Submitted:
03 April 2024
Posted:
04 April 2024
You are already at the latest version
Abstract
Keywords:
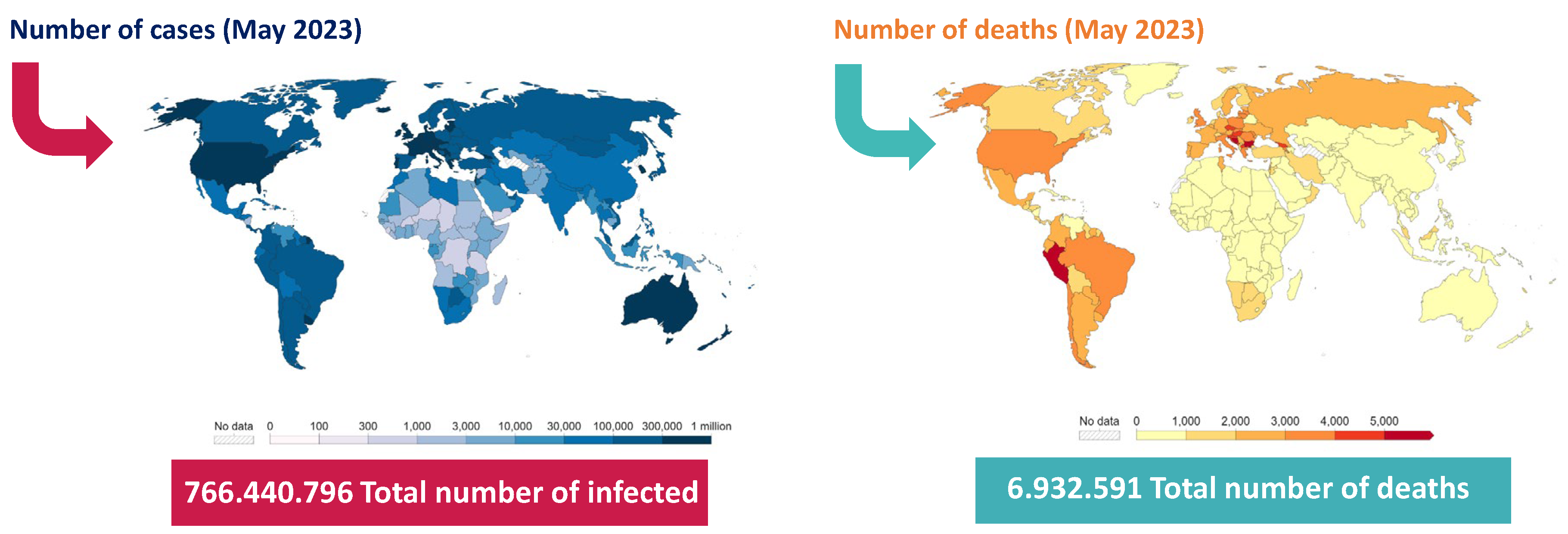
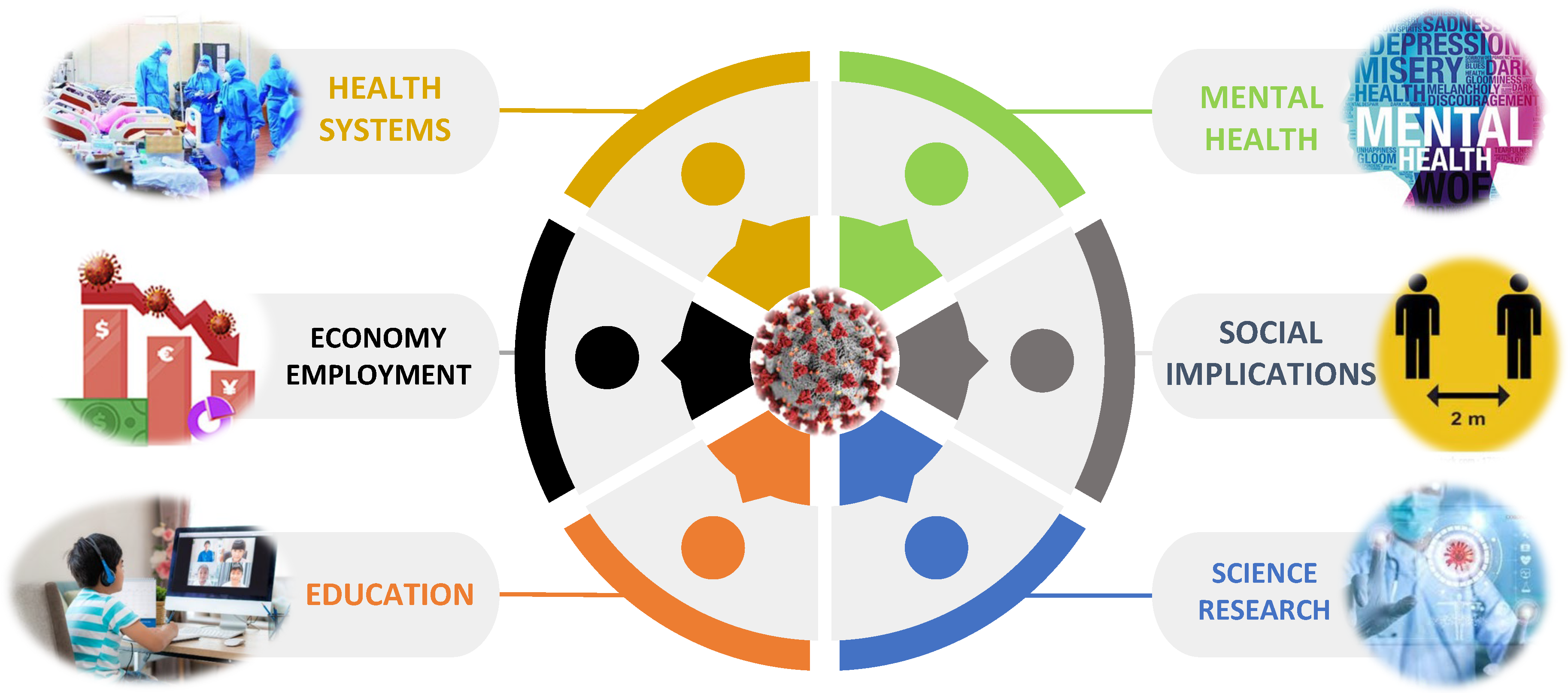
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Study Design and Samples
2.3. Urinary Volatilome Analysis and Data Processing
2.4. Statistical Analyses
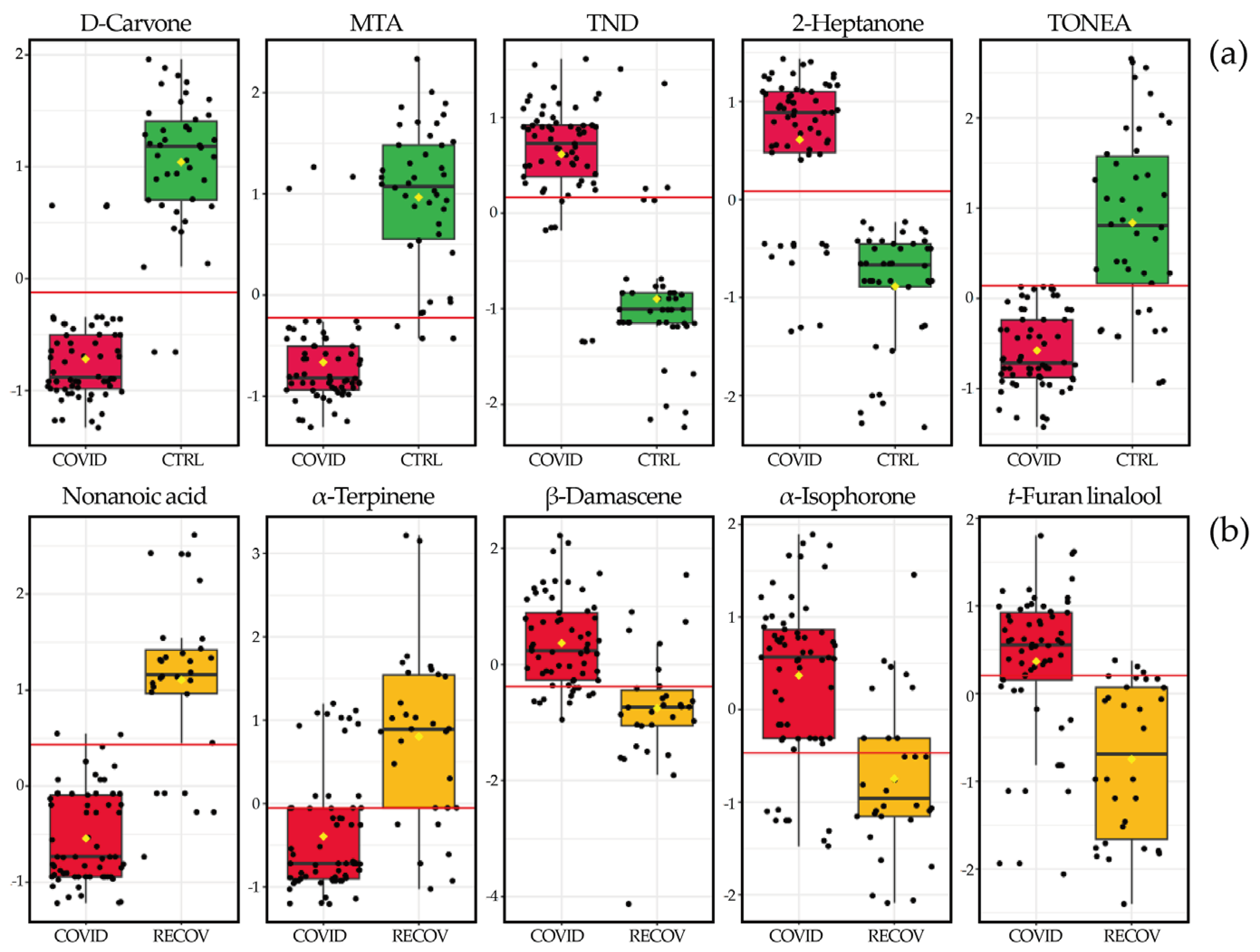
3. Results
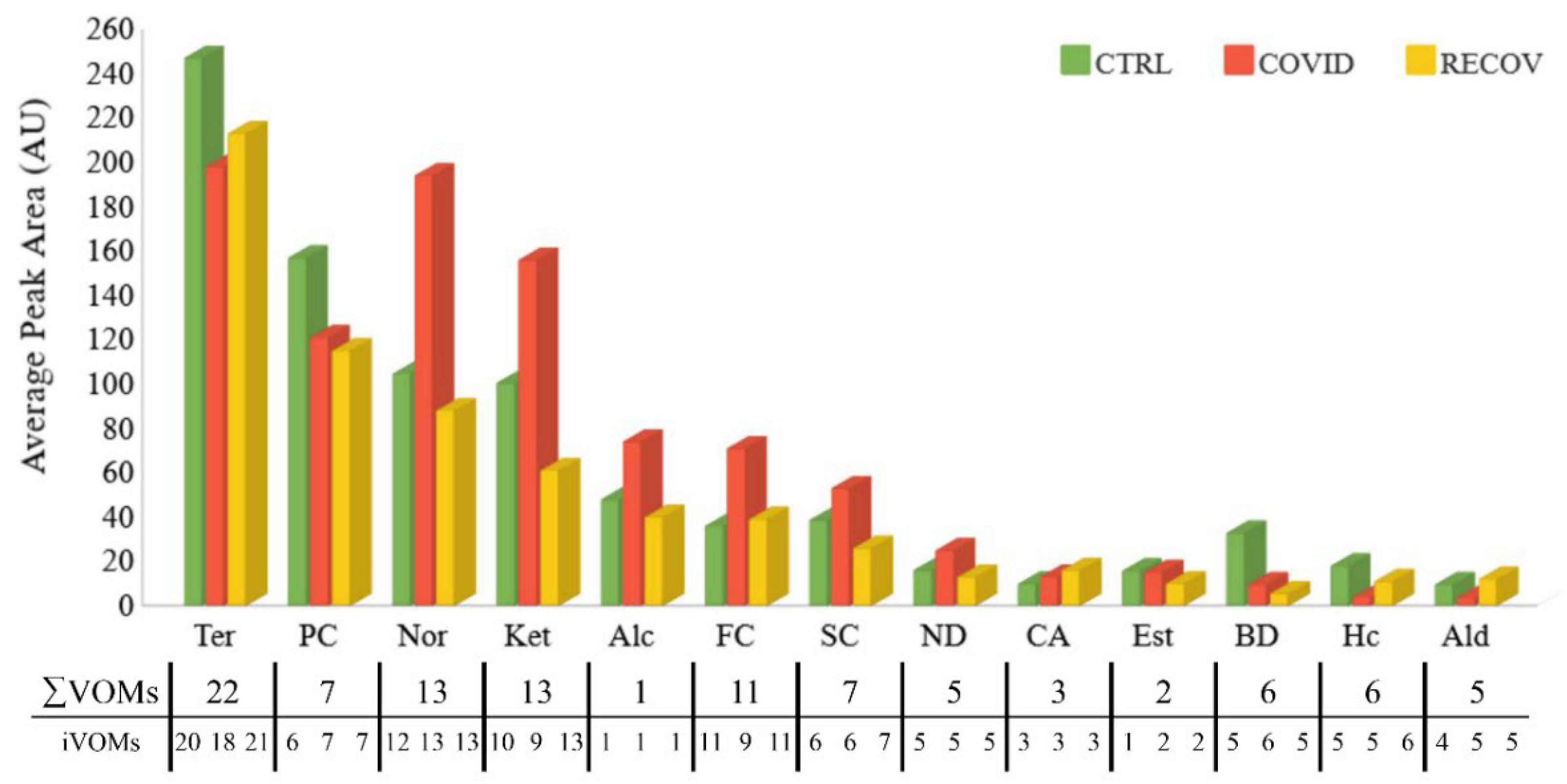
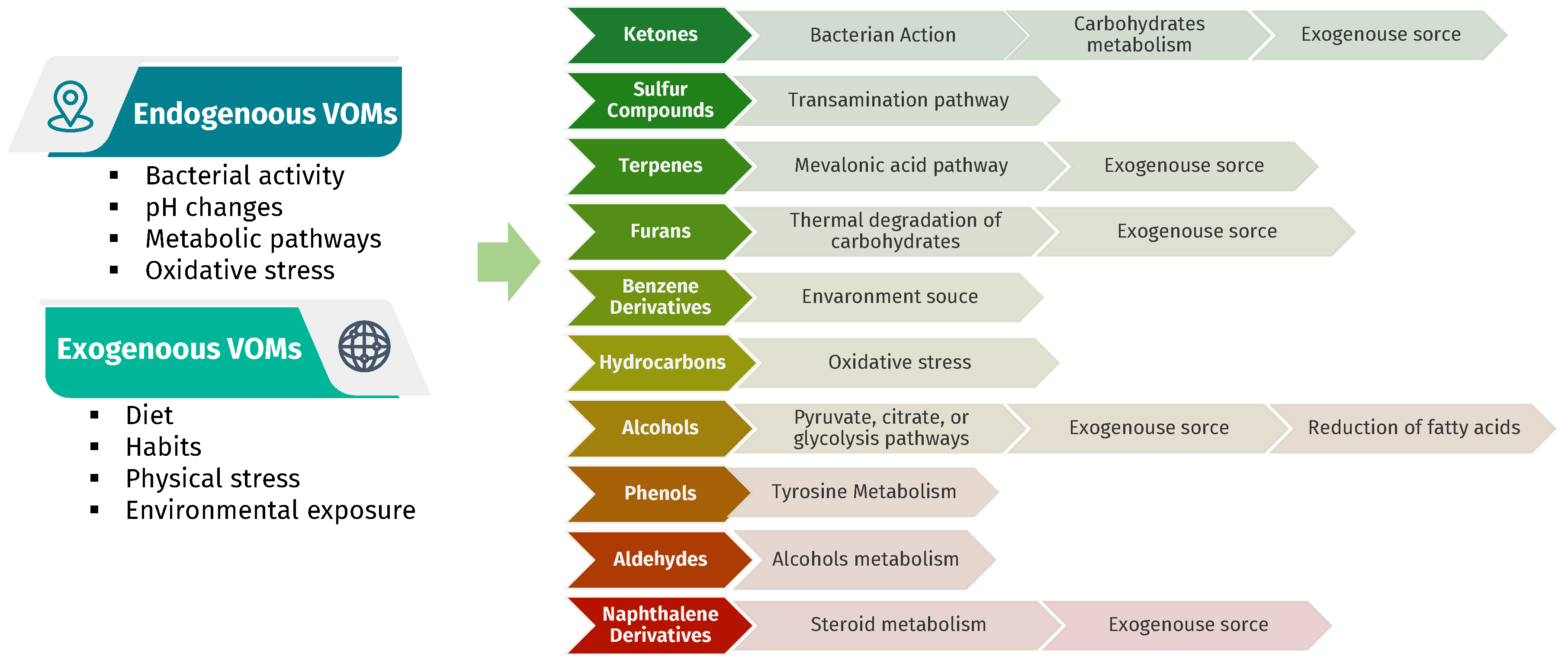
3.1. Characterization of the Urinary Volatilome
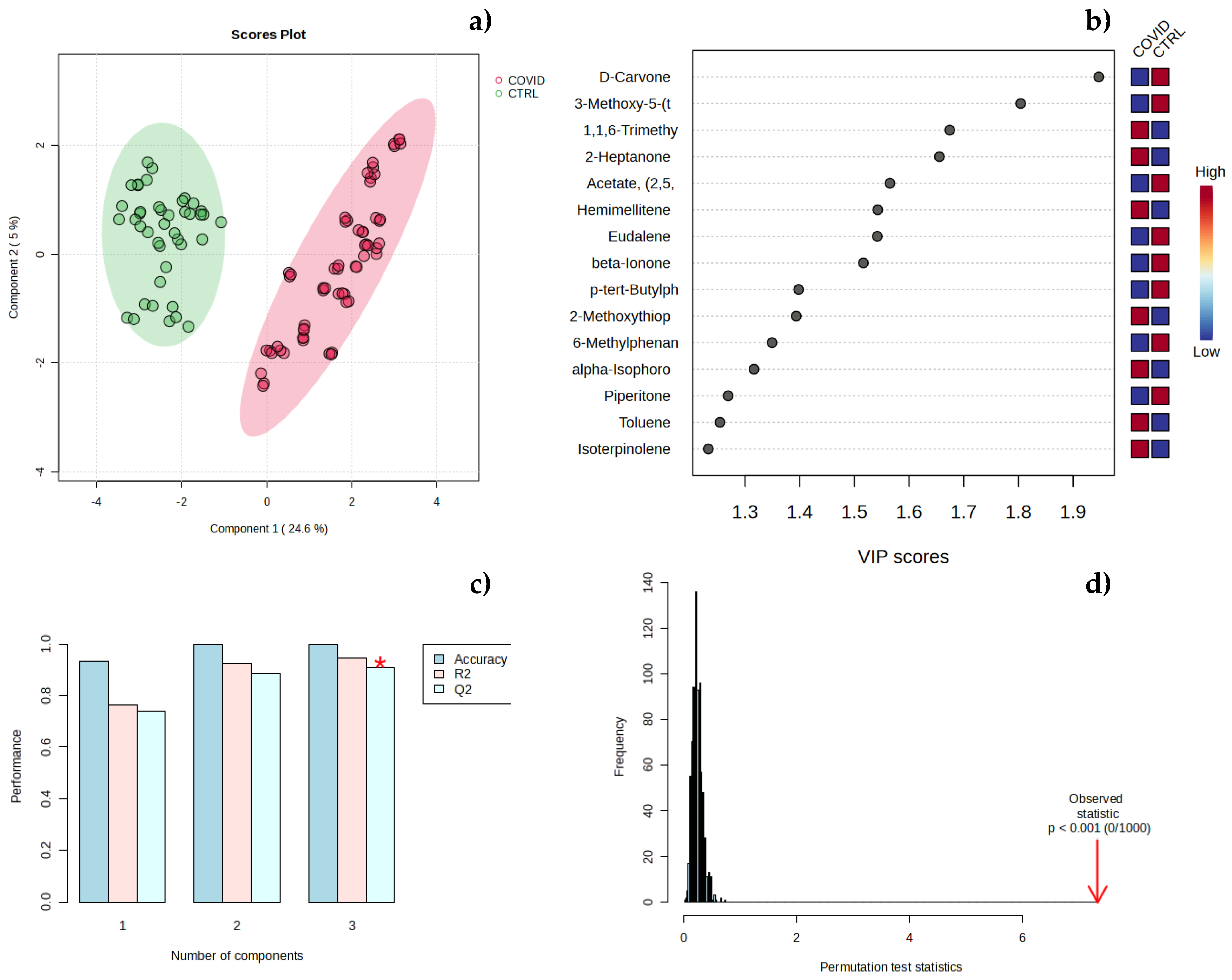
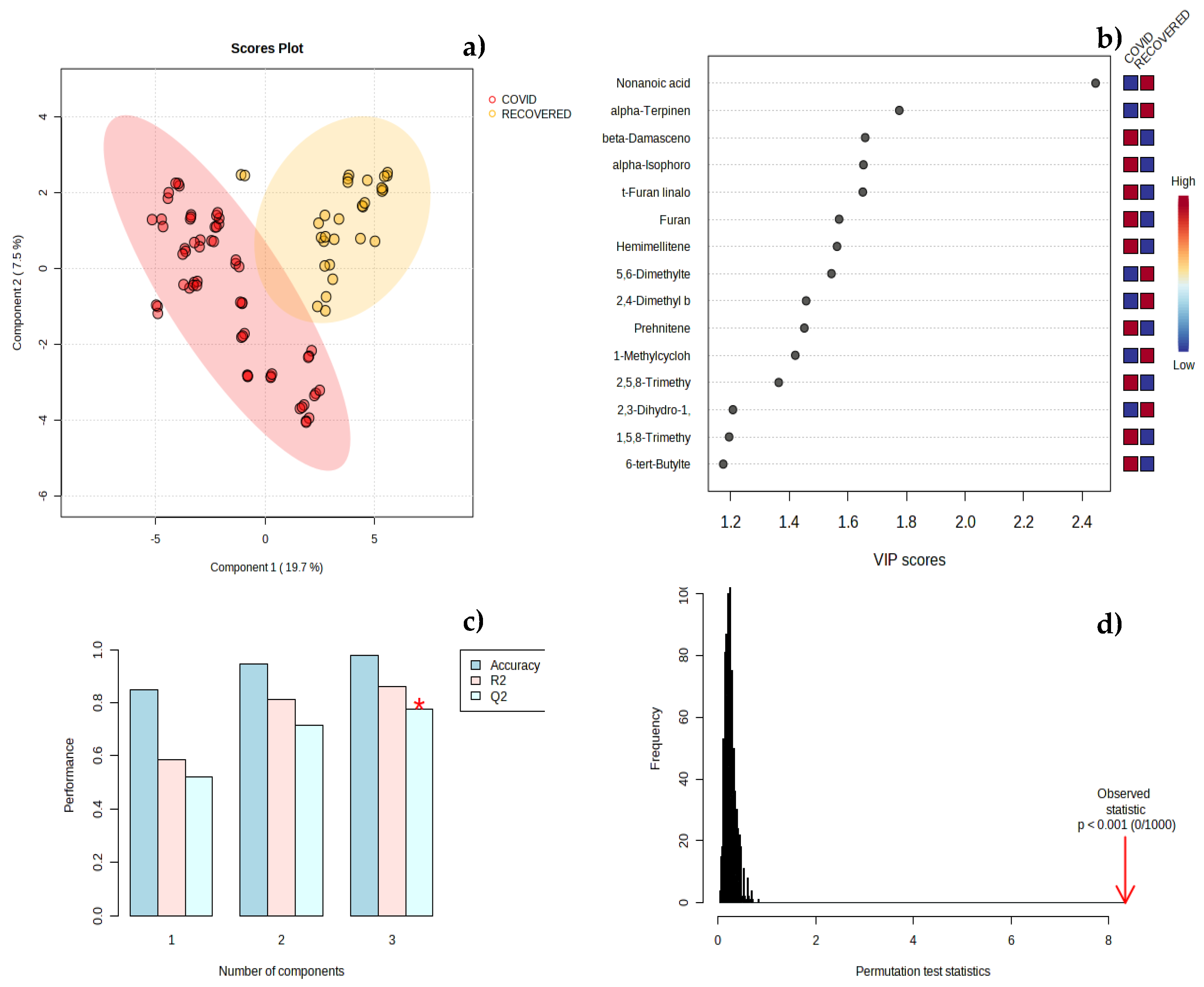
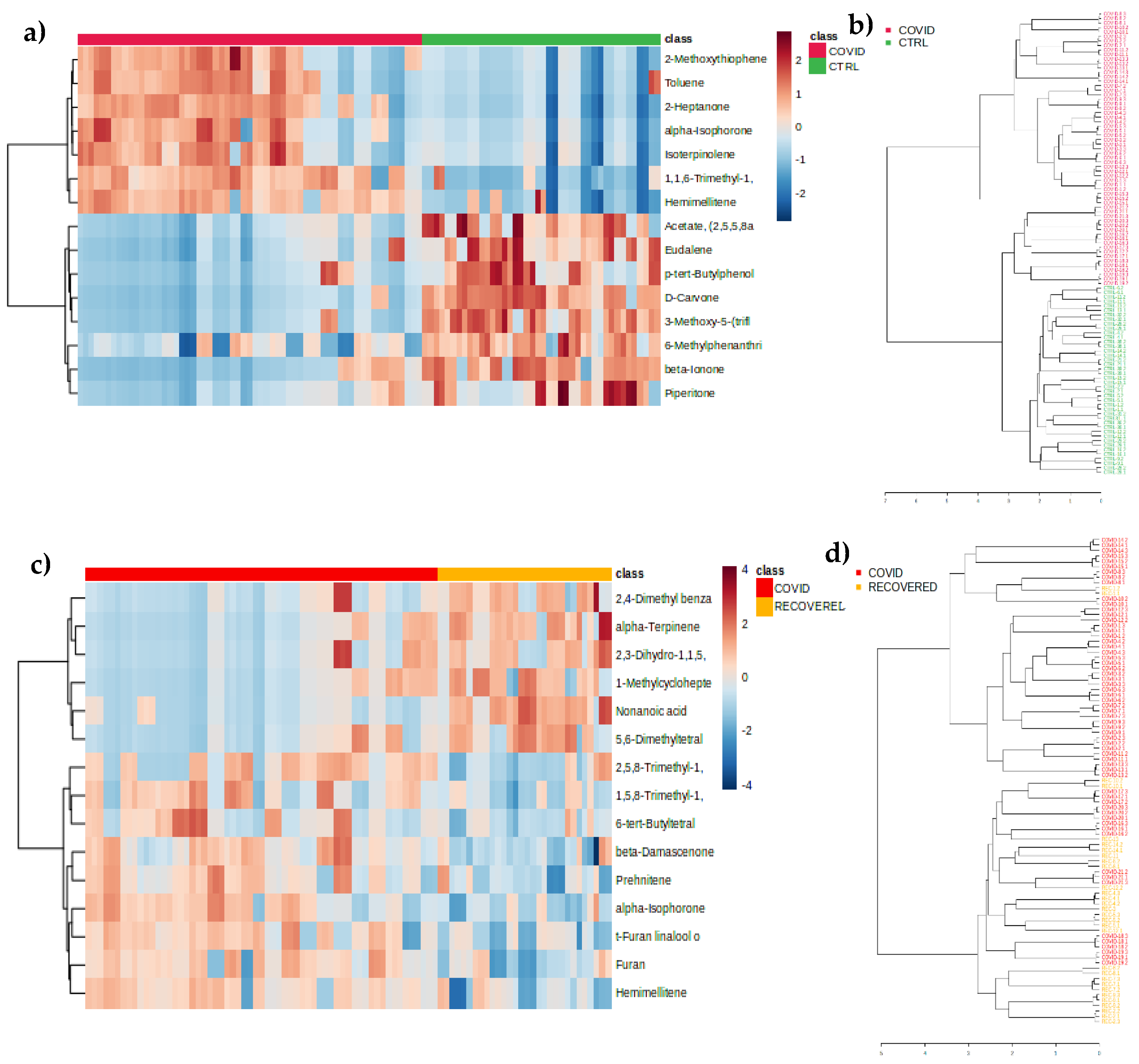
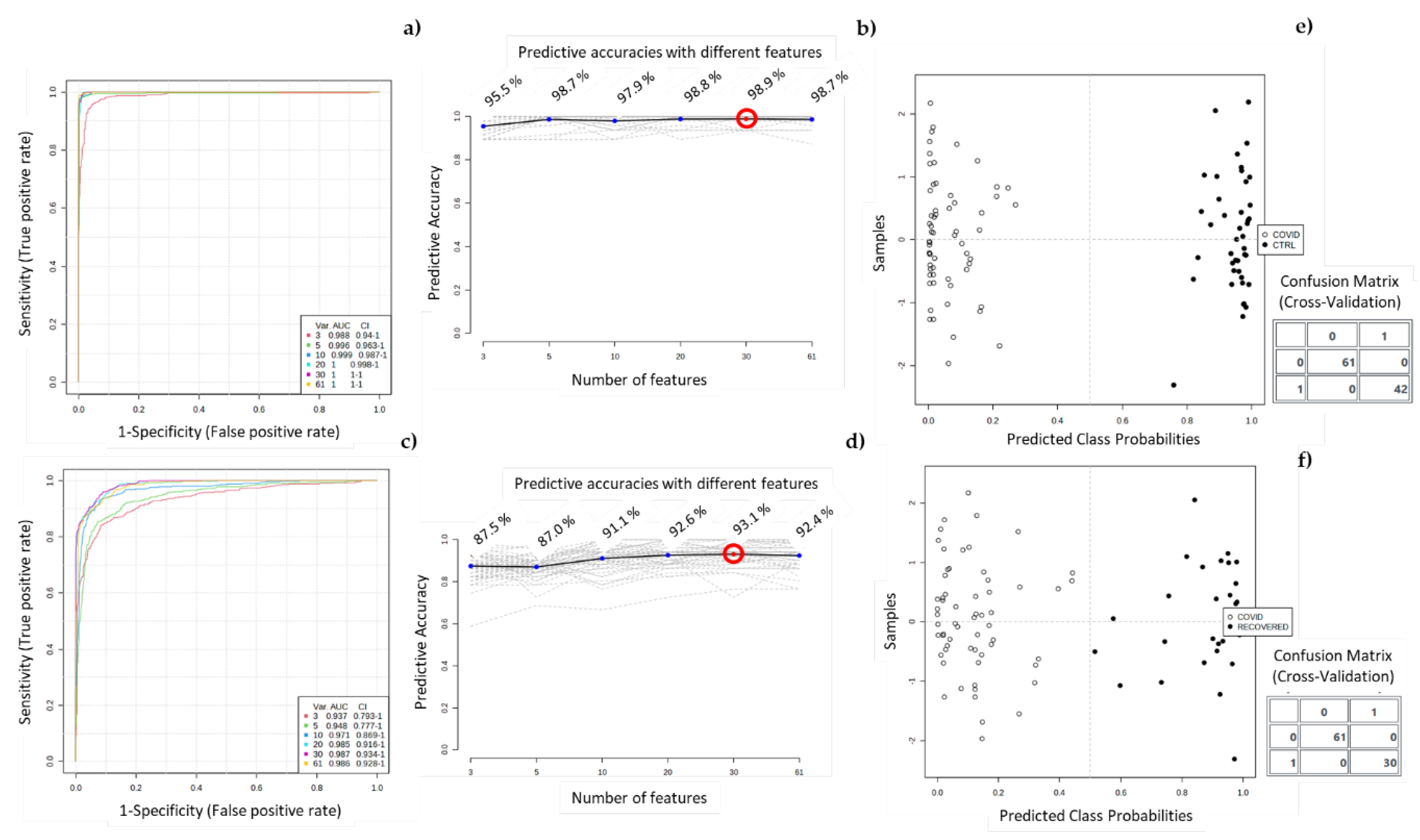
3.2. Chemometric Analysis of Urine Samples
3. Discussion
4. Conclusion
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Huang, C.; Wang, Y.; Li, X.; Ren, L.; Zhao, J.; Hu, Y.; Zhang, L.; Fan, G.; Xu, J.; Gu, X.; et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020, 395, 497–506. [Google Scholar] [CrossRef] [PubMed]
- Vasbinder, A.; Padalia, K.; Pizzo, I.; Machado, K.; Catalan, T.; Presswalla, F.; Anderson, E.; Ismail, A.; Hutten, C.; Huang, Y.; et al. SuPAR, biomarkers of inflammation, and severe outcomes in patients hospitalized for COVID-19: The International Study of Inflammation in COVID-19. J. Med. Virol. 2024, 96, e29389. [Google Scholar] [CrossRef] [PubMed]
- Kabir, M.A.; Ahmed, R.; Chowdhury, R.; Iqbal, S.M.A.; Paulmurugan, R.; Demirci, U.; Asghar, W. Management of COVID-19: current status and future prospects. Microbes Infect. 2021, 23, 104832. [Google Scholar] [CrossRef] [PubMed]
- Talic, S.; Shah, S.; Wild, H.; Gasevic, D.; Maharaj, A.; Ademi, Z.; Li, X.; Xu, W.; Mesa-Eguiagaray, I.; Rostron, J.; et al. Effectiveness of public health measures in reducing the incidence of covid-19, SARS-CoV-2 transmission, and covid-19 mortality: systematic review and meta-analysis. BMJ 2021, 375, e068302. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Undurraga, E.A.; Zubizarreta, J.R. Effectiveness of Localized Lockdowns in the COVID-19 Pandemic. Am. J. Epidemiol. 2022, 191, 812–824. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. WHO Coronavirus (COVID-19) Dashboard. Availabe online: (accessed on 21 June 2023).
- Jones, E.A.K.; Mitra, A.K.; Bhuiyan, A.R. Impact of COVID-19 on Mental Health in Adolescents: A Systematic Review. Int. J. Environ. Res. Public. Health 2021, 18. [Google Scholar] [CrossRef] [PubMed]
- Ventriglio, A.; Castaldelli-Maia, J.M.; Torales, J.; Chumakov, E.M.; Bhugra, D. Personal and social changes in the time of COVID-19. Ir J Psychol Med 2021, 38, 315–317. [Google Scholar] [CrossRef] [PubMed]
- May, T.; Aughterson, H.; Fancourt, D.; Burton, A. ‘Stressed, uncomfortable, vulnerable, neglected’: a qualitative study of the psychological and social impact of the COVID-19 pandemic on UK frontline keyworkers. BMJ Open 2021, 11, e050945. [Google Scholar] [CrossRef] [PubMed]
- Miyah, Y.; Benjelloun, M.; Lairini, S.; Lahrichi, A. COVID-19 Impact on Public Health, Environment, Human Psychology, Global Socioeconomy, and Education. Scientific World Journal 2022, 2022, 5578284. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Clarke, S.P.; Wu, H.; Li, W.; Zhou, C.; Lin, K.; Wang, J.; Wang, J.; Liang, Y.; Wang, X.; et al. A comprehensive overview on the transmission, pathogenesis, diagnosis, treatment, and prevention of SARS-CoV-2. J. Med. Virol. 2023, 95, e28776. [Google Scholar] [CrossRef] [PubMed]
- Abusrewil, Z.; Alhudiri, I.M.; Kaal, H.H.; El Meshri, S.E.; Ebrahim, F.O.; Dalyoum, T.; Efrefer, A.A.; Ibrahim, K.; Elfghi, M.B.; Abusrewil, S.; et al. Time scale performance of rapid antigen testing for SARS-CoV-2: Evaluation of 10 rapid antigen assays. J. Med. Virol. 2021, 93, 6512–6518. [Google Scholar] [CrossRef] [PubMed]
- Pasomsub, E.; Watcharananan, S.P.; Boonyawat, K.; Janchompoo, P.; Wongtabtim, G.; Suksuwan, W.; Sungkanuparph, S.; Phuphuakrat, A. Saliva sample as a non-invasive specimen for the diagnosis of coronavirus disease 2019: a cross-sectional study. Clin. Microbiol. Infect. 2021, 27, 285 e281–285 e284. [Google Scholar] [CrossRef]
- Teymouri, M.; Mollazadeh, S.; Mortazavi, H.; Naderi Ghale-Noie, Z.; Keyvani, V.; Aghababaei, F.; Hamblin, M.R.; Abbaszadeh-Goudarzi, G.; Pourghadamyari, H.; Hashemian, S.M.R.; et al. Recent advances and challenges of RT-PCR tests for the diagnosis of COVID-19. Pathol. Res. Pract. 2021, 221, 153443. [Google Scholar] [CrossRef] [PubMed]
- Merino, P.; Guinea, J.; Munoz-Gallego, I.; Gonzalez-Donapetry, P.; Galan, J.C.; Antona, N.; Cilla, G.; Hernaez-Crespo, S.; Diaz-de Tuesta, J.L.; Gual-de Torrella, A.; et al. Multicenter evaluation of the Panbio COVID-19 rapid antigen-detection test for the diagnosis of SARS-CoV-2 infection. Clin. Microbiol. Infect. 2021, 27, 758–761. [Google Scholar] [CrossRef] [PubMed]
- Berenguer, C.V.; Pereira, F.; Pereira, J.A.M.; Camara, J.S. Volatilomics: An Emerging and Promising Avenue for the Detection of Potential Prostate Cancer Biomarkers. Cancers (Basel) 2022, 14. [Google Scholar] [CrossRef] [PubMed]
- Silva, C.; Perestrelo, R.; Silva, P.; Tomas, H.; Camara, J.S. Breast Cancer Metabolomics: From Analytical Platforms to Multivariate Data Analysis. A Review. Metabolites 2019, 9, 102. [Google Scholar] [CrossRef] [PubMed]
- Bond, A.; Greenwood, R.; Lewis, S.; Corfe, B.; Sarkar, S.; O’Toole, P.; Rooney, P.; Burkitt, M.; Hold, G.; Probert, C. Volatile organic compounds emitted from faeces as a biomarker for colorectal cancer. Alimentary Pharmacology & Therapeutics 2019, 49, 1005–1012. [Google Scholar] [CrossRef]
- Markar, S.R.; Brodie, B.; Chin, S.-T.; Romano, A.; Spalding, D.; Hanna, G.B. Profile of exhaled-breath volatile organic compounds to diagnose pancreatic cancer. BJS (British Journal of Surgery) 2018, 105, 1493–1500. [Google Scholar] [CrossRef] [PubMed]
- Taunk, K.; Porto-Figueira, P.; Pereira, J.A.M.; Taware, R.; da Costa, N.L.; Barbosa, R.; Rapole, S.; Câmara, J.S. Urinary Volatomic Expression Pattern: Paving the Way for Identification of Potential Candidate Biosignatures for Lung Cancer. Metabolites 2022, 12. [Google Scholar] [CrossRef] [PubMed]
- Opitz, P.; Herbarth, O. The volatilome - investigation of volatile organic metabolites (VOM) as potential tumor markers in patients with head and neck squamous cell carcinoma (HNSCC). J Otolaryngol Head Neck Surg 2018, 47, 42. [Google Scholar] [CrossRef] [PubMed]
- Sethi, S.; Nanda, R.; Chakraborty, T. Clinical application of volatile organic compound analysis for detecting infectious diseases. Clin. Microbiol. Rev. 2013, 26, 462–475. [Google Scholar] [CrossRef] [PubMed]
- Gallagher, M.; Wysocki, C.J.; Leyden, J.J.; Spielman, A.I.; Sun, X.; Preti, G. Analyses of volatile organic compounds from human skin. Br. J. Dermatol. 2008, 159, 780–791. [Google Scholar] [CrossRef] [PubMed]
- Neerincx, A.H.; Vijverberg, S.J.H.; Bos, L.D.J.; Brinkman, P.; van der Schee, M.P.; de Vries, R.; Sterk, P.J.; Maitland-van der Zee, A.-H. Breathomics from exhaled volatile organic compounds in pediatric asthma. Pediatr. Pulmonol. 2017, 52, 1616–1627. [Google Scholar] [CrossRef]
- Kusano, M.; Mendez, E.; Furton, K.G. Comparison of the Volatile Organic Compounds from Different Biological Specimens for Profiling Potential*. J. Forensic Sci. 2013, 58, 29–39. [Google Scholar] [CrossRef] [PubMed]
- Sola-Martínez, R.A.; Sanchez-Solis, M.; Lozano-Terol, G.; Gallego-Jara, J.; García-Marcos, L.; Cánovas Díaz, M.; de Diego Puente, T.; Group, T.N.S. Relationship between lung function and exhaled volatile organic compounds in healthy infants. Pediatr. Pulmonol. 2022, 57, 1282–1292. [Google Scholar] [CrossRef] [PubMed]
- Riccio, G.; Berenguer, C.V.; Perestrelo, R.; Pereira, F.; Berenguer, P.; Ornelas, C.P.; Sousa, A.C.; Vital, J.A.; Pinto, M.d.C.; Pereira, J.A.M.; et al. Differences in the Volatilomic Urinary Biosignature of Prostate Cancer Patients as a Feasibility Study for the Detection of Potential Biomarkers. Cur. Oncol. 2023, 30, 4904–4921. [Google Scholar] [CrossRef] [PubMed]
- Silva, C.L.; Passos, M.; Camara, J.S. Solid phase microextraction, mass spectrometry and metabolomic approaches for detection of potential urinary cancer biomarkers--a powerful strategy for breast cancer diagnosis. Talanta 2012, 89, 360–368. [Google Scholar] [CrossRef]
- Arthur, C.L.; Pawliszyn, J. Solid-Phase Microextraction with Thermal-Desorption Using Fused-Silica Optical Fibers. Anal. Chem. 1990, 62, 2145–2148. [Google Scholar] [CrossRef]
- Camara, J.S.; Perestrelo, R.; Berenguer, C.V.; Andrade, C.F.P.; Gomes, T.M.; Olayanju, B.; Kabir, A.; C, M.R.R.; Teixeira, J.A.; Pereira, J.A.M. Green Extraction Techniques as Advanced Sample Preparation Approaches in Biological, Food, and Environmental Matrices: A Review. Molecules 2022, 27. [Google Scholar] [CrossRef]
- Pang, Z.; Chong, J.; Zhou, G.; de Lima Morais, D.A.; Chang, L.; Barrette, M.; Gauthier, C.; Jacques, P.E.; Li, S.; Xia, J. MetaboAnalyst 5.0: narrowing the gap between raw spectra and functional insights. Nucleic Acids Res. 2021, 49, W388–W396. [Google Scholar] [CrossRef] [PubMed]
- Câmara, J.S.; Perestrelo, R.; Berenguer, C.V.; Andrade, C.F.P.; Gomes, T.M.; Olayanju, B.; Kabir, A.; Rocha, C.M.R.; Teixeira, J.A.; Pereira, J.A.M. Green Extraction Techniques as Advanced Sample Preparation Approaches in Biological, Food, and Environmental Matrices: A Review. Molecules 2022, 27. [Google Scholar] [CrossRef] [PubMed]
- de Lacy Costello, B.; Amann, A.; Al-Kateb, H.; Flynn, C.; Filipiak, W.; Khalid, T.; Osborne, D.; Ratcliffe, N.M. A review of the volatiles from the healthy human body. J. Breath Res. 2014, 8, 014001. [Google Scholar] [CrossRef] [PubMed]
- Pang, Z.; Chong, J.; Zhou, G.; de Lima Morais, D.A.; Chang, L.; Barrette, M.; Gauthier, C.; Jacques, P.; Li, S.; Xia, J. MetaboAnalyst 5.0: narrowing the gap between raw spectra and functional insights. Nucleic Acids Res. 2021, 49, W388–W396. [Google Scholar] [CrossRef] [PubMed]
- Wishart, D.S.; Guo, A.; Oler, E.; Wang, F.; Anjum, A.; Peters, H.; Dizon, R.; Sayeeda, Z.; Tian, S.; Lee, B.L.; et al. HMDB 5.0: the Human Metabolome Database for 2022. Nucleic Acids Res. 2022, 50, D622–D631. [Google Scholar] [CrossRef] [PubMed]
- Riccio, G.; Berenguer, C.; Perestrelo, R.; Pereira, F.; Berenguer, P.; Ornelas, C.; Sousa, A.; Vital, J.; Pinto, M.; Pereira, J.; et al. Differences in the Volatilomic Urinary Biosignature of Prostate Cancer Patients as a Feasibility Study for the Detection of Potential Biomarkers. Cur. Oncol. in press. 2023. [CrossRef] [PubMed]
- Jala, A.; Dutta, R.; Josyula, J.V.N.; Mutheneni, S.R.; Borkar, R.M. Environmental phenol exposure associates with urine metabolome alteration in young Northeast Indian females. Chemosphere 2023, 317, 137830. [Google Scholar] [CrossRef] [PubMed]
- Buszewski, B.; Ulanowska, A.; Ligor, T.; Jackowski, M.; Klodzinska, E.; Szeliga, J. Identification of volatile organic compounds secreted from cancer tissues and bacterial cultures. J. Chromatogr. B Analyt. Technol. Biomed. Life. Sci. 2008, 868, 88–94. [Google Scholar] [CrossRef]
- Mills, G.A.; Walker, V. Headspace solid-phase microextraction profiling of volatile compounds in urine: application to metabolic investigations. J. Chromatogr. B Biomed. Sci. Appl. 2001, 753, 259–268. [Google Scholar] [CrossRef]
- Zhang, J.J.; Dong, X.; Liu, G.H.; Gao, Y.D. Risk and Protective Factors for COVID-19 Morbidity, Severity, and Mortality. Clin. Rev. Allergy Immunol. 2023, 64, 90–107. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




