1. Introduction
Coronavirus disease (COVID-19) is a disease that infects the respiratory system, caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). The transmission of the virus can be directly person to person by aerosol, small droplets or indirectly by contact with contaminated surfaces [
1]. Thus, it spreads widely and highly contagious. Coronavirus disease (COVID-19) has been declared by World Health Organization (WHO) in March, 2020 as a global pandemic, as it contributes to increase mortality rate and impacts various sectors in the whole world [
2].
COVID-19 vaccines have been found to be effective for preventing severe disease. Regarding to the policies by the WHO and Health Ministries, vaccination must be given to protect people against severe symptoms and minimize the risk of transmission and severe disease [
3]. Current situation has shown that COVID-19 vaccines have contributed significantly to controlling COVID-19 pandemic [
4]. However, the efficacy of COVID-19 vaccines among patients with comorbidity has been known to be lower than normal patients. The suboptimal performance of humoral as well as cellular immunity can be reduced at varying level in patient with Comorbidities [
5].
The best examination to measure the level of immune response is using the plaque reduction neutralization test, but this test is challenging and can only be done in a well-resourced laboratories. Surrogate Viral Neutralization Test (SVNT) is an easier alternative that can be conducted in standard immunological lab and has been well received as reference [
6]. The measurement of antibody level against SARS-CoV2-S-RBD antigen test is another examination that is regarded as being simple to perform. The fluorescent-based rapid anti-S-RBD test can even be utilized in a field laboratory. The accuracy of this test has been well reported [
7]. However, due to the known effect of comorbidities associated with lower immune response, we want to know whether the presence of comorbid conditions affects its accuracy performance [
8].
The study aimed to determine the accuracy of Anti SARS-CoV2-RBD antibody test against surrogate viral neutralization test among persons living with HIV (PLWH) Systemic lupus erythematosus (SLE) and chronic kidney disease (CKD).
2. Materials and Methods
2.1. Study Design
This is a diagnostic study comparing the accuracy of a point-of-care anti SARS-CoV2-RBD test (FastBio-RBD) compared to Genscript SVNT as the reference standard among subjects with comorbidity. We enrolled subjects consisting of person with no comorbidities, PLWH, SLE, and CKD in stable condition. The PLWH group involved subjects who were diagnosed with HIV and had received the anti retroviral therapy at the HIV Clinic, Hasan Sadikin General Hospital. Subjects with SLE were registered patients who has already diagnosed with SLE and had routine follow-up at the rheumatology clinic at Hasan Sadikin General Hospital, The CKD group consists of End Stage Renal Disease patients on Hemodialysis at Hasan Sadikin General Hospital and Slamet General Hospital, Garut, West Java, Indonesia. Enrollment of each group were conducted at different time points, the non-comorbid subjects were enrolled on month April 2021 to August 2022. PLWH group were enrolled on September 2021, SLE group on November 2021 to February 2022, and CKD group on September to December 2021. All subjects were enrolled at a single time point and consecutively based on their visit to the clinic at their convenience.
2.2. Data Collected
Data regarding subject characteristics collected were sex, age, and current comorbidity. We also collected data regarding history of previous COVID-19 infection, and vaccination acquired. Previous COVID-19 infection was defined as having respiratory symptoms with positive PCR result or a positive antibody test in the past. It is only assigned when COVID-19 is documented as no longer current. Patient acquiring COVID-19 vaccines were clasified into several categories, which are unvaccinated, Sinovac only, AstraZeneca only, or mRNA (Pfizer or Moderna) vaccines. Subjects who acquired any number of sinovac-vaccine with subsequent mRNA booster were classified in he mRNA group.
2.3. Anti RBD: The Point-of-Care Anti SARS-CoV-2-RBD Antibody Test
To detect anti SARS-CoV2-RBD antibody (anti RBD), we used FastBio-RBD test, which was produced by Wondfo (Wondfo Biotech Co., Ltd.,Guangzhou, China) for distribution in Indonesia by PT Biofarma Indonesia (persero). The test was performed according to the manufacturer’s instructions [
9]. FastBio-RBD test is a point-of-care SARS-CoV-2 RBD antibody test using fluorescence immunoassay technology. The platform is based on a sandwich reaction where the test line contained the S-RBD antigen. The serum sample was added to the detection buffer, mixed, and added to the sample well. The patient’s anti-S-RBD antibodies in the serum would bind with the RBD antigen conjugated with a phosphorescent marker and form immune complexes. The immune complexes then migrate on the nitrocellulose membrane, which is then captured by the RBD antigen in the test line. The resulting complex would be detected using the related fluorescent immuno-assay (FIA) meter. Quantification was enabled by the fluorescence intensity exited from the immunochromatographic test. The anti-RBD level was reported by Arbitrary Unit (AU/mL) ranging from 0 – 200 AU/mL representing minimum to maximum concentration respectively. A measure of > 1 AU was regarded having positive result [
9].
2.4. The Reference Test: GenScript cPass SARS-CoV-2 Neutralization Antibody Detection Kit
The GenScript cPass SARS-CoV-2 Neutralization Antibody Detection Kit (Genscript Biotech, Leiden, Netherlands) as Surrogate viral neutralization test (SVNT) was used as the reference test. The test was performed according to the manufacturer’s instructions [
10]. The GenScript SARS-CoV-2 SVNT kit detects the presence of neutralizing antibodies against SARS-CoV-2 circulating in human serum or plasma by competitive mechanism. Presence of neutralizing antibodies against SARS-CoV-2 will block the interaction between receptor binding domain (RBD) of viral spike glycoprotein with the ACE2 cell surface receptor available within the reaction. The degree of the inhibition was measured by enzyme linked serological assay were recognized as the level of neutralizing antibody. The kit contains two components: the Horseradish peroxidase (HRP) conjugated recombinant SARS-CoV-2 RBD fragment (HRP-RBD) and the human ACE2 receptor protein (hACE2). The antibody level is measured by % inhibition with a range from minimum to maximum inhibition of 0-100% respectively. A measure of > 30% inhibition level is regarded as positive result.
2.5. Statistical Analysis
We described subject characteristics which are age, gender, comorbidity, history of COVID-19 infection, and vaccination status using frequency distribution tabulation. We describe the distribution of the anti-RBD and % inhibition in the table and plotted their values stratified based on co-morbidity groups and their vaccination status.
We also plotted and analyzed the Spearman’s ranked correlation between anti-RBD titer measured with the anti-RBD AU value and the % inhibition measured with SVNT. The R values were compared between subjects with comorbidity against those non-comorbid groups. Next, we performed the ROC analyses of the Anti-RBD level at 30%, 60%, and 90% of SVNT (% inhibition) level to determine the best cut-off point with the best accuracy among subjects with no comorbidity. Using these values, we measured the sensitivity, specificity, positive predictive value, and negative predictive value for each group of PLWH, SLE, and CKD subjects. The accuracy performance of each group was finally compared with the non-comorbid subjects.
2.6. Ethical Clearance
The study was approved by the Health Research Ethics Committee of Universitas Padjadjaran with ethics number 410/UN6.KEP/EC/2021, 17th May 2021. The study was conducted in accordance with the Declaration of Helsinki, and all data were kept anonymous.
3. Results
3.1. Study Subjects
A total of 517 subjects were included in this study. Subjects were divided into four groups consisting of those with no comorbidity (n=182), PLWH (n=100), SLE (n=92), and CKD (n=143). Based on age, the CKD group had the highest age distribution, while the PLWH group had the lowest age distribution. Gender wise, the female proportion was slightly higher than the male, this is especially prominent in the SLE group while the PLWH group were predominantly male. A total 113 (21.86%) subjects had a history of COVID-19 infection. Among 517 subjects, 216 subjects (41.8%) were unvaccinated and 301 subjects (58,2%) were vaccinated at the time of the survey. Most of the subjects were vaccinated using Sinovac. At the later stage when mRNA vaccines were available, some non-comorbid subjects and CKD acquired mRNA vaccines. The enrollment time varied widely. There were two survey time points for the non-comorbid group, which were in the 1st and 2nd quarter of 2021, and the 3rd quarter of 2022. The PLWH group were enrolled in the second quarter of 2021. Most SLE and CKD subjects were enrolled in the 4th quarter of 2021.
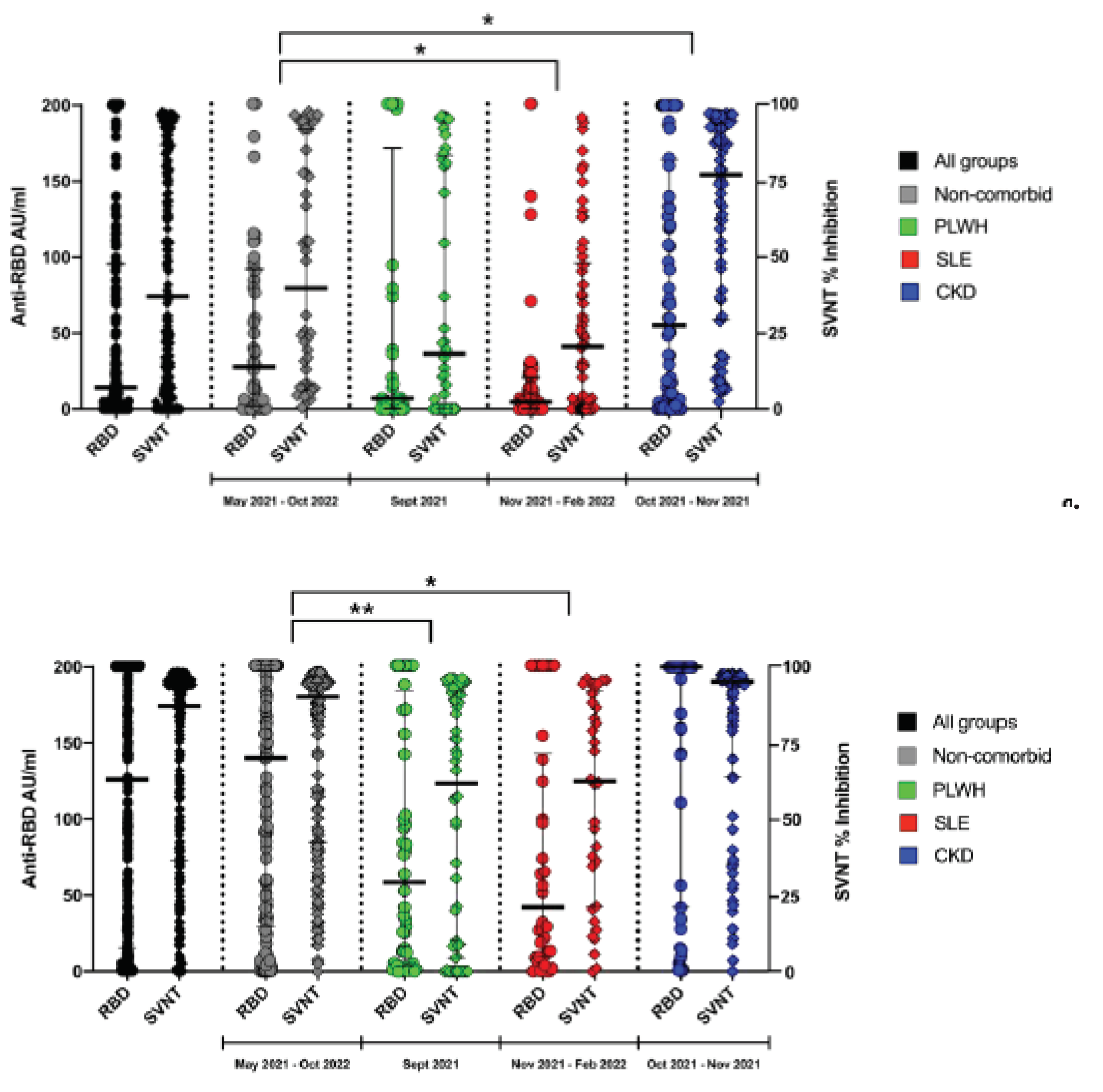
3.2. SVNT Is More Sensitive to Detect Neutralization Capacity Than Anti-RBD
In general, the % inhibition using SVNTs were distributed at a higher-level result compared to the anti-RBD
(Figure 1a). By using SVNT, overall 304 subjects (58.80%) have reached over the middle value of 50% inhibition while only 214 subjects (41.39%) reached above the middle value of 100 AU/mL of anti-RBD assay (p=0.0001). These distribution patterns were found in all of the observed groups as well
(Figure 1a,b).
3.3. The Different Level of Anti-RBD and SVNT in Each Group of Subjects
The serosurveys were not conducted at the same time among all of the subjects. It started with the non-comorbid subjects in the second and third quarter of 2021, followed by the PLWH group. The SLE and CKD group were conducted at similar time points. In
Figure 1a, we can see that the distribution of anti-RBD titer and sVNT % inhibition among unvaccinated group were higher in the non-comorbid compared to the PLWH and SLE. We think that this may be due to their comorbid condition in these groups. Interestingly, while still unvaccinated, the CKD group showed highest serological level. We think that this was due to the frequent visit to the Hospital for Hemodialysis. The highest proportion of having COVID-19 illness history may also support this hypothesis
(Table 1). In the vaccinated situation, we observed highest serological level in the non-comorbid group and CKD group who acquired mRNA vaccines
(Figure 1b).
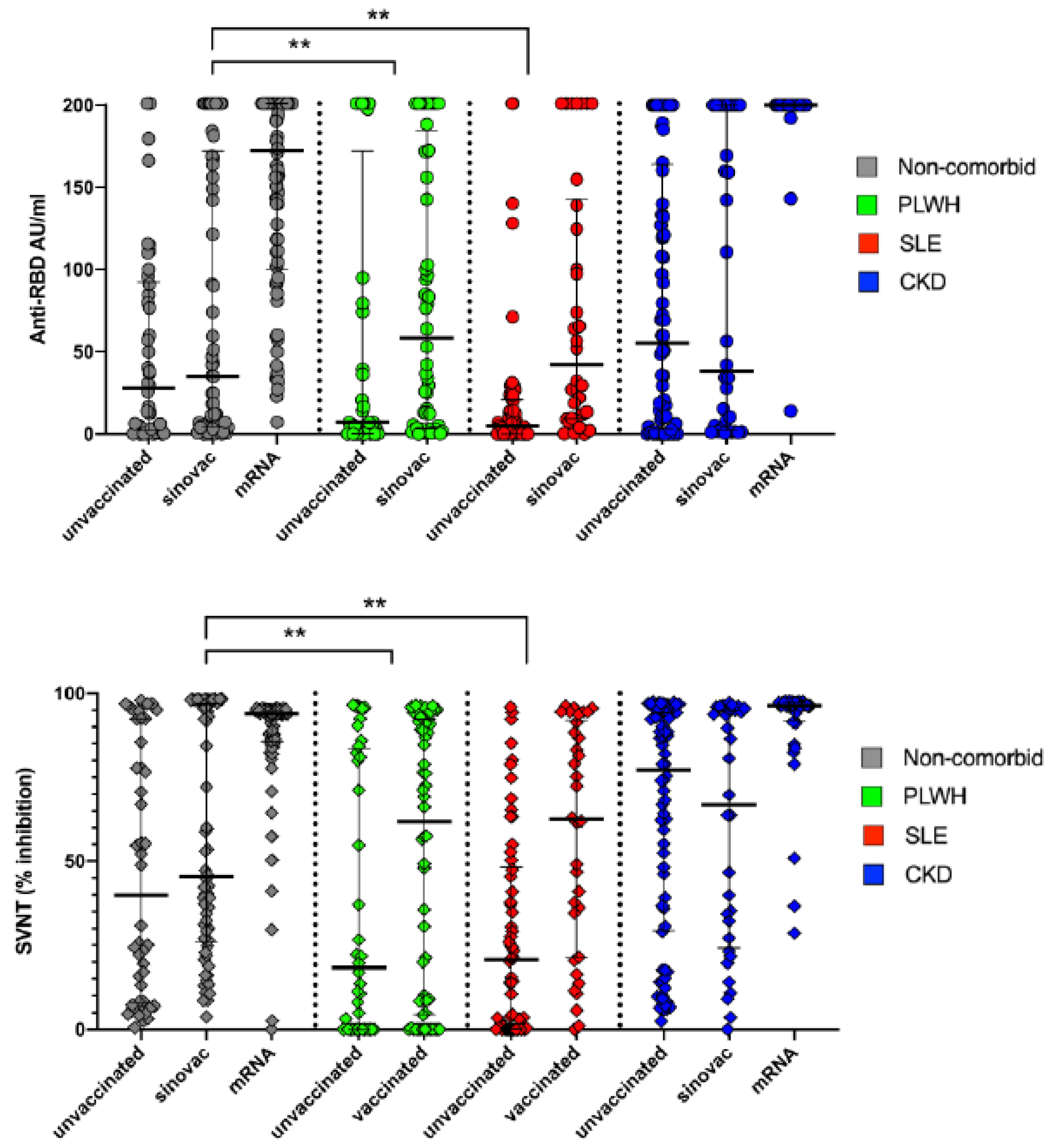
3.4. The Effect of Vaccination on the Distribution of Anti-RBD and SVNT Level
In
Figure 2a,b we observed that vaccination effects were prominent on all group, both shown by anti-RBD as well as the SVNT values. Some of the non-comorbid and CKD group, and all of the PLWH, and SLE groups were vaccinated with CoronaVac. With Sinovac, we could see a modest increase of the anti-RBD as well as the SVNT level. While most prominent increased were observed in some of the non-comorbid group and the CKD group who acquired m-RNA vaccines.
3.5. The effect of Vaccination on the Distribution of Anti-RBD and SVNT
In
Figure 2a,b we observed that vaccination effects were prominent on all group, both shown by anti-RBD as well as the SVNT values. Some of the non-comorbid and CKD group, and all of the PLWH, and SLE groups were vaccinated with CoronaVac. With Sinovac, we could see a modest increase of the anti-RBD as well as the SVNT level. While most prominent increased were observed in some of the non-comorbid group and the CKD group who acquired m-RNA vaccines.
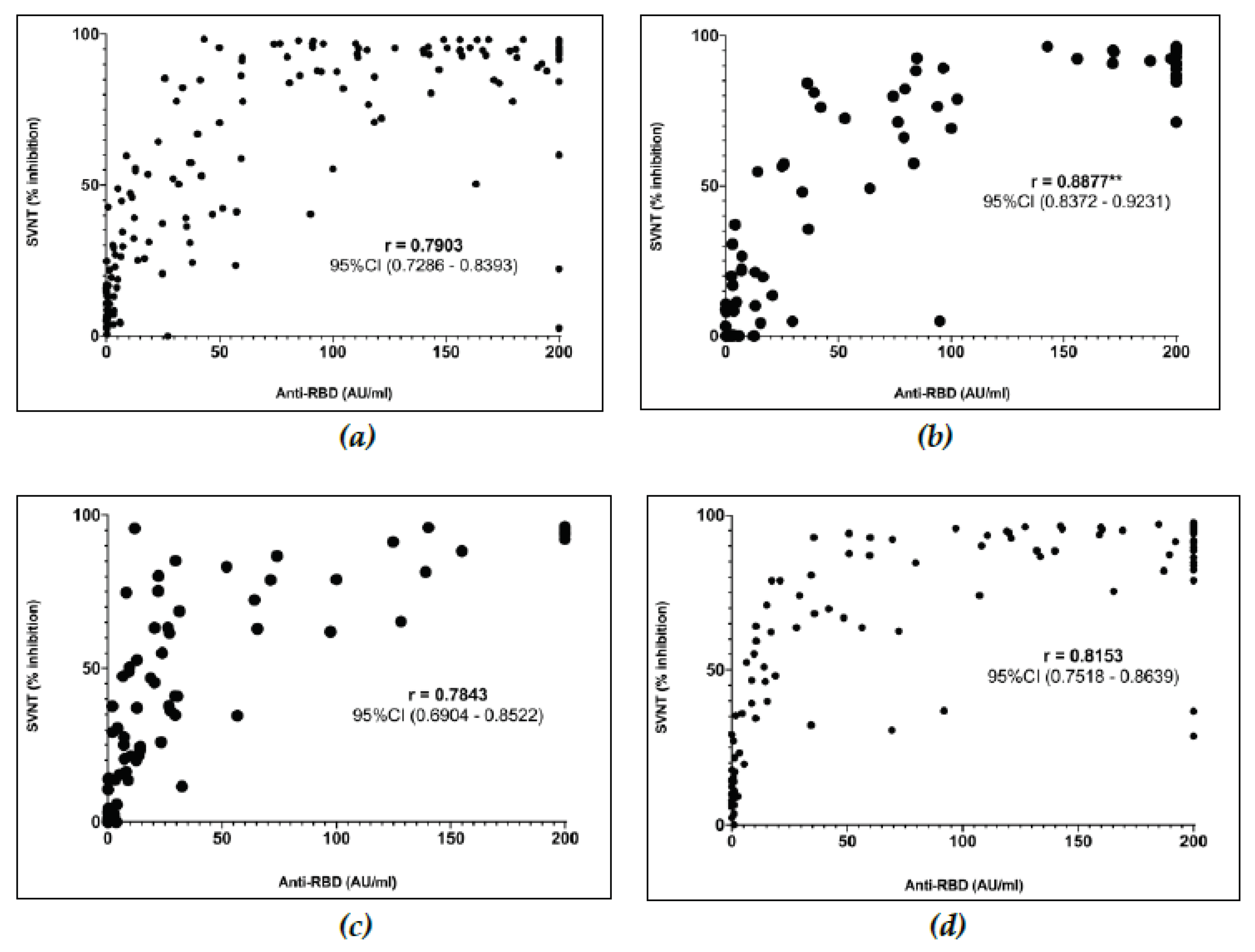
3.6. Correlation between Anti-RBD Titer and Percentage Inhibition
In
Figure 3a, we demonstrate the linear Spearman ranked correlation test between anti-RBD titer and the % inhibition measured with SVNT among each group of subjects. We observed a correlation of R=0.7903, (95% CI; 0.7286 – 0.8393) among the non-comorbid group. The correlation test for the PLWH group demonstrated an R = 0.8877, (95% CI; 0.8372 – 9.9231)
(Figure 3b) which was significantly higher (p=0.0072) compared to the non-comorbid group. The correlation test among SLW and CKD group were R = 0.7843 (95% CI; 0.6904 – 0.8522) and R = 0.8153, (95% CI; 0.7518 – 0.8639) respectively. These correlation test results among the SLE and CKD groups were not significantly different with the non-comorbid group
(Figure 3c,d).
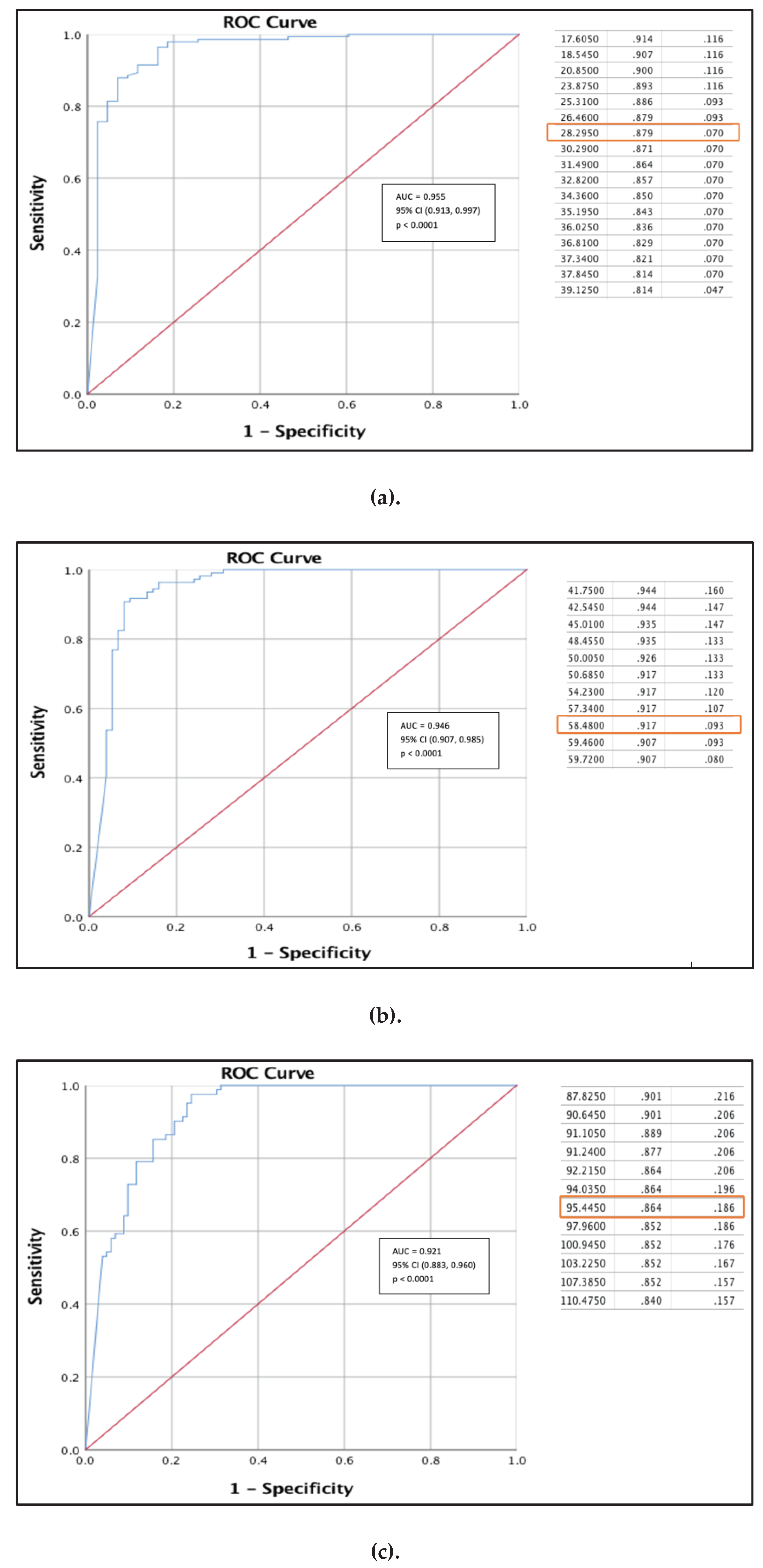
3.7. ROC and Cutoff Value to Detect 30%, 60%, and 90% Inhibition Level of SVNT
We performed ROC analyses to determine the best cutoff of the Anti-RBD against SVNT inhibition at 30%, 60%, and 90% level among the non-comorbid group. At 30% inhibition level, we obtained an excellent AUC of 0.955, (95% CI; 0.913, 0.997). Using this curve, we obtained an anti-RBD value of 28.30 AU/mL as the best cut-off with a sensitivity and specificity of 87.9% and 93.0% respectively
(Figure 4a). At 60% SVNT inhibition level, we obtained an excellent AUC of 0.946, (95% CI; 0.907, 0.985) with best cutoff point for an anti-RBD value of 58.48 AU/mL. With this value, we achieved sensitivity and specificity of 91.7% and 90.7%
(Figure 4b). While at 90% SVNT inhibition level, we achieved an great value of AUC 0.921, (95% CI; 0.883, 0.960) and obtained the best anti-RBD cut-off value of 95.45 AU/mL. With this value we obtained sensitivity and specificity of 86.4% and 81.4%, respectively
(Figure 4c).
3.8. Sensitivity and Sensitivity of AntiRBD in Various Comorbid Condition
In
Table 2, we presented the performance of anti RBD test at the 30%, 60% and 90% SVNT inhibition level for all of the comorbid group compared to non-comorbid group. At anti-RBD cut-off level of 28.3 AU/mL we found almost no significant different of sensitivity and specificity to detect 30% SVNT inhibition level, except for the SLE group. Significantly lower sensitivity was observed in the SLE group (p=0.000014) while the specificity remains similar. We also observed similar pattern using the Anti RBD cut-off level of 58.8AU/mL to detect 60% SVNT inhibition level. Similar sensitivity and specificity were found except for the SLE group which showed significantly lower sensitivity (p=0.000326). While at RBD 95.4 AU/mL there were no significant difference of sensitivity across all the comorbid group to detect 90% SVNT inhibition level. However, the specificity was significantly higher in SLE group (p=0.007631). With the difference mainly observed in the sensitivity of the tests but specificity remain high, negative predictive value of the test shown to be adequately high in all the groups. Meaning that there would be minimal number of cases missed classified as having low serological level.
Table 2. a.
Accuracy of anti-RBD with specific cut-off at 30% inhibition level of SVNT for each comorbidity groups.
Table 2. a.
Accuracy of anti-RBD with specific cut-off at 30% inhibition level of SVNT for each comorbidity groups.
| |
Accuracy to detect 30% inhibition using anti-RBD cutoff of 28.30 AU/ml |
| |
|
Non-comorbid |
HIV |
SLE |
CKD |
| Sensitivity |
n/total |
121/138 |
48/53 |
27/46 |
97/115 |
| % |
87.7% |
90.6% |
58.7%** |
84.4% |
| 95% CI |
(81.0 – 92.7) |
(79.3 – 96.9) |
(43.2 – 73.0) |
(76.4 – 90.4) |
| Specificity |
n/total |
40/44 |
45/47 |
45/46 |
27/28 |
| % |
90.9% |
95.7% |
97.8% |
96.4% |
| 95% CI |
(78.3 – 97.5) |
(85.5 – 99.5) |
(88.5 – 99.9) |
(81.6 – 99.9) |
| PPV |
n/total |
121/125 |
48/50 |
27/28 |
97/98 |
| % |
96.8% |
96.0% |
96.4% |
99.0% |
| 95% CI |
(92.2 – 98.7) |
(86.0 – 98.9) |
(79.28 – 99.5) |
(93.39 – 99.8) |
| NPV |
n/total |
40/57 |
45/50 |
45/64 |
27/45 |
| % |
70.3% |
90.00%** |
70.31% |
60.00% |
| 95% CI |
(59.9 – 78.8) |
(79.6 – 95.4) |
(62.6 – 77.0) |
(49.4 – 69.8) |
Table 2. b.
Accuracy of anti-RBD with specific cut-off at 60% inhibition level of SVNT for each comorbidity groups.
Table 2. b.
Accuracy of anti-RBD with specific cut-off at 60% inhibition level of SVNT for each comorbidity groups.
| |
Accuracy to detect 60% inhibition using anti-RBD cutoff of 58.48 AU/ml |
| |
Non-comorbid |
HIV |
SLE |
CKD |
| Sensitivity |
n/total |
98/107 |
40/44 |
20/30 |
84/99 |
| % |
91.6% |
90.9% |
66.7%** |
84.9% |
| 95% CI |
(84.6 – 96.1) |
(78.3 – 97.5) |
(47.2 – 82.7) |
(76.2 – 91.3) |
| Specificity |
n/total |
68/75 |
53/56 |
62/62 |
40/44 |
| % |
90.7% |
94.6% |
100.0% |
90.9% |
| 95% CI |
(81.7 – 96.2) |
(85.1 – 98.9) |
(94.2 – 100.0) |
(78.3 – 97.5) |
| PPV |
n/total |
98/105 |
40/43 |
20/20 |
84/88 |
| % |
93.3% |
93.0% |
100.0% |
95.5% |
| 95% CI |
(87.3 – 96.6) |
(81.5 – 97.6) |
(83.2 – 100.0) |
(89.2 – 98.2) |
| NPV |
n/total |
68/77 |
53/57 |
62/72 |
40/55 |
| % |
88.3% |
93.0% |
86.11% |
72.7%* |
| 95% CI |
(80.1 – 93.4) |
(83.9 – 97.1) |
(78.9 – 91.1) |
(62.4 – 81.1) |
Table 2. c.
Accuracy of anti-RBD with specific cut-off at 90% inhibition level of SVNT for each comorbidity groups.
Table 2. c.
Accuracy of anti-RBD with specific cut-off at 90% inhibition level of SVNT for each comorbidity groups.
| |
Accuracy to detect 90% inhibition using anti-RBD cutoff of 95.45 AU/ml |
| |
|
Non-comorbid |
HIV |
SLE |
CKD |
| Sensitivity |
n/total |
69/80 |
25/26 |
11-Dec |
65/69 |
| % |
86.3% |
96.2% |
91.7% |
94.2% |
| 95% CI |
(76.7 – 92.9) |
(80.3 – 99.9) |
(61.5 – 99.8) |
(85.8 – 98.4) |
| Specificity |
n/total |
83/102 |
66/74 |
75/80 |
58/74 |
| % |
81.4% |
89.2% |
93.8%** |
78.4% |
| 95% CI |
(72.5 – 88.4) |
(79.8 – 95.2) |
(86.0 – 97.9) |
(67.3 – 87.1) |
| PPV |
n/total |
69/88 |
25/33 |
Nov-16 |
65/81 |
| % |
78.4% |
75.8% |
68.8% |
80.3% |
| 95% CI |
(70.6 – 84.6) |
(61.8 – 85.8) |
(48.1 – 83.9) |
(72.4 – 86.3) |
| NPV |
n/total |
83/94 |
66/67 |
75/76 |
58/62 |
| % |
88.3% |
98.5%** |
98.7%** |
93.6% |
| 95% CI |
(77.3 – 88.6) |
(90.6 – 99.8) |
(92.0 – 99.8) |
(84.8 – 97.4) |
4. Discussion
We have conducted a head to head comparison of a point-of-care Anti-SARS-CoV2-S-RBD antibody test versus Surogate Viral Neutralization test in subjects living with HIV, SLE and CKD. The effect of the previous infection and the acquired vaccines were significant and clearly shown. We have found that the in general, the results of Anti-RBD were well correlated with SVNT both in non-comorbid as well as in subjects with comorbid. However, we observed a slight change in the accuracy. The sensitivity and specificity of the Anti-RBD test among PLWHA and CKD were comparable with the non-comorbid subjects. But among SLE, Anti-RBD sensitivity to detect 30% and 60% SVNT inhibition level were lower while specificity is to detect 90% SVNT inhibition level was higher. However overall, specificity and negative predictive value remained at high level, confirming that the test should still be beneficial on comorbid subjects to detect low serological level. To our knowledge, our evaluation has not been reported by other researchers.
One of the method to measure Immunological response to a disease and vaccine is by measuring the level of specific antibodies or its neutralization capacity [
11]. In COVID-19, High level of anti-RBD or neutralizing antibody titers are associated with lower risk of symptomatic and severe disease [
12]. A point-of-care Anti-SARS-CoV2-RBD tests are available to simplify the method so it can readily be utilized in resource limited settings. The correlation of neutralizing activity with anti-SARS-CoV2-RBD antibody level is known to be high. But due to the various method and variation in reagents, an absolute conversion factor could not be established [
13].
4.1. SVNT Is More Sensitive Than Anti-RBD
Our study showed that the SVNT results were distributed at a higher range than Anti-RBD in the same blood sample. Meaning that SVNT is more sensitive to detect the presence of neutralization capacity. This situation may be due to the nature of the test which identify total immunodominant neutralizing antibodies that blocked the interaction between the SARS-CoV2 surface receptor-binding domain and the angiotensin-converting enzyme 2 (ACE2) receptor protein [
12]. The anti-RBD test was less sensitive because it only detects specific antibodies against the human receptor binding domain (S-RBD) [
14]. Anti-RBD test results do not fully represent the whole spectrum of possible neutralizing antibodies. There could be other additional neutralizing antibodies which reinforced % inhibition detected by SVNT [
11,
15].
4.2. The Effect of Natural Transmission and Vaccines
Among the unvaccinated subjects, we have found a significantly higher anti-RBD and SVNT % inhibition levels in the CKD group as compared to the other groups. We think that this condition can be due to several factors. Firstly, the serosurvey of the CKD patient were conducted latter in the pandemic time. The severely impacting SARS-CoV-2 Delta outbreak occurred between July-September 2021. While the CKD patient were tested on the October to November 2021 [
16]. While secondly, the CKD subjects had at least twice weekly visits to hospital for their Hemodyalisis, which were more frequent than the other group. As we also know that COVID-19 is very widely transmitted especially during the outbreaks and even higher in the hospitals [
17,
18]. As such, CKD patients may be more exposed to COVID-19 transmission than most other patients registered in a Hospital.
The effect of vaccination was clearly shown in the distribution of the anti-RBD and SVNT result. Sinovac provide modest increase in antibody level in all of the groups. But mRNA vaccine showed greater antibody distribution to the highest level in the non-comorbid and CKD groups. In Indonesia, the Sinovac was delivered starting in March 2021, but the mRNA vaccine was only delivered in August/ September 2021. Some of the non-comorbid subjects manage to acquire mRNA booster vaccine, while in some of the CKD subjects, mRNA was given as their primary vaccine. The modest effects of Sinovac and higher stimulation effects of mRNA vaccine has been well documented elsewhere [
19,
20].
4.3. Correlation of SVNT vs Anti-RBD
Our study has shown that the correlation remains excellent between SVNT and point-of-care Anti-RBD test. We found correlations coefficient of R>0,7 in all subject groups. Other reports have shown similar findings. Malipiero et al. have tested other point of care anti-RBD test and acquired a correlation coefficient of R = 0.5887 to 0.7332 [
13]. However, a more sophisticated laboratory methods using ECLIA/ELISA have been known to demonstrate higher level of correlation coefficients at 0.8425 to 0.9736 [
21]. It is interesting that we found significantly higher correlation among PLWH. We hypothesized that the SVNT which utilized all predominant neutralizing antibodies in the reaction were more reduced than the Anti-RBD test which only measure specific antibodies. Therefore the lower SVNT result may contribute to the higher correlation coefficient. Supporting facts so far have shown that seroconversion rates and immunologic titers were lower in PLWH with lower baseline CD4 counts, lower baseline CD4/CD8 ratios, and higher baseline viral loads [
22].
4.4. Accuracy to Detect Specific SVNT Inhibition Level
The study started by a hypothesis that the accuracy of the anti-RBD test would be affected by the comorbid status. However, we have found that the comorbid effect to sensitivity and specificity were only found in the SLE group. The sensitivity of the Anti-RBD test to detect 30% and 60% SVNT inhibition level were lower, and the specificity was higher at 90% inhibition level. From other studies, we have learned that COVID-19 vaccination recipients with SLE show a reduced antibody response, even in the absence of immune-suppressing medicines. After receiving the COVID-19 immunization, auto-reactive T cells showed decreased activation [
23]. On the other hand, immune suppression provided by SLE therapy were mostly directed to decrease activity of the humoral immunity [
24]. Perhaps in this group we have observed the predominating lack of humoral immunity in contrast to the PLWH group which were lacking cellular immunity.
Our observation among CKD subjects did not show difference either in the correlation nor the accuracy of the Anti-RBD against non-comorbid subjects. Several studies have pointed out that the renal impairment among persons attending hemodialysis lower anti-SARS-CoV-2-S antibody titers [
25]. This difference were not seen in our observation perhaps because many patient in this group acquired mRNA vaccine and had maximum anti-RBD as well as the SVNT inhibition level. Another possibility was that if both Anti-RBD and Antibody neutralization level were smililarly reduced by the the comorbidity condition, there will be no effect to the sensitivity and specificity.
4.5. Weakness/Limitation
Since the enrollment of subjects were conducted cross sectionally and at various points of time, our observation may be subjected to pandemic phases, vaccine type and duration between vaccination and assessment. Because of this, we were unable to exactly compare or make assessment of the specific effect of the comorbidity to the antibody response. With this convenient design as well, higher range of antibody titers in some groups who acquired mRNA vaccines may affect the comparison. The reference standard utililzed in this study were SVNT while the best standard for neutralizing antibody test would be the placque readuction neutralization assays (PRNT). The PRNT test was not available in our area. Lastly, the SVNT, which we us as the reference standard can as well be affected by the comorbidity. Therefore it is not a perfect gold standard with a presistent result.
5. Conclusions
The percent inhibition values of SVNT were more sensitive than anti-RBD titers in detecting the presence of neutralizing capacity. Anti-RBD had an excellent correlation to SVNT in PLWH, SLE as well as CKD. Higher correlation coefficient among PLWH was hypothesized to be caused by the lower detection capacity by the SVNT. The anti-RBD has high accuracy with sensitivity > 80% and specificity >90% in most group. Lower sensitivity and higher specificity among the person with SLE made us consider that this group has the most compromised humoral immunity capacity related to their illness and immunotherapy. However, overall, the consistently high specificity of anti-RBD in all groups showed that the Anti-RBD test can be reliably utilized to detect the low antibody responses or neutralization capacity in subjects with these comorbidity. This may sugests the need for additional COVID-19 vaccination booster.
Author Contributions
Conceptualization, M.R.T., B.A., H.D., R.W., and A.R.I; data curation, M.R.T., H.D., C.B.P., T.T.M., B.A., R.W., L.H., R.S., and A.R.I; investigation, M.R.T, H.D., C.B.P., T.T.M., B.A., R.W., L.H., R.S., and A.R.I; writing – original draft preparation, M.R.T, B.A., C.B.P., T.T.M., R.W., and A.R.I; review, M.R.T, B.A., C.B.P., D.S., R.W., L.H., R.S., and A.R.I; editing, M.R.T, B.A., C.B.P, R.W., L.H., R.S., and A.R.I; supervision, B.A., D.S., R.W., L.H., R.S., and A.R.I. All authors have read and agreed to the published version of the manuscript.
Funding
We received funding from the Indonesian National Research and Innovation Agency (BRIN) to conduct the study. Support for FastBio-RBDTM test kits was kindly provided by PT Biofarma Indonesia (Persero) and Wondfo, Guangzhou Biotech, Co., Ltd., China..
Institutional Review Board Statement
The study was conducted by the Declaration of Helsinki and approved by the Research Committee of Universitas Padjadjaran (Ethical Approval No. 410/UN6.KEP/EC/2021).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data used to support the findings of this study are included in the article.
Acknowledgments
We are grateful for the support of the Indonesian National Research and Innovation Agency (BRIN) for providing the operational support for conducting the study. We are also thankful for the contributions from Wondfo, Guangzhou Biotech, Co., Ltd., China, and PT. Biofarma Indonesia (Persero) for providing the FastBioRBD™ fluorescent immunoassay reader and the rapid test reagents for our study.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Lotfi, M.; Hamblin, M.R.; Rezaei, N. COVID-19: Transmission, Prevention, and Potential Therapeutic Opportunities. Clin Chim Acta 2020, 508, 254–266. [Google Scholar] [CrossRef] [PubMed]
- Cucinotta, D.; Vanelli, M. WHO Declares COVID-19 a Pandemic. Acta Biomed 2020, 91, 157–160. [Google Scholar] [CrossRef] [PubMed]
- Wilder-Smith, A. What Is the Vaccine Effect on Reducing Transmission in the Context of the SARS-CoV-2 Delta Variant? Lancet Infect Dis 2022, 22, 152. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Qin, C.; Liu, M.; Liu, J. Effectiveness and Safety of SARS-CoV-2 Vaccine in Real-World Studies: A Systematic Review and Meta-Analysis. Infect Dis Poverty 2021, 10. [Google Scholar] [CrossRef] [PubMed]
- Lee, A.R.Y. Bin; Wong, S.Y.; Chai, L.Y.A.; Lee, S.C.; Lee, M.X.; Muthiah, M.D.; Tay, S.H.; Teo, C.B.; Tan, B.K.J.; Chan, Y.H.; et al. Efficacy of Covid-19 Vaccines in Immunocompromised Patients: Systematic Review and Meta-Analysis. BMJ 2022, 376, e068632. [Google Scholar] [CrossRef]
- Kweon, O.J.; Bae, J.Y.; Lim, Y.K.; Choi, Y.; Lee, S.; Park, M.S.; Suh, I.B.; Kim, H.; Jee, Y.S.; Lee, M.K. Performance Evaluation of Newly Developed Surrogate Virus Neutralization Tests for Detecting Neutralizing Antibodies against SARS-CoV-2. Sci Rep 2023, 13. [Google Scholar] [CrossRef] [PubMed]
- Tiara, M.R.; Djauhari, H.; Rachman, F.R.; Rettob, A.C.; Utami, D.; Pulungan, F.C.S.; Purwanta, H.; Wisaksana, R.; Alisjahbana, B.; Indrati, A.R. Performance of a Point-of-Care Fluorescence Immunoassay Test to Measure the Anti-Severe Acute Respiratory Syndrome Corona Virus 2 Spike, Receptor Binding Domain Antibody Level. Diagnostics (Basel) 2023, 13. [Google Scholar] [CrossRef] [PubMed]
- Lo Sasso, B.; Giglio, R.V.; Vidali, M.; Scazzone, C.; Bivona, G.; Gambino, C.M.; Ciaccio, A.M.; Agnello, L.; Ciaccio, M. Evaluation of Anti-SARS-Cov-2 S-RBD IgG Antibodies after COVID-19 MRNA BNT162b2 Vaccine. Diagnostics 2021, 11. [Google Scholar] [CrossRef] [PubMed]
- Wondfo Biotech Co.(China), G. W. B. Result Report on Finecare 2019-NCoV RBD Antibody Test. Guangzhou Wondfo Biotech Co.: Guangzhou, China, 2019. [Google Scholar]
- GenScript. CPass SARS-CoV-2 Neutralization Antibody Detection Kit Instruction for Use; GenScript: Piscataway, NJ, USA, 2022. [Google Scholar]
- Malipiero, G.; D’Agaro, P.; Segat, L.; Moratto, A.; Villalta, D. Long-Term Decay of Anti-RBD IgG Titers after BNT162b2 Vaccination Is Not Mirrored by Loss of Neutralizing Bioactivity against SARS-CoV-2. Clinica Chimica Acta 2022, 524, 11–17. [Google Scholar] [CrossRef]
- Tan, C.W.; Chia, W.N.; Qin, X.; Liu, P.; Chen, M.I.C.; Tiu, C.; Hu, Z.; Chen, V.C.W.; Young, B.E.; Sia, W.R.; et al. A SARS-CoV-2 Surrogate Virus Neutralization Test Based on Antibody-Mediated Blockage of ACE2–Spike Protein–Protein Interaction. Nat Biotechnol 2020, 38, 1073–1078. [Google Scholar] [CrossRef]
- Malipiero, G.; D’Agaro, P.; Segat, L.; Moratto, A.; Villalta, D. Long-Term Decay of Anti-RBD IgG Titers after BNT162b2 Vaccination Is Not Mirrored by Loss of Neutralizing Bioactivity against SARS-CoV-2. Clin Chim Acta 2022, 524, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Premkumar, L.; Segovia-Chumbez, B.; Jadi, R.; Martinez, D.R.; Raut, R.; Markmann, A.J.; Cornaby, C.; Bartelt, L.; Weiss, S.; Park, Y.; et al. The Receptor Binding Domain of the Viral Spike Protein Is an Immunodominant and Highly Specific Target of Antibodies in SARS-CoV-2 Patients. Sci Immunol 2020, 5. [Google Scholar] [CrossRef] [PubMed]
- Sariol, C.A.; Pantoja, P.; Serrano-collazo, C.; Rosa-arocho, T.; Armina-rodríguez, A.; Cruz, L.; Stone, E.T.; Arana, T.; Climent, C.; Latoni, G.; et al. Function Is More Reliable than Quantity to Follow up the Humoral Response to the Receptor-binding Domain of Sars-cov-2-spike Protein after Natural Infection or Covid-19 Vaccination. Viruses 2021, 13. [Google Scholar] [CrossRef]
- Hartantri, Y.; Debora, J.; Widyatmoko, L.; Giwangkancana, G.; Suryadinata, H.; Susandi, E.; Hutajulu, E.; Hakiman, A.P.A.; Pusparini, Y.; Alisjahbana, B. Clinical and Treatment Factors Associated with the Mortality of COVID-19 Patients Admitted to a Referral Hospital in Indonesia. The Lancet Regional Health - Southeast Asia 2023, 11, 100167. [Google Scholar] [CrossRef] [PubMed]
- Tang, H.; Tian, J.B.; Dong, J.W.; Tang, X.T.; Yan, Z.Y.; Zhao, Y.Y.; Xiong, F.; Sun, X.; Song, C.X.; Xiang, C.G.; et al. Serologic Detection of SARS-CoV-2 Infections in Hemodialysis Centers: A Multicenter Retrospective Study in Wuhan, China. American Journal of Kidney Diseases 2020, 76, 490–499.e1. [Google Scholar] [CrossRef] [PubMed]
- Clarke, C.; Prendecki, M.; Dhutia, A.; Ali, M.A.; Sajjad, H.; Shivakumar, O.; Lightstone, L.; Kelleher, P.; Pickering, M.C.; Thomas, D.; et al. High Prevalence of Asymptomatic COVID-19 Infection in Hemodialysis Patients Detected Using Serologic Screening. J Am Soc Nephrol 2020, 31, 1969–1975. [Google Scholar] [CrossRef] [PubMed]
- Lau, C.S.; Oh, M.L.H.; Phua, S.K.; Liang, Y.L.; Li, Y.; Huo, J.; Huang, Y.; Zhang, B.; Xu, S.; Aw, T.C. Kinetics of the Neutralizing and Spike SARS-CoV-2 Antibodies Following the Sinovac Inactivated Virus Vaccine Compared to the Pfizer MRNA Vaccine in Singapore. Antibodies 2022, 11. [Google Scholar] [CrossRef] [PubMed]
- Muslimah, A.H.; Tiara, M.R.; Djauhari, H.; Dewantara, M.H.; Susandi, E.; Indrati, A.R.; Alisjahbana, B.; Soeroto, A.Y.; Wisaksana, R. High Levels of Anti-SARS-CoV-2 Receptor-Binding Domain (RBD) Antibodies One Year Post Booster Vaccinations among Hospital Workers in Indonesia: Was the Second Booster Needed? Vaccines (Basel) 2023, 11, 1300. [Google Scholar] [CrossRef] [PubMed]
- Tan, C.W.; Chia, W.N.; Qin, X.; Liu, P.; Chen, M.I.C.; Tiu, C.; Hu, Z.; Chen, V.C.W.; Young, B.E.; Sia, W.R.; et al. A SARS-CoV-2 Surrogate Virus Neutralization Test Based on Antibody-Mediated Blockage of ACE2-Spike Protein-Protein Interaction. Nat Biotechnol 2020, 38, 1073–1078. [Google Scholar] [CrossRef]
- Chun, H.M.; Milligan, K.; Agyemang, E.; Ford, N.; Rangaraj, A.; Desai, S.; Wilder-Smith, A.; Vitoria, M.; Zulu, I. A Systematic Review of COVID-19 Vaccine Antibody Responses in People With HIV. Open Forum Infect Dis 2022, 9. [Google Scholar] [CrossRef]
- Izmirly, P.M.; Kim, M.Y.; Samanovic, M.; Fernandez-Ruiz, R.; Ohana, S.; Deonaraine, K.K.; Engel, A.J.; Masson, M.; Xie, X.; Cornelius, A.R.; et al. Evaluation of Immune Response and Disease Status in Systemic Lupus Erythematosus Patients Following SARS–CoV-2 Vaccination. Arthritis and Rheumatology 2022, 74, 284–294. [Google Scholar] [CrossRef] [PubMed]
- Pappa, M.; Panagiotopoulos, A.; Thomas, K.; Fanouriakis, A. Systemic Lupus Erythematosus and COVID-19. Curr Rheumatol Rep 2023, 25. [Google Scholar] [CrossRef] [PubMed]
- Matsunami, M.; Suzuki, T.; Fukuda, J.; Terao, T.; Ukai, K.; Sugihara, S.; Toishi, T.; Nagaoka, K.; Nakata, M.; Ohara, M.; et al. Comparison of Antibody Response Following the Second Dose of SARS-CoV-2 MRNA Vaccine in Elderly Patients with Late-Stage Chronic Kidney Disease. Ren Replace Ther 2022, 8, 1–11. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).