pelsor 2001; MacDougall & Turkington 2005). Additionally, the regeneration of these sites is clearly quite limited: the presence of weeds and alien species in the seed bank (Rusvai et al. 2021) clearly shows that in the event of a possible new disturbance, the previously abundant species, or even other invasive species, may appear again on open and disturbed, nutrient-rich habitats (Davis & Pelsor 2001; Devlaeminck et al. 2005).
Considering, that there is a very large number of bait sites in the country (about 30,000), and they are used regularly and very intensive (Nagy 2004), they can also serve as major infection hotspots in a network. Thus, although, weed infestation typically remains local, and the light-demanding weed species that become abundant in small clearings are presumably not expected to spread to neighboring forest areas (Burst et al. 2017), their effect may occur even further away in the case of suitable habitat patches (e.g. disturbed clearings, open forest patches, unclosed regeneration patches) by spreading via the road network and on other ways (Sukopp 1962; Kleijn & Sutherland 2003). The reality of this assumption is indicated by the fact that, for example, in Slovakia, where feeding activities take place in a similar way and to the same extent, the number of non-native plant species in the national parks is increasing every year, and some researchers mention these hunting facilities as one of the main sources of alien plants (Kochjarová & Blanár 2018). What is more, thanks to the climate change, segetal weeds was found at increasing altitudes above sea level along these hunting facilities (Kochjarová et al. 2023). It has been also proven in Hungary, according to which Abutilon thepohrasti Medik. and Datura stramonium L. was found above 900 m at a bait site located in a beech forest (Rusvai 2023). In this research, an increase in the abundance of invasive alien species over the years was demonstrated. It means that thanks to the effects of climate change and the increasing anthropogenic disturbances, the threat to natural habitats is increasing and mountainous areas are also endangered.
All in all, it can be said that the least environmental damage would be achieved by the prohibition or significant limitation of bait sites in nature conservation areas. In terms of scientific part, it would be also worthwhile to plan more comprehensive studies that focus on the role of bait sites in changes at local and landscape level as well, regarding the spread and establishment of invasive and weed species.
Author Contributions
Conceptualization, J.H., K.R. and Sz.Cz.; methodology, K.R.; software, J.H.; validation, K.R., J.H. and Sz.Cz formal analysis, K.R.; investigation, K.R.; resources, K.R; data curation, K.R., J.H.; writing—original draft preparation, K.R.; writing—review and editing, J.H. and Sz.Cz visualization, K.R.; supervision, J.H. and Sz.Cz. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
The data presented in this study are available on request from the first author.
Acknowledgments
We sincerely thank the editors and the anonymous reviewers for their insightful comments and suggestions.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- HungaroMet 2020: https://odp.met.hu/ [Lekérdezés időpontja: 2021-06-20].
- Rusvai K.; Saláta D.; Falvai D.; Czóbel Sz. Assesment of weed invasion at bait sites in a Central European lower montane zone. Perspect. Plant Ecol. Evol. Syst. 2022 a, 55, 125667. [CrossRef]
- Rusvai K.; Wichmann B., Saláta D., Grónás V., Skutai J., Czóbel Sz. Changes in the Vegetation, Soil Seed Bank and Soil Properties at Bait Sites in a Protected Area of the Central European Lower Montane Zone. Sustainability 2022 b, 14(20), 13134. [CrossRef]
- Rusvai, K.; Czóbel, Sz. Changes in Soil Seed Bank and Vegetation at Abandoned Bait Sites in a Central European Hilly Area. Biol. Life Sci. Forum 2021, 2, 15. [CrossRef]
- Rusvai K. (2023): The effects of feeding places for hunting purposes on vegetation, seed bank and soil in the Mátra Mountains. Doctoral dissertation. Hungarian University of Agriculture and Life Sciences, PhD School of Environmental Sciences, Hungary, Gödöllő. 189 p.
- Hulme, P. E. (2016). Climate change and biological invasions: evidence, expectations, and response options. Biological Reviews, 92(3), 1297–1313. [CrossRef]
- Wainwright, C. E., & Cleland, E. E. (2013). Exotic species display greater germination plasticity and higher germination rates than native species across multiple cues. Biological Invasions, 15(10), 2253–2264. [CrossRef]
- Inslerman R.A., Baker D.L., Cumberland R., Doerr P., Miller J.E., Kennamer J.E., Stinson E.R., Williamson S.J. (2006): Baiting and Supplemental Feeding of Game Wildlife Species. The Wildlife Society. Technical Review 06-1, Washington, D.C., USA.
- Richardson C. (2006): Supplemental feeding of deer in west texas. Trans-pecos wildlife management series. Leaflet No.9, 10.
- Milner J.M., Van Beest F.M., Schmidt K.T., Brook R.K., Storaas T. (2014) To feed or not to feed? Evidence of the intended and unintended effects of feeding wild ungulates. Journal of Wildlife Management 78 (8): 1322–1334. [CrossRef]
- Apollonio M., Andersen R., Putman R. (2010): European ungulates and their management in the 21st century. New York: Cambridge University Press. 618 p.
- Selva N., Berezowska-Cnota T., Elguero-Claramunt I. (2014): Unforeseen Effects of Supplementary Feeding: Ungulate Baiting Sites as Hotspots for Ground-Nest Predation. PlosOne 9(3): e90740. [CrossRef]
- Wilson C.E., Castro, K.L., Thurston G.B., Sissons A. (2016): Pathway risk analysis of weed seeds in imported grain: A Canadian perspective. NeoBiota 30: 49–74.
- Gervilla C., Rita J., Cursach J. (2019): Contaminant seeds in imported crop seed lots: a non-negligible human-mediated pathway for introduction of plant species to islands. Weed Research. 59: 245–253. [CrossRef]
- Hobbs R., Huenneke L. (1992): Disturbance, diversity, and invasion: implications for conservation. Conservation Biology 6(3): 324–337. [CrossRef]
- Hirka A., Csóka Gy. (2009): Annual ragweed (Ambrosia artemisiifolia L.) in Hungarian forests /in Hungarian/. Növényvédelem 45(8): 438–439.
- MacDougall A.S., Turkington R. (2005): Are invasive species the drivers or passengers of change in degraded ecosystems? Ecology 86:42–55.
- Kochjarová J., Blanár D. (2018): Anthropophytes in protected mountain area: past, present and risk to diversity – a case study from the Muránska planina National park (Western Carpathians). Acta Oecol Carpatica 11(2): 75–90.
- Kochjarová J., Blanár D., Jarolímek I., Slezák M. (2023): Wildlife supplementary feeding facilitates spread of alien plants in forested mountainous areas: a case study from the Western Carpathians. Biologia 2: 1–19. [CrossRef]
- Borhidi A. (1995): Social behaviour types, the naturalness and relative ecological indicator values of the higher plants in the Hungarian Flora. Acta Botanica Hungarica 39(1-2): 97–181.
- Pinke G., Karácsony P., Czúcz B., Botta-Dukát Z., Lengyel A. (2012): The influence of environment, management and site context on species composition of summer arable weed vegetation in Hungary. Applied Vegetation Science 15(1): 136–144. [CrossRef]
- Vajdai I. (Szerk.) (1996): Fontosabb szántóföldi gyomok ismerete és a védekezés ellenük. GATE Mezőgazdasági Szaktanácsadási és Kutatásszervi Intézete, Gödöllő 222 p.
- Lambdon P. W., Pyšek P., Basnou C., Hejda M., Arianoutsou M., Essl F., Jarošík V., Pergl J., Winter M., Anastasiu P., Andriopoulos P., Bazos I., Brundu G., Celesti-Grapow L., Chassot P., Delipetrou P., Josefsson M., Kark S., Klotz S., Kokkoris Y., Kühn I., Marchante H., Perglová I., Pino J., Vila M., Zikos A., Roy D. & Hulme P. E. (2008) Alien flora of Europe: species diversity, temporal trends, geographical patterns and research needs. – Preslia 80: 101–149.
- Pauchard, A., Kueffer, C., Dietz, H., Daehler, C. C., Alexander, J., Edwards, P. J., Arévalo J.R., Cavieres L., Guisan A., Haider S., Jakobs G., Mcdougall K., Millar C., Naylor B., Parks C., Rew L., Seipel T. (2009): Ain’t no mountain high enough: plant invasions reaching new elevations. Frontiers in Ecology and the Environment 7(9): 479–486. [CrossRef]
- Pinke G., Pál R., Botta-Dukát Z. (2010): Effects of environmental factors on weed species composition of cereal and stubble fields in western Hungary. Central European Journal of Biology 5(2): 283–292. [CrossRef]
- Bíró I. (1998): A vadászat és vadgazdálkodás természetvédelmi vonatkozásai Békés megyében. A Puszta 1998. 1/15, 73–96.
- Molnár V.A. (2014): Természetvédelmi botanika. Oktatási segédanyag a Debreceni Egyetem kurzusához. Debreceni Egyetem TTK Növénytani Tanszék, Debrecen 65 p.
- Pinke G., Pál R., Botta-Dukát Z. (2010): Effects of environmental factors on weed species composition of cereal and stubble fields in western Hungary. Central European Journal of Biology 5(2): 283–292. [CrossRef]
- Berzsenyi Z. 2000: Gyomnövények, gyomirtás, gyombiológia. Szerk.: Hunyadi K. Mezőgazda Kiadó, Bp., 347.
- Von Holle B. (2013): Environmental stress alters native-nonnative relationships at the community scale. Biological Invasions 15: 417–427.
- Szabó G. (Szerk.) (2021): „Szubjektív élményföldrajz” Tanulmánykötet Aubert Antal professzor tiszteletére. Pécsi Tudományegyetem, Természettudományi Kar, Földrajzi és Földtudományi Intézet, Pécs. 247 p.
- Sauter-Louis, C.; Conraths, F.J.; Probst, C.; Blohm, U.; Schulz, K.; Sehl, J.; Fischer, M.; Forth, J.H.; Zani, L.; Depner, K.; Mettenleiter, T.C.; Beer, M.; Blome S. African Swine Fever in Wild Boar in Europe—A Review. Viruses 2021, 13, 1717. [CrossRef]
- Milad M., Schaich H., Konold, W. (2011): How is adaptation to climate change reflected in current practice of forest management and conservation? A case study from Germany. Biodiversity and Conservation 22: 1181–1202. [CrossRef]
- Dale V.H., Joyce L.A., McNulty S., Neilson R.P., Ayres M.P., Flanningan M.D., Hanson P.J., Irland L.C., Lugo A.E., Peterson C.J., Simberloff D., Swanson F.J., Stocks B.J., Wotton B.M. (2001). Climate Change and Forest Disturbances. BioScience 51(9): 723–734.
- Kueffer C., McDougall K., Alexander J., Daehler C., Edwards P., Haider S., Milbau A., Parks C., Pauchard A., Reshi Z.A., Rew L.J., Schroder M., Seipel, T. (2013): Plant Invasions into Mountain Protected Areas: Assessment, Prevention and Control at Multiple Spatial Scales. Plant Invasions in Protected Areas. 89–113.p. In: Foxcroft L.C., Pyšek P., Richardson D.M., Genovesi P. (Eds.): Plant invasions in protected areas. Patterns, problems and challenges. Invading Nature – Springer Series in Invasion Ecology. Volume 7. 656 p.
- Laurence W.F., Yensen E. (1991): Predicting the impacts of edge effects in fragmented habitats. Biological Conservation 55: 77–92.
- Martin P.H., Canham C.D., Marks P.L. (2009): Why forests appear resistant to exotic plant invasions: Intentional introductions, stand dynamics, and the role of shade tolerance. Frontiers in Ecology and the Environment 7: 142–149.
- Rejmánek M., Richardson D.M., Pyšek P. (2013): Plant Invasions and Invasibility of Plant Communities. 387–424. p. In: van der Maarel E., Franklin J. (Eds.): Vegetation Ecology. 2nd edn. Wiley-Blackwell, Oxford., 576 p.
- Spurrier C., Drees L. (2000): Hostile takeovers in America: invasive species in wildlands and waterways. Transactions of the 65th North American Wildlife and Natural Resources Conference 65: 315–325.
- Davis M.A., Pelsor M. (2001): Experimental support for a resource - based mechanistic model of invasibility. Ecology Letters 4: 421–428.
- Devlaeminck R., Bossuyt B., Hermy M. (2005) Inflow of seeds through the forest edge: evidence from seed bank and vegetation patterns. Plant Ecology 176: 1–17. [CrossRef]
- Burst M., Chauchard S., Dupouey J.-L., Amiaud, B. (2017): Interactive effects of land-use change and distance-to-edge on the distribution of species in plant communities at the forest-grassland interface. Journal of Vegetation Science 28(3): 515–526.
- Sukopp H. (1962): Neophyten in natürlichen Pflanzengesellschaften Mitteleuropas. Berichte der Deutschen Botanischen Gesellschaft, Band 75, Heft 6: 193–205.
- Kleijn D., Sutherland W.J. (2003): How effective are European agrienvironment schemes in conserving and promoting biodiversity? Journal of Applied Ecology 40: 947–969.
- Blossey B., Gorchov D.L. (2017): Introduction to the special issue: ungulates and invasive species: quantifying impacts and understanding interactions. AoB Plants 9: plx063. [CrossRef]
- Király, G. [ed.] Új magyar füvészkönyv. Magyarország hajtásos növényei. Határozókulcsok [New Hungarian herbal. The vascular plants of Hungary. Identification key]. ANP Igazgatóság: Jósvafő, Hungarian, 2009; pp. 3–456.
- Hammer, Ø.; Harper, D. A. T.; Ryan, P. D. PAST: Paleontological Statistics Software Package for Education and Data Analysis. Palaeontologia Electronica 2001, 4. https://palaeo-electronica.org/2001_1/past/issue1_01.htm.
- R Development Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2009 ISBN 3-900051-07-0. Available online: http://www.R-project.org (accessed on 20 October 2023).
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).