1. Introduction
Tambaqui
Colossoma macropomum (Cuvier, 1816) is a good option for national fish farming, presenting several characteristics favorable to confinement, highlighting its excellent rusticity, tolerance to low concentrations of dissolved oxygen in water, good acceptance of agro-industrial by-products, good food conversion, adaptation to captive cultivation, rapid growth and good acceptance by consumers [
1]. Thus, tambaqui breeding has been intensifying in recent years, and today, it is the second most commercially produced species in Brazil [
1]. This fact has boosted studies on the species, from its physiology, genetics, preservation, and ecology to production technologies [
2].
Among the biological tools used to monitor animal welfare conditions, hematological studies and biochemical profiles are used to monitor the physiological expressions of fish and contribute to certifying the animals health, and can be a good bioindicator that can help in the improvement of the tambaqui production chain [
3,
4,
5].
Factors such as the quality of the cultivation water, daily management, feeding system, form of slaughter, property infrastructure, and the form of acquisition and handling contribute to animal welfare and, consequently, affect yield and productivity. When these factors are not performed correctly, fish are predisposed to bacterial infections, parasite infestation and physiological conditions of extreme weakness, thus reducing food conversion rates and animal growth, which can even cause their death, generating production losses [
6]. The most appropriate physical and chemical variables for the qualification of pond water are dissolved oxygen, pH, free carbon dioxide, total alkalinity, hardness, electrical conductivity, temperature, transparency, nutrients, and abundance of plankton [
6]. This study aimed to determine and compare the hematological and biochemical parameters of tambaqui from fish farms in municipalities in the Metropolitan Region of Manaus (MRM), Amazonas, Brazil.
2. Materials and Methods
2.1. Animal Ethics
The study was developed following the regulations of ethical principles in animal experimentation considered by the National Council for the Control of Animal Experimentation (NCCAE) upon approval by the Ethics Committees on the Use of Animals—CUA of the Federal University of Amazonas under protocol nº 005/ 2018. All experiments performed were conducted following Percie et al. [
7].
2.2. Study and Sampling Area
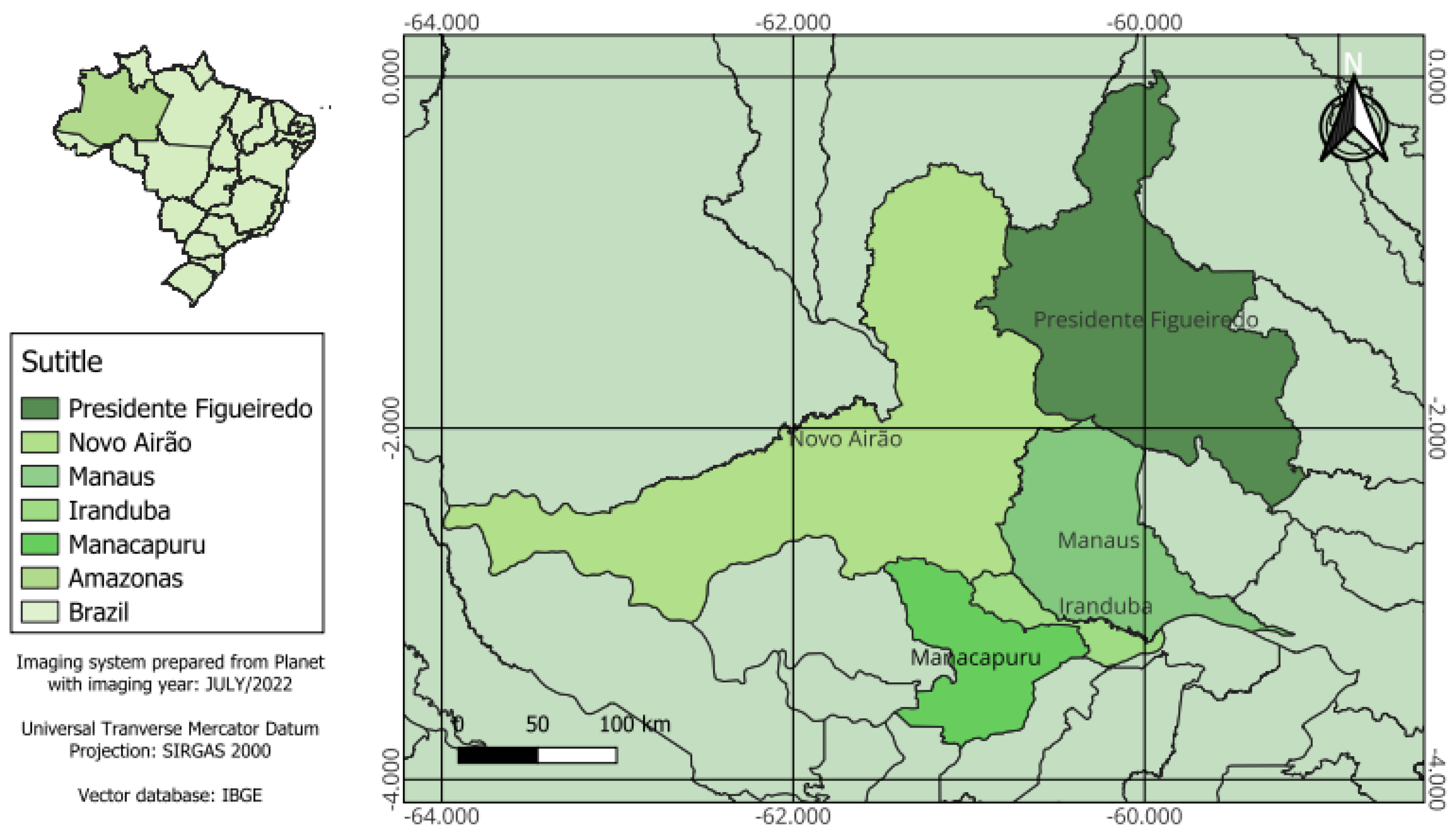
The Metropolitan Region of Manaus (MRM), also known as Greater Manaus, is the second largest metropolitan region in the North of Brazil, according to the estimate of the Brazilian Institute of Geography and Statistics (IBGE) [
8]. Its population was 2,676,936 inhabitants. Established in 2007 by State Complementary Law No. 52, it brings together 13 municipalities in the state of Amazonas in the process of conurbation, namely Autazes, Careiro, Careiro of Várzea, Iranduba, Itacoatiara, Itapiranga, Manacapuru, Manaquiri, Manaus, Novo Airão, Presidente Figueiredo, Novo Airão, Rio Preto da Eva and Silves. For the development of this research, two semi-excavated fish farms were sampled in each of the five municipalities of the MRM, namely Presidente Figueiredo, Manacapuru, Iranduba, Manaus and Novo Airão, totaling ten fish farms. Ten (n= 10) tambaqui specimens were collected on each farm sampled, constituting one hundred (N= 100) tambaqui.
Figure 1.
Map showing the sampling locations in the Manaus Metropolitan Region.
Figure 1.
Map showing the sampling locations in the Manaus Metropolitan Region.
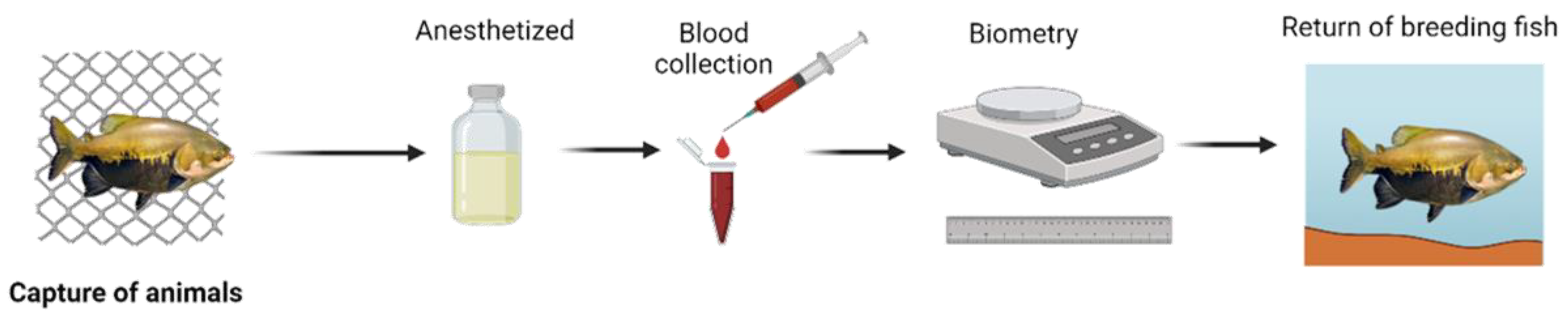
2.3. Animal Capture
The fish were confined in excavated fattening ponds and fed with commercial extruded feed containing 32% crude protein in all locations; they were captured in the early hours of the morning with the aid of the trawl net (Fio 210/6 5mn 400 meshes ) and had previously been fasting for 24 hours [
9].
2.3. Blood Collection and Biometric Analysis
After capture, the animals (N= 100) were anesthetized using eugenol (0.2 g.L
-1) and blood samples were taken by caudal puncture with disposable syringes (1 mL) containing heparin anticoagulant (5000 IU). The collected blood was divided into two aliquots, one for determining the erythrogram, leukogram and thrombogram, and the other for obtaining plasma and subsequent measurement of biochemical constituents [
10,
11]. After blood collection, the biometrics of each animal were determined, such as total length (TL, cm) using a measuring tape and body weight (g) using portable scales. After the handling procedures, the animals were returned to the cultivation environment, as shown in (
Figure 2).
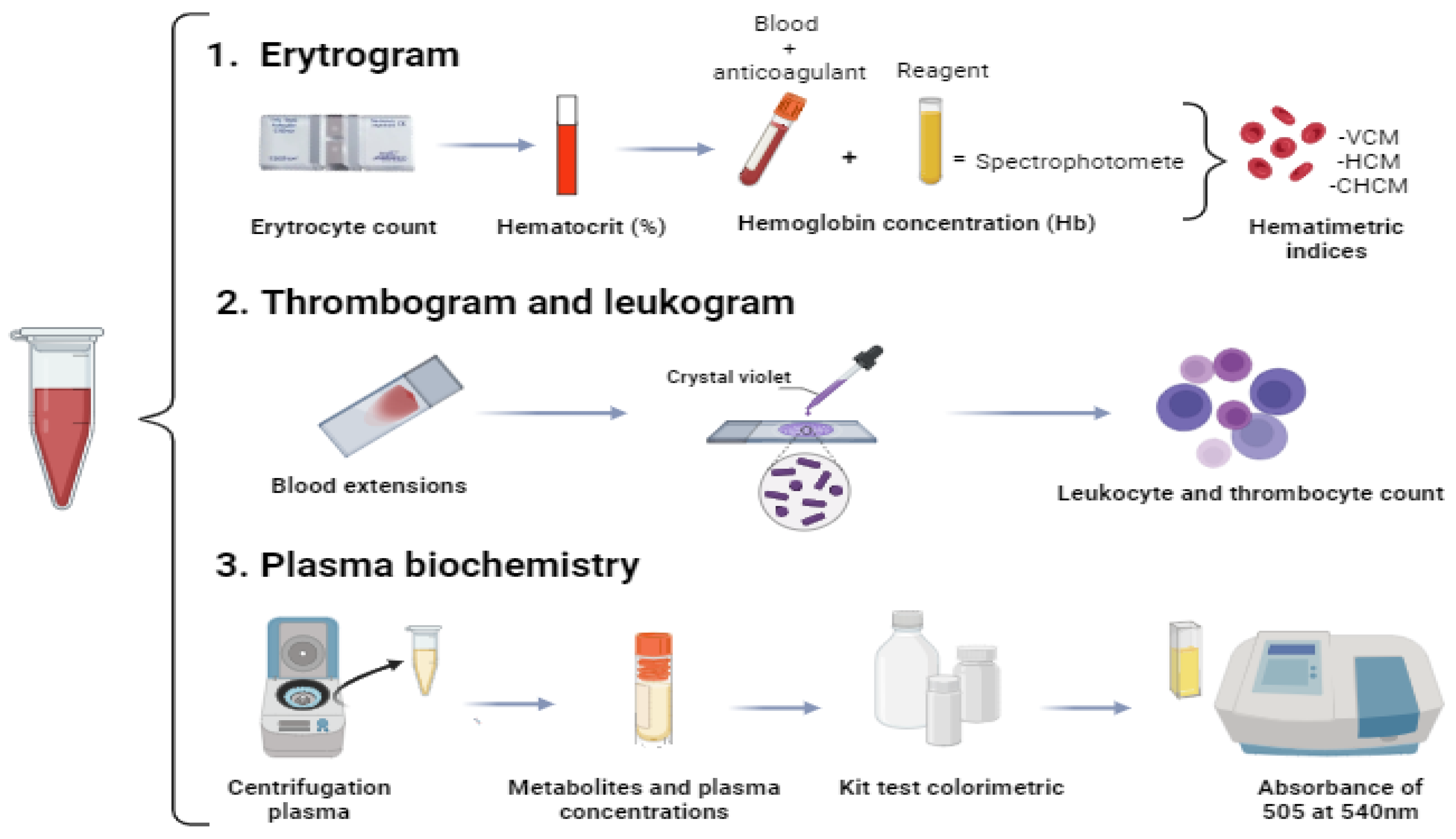
2.4. Erythrogram
The erythrocyte count (RBC) was performed in a Neubauer chamber with the aid of a microscope (Leica®, DM 500, Wetzlar, Germany) after fixing the samples in a formaldehyde-citrate solution (1:200). The hematocrit percentage (Ht) was determined by the microhematocrit method, using samples centrifuged in microcapillary tubes. To determine the hemoglobin concentration (Hb), the verified blood was included in Drabklin's solution and analyzed using the cyanmethemoglobin method [
12,
13]. From these data, hematimetric indices were calculated: mean corpuscular volume (MCV), mean corpuscular hemoglobin (MCH) and mean corpuscular hemoglobin concentration (MCHC), using equations available in the literature [
12,
14].
2.5. Leukogram
Slides (n: 3/collected fish) with blood extensions were prepared and stained with May-Grunwald, Giemsa, Wright and methanol (MGGW) [
15]. The material was used to count total leukocytes and thrombocytes [
16] and for the differential count of leukocytes based on 200 leukocyte types.
2.6. Plasma Biochemistry
Blood plasma was obtained after centrifugation at 750 G, frozen in liquid nitrogen (-86
oC) until biochemical analysis. The concentrations of glucose, triglycerides, total cholesterol, total proteins, urea and lactate were determined by enzymatic-colorimetric methods quantified by specific commercial kits (LabTest®, Belo Horizonte, Brazil), and processed in a spectrophotometer (Thermo Fisher Scientific®, Waltham, USA). The dosage of sodium (Na
+), potassium (K
+) and chloride (Cl
-) ions was determined by colorimetric method using a commercial kit (LabTest®, Belo Horizonte, Brazil).
Figure 3 shows a simplified scheme of blood and plasma processing.
2.5. Water Analysis
The evaluation of the physical-chemical properties of water, such as temperature (°C), pH, conductivity (μS.cm-1) and dissolved oxygen (mg.L-1), were determined in each fish farm sampled in the morning and afternoon using a multi-parametric digital device. Other parameters, such as hardness (mg.L-1), alkalinity (mg.L-1), total ammonia (mg.L-1) and nitrite (mg.L-1), were determined using a colorimetric kit (Alfakit®, Florianópolis, Brazil).
2.6. Statistical Analysis
Data were analyzed for normality using the Shapiro-Wilk test and homogeneity of variance using Levene's test. Analysis of Variance (ANOVA) was followed by the Tukey test, which was used to compare fish farms in the same municipality and between municipalities. The significance level used in all tests was 95% (p-value ≥0.05). For this, Software R version 4.0.2 was used.
3. Results
The biometric parameters demonstrate similarities in the total length and weight of the tambaqui in the five sampled municipalities. However, dominant individuals were accentuated in Iranduba and Novo Airão (
Table 1).
For the erythrogram, significant statistical differences were observed with lower values in the Iranduba and Novo Airão municipalities, mainly for hematocrit, Hb, RBC and MCHC (
Table 2).
Six cell types were found in the leukogram and thrombogram: thrombocytes, lymphocytes, eosinophils, monocytes, neutrophils, and LG-PAS (
Table 3). Significant statistical differences were found in total leukocytes, thrombocytes, lymphocytes, eosinophils, and monocytes associated with tambaqui from Iranduba and Novo Airão (
Table 3).
In plasma biochemistry, the total proteins, glucose, cholesterol, triglycerides and urea levels presented lower values in tambaqui from Iranduba and Novo Airão (
Table 4). The phosphorus, chlorides, sodium and potassium levels did not differ significantly between the five municipalities investigated (
Table 4).
In the physical and chemical properties of the water from the sampled excavated ponds, it was possible to observe changes between the municipalities, mainly in the levels of dissolved oxygen, total ammonia and nitrite, which presented high values in Iranduba and Novo Airão (
Table 5).
4. Discussion
The biometric data of the tambaqui between the sampled municipalities did not show statistical differences; the specimens investigated are juveniles. However, it is possible to observe lower values of the biometrics of the tambaqui, mainly about weight in the fish farms of Iranduba and Novo Airão. The results in
Table 1 corroborate the studies conducted by Aride et al. [
10] and Ranzani et al. [
11], who conducted research with tambaqui under experimental conditions that differ from those in the present study, which took place in excavated ponds.
The tambaqui erythrogram values (
Table 2), the Presidente Figueiredo, Manaus and Manacapuru specimens present similar physiological conditions. On the other hand, it should be noted that the tambaqui from Iranduba and Novo Airão present signs of anemic processes caused by low Ht and Hb values; in addition, there is an attempt at physiological compensation due to the reduction in Ht values, which was directly related to the decrease in erythropoiesis (low RBC value) observed.
The white blood series (leukogram and thrombogram) mainly indicates fishes responses to disturbances of abiotic or biotic factors [
9]. In the present study, thrombocytes, lymphocytes, eosinophils, neutrophils, and LG-PAS were identified with morphology similar to studies by Oliveira et al. [
14]. In that study, there was a warning about the difficulty of finding a leukocyte type called LG-PAS in blood samples.
In the comparative analysis of white cells, there was a marked intraspecific variation, which resulted in high standard deviation values, directly reflected in the statistical tests for comparing means. Despite this characteristic, total leukocyte values in tambaqui at Iranduba were higher than at other fish farms. Thrombocytopenia was identified in tambaqui from fish farms in Iranduba and Novo Airão; the animals showed paleness and hemorrhagic spots on the body [
15]. These characteristics may indicate that low numbers of thrombocytes are caused by diseases, parasites, or other environmental conditions imposed on the organism. There was also lymphocytosis, eosinocytosis and monocytosis among tambaqui from excavated ponds in Iranduba and Novo Airão, unlike other studies conducted with tambaqui
C. macropomum that did not observe changes in the values of leukocyte types [
16,
17,
18,
19].
Total protein levels were high in tambaqui from Presidente Figueiredo and Novo Airão (
Table 4). Fish from Presidente Figueiredo and Novo Airão have total plasma protein values similar to tambaqui fish investigated under experimental conditions [
11,
12,
20].
Higher glucose values were found in Manaus fish and lower in Iranduba and Novo Airão (
Table 4). However, the tambaqui from Manaus may be overfed and in the process of accumulating energy in the form of glucose; on the other hand, the fish from Iranduba and Novo Airão may be malnourished and in physiological difficulty within the ponds [
20,
21,
22,
23] reiterate that the main difficulty for fish producers in Amazonas is acquiring feed, given the farming systems' high prices and logistical challenges.
The lipid compounds (cholesterol and triglycerides) investigated reinforce low levels in the blood of fish from Iranduba and Novo Airão, such as total proteins and glucose. This indicates low levels of fat accumulation in these specimens, which will naturally reflect on the flavor of their meats.
Phosphorus was similar in the tambaqui of the five locations investigated, and its values were within those found in other research [
16].
There was no significant statistical difference for chloride, sodium, and potassium ions; the values were within those described for the same species [
11,
12,
16]. Thus, this indicates good conditions of ionic regulation in the environments of excavated nurseries from MRM tambaqui.
Temperature levels were similar between the sampled locations, and values were identical to those described by Mariano et al. [
21] and Porto et al. [
24] when they analyzed this variable in tanks excavated in Amazonas. For pH, there was a significant statistical difference in tambaqui from Presidente Figueiredo, explained by the fact that most of the waters from excavated ponds in the locality come from stream waters, which are black and more acidic [
25,
26]. Dissolved oxygen levels in water from excavated ponds were significantly low in Iranduba and Novo Airão. These values were low compared to the recommendations described by Mariano et al. [
21]. However, Porto et al. [
24], Pantoja-Lima et al. [
26], and Santos et al. [
27] also described these values in a production system located in the MRM. The hardness and alkalinity levels did not present a clearly defined pattern compared to the other parameters, and they were explained by the type of river water that supplies these municipalities. However, the values were within the findings of Soares et al. [
22]. For the levels of total ammonia and nitrite, there were accentuated values in Iranduba and Novo Airão, although not predominant [
22]. Also found some excavated ponds with similar values.
5. Conclusions
Specimens of juvenile tambaqui cultivated in excavated nurseries in the MRM have different hematological and biochemical variables, physical properties and water chemistry. It is essential when proposing physiological reference ranges for tambaqui, as well as the physical and chemical quality of the water that it is considered in addition to nutritional issues, management and other factors associated with cultivation, location and origin of the water. Furthermore, water quality is fundamental to the excellent health of the tambaqui, so the fish from Iranduba and Novo Airão are in low physiological conditions. On the other hand, fish from fish farms in Manaus have an inappropriate diet, as indicated by the high cholesterol levels and triglycerides in their blood. However, these data may be associated with other factors such as seasonality, sex, increase in carbon dioxide and temperature, nutritional status, genetic variation, high stocking densities and mainly inadequate management practices, resulting in changes in hematological and biochemical variables and animal immunity.
Author Contributions
FDFF and ATO; methodology, ACT; software, MWSR; validation, FDFF, ATO, PHRA and ARSL; formal analysis, ATO, AAB, NPA and TCN; investigation, FDFF and ATO; resources, ATO and PHRA; data curation, FDFF, ATO and ARSL; writing – preparation of the original draft, FDFF and ARSL; writing – review and editing, ATO, ARSL and FDFF; visualization, ATO and ARSL; supervision, FDFF; project administration, ATO; acquisition of finance, ATO and PHRA. All authors read and agreed to the published version of the manuscript.
Financing
To the Federal Institute of Education, Science and Technology of Amazonas (IFAM) through the Scientific and Technological Development Support Program (PADCIT). The Amazonas State Research Support Foundation (FAPEAM) and the Scientific and Technological Development Council (CNPq), through the First Projects Program (PPP) notice, granted the resources to carry out this work. FDFF received a master's scholarship granted by FAPEAM and ATO for a productivity scholarship granted by CNPq.
Statement from the Research Ethics Board
This study's procedures were by the UFAM Research Ethics Committee on the Use of Animals (nº 005/2018).
Free and Informed Consent Form
Not applicable.
Data Availability Statement
Study data can be requested from the corresponding authors upon request.
Thanks
They would like to thank the Federal Institute of Education, Science and Technology team of Amazonas (IFAM) and all the students at the Center for Invertebrate and Vertebrate Studies of the Amazon (NEIVA) for their assistance.
Interest Conflicts
The authors declare that there are no conflicts of interest.
References
- Aride, P.H.R.; Batista, R.B.; Ferreira, M.S.; Pantoja-Lima, J.; Ladislau, D.S.; Castro, P.D.S.; Oliveira, A.T. Changes on physiological parameters of tambaqui (Colossoma macropomum) fed with diets supplemented with Amazonian fruit Camu camu (Myrciaria dubia). Braz. J. Biol. 2017, 78, 360–367. [CrossRef]
- Peixe BR - Anuário brasileiro de piscicultura. 2023. 65p. Disponível em:.
- Hesser, E.F. Methods for Routine Fish Hematology. Progress. Fish-Culturist 1960, 22, 164–171. [CrossRef]
- Higuchi, L. H.; Feiden, A.; Maluf, M. L. F.; Dallagnol, J. M.; Zaminhan, M., & Boscolo, W. R. (2011). Erythrocyte and biochemical evaluation of catfish (Rhamdia quelen) submitted to a diet with different protein and energy levels. Brazilian Animal Science, 12(1), 70-75. [CrossRef]
- Pereira, D. S. P.; Guerra-Santos, B.; Moreira, E. L. T.; Albinati, R. C. B. & Ayres, M. C. C. (2018). Hematological and histological parameters of Nile tilapia in response to the challenge of different salinity levels. Fisheries Institute Bulletin, 42(3), 635-647. [CrossRef]
- Oliveira, A. C. B.; Miranda, E.; Correa, R. Nutritional requirements and feeding of the tambaqui. In: Fracalossi, D. M., Cyrino, J. E. P. Nutrition and feeding of species of interest to Brazilian aquaculture. Florianópolis: Aquabio, 2013. p. 231-240.
- Sipaúba Tavares, L.H. Limnologia Aplicada a Aquicultura. Jaboticabal: FUNEP, 1994. 70 p.
- du Sert, N.P.; Hurst, V.; Ahluwalia, A.; Alam, S.; Avey, M.T.; Baker, M.; Browne, W.J.; Clark, A.; Cuthill, I.C.; Dirnagl, U.; et al. The ARRIVE guidelines 2.0: Updated guidelines for reporting animal research. PLoS Biol. 2020, 18, e3000410. [CrossRef]
- Instituto Brasileiro de Geografia e Estatística (IBGE), 2019. Available at: http://www.ibge.gov.br/estatística/ (access in 26 feb. 2024).
- Oliveira, A. M.; Silva, M. De N. P.; Almeida-Val, V. M. F. De; Val, A. L. Characterization of fish farming activity in the mesoregions of the State of Amazonas, Brazilian Amazon. Colombian Journal of Animal Science, v. 4, n. 1, p. 154-162, 2012.
- Aride, P.H.R.; Ferreira, M.S.; Liebl, A.R.S.; Comassetto, L.E.; Ladislau, D.S.; Bassul, L.A.; Silva, B.R.; Mattos, D.C.; Lavander, H.D.; Souza, A.B.; et al. Growth and hematological responses of tambaqui, Colossoma macropomum fed different levels of rice, Oryza spp.. Braz. J. Biol. 2021, 81, 962–968. [CrossRef]
- Razani, M.; Yazdani-Chamzini, A.; Yakhchali, S.H. A novel fuzzy inference system for predicting roof fall rate in underground coal mines. Saf. Sci. 2013, 55, 26–33. [CrossRef]
- Nascimento, M. Dos S.; Mattos, B. O.; Bussons, M. R. F. M.; Oliveira, A. T.; Liebl, A. R. Da S.; Carvalho, T. B. Supplementation of citric acid in plant protein-based diets for juvenile tambaqui, Colossoma macropomum . Journal Of The World Aquaculture Society, v. 1, p. 1, 2020.
- Wintrobe, M. M. Variations on the size and haemoglobin content of erythrocytes in the blood of various vertebrates. Folia Haematologica, Leipzig, v.51, p.32-49, 1934.
- de Oliveira, A.T.; de Lemos, J.R.G.; Santos, M.Q.d.C.; Pantoja-Lima, J.; Aride, P.H.R.; de Araújo, M.L.G.; Tavares-Dias, M.; Marcon, J.L. Morphological, cytochemical and ultrastructural aspects of blood cells in freshwater stingray species in the middle Rio Negro basin of Amazonian Brazil. Sci. Rep. 2021, 11, 1–11. [CrossRef]
- Tavares-Dias, M.; Moraes, F. R. (2010). Biochemical Parameters for Piaractus mesopotamicus, Colossoma macropomum (Characidae) and hybrid tambacu (P. mesopotamicus X C. macropomum). Ci. Anim., Bras., Goiânia, v. 11, n. 2, p. 363-368, abr./jun. 2010.
- Tavares-Dias, M.; Moraes, F. R. Hematologia de peixes teleósteos. Villimpress: Ribeirão Preto, SP. 144p, 2004.
- Bussons, I.N.B.; Sousa, E.d.S.; Aride, P.H.R.; Duncan, W.L.P.; Pantoja-Lima, J.; Furuya, W.M.; de Oliveira, A.T.; Bussons, M.R.F.M.; Faggio, C. Growth performance, hematological responses and economic indexes of Colossoma macropomum (Cuvier, 1818) fed graded levels of glycerol. Comp. Biochem. Physiol. Part C: Toxicol. Pharmacol. 2021, 249, 109122. [CrossRef]
- Liebl, A.R.d.S.; Cáo, M.A.; Nascimento, M.d.S.; Castro, P.D.d.S.; Duncan, W.L.P.; Pantoja-Lima, J.; Aride, P.H.R.; Bussons, M.R.F.M.; Furuya, W.M.; Faggio, C.; et al. Dietary lysine requirements of Colossoma macropomum (Cuvier, 1818) based on growth performance, hepatic and intestinal morphohistology and hematology. Veter- Res. Commun. 2022, 46, 9–25. [CrossRef]
- de Oliveira, A.T.; Santos, M.Q.d.C.; de Araújo, M.L.G.; de Lemos, J.R.G.; Sales, R.S.d.A.; Aride, P.H.R.; Pantoja-Lima, J.; Tavares-Dias, M.; Marcon, J.L. Hematological parameters of three freshwater stingray species (Chondrichthyes: Potamotrygonidae) in the middle Rio Negro, Amazonas state. Biochem. Syst. Ecol. 2016, 69, 33–40. [CrossRef]
- Tavares-Dias, M.; Moraes, F. R. (2010). Biochemical Parameters for Piaractus mesopotamicus, Colossoma macropomum (Characidae) and hybrid tambacu (P. mesopotamicus X C. macropomum). Ciência Animal Brasileira, Goiânia, v. 11, n. 2, p. 363-368, abr./jun. 2010.
- Mariano, W. S.; Souza, F. L. G.; Tavares-Dias, M.; Rodrigues, R. L. C.; Azevedo, S. B.; Lima, J. O. Hematological study of tambaqui (Colossoma macropomum) subjected to sublethal concentration of biopesticide based on Bacillus thuringiensis. Magazine SODEBRAS, v. 12, p. 54-58, 2017.
- Soares, B.V.; Neves, L.R.; Ferreira, D.O.; Oliveira, M.S.B.; Chaves, F.C.M.; Chagas, E.C.; Gonçalves, R.A.; Tavares-Dias, M. Antiparasitic activity, histopathology and physiology of Colossoma macropomum (tambaqui) exposed to the essential oil of Lippia sidoides (Verbenaceae). Veter- Parasitol. 2017, 234, 49–56. [CrossRef]
- Oliveira, L.C.D.; Brasiliense, A.R.P.; Dias, M.K.R.; Yoshioka, E.T.O.; Tavares-Dias, M. Toxicological, hematological and immunological effects of levamisole and ivermectin diet supplementation on Colossoma macropomum (Serrasalmidae). Dis. Aquat. Org. 2019, 136, 255–263. [CrossRef]
- Porto, E. L.; da Cruz, M. G.; Bolson, M. A.; Junior, É. S.; Martins, M. L.; & Jerônimo, G. T. (2024). Trace metal biomonitoring in farming tambaqui (Colossoma macropomum), an Amazonian neotropical fish. Environmental Science and Pollution Research, 31(5), 7664-7679. [CrossRef]
- Izel, A. C. U.; Melo, L. A. S. (2004). Breeding tambaqui (Colossoma macropomum) in excavated tanks in the State of Amazonas. Manaus: Embrapa Western Amazon, 2004. 20 p.
- Pantoja-Lima, J., Santos, S.M., Oliveira, A. T., Araújo, R. L., Silva Junior, J. A. L.; Bernardino, G.; Alves, R. R. S.; Ferraz Filho, A.; Gomes, A. L.; Aride, P. H. R. Research and transfer of technology combined for the development of aquaculture in the State of Amazonas. In: Marcos Tavares Dias, Wagner dos Santos Mariano. (Org.). Aquaculture in Brazil: new perspectives. 2ed.São Carlos: Pedro & João, 2015, v. 2, p. 313-332.
- Santos, S.M.; Oliveira, A.T.; Aride, P.H.R.; Liebl, A.R.S.; Mendonça, F.P.; Zuanon, J.; Pantoja-Lima, J. Environmental and ichthyofaunistic characteristics of Amazonian streams with and without fish farm. Braz. J. Biol. 2023, 83, e272623. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).