1. Introduction
Congenital heart disease (CHD) is a prevalent congenital disorder diagnosed with high frequency, affecting approximately 0.8% to 1.2% of all live births [
1]. CHD encompasses a comprehensive range of abnormalities that manifest at birth, including numerous defects that may manifest individually or in conjunction. The atypical configuration of the cardiac chambers, valves, or major blood vessels in individuals diagnosed with congenital heart disease disrupts the typical hemodynamic pattern [
2]. While some defects are mild and may not require intervention, others are severe and need treatment shortly after birth [
3].
Although there are now more clearly defined risk factors, the cause of most CHDs is still unknown. A well-established causal relationship can only be defined in 10 to 15% of cases [
4]. The majority of adverse pregnancy outcomes can be attributed to gene-environment interactions. The results of this thorough examination of risk factors and responses to congenital heart defects reveal a complex network of functional interactions between genomic variations and environmental exposures. These interactions play a crucial role in regulating essential biological systems during heart development [
5].
Studies often highlight risk factors for congenital heart disease that are linked to extreme maternal and paternal ages, particularly those under 21 years and over 35 years of age, as well as unfavorable socioeconomic status. In addition, maternal health status plays a crucial role and diseases such as diabetes with insulin use, autoimmune pathology such as lupus, hypertension, obesity, and unspecified febrile illness contribute [
6]. Specific infectious diseases such as rubella and parvovirus, smoking, and gestational stress are also included. Moreover, there is a correlation between maternal and paternal exposure to alcohol and other substances and congenital heart disease [
7].
Over the past decades, mortality for this category of patients has decreased significantly [
8]. Nevertheless, mortality rate of congenital heart disease exhibits important global variability. In countries with limited access to healthcare services the mortality rates are higher compared to developed nations [
9].
Research has demonstrated that the utilization of pulse oximetry screening (POS) in neonates can effectively improve the identification of critical congenital heart disease (CCHD) [
10]. Critical congenital heart disease (CHD) has been characterized in the literature as duct-dependent CHD that poses a significant risk to life if left untreated during the neonatal period. These conditions include duct-dependent pulmonary and systemic anomalies. Pulmonary anomalies include pulmonary atresia with an intact ventricular septum, pulmonary stenosis, tetralogy of Fallot, total anomalous pulmonary venous return, transposition of the great arteries (TGA), tricuspid atresia, truncus arteriosus. Duct-dependent systemic abnormalities, including coarctation of the aorta, interrupted aortic arch, hypoplastic left heart syndrome, and aortic stenosis [
11]. Pulse oximetry is an easily accessible method of screening, cost-efficient, and a noninvasive tool. It remains an underutilized screening method in newborns, although it can be easily performed by nurses or doctors at patient’s bedside [
12]. Furthermore, postnatal detection by pulse oximetry combined with clinical assessment are the useful methods for CHD screening in most areas. Clinical assessment includes standard physical examination (observation, palpation, and auscultation), chest radiograph, electro- and echocardiography [
13].
The primary objective of our comprehensive study was to evaluate various screening methods utilized for all newborns admitted to intensive care units. This meticulous approach serves as a vital tool in aiding the early and accurate diagnosis of congenital heart disease, thus ensuring timely intervention and improved outcomes for these vulnerable infants.
2. Materials and Methods
We conducted a longitudinal restrospective study to evaluate the prevalence of CHD in a tertiary referral center in Bucharest, Romania. The study focused on infants born with complex cardiovascular malformations admitted to our clinic over a 3-year period (January 2021 - December 2023). Additionally, we assessed the effectiveness of screening methods in identifying patients with CHD and their survival rates. During the period mentioned above, 11,013 patients were born into our clinic, of which 1188 newborns were admitted to the NICU and included in the study.
The inclusion criteria were represented by patients born in our clinic and admitted to the neonatal intensive care unit (NICU), antenatal suspicion of congenital heart disease, need for pulse oximetry monitoring. Those with co-existing medical conditions such as congenital anomalies not related to congenital disease, those born in other maternities and transferred into our clinic were excluded.
Screening methods for CHD detection included pulse oximetry (POS) and clinical assessment (observation, auscultation). All positive screening tests were confirmed using echocardiography. The pulse oximetry and clinical assessment were performed by a trained neonatologist, and cardiac ultrasonography was done by a cardiologist specialized in pediatric cardiology.
A positive pulse oximetry screen was determined by a SpO2 level below 90% in the right hand, or a SpO2 level between 90% and 94% in either location (preductal or postductal location), or a difference of more than 3% between the two locations (measured twice with a one-hour interval). A negative pulse oximetry screen was determined by an oxygen saturation (SpO2) level of 95% or higher in the right hand, with a difference of 3% or less between the preductal and postductal measurements.
The statistical analysis used an array of essential metrics such as sensitivity, specificity, positive predictive value, prevalence, and likelihood ratio, which were employed to evaluate the accuracy of the screening method. Furthermore, the Kaplan-Meier function was applied to assess the survival rates of patients diagnosed with congenital heart disease (CHD), offering valuable insights into their prognosis. It is worth noting that all statistical analysis was performed using R Studio software (Version 2023.09.1+494 for Mac). The following libraries were used: “tidyverse”, “pROC”, “survival”, “epiR”.
3. Results
During the studied period (January 2021 – December 2023) 11,013 patients were delivered in our clinic. 948 (79.7%) neonates were born from personalized follow-up pregnancies (PPUP), while 192 (16.16%) were partially followed-up, and 48 (4.13%) pregnant women did not go to the obstetrician up until admission for labor and birth. Throughout the analyzed timeframe, less than a quarter of newborns required admission in the NICU (1188 patients, 10.7%). Of them, 1102 (92.7%) were preterm and 86 (7.3%) were term neonates.
Pulse oximetry screening (both pre- and postductal screening) and clinical examination on admission and up to 72 hours of life were performed for all patients admitted into NICU. Although 23 patients had a prenatal diagnosis of CHD, we included them in the initial pulse oximetry screening as well.
Clinical examination consisted of identification of cyanosis, SpO2<95% or major clinical signs of cardiorespiratory distress, systolic murmur on auscultation or cardiogenic shock (for complex CHDs).
Over the three-year period, 201 newborns were diagnosed with CHD. A great number (n=198, 98.5%) had a positive screening test during the 72-hour period, while 3 had a negative screening test and were diagnosed using echocardiography. Patient characteristics are described in
Table 1.
The prevalence rate of CHD in the screened population was approximately 16.67%. A confusion matrix was constructed with 198 true positives (TP), 3 false negatives (FN), no false positives (FP), and 987 true negatives (TN). The sensitivity of the screening test (the ability to correctly identify those with CHD) is about 98.51%, while the specificity (the ability to correctly identify those without CHD) is 100%, as there are no false positives.
The likelihood ratio was evaluated as well. For our study, the Positive Likelihood Ratio (LR+) is infinite. In practice, this suggests that a positive screening result is a strong indicator of the disease, given there are no false positives. However, the Negative Likelihood Ratio (LR-) is approximately 0.015, indicating that a negative screening result moderately decreases the likelihood of CHD.
The high sensitivity and specificity values of the screening test demonstrate its effectiveness in accurately identifying individuals with and without congenital heart disease with no false positives reported, the test's reliability is commendable. The infinite Positive Likelihood Ratio (LR+) underscores the strong predictive value of a positive screening result. Conversely, the low Negative Likelihood Ratio (LR-) suggests that a negative result can moderately reduce the probability of CHD. These results highlight the importance of using this screening test as a valuable tool in assessing CHD risk and making informed healthcare decisions.
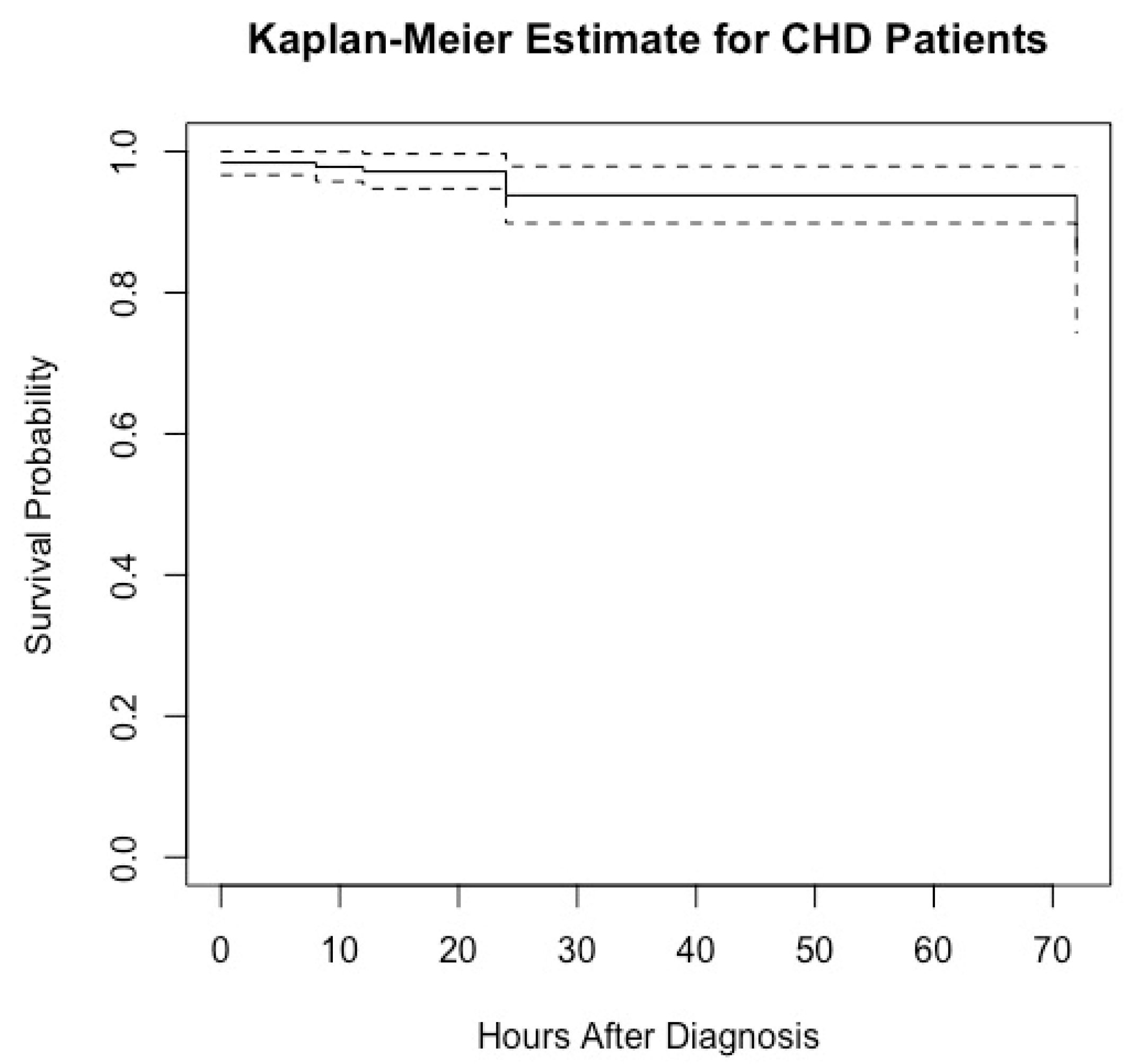
The Kaplan-Meier estimate is a widely employed statistical method for comprehending the survival distribution of a population. It is frequently utilized in clinical research to estimate the probabilities of patients' survival over a given period. In our study, a total of 9 deaths occurred. All patients began with a survival probability of 1 (100%) at the time of diagnosis (0 hours). The Kaplan-Meier graph (
Figure 1) for CHD patients illustrates the survival probability over time, measured in hours after diagnosis. There is a noticeable decrease in the survival probability within the first 12 hours, reflecting 3 deaths. Another decline is observed at 24 hours, corresponding to an additional 4 deaths, further reducing the survival probability. The final decrease occurs by 72 hours, with 2 more deaths accounted for, further reducing the survival probability. Between these drops, the survival probability remains constant, indicating periods where no deaths were recorded. The dashed lines after the last drop at 72 hours suggest the presence of censored data. Censored data indicates that some patients did not experience the event (death) by the end of the observation period or were lost to follow-up.
By the end of the study period (72 hours), the survival probability appears to be slightly above 0.9 (95.5%), indicating that approximately 90% of the patients survived past 72 hours after being diagnosed with CHD. The Kaplan-Meier graph provides a detailed and insightful visualization of the survival probabilities for CHD patients following their diagnosis. It clearly illustrates the impact of time on survival rates and highlights the critical periods where a decline in survival probability occurs due to patient deaths. The presence of censored data is also acknowledged, indicating that not all patients experienced the event (death) within the observation period. Overall, by analyzing this data, researchers can gain valuable insights into the progression of CHD and better understand the factors influencing patient outcomes over time.
The pulse oximetry screening test and clinical examination were followed by an echocardiography which was performed by a skilled Pediatric Cardiologist. All diagnoses can be observed in
Table 2. The most frequent anomaly was represented by patent ductus arteriosus (PDA). While 19 (16.9%) required pharmacological closure, most of them (n=93, 83.1%) did not require neither medication nor surgical correction. Almost a quarter of patients were diagnosed with ventricular septal defect (VSD) (n=34, 16.9%) and a similar percentage with pulmonary stenosis (n=27, 13.4%). Several cases of transposition of the great vessels, coarctation of the aorta, double aortic arch, tetralogy of Fallot, bicuspid aortic valve, hypoplastic left heart syndrome, or pulmonary valve atresia were also found.
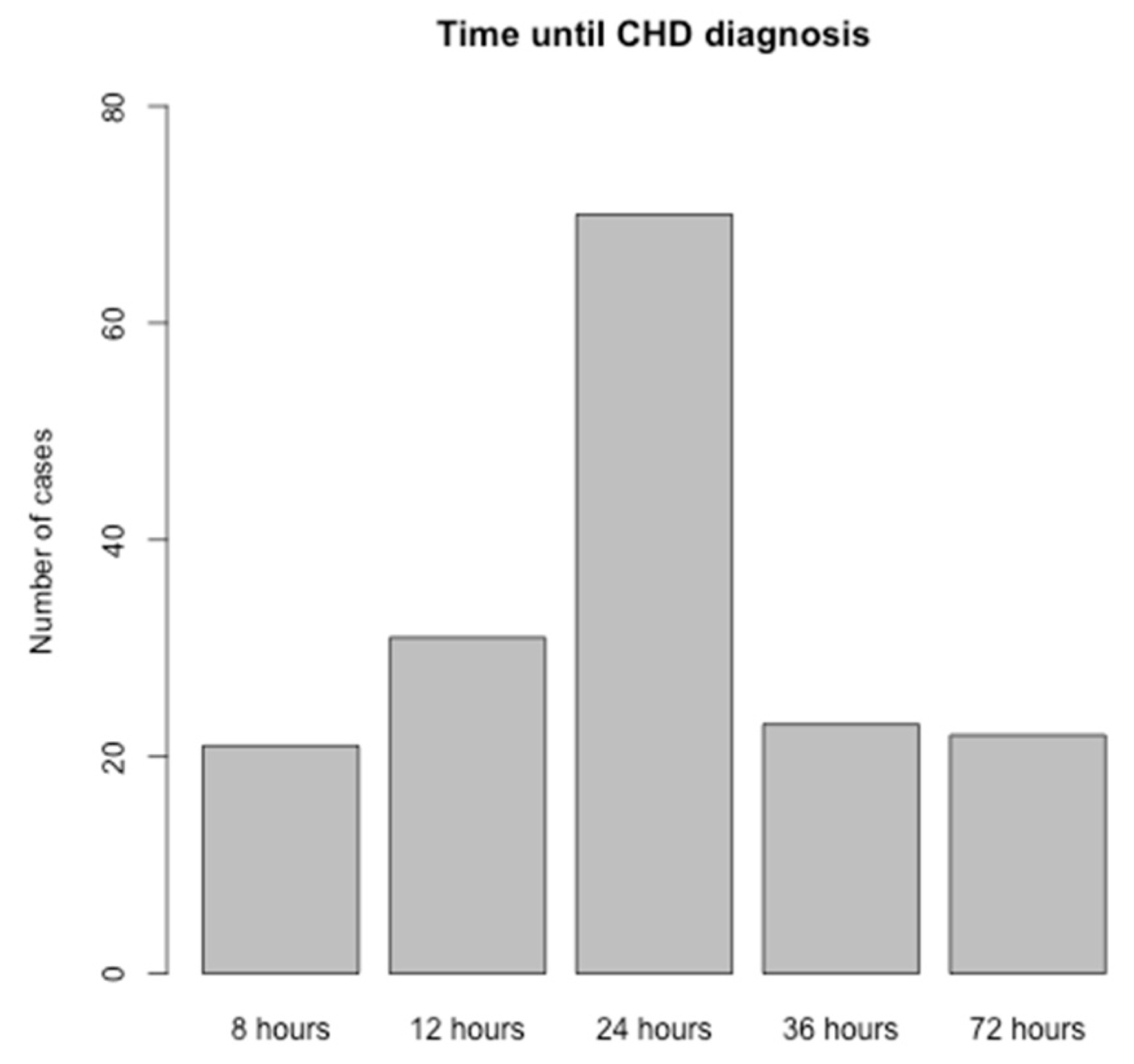
The timeline between the screening test and diagnosis is depicted in
Figure 2. More than a half of cases were diagnosed by 72 hours of life (n=122). The graph does not include the 23 cases with prenatal diagnosis, emphasizing the focus on postnatal diagnostic procedures.
A small percentage (n=16, 7.9%) required transfer and surgical correction of the identified CHD. All 16 of them underwent surgery in our country (Bucharest, Cluj-Napoca, and Târgu-Mureș). Several diseases can be observed in the echocardiographic exams shown in
Figure 3 and
Figure 4.
This study focused on the implementation of a screening program for congenital heart disease (CHD) in newborns and subsequent monitoring of early-life patient outcomes. Future research should prioritize longitudinal follow-up to understand long-term outcomes beyond the initial 72-hour window. Detailed investigation into the causes of mortality and critical periods for intervention could offer insights for improving care. The ultimate goal is to enhance early detection and treatment to improve survival rates and quality of life for infants with CHD.
The main limitations of our study are represented by a sample size drawn from a single NICU, which may not be representative of the broader neonatal population, along with the short follow-up period leading to limited understanding of the long-term outcomes. Additionally, without linking screening outcomes to specific medical interventions, the study overlooks evaluating the effectiveness of post-diagnosis treatment. Future studies should address these limitations to enhance the validity and applicability of the research findings.
4. Discussion
During the three-year study period (January 2021- December 2023), 11,013 newborns were born into our clinic and 1188 were admitted to the NICU. All these patients were screened using pulse oximetry and clinical assessment for 72 hours and 201 were diagnosed with congenital heart disease (198 had a positive screening test and 3 had a negative screening test but were later diagnosed with CHD). There is a growing body of literature that presents evidence supporting the effectiveness of pulse oximetry as a screening test for critical congenital heart disease (CCHD) in newborns [
14].
The implementation of CCHD screening during the first hours of life in tertiary maternities has shown promising results. In our study, pulse oximetry screening during the first 72 hours of life has proven a sensitivity of 98.51%. Gopalakrishnan S. et al. reports a 75% sensitivity, 99.29% specificity, with a false positive rate of 0.81% [
11], while Singh et al. describes a higher sensitivity (85.7%) and 99.3% specificity [
15].
Hu X. et al. underline the importance of pulse oximetry screening in the NICU, but additionally, the significance of both pre- and postductal monitoring of saturations. The authors highlighted the fact that without simultaneous monitoring of SpO2, the screen for CCHD would have failed to identify one case of transposition of the great vessels and 6 cases of persistent pulmonary hypertension, with delayed treatment and grave consequences [
16]. Likewise, our screening consisted of both preductal and postductal saturation 72 hours assessment, which led to early detection and echocardiographic diagnosis.
Ma X. et al. conducted a 5-year screening program for detecting congenital heart disease using pulse oximetry and cardiac murmur auscultation for 801,831 newborns from 6 to 72 hours of life. High sensitivity and specificity of the cardiac murmur auscultation method in clinical practice were observed for both critical (100.00% and 97.72%) and major CHD (98.47% and 97.76%) [
17]. In our study, only 133 had a systolic heart murmur (66.1%), but they had a positive pulse oximetry screening test.
The pediatric cardiologist plays a major role in congenital heart disease diagnosis confirmation. Nevertheless, neonatologists can detect structural CHDs using cardiac ultrasonography. The goal is to involve a pediatric cardiologist for a precise and early diagnosis. On the other hand, if a scan shows a heart that seems to be structurally normal, it could decrease the number of referrals to pediatric cardiology and potentially prevent the need for patient transfers [
18].
The implementation of oximetry to the standard physical examination aids in identifying a greater number of newborns with critical congenital heart defects (CCHDs), without significantly raising the rate of false-positive results. This discovery has the potential to decrease the morbidity and mortality rates linked to discharging newborns from the hospital without a timely diagnosis. This leads to a more rapid diagnosis and transfer for surgical correction and better outcomes [
19].
A key component of the treatment approach is to promptly detect CCHD. This includes prenatal diagnosis. In our study it was present only for 23 out of 201 cases, as only 79.7% newborns came from correctly followed-up pregnancies. Prenatal diagnosis and newborn screening are two methods used to detect these conditions at an early stage. Prenatal diagnosis is particularly useful as it allows for careful planning of management strategies in collaboration with family and care providers [
20].
Pulse oximetry represents a relatively low-cost, simple to use and effective method for CCHD screening. However, for long-term implementation, combining pulse oximetry with clinical assessment is a viable approach worth considering. Enhancing the accessibility of treatment is essential for maximizing the potential health advantages of screening [
21].
The primary objective of our comprehensive study was to showcase the remarkable impact of integrating pulse oximetry screening, clinical assessment, and cardiac ultrasonographic examination in enhancing the early detection rates of congenital heart defects (CHD) within the crucial 72-hour window following birth. This approach not only facilitates swifter interventions but also significantly improves neonatal outcomes, thereby reducing hospital NICU stays, mitigating morbidity rates, and ultimately lowering mortality risks within the vulnerable neonatal cohort.
5. Conclusions
This study assessed neonatal screening using pulse oximetry and clinical evaluation for congenital heart disease (CHD) in a NICU setting, and revealed a high prevalence rate and excellent initial diagnostic accuracy, with the screening test demonstrating a sensitivity of 98.51% and a specificity of 100%. The Kaplan-Meier survival analysis within the first 72 hours post-diagnosis indicated a promising survival probability, with over 90% of infants surviving beyond this critical period. However, the study's scope, constrained by a single-center design and a short follow-up duration, highlights the necessity for extended research to capture long-term outcomes. Future research should expand to multicenter trials with diverse populations and longer follow-up to fully ascertain the benefits of early CHD detection and to refine intervention strategies to improve survival rates and quality of life for affected neonates.
Author Contributions
Conceptualization, I.R. and D.E.P.; methodology, A.T.; software, A.M.C.J.; validation, I.R., D.E.P. and C.F.; formal analysis, A.T.C.; investigation, C.F.; resources, A.D.; data curation, I.R.; writing—original draft preparation, I.R.; writing—review and editing, A.M.C.J.; visualization, D.E.P.; supervision, I.R. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Ethical review and approval were waived for this study due to the fact that the study was an observational study.
Informed Consent Statement
Not applicable.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Wu, W.; He, J.; Shao, X. Incidence and Mortality Trend of Congenital Heart Disease at the Global, Regional, and National Level, 1990–2017. Medicine (Baltimore) 2020, 99, e20593. [Google Scholar] [CrossRef] [PubMed]
- Criteria, I. of M. (US) C. on S.S.C.D. Congenital Heart Disease. In Cardiovascular Disability: Updating the Social Security Listings; National Academies Press (US), 2010.
- Congenital Heart Defects - What Are Congenital Heart Defects? | NHLBI, NIH Available online:. Available online: https://www.nhlbi.nih.gov/health/congenital-heart-defects (accessed on 26 March 2024).
- Lopes, S.A.V. do A.; Guimarães, I.C.B.; Costa, S.F.O.; Acosta, A.X.; Abe-Sandes, K.; Mendes, C.M.C. Risk Factors for Critical and Complex Congenital Heart Diseases: Case-Control Study. Progress in Pediatric Cardiology 2023, 68, 101612. [Google Scholar] [CrossRef]
- Lage, K.; Greenway, S.C.; Rosenfeld, J.A.; Wakimoto, H.; Gorham, J.M.; Segrè, A.V.; Roberts, A.E.; Smoot, L.B.; Pu, W.T.; Pereira, A.C.; et al. Genetic and Environmental Risk Factors in Congenital Heart Disease Functionally Converge in Protein Networks Driving Heart Development. Proc Natl Acad Sci U S A 2012, 109, 14035–14040. [Google Scholar] [CrossRef] [PubMed]
- Dolk, H.; Loane, M.; Garne, E. ; European Surveillance of Congenital Anomalies (EUROCAT) Working Group Congenital Heart Defects in Europe: Prevalence and Perinatal Mortality, 2000 to 2005. Circulation 2011, 123, 841–849. [Google Scholar] [CrossRef] [PubMed]
- Wen, Z.; Yu, D.; Zhang, W.; Fan, C.; Hu, L.; Feng, Y.; Yang, L.; Wu, Z.; Chen, R.; Yin, K.; et al. Association between Alcohol Consumption during Pregnancy and Risks of Congenital Heart Defects in Offspring: Meta-Analysis of Epidemiological Observational Studies. Ital J Pediatr 2016, 42, 12. [Google Scholar] [CrossRef] [PubMed]
- Bouma, B.J.; Mulder, B.J.M. Changing Landscape of Congenital Heart Disease. Circulation Research 2017, 120, 908–922. [Google Scholar] [CrossRef] [PubMed]
- Lopes, S.A.V. do A.; Guimarães, I.C.B.; Costa, S.F. de O.; Acosta, A.X.; Sandes, K.A.; Mendes, C.M.C. Mortality for Critical Congenital Heart Diseases and Associated Risk Factors in Newborns. A Cohort Study. Arq Bras Cardiol 2018, 111, 666–673. [Google Scholar] [CrossRef] [PubMed]
- Narvey, M.; Wong, K.K.; Fournier, A. Pulse Oximetry Screening in Newborns to Enhance Detection of Critical Congenital Heart Disease. Paediatr Child Health 2017, 22, 494–498. [Google Scholar] [CrossRef] [PubMed]
- Gopalakrishnan, S.; Karmani, S.; Pandey, A.; Singh, N.; Ratheesh Kumar, J.; Praveen, R.; Sodhi, K. Pulse Oximetry Screening to Detect Critical Congenital Heart Diseases in Asymptomatic Neonates. Medical Journal Armed Forces India 2021, 77, 214–219. [Google Scholar] [CrossRef] [PubMed]
- Chamsi-Pasha, M.A.; Chamsi-Pasha, H. Critical Congenital Heart Disease Screening. Avicenna J Med 2016, 06, 65–68. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Xu, W.; Yu, J.; Shu, Q. Screening for Congenital Heart Defects: Diversified Strategies in Current China. World Journal of Pediatric Surgery 2019, 2, e000051. [Google Scholar] [CrossRef]
- Frank, L.H.; Bradshaw, E.; Beekman, R.; Mahle, W.T.; Martin, G.R. Critical Congenital Heart Disease Screening Using Pulse Oximetry. The Journal of Pediatrics 2013, 162, 445–453. [Google Scholar] [CrossRef] [PubMed]
- Singh, Y.; Chen, S.E. Impact of Pulse Oximetry Screening to Detect Congenital Heart Defects: 5 Years’ Experience in a UK Regional Neonatal Unit. Eur J Pediatr 2022, 181, 813–821. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Zhao, Q.; Ma, X.; Yan, W.; Ge, X.; Jia, B.; Liu, F.; Wu, L.; Ye, M.; Huang, G. Pulse Oximetry Could Significantly Enhance the Early Detection of Critical Congenital Heart Disease in Neonatal Intensive Care Units. Acta Paediatrica 2016, 105, e499–e505. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Tian, Y.; Ma, F.; Ge, X.; Gu, Q.; Huang, M.; Zhang, Y.; Sun, K.; Hu, X.; Yang, M.; et al. Impact of Newborn Screening Programme for Congenital Heart Disease in Shanghai: A Five-Year Observational Study in 801,831 Newborns. Lancet Reg Health West Pac 2023, 33, 100688. [Google Scholar] [CrossRef] [PubMed]
- Priyadarshi, A.; Klimek, J. Neonatal Cardiac Ultrasound: How Accurate Are We? Australas J Ultrasound Med 2017, 20, 66–71. [Google Scholar] [CrossRef] [PubMed]
- Aranguren Bello, H.C.; Londoño Trujillo, D.; Troncoso Moreno, G.A.; Dominguez Torres, M.T.; Taborda Restrepo, A.; Fonseca, A.; Sandoval Reyes, N.; Chamorro, C.L.; Dennis Verano, R.J. Oximetry and Neonatal Examination for the Detection of Critical Congenital Heart Disease: A Systematic Review and Meta-Analysis. F1000Res 2019, 8, 242. [Google Scholar] [CrossRef] [PubMed]
- Bakker, M.K.; Bergman, J.E.H.; Krikov, S.; Amar, E.; Cocchi, G.; Cragan, J.; de Walle, H.E.K.; Gatt, M.; Groisman, B.; Liu, S.; et al. Prenatal Diagnosis and Prevalence of Critical Congenital Heart Defects: An International Retrospective Cohort Study. BMJ Open 2019, 9, e028139. [Google Scholar] [CrossRef] [PubMed]
- Tobe, R.G.; Martin, G.R.; Li, F.; Moriichi, A.; Wu, B.; Mori, R. Cost-Effectiveness Analysis of Neonatal Screening of Critical Congenital Heart Defects in China. Medicine (Baltimore) 2017, 96, e8683. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).