1. Introduction
Alzheimer's Disease (AD) has been considered a global public health priority by the World Health Organization (WHO). Our understanding of the causes and potential drug targets for AD is mainly theoretical, consisting of well-defined concepts and hypotheses. Following this framework, medications have been developed to mitigate the progression of the disease [
1].
AD is the leading cause of dementia in individuals aged 60 and above, representing 50–75% of all dementia. Globally, gathered statistical data shows a higher susceptibility to AD among females than males, with this risk further escalating with advancing age [
2]. The number of people live with AD, currently is approximately 50 million, and studies indicate that this number could double every 20 years [
3,
4,
5].
The progression of AD causes loss of neural extensions and impairment of their surroundings, leading to brain atrophy by reducing connectivity between synapses, cellular metabolism, and loss of neural recovery ability. Cognitive functions are affected in AD which are responsible for conducting daily activities, such as loss of ability to plan and capabilities of speaking and writing, making affected individuals unable to perform self-care activities [
6].
Oxidative stress has a pivotal role in AD, where the excessive production and release of reactive oxygen species (ROS) cause brain cells damage, contributing with the extensive neuronal loss. The search for a cure for AD is being widely explored to discover an early diagnosis and more effective treatments. It is already known that there are drugs that delay disease progression, promoting a better quality of life for the patient. There are also alternatives to prevent the progression of the disease, such as phototherapy and the use of antioxidants. The study by Lee et al., 2020 [
7] showed that taurine promotes neuroprotective effect on neural cells, characterized as an antioxidant. Recent studies also indicate that taurine at low concentrations has beneficial properties in neurodegenerative pathologies and that light emitting diode (LED) irradiation modulates cells. On the other hand, it is known that phototherapy performed by LED, or light amplification by stimulated emission of radiation (laser) is a non-invasive treatment possibility to improve brain function [
8]. Therefore, the present study evaluates the effects of taurine on neural cells under oxidative stress conditions induced by hydrogen peroxide (H
2O
2), an important hallmark of AD. Phototherapy with LED was also performed to verify cellular biomodulation. Thus, as illustrated in
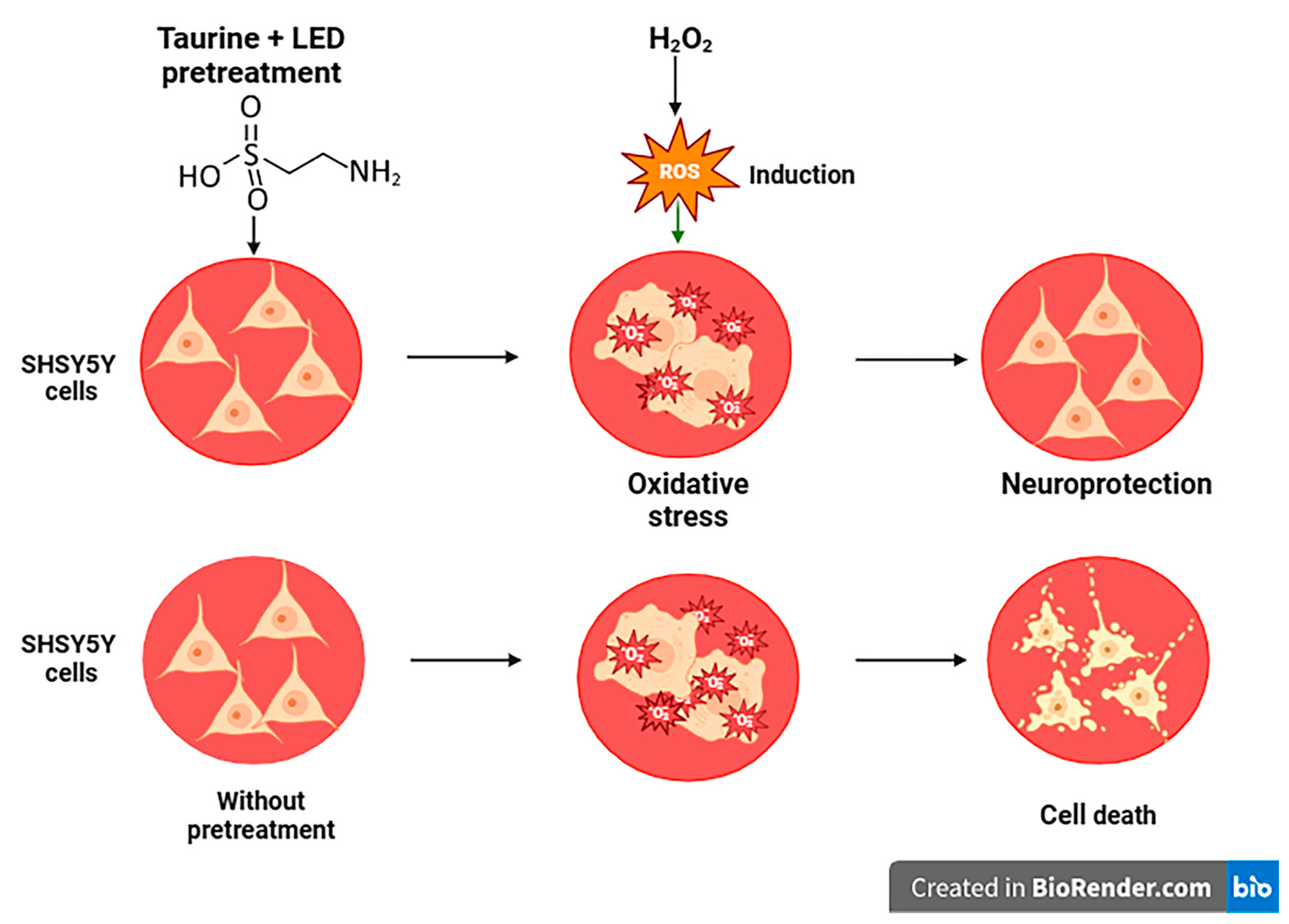
Figure 1, the objective of this study was to analyze the potential therapeutic effects of taurine combined with LED in an
in vitro model of AD, where neuron-like cells were exposed to H
2O
2, to induce oxidative stress.
Further, we aimed to establish the ideal concentration of H
2O
2 to induce oxidative stress, the ideal antioxidant concentration of taurine, the ideal fluence of LED irradiation, and to verify the neuroprotective and neurorestorative ability of taurine associated with LED in SH5Y5 human neuroblastoma cells, which are used as in vitro model for TAU pathology and Alzheimer’s disease [
9,
10] (
2. Materials and Methods
2.1. Reagents
Hydrogen peroxide (H2O2) induced oxidative stress in human neuroblastoma cells (SH-SY5Y) to simulate Alzheimer's conditions. Taurine, a neuroprotective amino acid, was employed for pre-treatment and treatment experiments. MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) was used for colorimetric mitochondrial activity assessment. MitoTracker Orange, a fluorescent dye, was used for live cell mitochondrial labeling. Trypan blue dye was employed for the evaluation of cell viability. Hydrogen peroxide, taurine, MTT, MitoTracker Orange, Trypan blue dye, and SEM supplies were sourced from Sigma-Merck (St. Louis, Missouri, United States). CM-H2DCFDA (5-(and -6)-Chloromethyl-2,7-dichlorodihydrofluorescein diacetate) and TMRM (tetramethyl rhodamine methyl ester) were acquired from Invitrogen- Thermo Fisher Scientific (Carlsbad, California, United States).
2.2. Cell Culture
The experiments were conducted with the cell line SH-SY5Y (Human Neuroblastoma ATCC - CRL-22660. Cell culture was conducted in 25 cm2 cell culture flasks with Dulbecco’s Modified Eagle Medium: Nutrient Mixture (DMEM/F12, Gibco), supplemented with 10% fetal bovine serum (FBS) and 1% antibiotic and antimycotic, at 37 °C, in an atmosphere of 5% CO2. SH-SY5Y cells were seeded at a density of 1x105 cells/well in 24-well plates. Cells were then incubated at 37°C and 5% CO2 overnight to allow cell adhesion.
2.3. Irradiation
Irradiation was performed using the Biopdi/Irrad-Led5 660 LED device (Biopdi – São Carlos, São Paulo, Brazil – Applied Photobiology Laboratory for Health – IP&D, UNIVAP). The irradiation parameters were at wavelength of 660 nm ± 5 nm. The Biopdi/Irrad-Led5 device consists of 54 LEDs for irradiation, with each LED having a power of 70 Mw, covering an area of 150 cm2 and providing a power density (irradiance) of up to 25 Mw/cm2. Following irradiation, cells were incubated for 24 hours at 37°C and 5% CO2.
2.4. Treatment
The optimal concentrations of H2O2 for inducing cellular stress in SH-SY5Y cells was standardized. Different energy densities of LED at 660 nm were compared to enhance cell viability. A dose-response curve for taurine was also performed. Pre-treatments involving LED and taurine with H2O2 were conducted. Cells were pre-treated with LED and/or taurine at optimal concentrations before H2O2-exposure. Additionally, treatment experiments were performed using LED and taurine together with H2O2-exposure.
2.5. Viability Assay
Cell viability was assessed using 0.2% Trypan Blue dye (Sigma). After the treatments, the culture medium was carefully removed, and 150 Μl of 0.2% Trypan Blue was added for 5 minutes. Cells were then washed with PBS, and 150 Μl of PBS was added for cell counting under a bright-field microscope Leica DLM.
2.6. MTT Assay
Mitochondrial activity was assessed using the colorimetric MTT (3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide) assay (Sigma). After the respective treatments, ells were incubated with 0.5 mg/mL MTT solution diluted in culture medium was added to each well and incubated for 3 hours, at 37°C in a 5% CO2 atmosphere. After incubation, the MTT solution was carefully aspirated, and cells were incubated with 400 µLl of dimethyl sulfoxide (DMSO) for 5 minutes, under constant agitation. Absorbance was measured at 570 nm using a spectrophotometer Packard SpectraCount BS10000.
2.7. Mitochondrial Membrane Potential Analysis
MitoTracker Orange chloromethyltetramethylrosamine (CMTMRos) (Invitrogen, Carlsbad, CA) was used to assess mitochondrial membrane potential (ΔΨm). After treatments, cells were incubated with MitoTracker Orange (1 mM) for 30 min, at 37°C in a 5% CO2 atmosphere. Cells were washed with PBS and imaged under a fluorescence microscope (EVOS FL Cell Imaging System, Thermo Fisher Scientific). Red fluorescence intensity was measured and analyzed to assess mitochondrial membrane potential.
2.8. Scanning Electron Microscopy (SEM)
SH-SY5Y cells were subjected to SEM to evaluate morphological changes after treatment. Cells were fixed in 0.1 M sodium cacodylate solution containing 2.5% glutaraldehyde and 4% paraformaldehyde for 1 h. Then, the fixative solution was removed, and cells were washed twice with PBS. Dehydration was conducted in a series of increasing ethanol concentrations (70% to 100%), exposed for 10 min each. Then, samples were dehydrated with 100% ethanol + hexamethyldisilane (HMDS) (1:1) for 10 minutes and pure HMDS for another 10 minutes. After HMDS complete evaporation, samples were metalized on a sputter coater (EMITECH K 550 X®). The analysis was conducted using an EVO-Zeiss® Scanning Electron Microscope, capturing images of the control, H2O2, LED + H2O2, taurine + H2O2, and LED + taurine + H2O2 exposure groups.
2.9. Statistical Analysis
Statistical analysis was performed using GraphPad Prism software (version 8.0). Data were expressed as mean ± standard deviation. One-way analysis of variance (ANOVA) followed by Tukey’s multiple comparisons test was used for comparisons between multiple groups. The level of significance was set at p < 0.05. All experiments were performed in triplicate with three repetitions.
3. Results
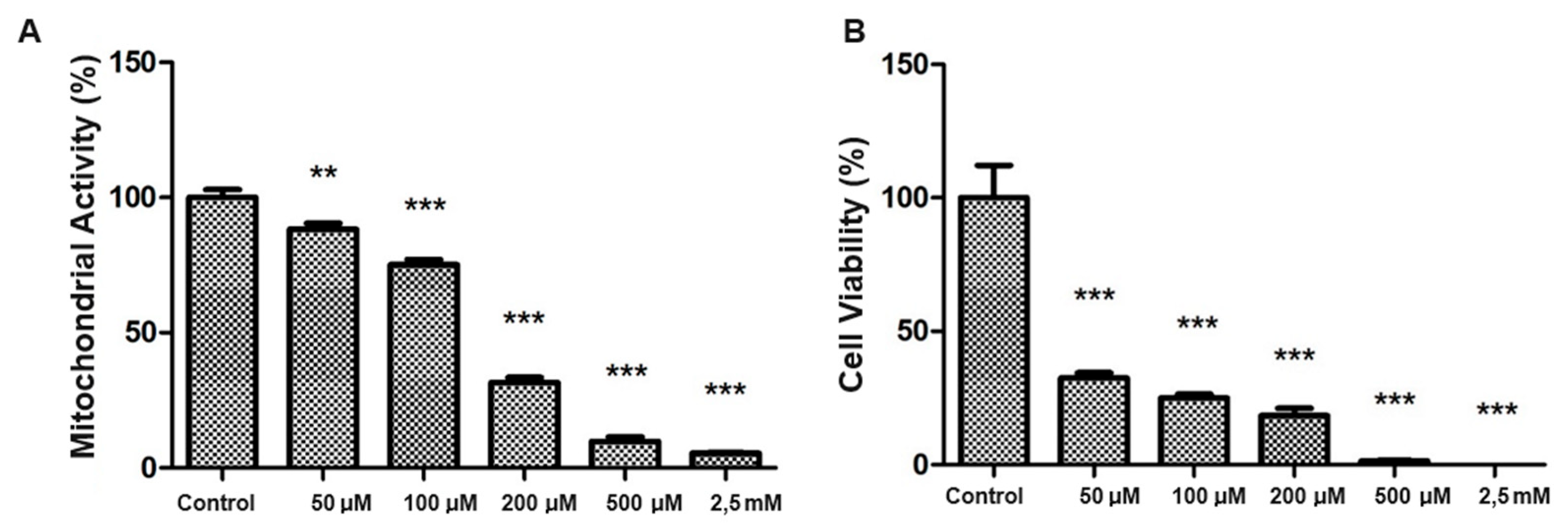
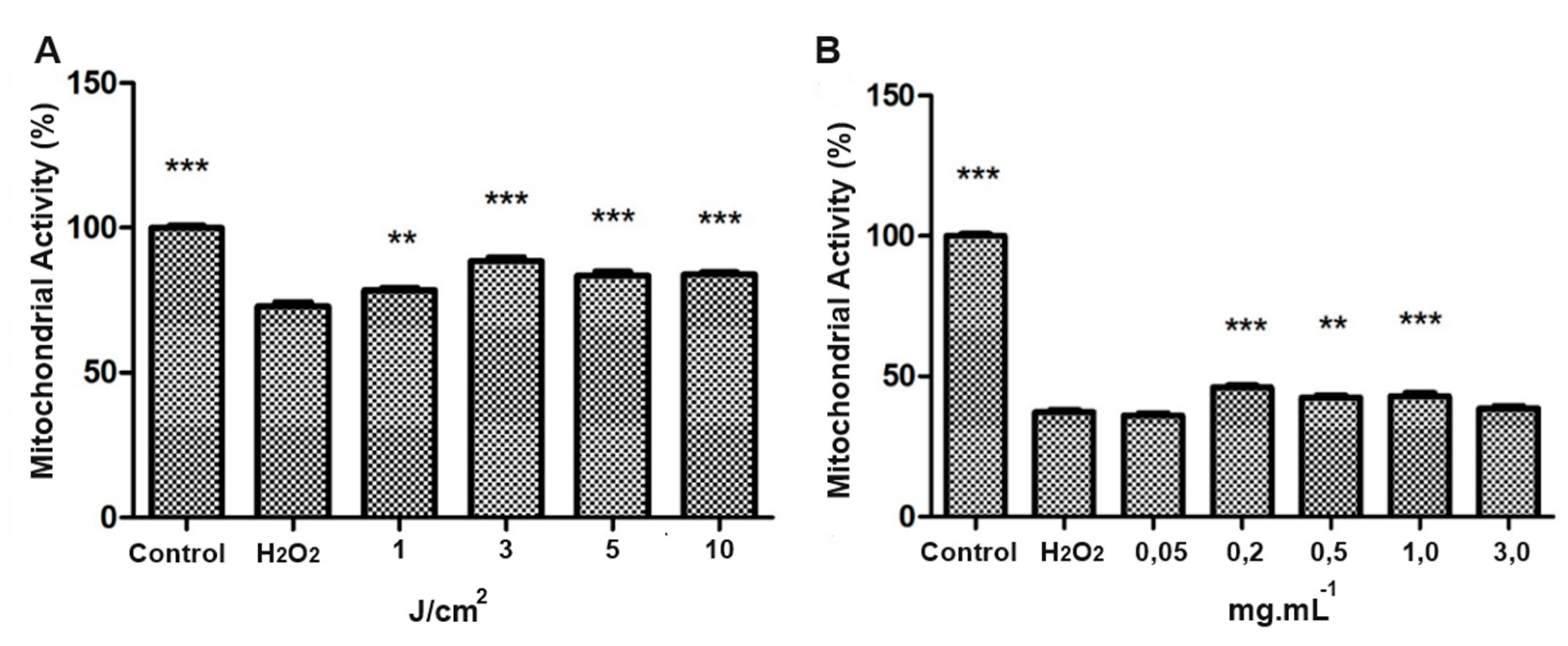
The generation of oxidative stress in SH-SY5Y cells was evaluated by standardizing the H₂O₂ dosage. Thus, exposures to various concentrations of H₂O₂ (50 µM to 2.5 Mm) were performed to standardize an effective dose of oxidative stress. We observed that 200 µM of H₂O₂ resulted in a significant reduction in mitochondrial activity (
Figure 2A) and cell viability (
Figure 2B) compared to the control group (p < 0.001).
Irradiation of SH-SY5Y cells was performed with LED at an energy density of 3 J/cm
2. The LED exposed group increased mitochondrial activity when compared to the control group (p < 0.01). At higher energy densities (5 J/cm
2 and 10 J/cm
2), mitochondrial activity was statistically reduced (
Figure 3).
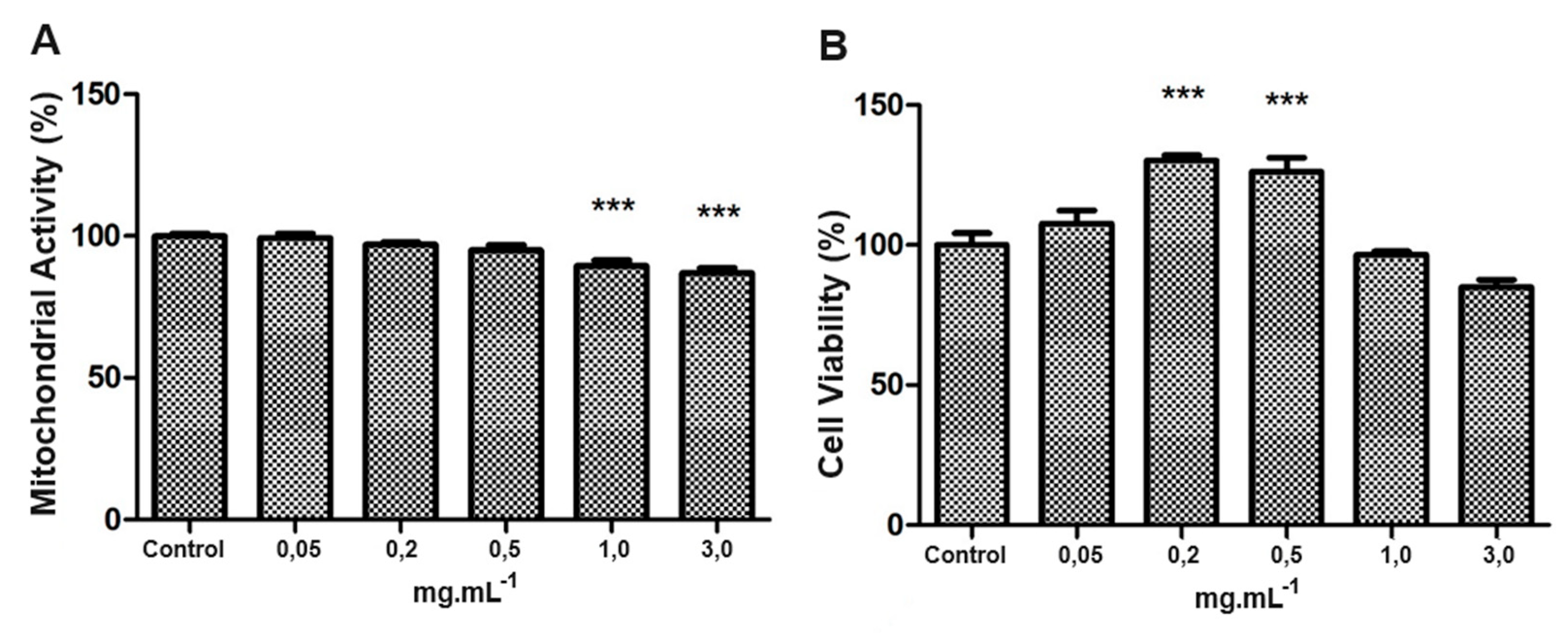
Regarding the use of taurine in SH-SY5Y cell culture, the concentrations up to 0.5 mg/mL did not negatively affect mitochondrial activity or cell viability. In comparison, concentrations of 1.0 mg/mL and 3. 0 mg/mL significantly reduced mitochondrial activity (
Figure 4A) and viability (
Figure 4B), compared with the control group (p < 0.001).
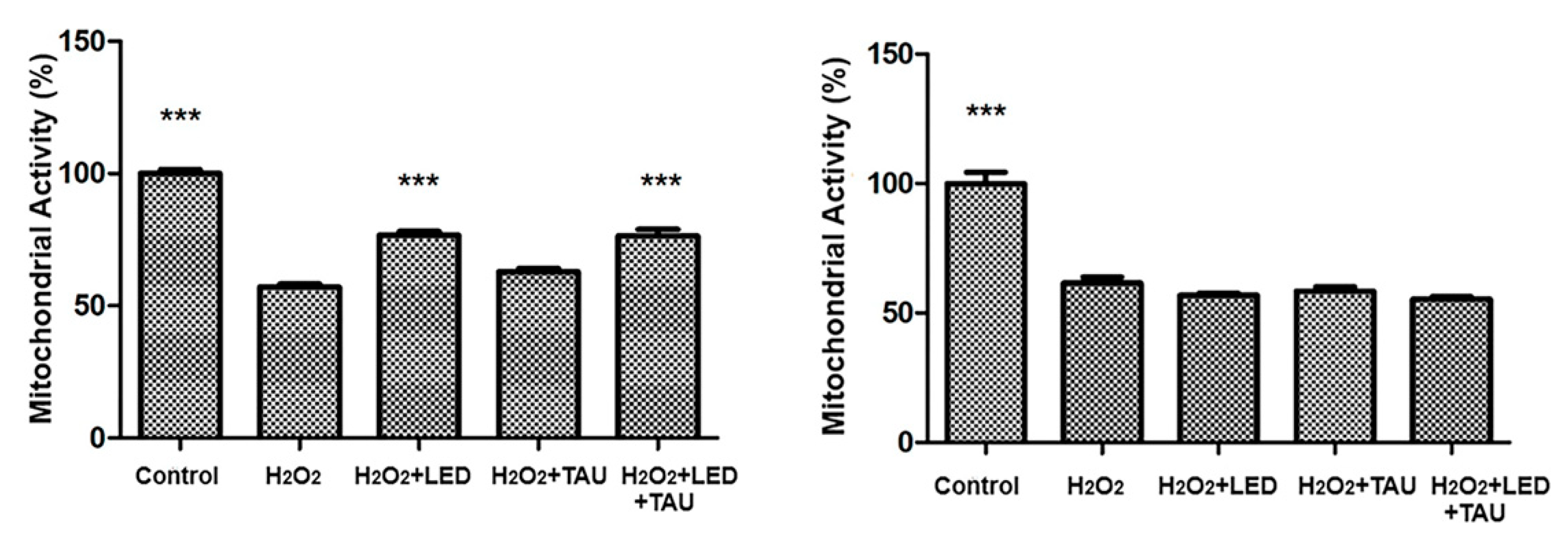
Regarding the pre-treatment of SH-SY5Y cells with LED before the induction of oxidative stress by H
2O
2, a significant neuroprotection effect was observed (
Figure 5A), preserving mitochondrial activity and cell viability after cells exposure to H₂O₂. It is triggered by H₂O₂, and the same effect is observed with pre-treatment using taurine (
Figure 5B).
The combination of LED + Taurine exposure before H₂O₂ exposure showed a synergistic neuroprotection, significantly preserving mitochondrial activity and cell viability compared to the group only exposed to H₂O₂ (p < 0.001), as shown in
Figure 6 A. However, when cells are first exposed to H₂O₂ and then treated with combined LED irradiation + taurine, no restorative effect was observed (
Figure 6B), indicating that the combined therapy is more protective than restorative, when evaluating mitochondrial activity and cell viability.
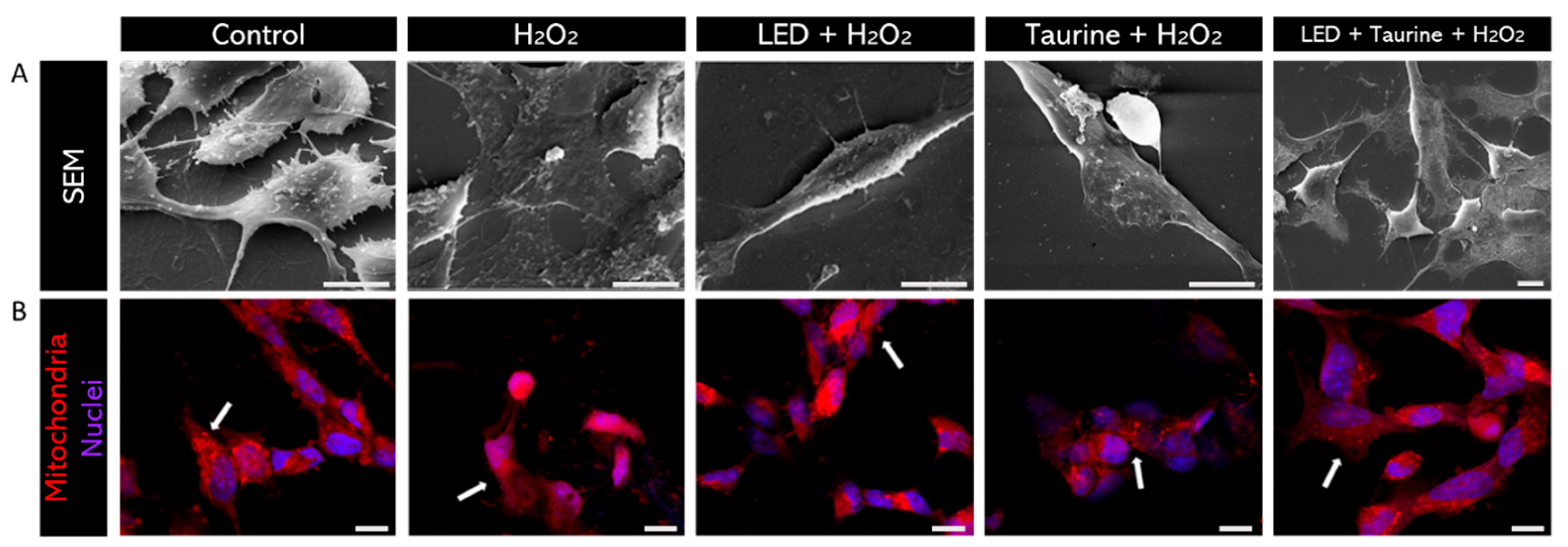
MitoTracker Orange labeling visually confirmed mitochondrial protection following pre-treatment with LED and taurine, preserving mitochondrial morphology and organization after induced oxidative stress. The images were obtained using a confocal microscope, and it is possible to verify the neuroprotective effect visually. Therefore, in this experiment, pre-treatment was conducted using LED (3 J/cm2) and taurine (0.5 mg/mL) and then added H2O2 (200 μM) to promote oxidative stress. The groups analyzed were Control; H2O2; LED + H2O2; taurine + H2O2; LED + taurine + H2O2. After exposure to H2O2, cell showed low SEM images clearly showed the morphological preservation of neural cells projections in the LED + Taurine + H₂O₂ group compared to the H₂O₂ group, indicating the effectiveness of the combination of LED and taurine as a neuroprotective pre-treatment Figure 7A.
MitoTracker staining emission and mitochondrial fragmentation, resulting from high ROS production (Figure 7B). However, in the LED + taurine pre-treatment group, mitochondria have a higher degree of organization compared to the H2O2 group.
Figure 8.
SEM and mitochondrial staining. (A) Morphological preservation of neural cells in the LED + Taurine + H₂O₂ group versus the H₂O₂-exposed group, demonstrating the neuroprotective potency of combining LED and Taurine as a pre-treatment. (B) After H2O2-exposure, cells reduced MitoTracker fluorescence emission and mitochondrial fragmentation is observed due to high ROS production (white arrows). However, with LED + taurine pre-treatment, mitochondria are more organized and distributed within the cytoplasm than in the H2O2-exposed only group. Scale bar: 10µm.
Figure 8.
SEM and mitochondrial staining. (A) Morphological preservation of neural cells in the LED + Taurine + H₂O₂ group versus the H₂O₂-exposed group, demonstrating the neuroprotective potency of combining LED and Taurine as a pre-treatment. (B) After H2O2-exposure, cells reduced MitoTracker fluorescence emission and mitochondrial fragmentation is observed due to high ROS production (white arrows). However, with LED + taurine pre-treatment, mitochondria are more organized and distributed within the cytoplasm than in the H2O2-exposed only group. Scale bar: 10µm.
4. Discussion
It is widely known that oxidative stress contributes with mitochondrial dysfunction and disorder, which is one of the key roles in the development and progression of AD. Moreover, oxidative stress results in damage of further cellular components, such as lipids, protein and DNA, accelerating the neurodegenerative process of the disease, contributing with the aggregation of amyloid beta plaques and neurofibrillary tangles, which are the main pathological features of AD. SH-SY5Y cells are an adequate model to study neurodegenerative processes induced by ROS. For instance, fruit and leaf extracts of mulberry were tested against H
2O
2-induced cytotoxicity [
11]
For in vitro studies, H
2O
2 is commonly used to induce cell oxidative stress [
8]. Our results showed that SH-SY5Y cells exposed to 200 µM H
2O
2, for 24h, had mitochondrial dysfunction and cellular apoptosis [
12,
13], resulting in a significant reduction in cell viability compared to the control group (p<0.001). For this reason, this concentration and period of H
2O
2 exposure was stablished for all the following assays, which corroborates the results of previous studies [
14].
Photobiomodulation uses monochromatic or nearly monochromatic light from a laser or LED to modify biological functions [
15]. This modulating effect is based on chromophores in cells and tissues, molecules capable of absorbing light whose excitation can influence other molecules and biochemical pathways with potential therapeutic effects [
16].
Sommer et al. (2012), reported that photobiomodulation reduced β-amyloid aggregates in SH-SY5Y cells and observed a reduction in β-amyloid-induced oxidative stress and inflammatory responses in primary rat cortical astrocytes [
17].
Zhang et al. (2012), specifically reported that protein kinase B was activated by photobiomodulation at an energy density of 2 J/cm
2, promoting a series of anti-apoptotic effects [
18]. Generally, energy densities between 0.5 J/cm
2 and 4 J/cm
2 applied to human neural cells have anti-apoptotic and proliferative effects and can reduce ROS accumulation [
15].
The MTT assay results showed (
Figure 3) that an energy density of 3 J/cm
2 under oxidative stress significantly increased mitochondrial activity compared to the H
2O
2-exposed group (p<0.001). This energy density was determined for further experiments, indicating that neuroprotective and proliferative effects are not observed at 4 J/cm
2, as the literature suggests [
15].
According to Che et al. (2018), the cytoprotective action of taurine is achieved, improving neural cell metabolism, and preventing cellular apoptosis [
19]. This cytoprotective action occurs mainly by taurine's ability to facilitate cellular permeability, preventing the formation of ROS and the damage resulting from them, also acting as a buffer, maintaining the medium's pH, and capturing electrically viable ions [
7].
As mentioned, excessive oxidative stress damages cells, potentially triggering necrotic or apoptotic cell death. Mitochondria are the primary site of ROS production, and excess ROS can damage them [207]. Mitochondria are cytoplasmic organelles in eukaryotic cells, responsible for most adenosine triphosphate (ATP) production. Studies report that mitochondrial dysfunction is associated with oxidative stress, contributing to structural abnormalities, synaptic loss, and cell degeneration [
21,
22].
Maintaining mitochondrial function is crucial, especially when exposed to chemical or physical stressors. Mitochondria play a fundamental role in triggering and sustaining the apoptotic pathway. When exposed to toxins, they may undergo organelle compromise, morphological alterations, and impaired mitochondrial dynamics [
23,
24].
Fluorescent staining with MitoTracker Orange is highly responsive to mitochondrial membrane potential and is considered an indicator for verifying oxidative stress in mitochondria [
25]. In the experiment conducted with pre-treatment using LED and taurine under H
2O
2 action, we observed that the control group exhibited well-organized mitochondria with visually more intense fluorescence than the H
2O
2 group. H
2O
2 caused mitochondrial fragmentation; a reduced MitoTracker fluorescence shows ROS production and is recognized as a reliable assay to examine oxidative stress, even with low-level ROS formed in mitochondria [
26]. The LED + taurine + H
2O
2 group showed a remarkable improvement in mitochondrial integrity, distribution, and organization (Figure 7B), with a considerable proportion of them remaining intact after treatment. To further understand these structural alterations, it was stated that ROS causes oxidative stress [
27], highly detrimental when produced excessively in our bodies, leading to structural alterations in proteins, lipids, and nucleic acids, leading to cell death. The deposition of β-amyloid aggregates, and the hyperphosphorylation of TAU protein, the main hallmarks of AD, leads to neural physiology alterations, especially in synapses, causing cellular impairment and death [
28]. In agreement, Monteiro et al. (2011) showed that this TAU protein hyperphosphorylation results in its deposition in cellular filaments responsible for the neurodegeneration process occurring in AD [
29].
5. Conclusions
This study elucidates the importance of combining LED irradiation and taurine as potential pre-therapeutic tools against the oxidative stress in AD. First, it was stablished 200 µM H₂O₂, exposed for 24h, as oxidative stressor agent in SH-SY5Y cells. Irradiating SH-SY5Y cells with LED at an energy density of 3 J/cm2 demonstrated a notable increase in mitochondrial activity, especially compared to the control group. When administered at up to 0.5 mg/mL, Taurine did not show adverse effects on mitochondrial activity or cell viability. Most importantly, the combination of pre-treatment with LED and Taurine exhibited a remarkable synergistic neuroprotective effect against oxidative stress, which was confirmed by MitoTracker Orange and SEM, demonstrating the preservation of mitochondrial morphology and organization post-oxidative stress, respectively-treatment with LED and Taurine offered notable protection; the combined treatment post oxidative stress did not fully restore mitochondrial activity or cell viability, underscoring the importance of early and preventive intervention. In summary, the findings of this study highlight the potential of LED and Taurine as neuroprotective agents against oxidative stress in SH-SY5Y cells. These insights may provide novel approaches for preventing and treating neurodegenerative diseases associated with oxidative stress, such as AD. However, further studies are needed to fully understand the underlying mechanisms and confirm these observations in in vivo models and clinical trials.
Author Contributions
Conceptualization R.C.R and C.P.S,; methodology, R.C.R and A.L.A.; validation, M.I.B.S. and A.E.C.G; formal analysis, G.R.S; investigation, R.C.R and A.L.A.;; writing—original draft preparation, C.P.S.; writing—review and editing, H.U..; visualization, M.A.P.; project administration, C.P.S.; funding acquisition,. C.P.S. and H.U. All authors have read and agreed to the published version of the manuscript.”.
Funding
C.P.S. acknowledges grant supported by the São Paulo Research Foundation [(FAPESP) Project No 2016/17984-1; HU acknowledges grant support for his work on purinergic signaling by the São Paulo Research Foundation [(FAPESP) Project No. 2018/07366-4 and 2012/50880-4] and the National Institute of Science and Technology in Regenerative Medicine (INCT-REGENERA), Brazil, as well as by the National Council of Scientific and Technological Development (CNPq Project No. 406396/2021 and 308012/2021-6). A.E.C.G. was supported by a postdoctoral fellowship awarded by the Coordination for the Improvement of Higher Education (CAPES), Brazil; and R.C.R was supported by a master's scholars by the Coordination for the Improvement of Higher Education (CAPES), Brazil.
Conflicts of Interest
“The authors declare no conflicts of interest.”.
References
- Downer, B.; Al Snih, S.; Chou, L.N.; et al. Changes in health care use by Mexican American Medicare beneficiaries before and after a diagnosis of dementia. J. Gerontol. A Biol. Sci. Med. Sci. 2021, 76, 534–542. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Torromino, G.; Maggi, A.; De Leonibus, E. Estrogen-dependent hippocampal wiring as a risk factor for age-related dementia in women. Prog. Neurobiol. 2021, 197, 101895. [Google Scholar] [CrossRef] [PubMed]
- Nandi, A.; Counts, N.; Chen, S.; Seligman, B.; Tortorice, D.; Vigo, D.; Bloom, D. Global and regional projections of the economic burden of Alzheimer's disease and related dementias from 2019 to 2050: A value of statistical life approach. E Clin. Med. 2022, 51, 101580. [Google Scholar] [CrossRef] [PubMed]
- Ferreira-Filho, S.F.D.; Borelli, W.V.; Sguario, R.M.; Biscaia, G.F.; Müller, V.S.; Vicentini, G.; Schilling, L.P.; Silveira, D.S.D. Prevalence of dementia and cognitive impairment with no dementia in a primary care setting in southern Brazil. Arq. Neuropsiquiatr. 2021, 79, 565–570. [Google Scholar] [CrossRef] [PubMed]
- Livingston, G.; Huntley, J.; Sommerlad, A.; Ames, D.; Ballard, C.; Banerjee, S.; Brayne, C.; Burns, A.; Cohen-Mansfield, J.; Cooper, C.; Costafreda, S.G.; Dias, A.; et al. Dementia prevention, intervention, and care: 2020 report of the Lancet Commission. Lancet 2020, 396, 413–446, Erratum in: Lancet. 2023, 402, 1132. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Furtado, C.M.; Félix, D.; Kihara, A.H.; Paschon, V. Terapia gênica recupera memória de ratos com Alzheimer: um novo passo em direção à cura. 2014. [CrossRef]
- Lee, W.J.; Lee, G.H.; Hur, J.; et al. Taurine and Ginsenoside Rf Induce BDNF Expression in SH-SY5Y Cells: A Potential Role of BDNF in Corticosterone-Triggered Cellular Damage. Molecules 2020, 25, 12. [Google Scholar] [CrossRef] [PubMed]
- Salehpour, F.; Ahmadian, N.; Rasta, S.H.; Farhoudi, M.; Karimi, P.; Sadigh-Eteghad, S. Transcranial low-level laser therapy improves brain mitochondrial function and cognitive impairment in D-galactose–induced aging mice. Neurobiol. Aging 2017, 58, 140–150. [Google Scholar] [CrossRef] [PubMed]
- Bell, M.; Zempel, H. SH-SY5Y-derived neurons: a human neuronal model system for investigating TAU sorting and neuronal subtype-specific TAU vulnerability. Rev. Neurosci. 2021, 33, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Agholme, L.; Lindström, T.; Kågedal, K.; Marcusson, J.; Hallbeck, M. An in vitro model for neuroscience: differentiation of SH-SY5Y cells into cells with morphological and biochemical characteristics of mature neurons. J. Alzheimers Dis. 2010, 20, 1069–1082. [Google Scholar] [CrossRef] [PubMed]
- Mairuae, N.; Palachai, N.; Noisa, P. The neuroprotective effects of the combined extract of mulberry fruit and mulberry leaf against hydrogen peroxide-induced cytotoxicity in SH-SY5Y Cells. BMC Complement. Med. Ther. 2023, 23, 117. [Google Scholar] [CrossRef] [PubMed]
- Yana, M.H.; Wang, X.; Zhu, X. Mitochondrial defects and oxidative stress in Alzheimer's disease and Parkinson's disease. Free Radic. Biol. Med. 2013, 62, 90–101. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Puspita, L.; Chung, S.Y.; Shim, J.W. Oxidative stress and cellular pathologies in Parkinson's disease. Mol. Brain 2017, 10, 1–12. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Niu, T.; Jin, L.; Niu, S.; Gong, C.; Wang, H. Lycium Barbarum Polysaccharides Alleviates Oxidative Damage Induced by H2O2 Through Down-Regulating MicroRNA-194 in PC-12 and SH-SY5Y Cells. Cell Physiol. Biochem. 2018, 50, 460–472. [Google Scholar] [CrossRef] [PubMed]
- Enengl, J.; Hamblin, M.R.; Dungel, P. Photobiomodulation for Alzheimer's Disease: Translating Basic Research to Clinical Application. J. Alzheimers Dis. 2020, 75, 1405–1416. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Rojas, J.C.; Gonzalez-Lima, F. Neurological and psychological applications of transcranial lasers and LEDs. Biochem. Pharmacol. 2013, 86, 447–457, Wai T, Langer T. Mitochondrial Dynamics and Metabolic Regulation. Trends in Endocrinology and Metabolism. 2016, 27(2), 105–117. PMID: 26754340 DOI: 10.1016/j.tem.2015.12.001. [Google Scholar] [CrossRef] [PubMed]
- Sommer, A.P.; Bieschke, J.; Friedrich, R.P.; Zhu, D.; Wanker, E.E.; Fecht, H.J.; Mereles, D.; Hunstein, W. 670 nm laser light and EGCG complementarily reduce amyloid-β aggregates in human neuroblastoma cells: Basis for treatment of Alzheimer's disease? Photomed. Laser Surg. 2012, 30, 54–60. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Wu, S.; Xing, D. Inhibition of Aβ25–35-induced cell apoptosis by low-power-laser-irradiation (LPLI) through promoting Akt-dependent YAP cytoplasmic translocation. Cell Signal. 2012, 24, 224–232. [Google Scholar] [CrossRef] [PubMed]
- Che, Y.; Hou, L.; Sun, F.; et al. Taurine protects dopaminergic neurons in a mouse Parkinson's disease model through inhibition of microglial M1 polarization. Cell Death Dis. 2018, 9, 4. [Google Scholar] [CrossRef] [PubMed]
- Wai, T.; Langer, T. Mitochondrial Dynamics and Metabolic Regulation. Trends Endocrinol. Metab. 2016, 27, 105–117. [Google Scholar] [CrossRef] [PubMed]
- Grimm, A.; Lim, Y.; Mensah-Nyagan, A.G.; Götz, J.; Eckert, A. Alzheimer's disease, estrogen, and mitochondria: An ambiguous relationship. Mol. Neurobiol. 2012, 46, 1. [Google Scholar] [CrossRef] [PubMed]
- Jang, J.Y.; Blum, A.; Liu, J.; Finkel, T. The role of mitochondria in aging. J. Clin. Invest. 2018, 128, 3662–3670. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Green, D.R.; Galluzzi, L.; Kroemer, G. Metabolic control of cell death. Science 2014, 345, 6203. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- De Oliveira, M.R.; Brasil, F.B.; Fürstenau, C.R. Evaluation of the Mitochondria-Related Redox and Bioenergetics Effects of Gastrodin in SH-SY5Y Cells Exposed to Hydrogen Peroxide. J. Mol. Neurosci. 2018, 64, 242–251. [Google Scholar] [CrossRef] [PubMed]
- Tomkova, S.; Misuth, M.; Lenkavska, L.; Miskovsky, P.; Huntosova, V. In vitro identification of mitochondrial oxidative stress production by time-resolved fluorescence imaging of glioma cells. Biochim. Biophys. Acta - Mol. Cell Res. 2018, 1865, 616–628. [Google Scholar] [CrossRef] [PubMed]
- Kesari, K.K.; Dhasmana, A.; Shandilya, S.; et al. Plant-derived natural biomolecule Picein attenuates menadione induced oxidative stress on neuroblastoma cell mitochondria. Antioxidants 2020, 9, 1–17. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Morales, G.I.; Farías, G.G.; Maccioni, R.B. La neuroinflamación como factor detonante del desarrollo de la enfermedad de Alzheimer. Rev. Chil. Neuro-Psiquiatr. 2010, 48, 49–57. [Google Scholar] [CrossRef]
- Kalagatur, N.K.; Abd_allah, E.F.; Poda, S.; et al. Quercetin mitigates the deoxynivalenol mycotoxin-induced apoptosis in SH-SY5Y cells by modulating the oxidative stress mediators. Saudi J. Biol. Sci. 2021, 28, 465–477. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Monteiro, M.R.; Kandratavicius, L.; Leite, J.P. The role of cytoskeleton proteins in normal cell physiology and in pathological conditions. J. Epilepsy Clin. Neurophysiol. 2011, 17, 17–23. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).