Submitted:
05 April 2024
Posted:
07 April 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Sampling and Single Species Pot Cultures
2.2. Morphological Analysis
2.3. Molecular Analysis
2.4. Phylogenetic Analyses
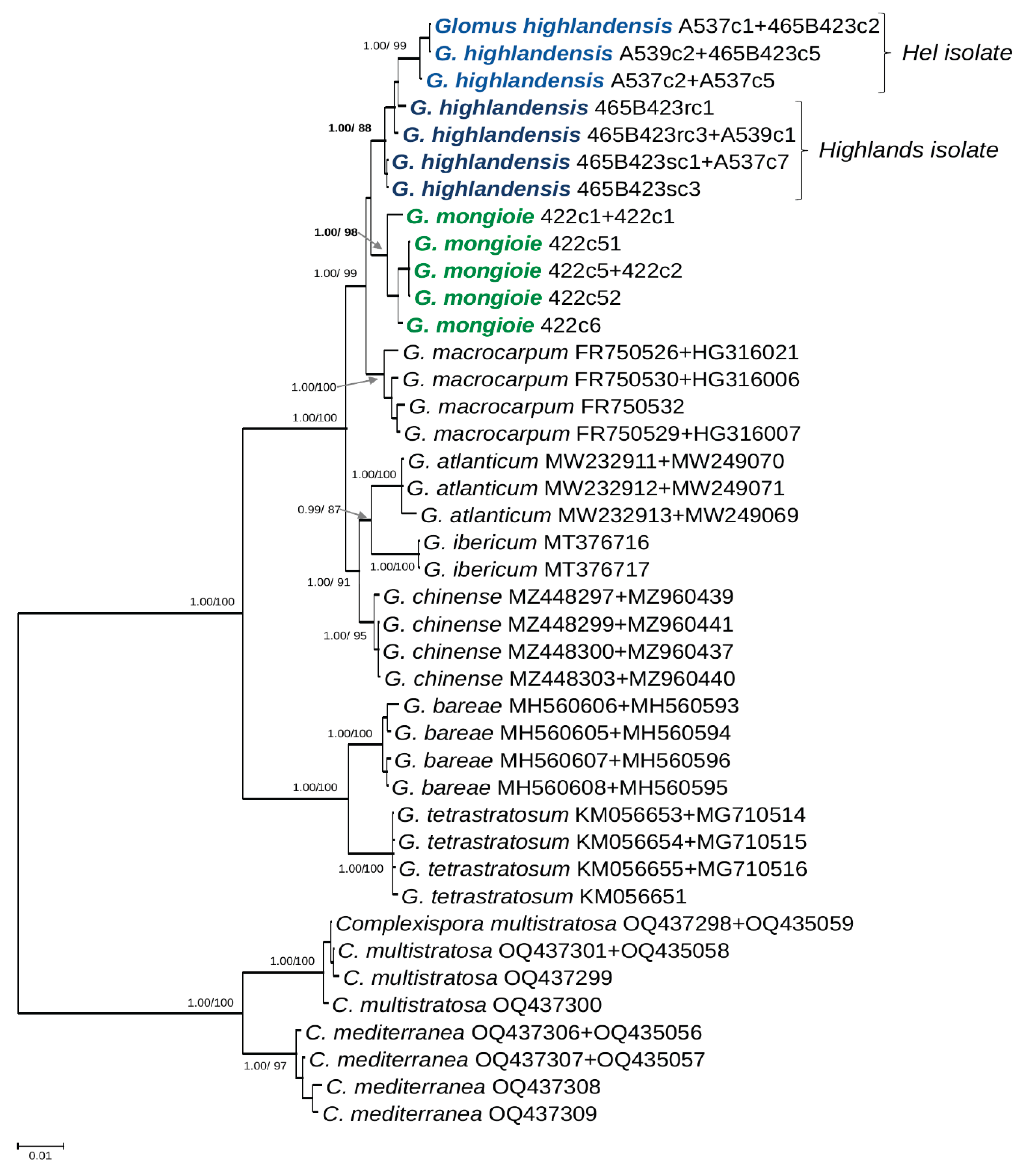
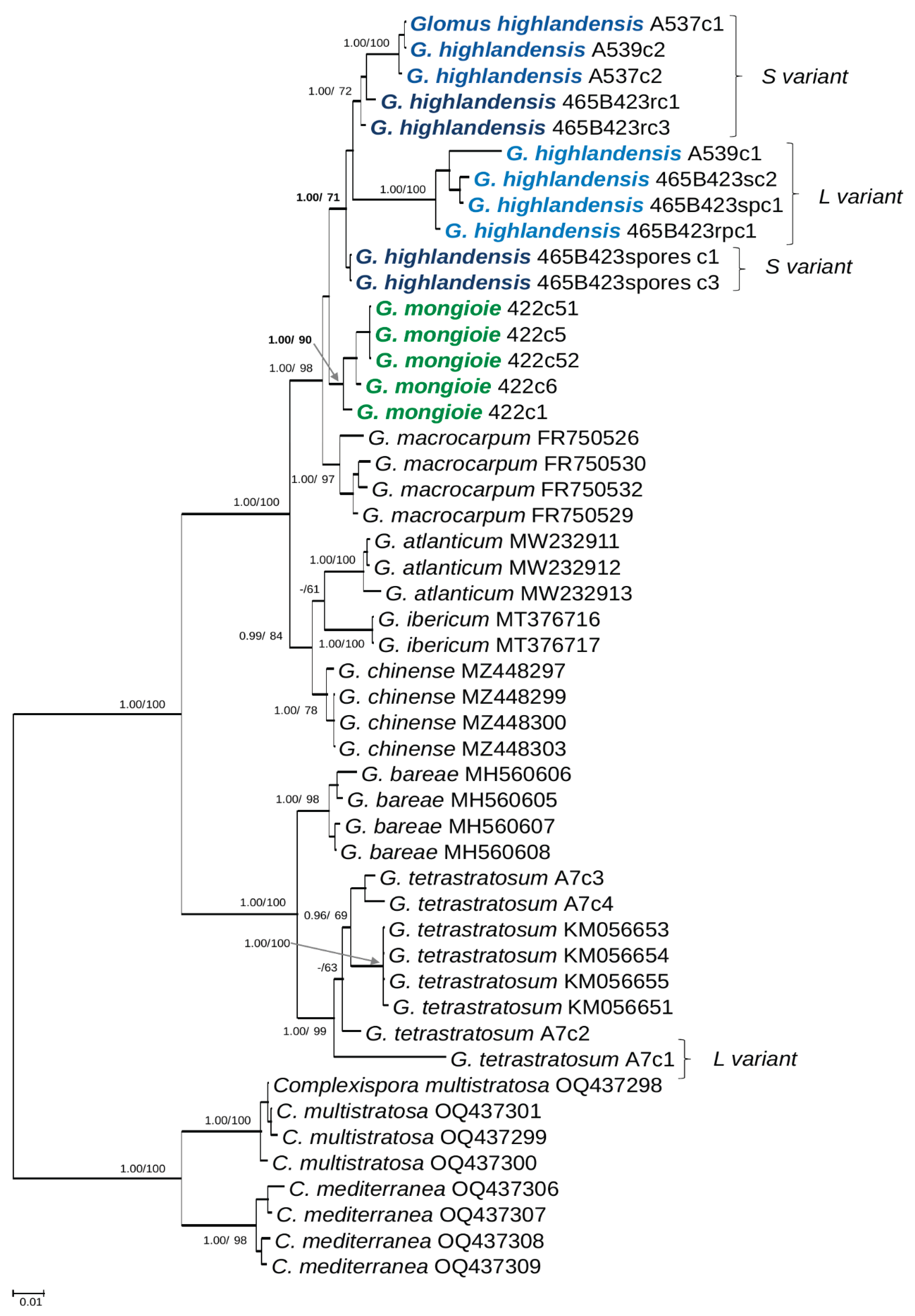
3. Results
3.1. Phylogenetic Analyses
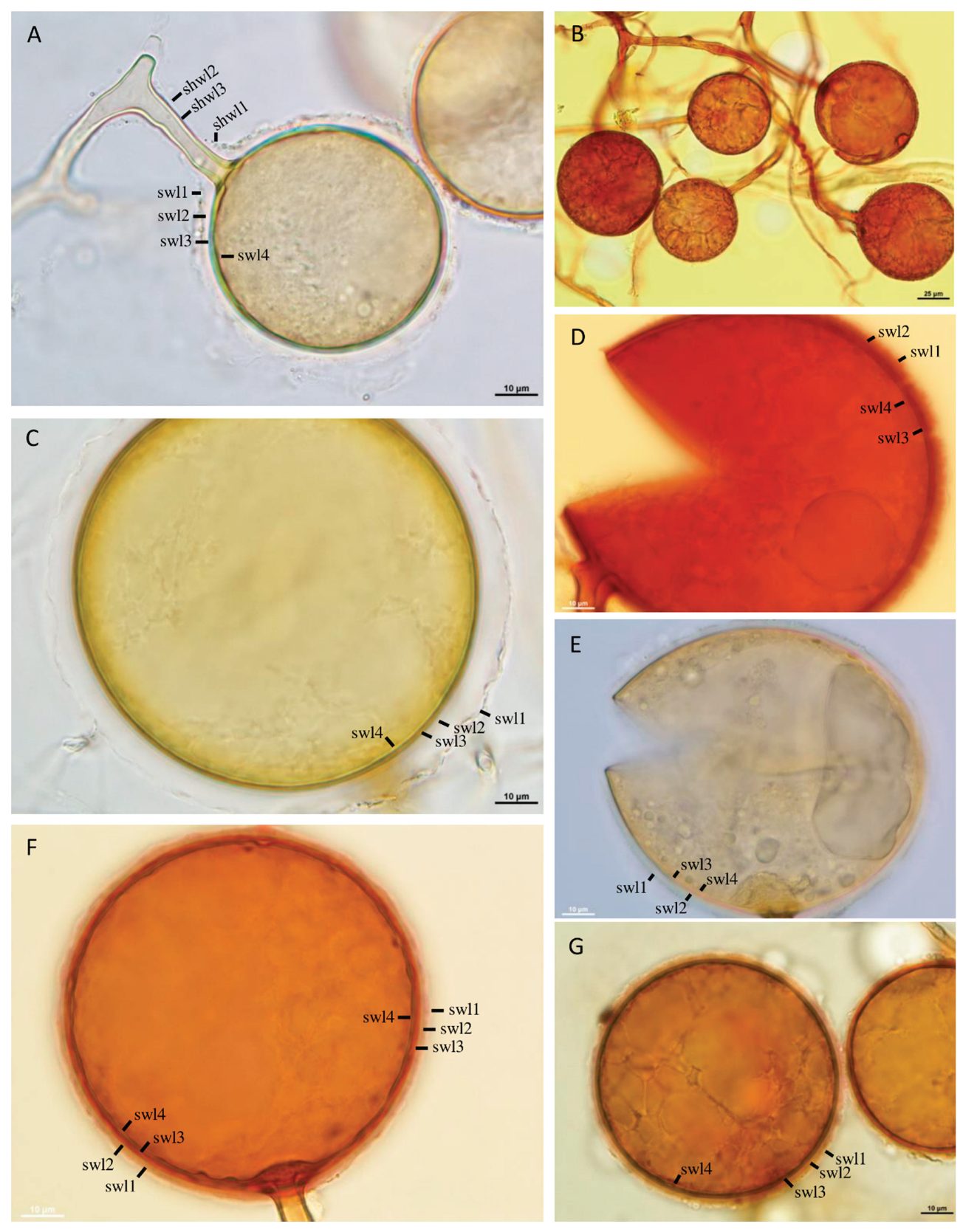
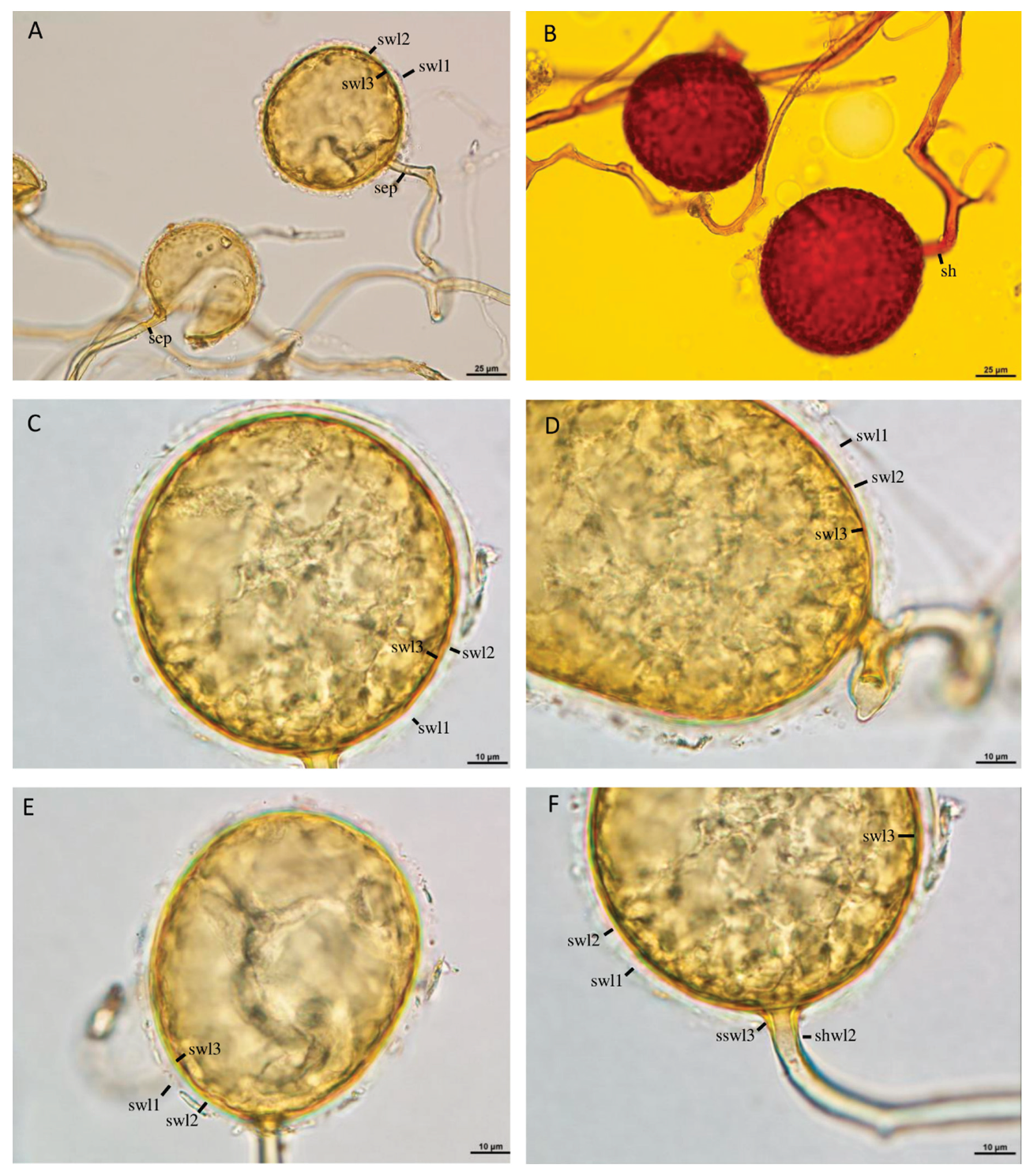
3.2. Taxonomy
4. Discussion
4.1. Comparison with Glomus Species
4.2. Ribosomal Variants: A “Forgotten” Issue
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wilkes, T.I.; Warner, D.J.; Edmonds-Brown, V.; Davies, K.G.; Denholm, I. Zero Tillage Systems Conserve Arbuscular Mycorrhizal Fungi, Enhancing Soil Glomalin and Water Stable Aggregates with Implications for Soil Stability. Soil Syst 2021, 5, 4. [Google Scholar] [CrossRef]
- Fall, A.F.; Nakabonge, G.; Ssekandi, J.; Founoune-Mboup, H.; Apori, S.O.; Ndiaye, A.; Badji, A.; Ngom, K. Roles of Arbuscular Mycorrhizal Fungi on Soil Fertility: Contribution in the Improvement of Physical, Chemical, and Biological Properties of the Soil. Front Fungal Biol 2022, 3, 723892. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Xiao, Q.; Geng, X.; Lin, K.; Li, Z.; Li, Y.; Chen, J.; Li, X. Arbuscular mycorrhizal fungi alter rhizosphere bacterial diversity, network stability and function of lettuce in barren soil. Sci Hortic 2024, 323, 112533. [Google Scholar] [CrossRef]
- Pandit, A.; Kochar, M.; Srivastava, S.; Johny, L.; Adholeya, A. Diversity and Functionalities of Unknown Mycorrhizal Fungal Microbiota. Microbiol Res 2022, 256, 126940. [Google Scholar] [CrossRef] [PubMed]
- Salomon, M.J.; Demarmels, R.; Watts-Williams, S.J.; McLaughlin, M.J.; Kafle, A.; Ketelsen, C.; Soupir, A.; Bücking, H.; Cavagnaro, T.R.; van der Heijden, M.G.A. Global evaluation of commercial arbuscular mycorrhizal inoculants under greenhouse and field conditions. Appl Soil Ecol 2022, 169, 104225. [Google Scholar] [CrossRef]
- Wijayawardene, N.N.; Hyde, K.; Dai, D.; Sánchez-García, M.; et al. Outline of Fungi and fungus-like taxa. Mycosphere 2022, 13, 53–453. [Google Scholar] [CrossRef]
- Błaszkowski, J.; Sánchez-García, M.; Niezgoda, P.; Zubek, S.; Fernández, F.; Vila, A.; Al-Yahya’ei, M.N.; Symanczik, S.; Milczarski, P.; Malinowski, R.; Cabello, M.; Goto, B.T.; Casieri, L.; Malicka, M.; Bierza, W.; Magurno, F. A new order, Entrophosporales, and three new Entrophospora species in Glomeromycota. Front Microbiol 2022, 13, 962856. [Google Scholar] [CrossRef] [PubMed]
- Silva, G.A.; Corazon-Guivin, M.A.; de Assis, D.M.A.; Oehl, F. Blaszkowskia, a new genus in Glomeraceae. Mycol Prog 2023, 22, 74. [Google Scholar] [CrossRef]
- Tulasne, L.R.; Tulasne, C. Fungi nonnulli hipogaei, novi v. minus cognito act. Giornale Botanico Italiano 1845, 2, 55–63. [Google Scholar]
- Tulasne, L.R.; Tulasne, C. Fungi hypogaei. Histoire et monographie des champignons hypogds. Paris, 1851.
- Gerdemann, J.W.; Trappe, J.M. The Endogonaceae of the Pacific Northwest. Micologie Memoir 1974, 5, 1–76. [Google Scholar]
- Schüßler, A.; Walker, C. The Glomeromycota. A species list with new families and new genera. Gloucester, England, 2010.
- Oehl, F.; Silva, G.A.; Goto, B.T.; Maia, L.C.; Sieverding, E. Glomeromycota: two new classes and a new order. Mycotaxon 2011, 116, 365–379. [Google Scholar] [CrossRef]
- Oehl, F.; Silva, G.A.; Goto, B.T.; Sieverding, E. Glomeromycota: three new genera and glomoid species reorganized. Mycotaxon 2011, 116, 75–120. [Google Scholar] [CrossRef]
- Błaszkowski, J.; Chwat, G.; Góralska, A.; Ryszka, P.; Kovács, G.M. Two new genera, Dominikia and Kamienskia, and D. disticha sp. nov. in Glomeromycota. Nova Hedwigia 2015, 1-2, 225–238. [Google Scholar]
- Sieverding, E.; Silva, G.A.; Reinhard, B.; Oehl, F. Rhizoglomus, a new genus of the Glomeraceae. Mycotaxon 2015, 129, 373–386. [Google Scholar] [CrossRef]
- Berch, S.M.; Fortin, J.A. Lectotypification of Glomus macrocarpum and proposal of new combinations: Glomus australe, Glomus versiforme, and Glomus tenebrosum (Endogonaceae). Canad J Bot 1983, 61, 2608–2617. [Google Scholar] [CrossRef]
- Błaszkowski, J.; Niezgoda, P.; Goto, B.T.; Kozłowska, A. Halonatospora gen. nov. with H. pansihalos comb. nov. and Glomus bareae sp. nov. (Glomeromycota; Glomeraceae). Botany 2018, 96, 737–748. [Google Scholar] [CrossRef]
- Guillén, A.; Serrano-Tamay, F.J.; Peris, J.B.; Arrillaga, I. Glomus ibericum, Septoglomus mediterraneum, and Funneliformis pilosus, three new species of arbuscular mycorrhizal fungi. Mycologia 2020, 1–10. [Google Scholar] [CrossRef]
- Błaszkowski, J.; Niezgoda, P.; Zubek, S.; Meller, E.; Milczarski, P.; Malicka, M.; Goto, B.T.; Woźniak, G.; Moreira, H.; Magurno, F. Dominikia bonfanteae and Glomus atlanticum, two new species in the Glomeraceae (phylum Glomeromycota) with molecular phylogenies reconstructed from two unlinked loci. Mycol Prog 2021, 20, 131–148. [Google Scholar] [CrossRef]
- Yu, F.; Goto, B.T.; Magurno, F.; Błaszkowski, J.; Wang, J.; Ma, W.; Feng, H.; Liu, Y. Glomus chinense and Dominikia gansuensis, two new Glomeraceae species of arbuscular mycorrhizal fungi from high altitude in the Tibetan Plateau. Mycol Prog 2022, 21, 32. [Google Scholar] [CrossRef]
- Firth, C.; Fleet, L. Information Sheet on Ramsar Wetlands (RIS), 2006.
- Gunn, G.F. Measuring nocturnal near-surface urban heat island intensity in the small, mid-latitude city of Inverness, Scotland. Scott Geogr J 2023, 1–18. [Google Scholar] [CrossRef]
- Polderman, P.J.; Polderman-Hall, R.A. Algal communities in Scottish saltmarshes. Brit Phycol J 1980, 15, 59–71. [Google Scholar] [CrossRef]
- Woch, M.W.; Kapusta, P.; Stanek, M.; Możdżeń, K.; Grześ, I.M.; Rożej-Pabijan, E.; Stefanowicz, A.M. Effects of invasive Rosa rugosa on Baltic coastal dune communities depend on dune age. NeoBiota 2023, 82, 163–187. [Google Scholar] [CrossRef]
- Fratianni, S.; Acquaotta, F. The climate of Italy. In: Landscapes and landforms of Italy, Eds. Soldati M., Marchetti M., Springer, 2017, pp 29-38.
- Kornerup, A.; Wanscher, J. H. Methuen handbook of colour, 3rd ed.; E. Methuen: London, UK, 1983. [Google Scholar]
- Furrazola, E.; Torres-Arias, Y.; Ferrer, R.L.; Herrera, R.A.; Berbara, R.; Goto, B.T. Glomus crenatum (Glomeromycetes), a new ornamented species from Cuba. Mycotaxon 2011, 116, 143–132. [Google Scholar] [CrossRef]
- Goto, B.T.; Jardim, J.G.; da Silva, G.A.; Furrazola, E.; Torres-Arias, Y.; Oehl, F. Glomus trufemii (Glomeromycetes), a new sporocarpic species from Brazilian sand dunes. Mycotaxon 2012, 120, 1–9. [Google Scholar] [CrossRef]
- Błaszkowski, J.; Jobim, K.; Niezgoda, P.; Meller, E.; Malinowski, R.; Milczarski, P.; Zubek, S.; Magurno, F.; Casieri, L.; Bierza, W.; Błaszkowski, J.; Crossay, T.; Goto, B.T. New Glomeromycotan Taxa, Dominikia glomerocarpica sp. nov. and Epigeocarpum crypticum gen. nov. et sp. nov. From Brazil, and Silvaspora gen. nov. From New Caledonia. Front Microbiol 2021, 12, 655910. [Google Scholar] [CrossRef] [PubMed]
- Walker, C. Taxonomic concepts in the Endogonaceae: spore wall characteristics in species descriptions. Mycotaxon 1983, 18, 443–455. [Google Scholar]
- Błaszkowski, J. Glomeromycota; W. Szafer Institute of Botany, Polish Academy of Sciences: Kraków, Poland, 2012. [Google Scholar]
- Goto, B.T.; Maia, L.C. Glomerospores: a new denomination for the spore of Glomeromycota, a group molecularly distinct from the zygomycota. Mycotaxon 2006, 96, 129–132. [Google Scholar]
- Jobim, K.; Błaszkowski, J.; Niezgoda, P.; Kozłowska, A.; Zubek, S.; Mleczko, P.; Chachuła, P.; Ishikawa, N.K.; Goto, B.T. New sporocarpic taxa in the phylum Glomeromycota: Sclerocarpum amazonicum gen. et sp. nov. in the family Glomeraceae (glomerales) and Diversispora sporocarpia sp. nov. in the Diversisporaceae (Diversisporales). Mycol Prog 2019, 18, 369–384. [Google Scholar] [CrossRef]
- Krüger, M.; Stockinger, H.; Krüger, C.; Schüßler, A. DNA-based species level detection of Glomeromycota: one PCR primer set for all arbuscular mycorrhizal fungi. New Phytol 2009, 183, 212–223. [Google Scholar] [CrossRef] [PubMed]
- Błaszkowski, J.; Yamato, M.; Niezgoda, P.; Zubek, S.; Milczarski, P.; Malinowski, R.; Meller, E.; Malicka, M.; Goto, B.T.; Uszok, S.; Casieri, L.; Magurno, F. A new genus, Complexispora, with two new species, C. multistratosa and C. mediterranea, and Epigeocarpum japonicum sp. nov. Mycol Prog 2023, 22, 34. [Google Scholar] [CrossRef]
- Miller, M.A.; Pfeiffer, W.; Schwartz, T. Creating the CIPRES science gateway for inference of large phylogenetic trees. In: Proceedings of the Gateway Computing Environments Workshop, New Orleans, LA: IEEE 2010, 1-8.
- Abadi, S.; Azouri, D.; Pupko, T.; Mayrose, I. Model selection may not be a mandatory step for phylogeny reconstruction. Nat Commun 2019, 10, 934. [Google Scholar] [CrossRef]
- Ronquist, F.; Teslenko, M.; Mark, P.V.D.; Ayres, D.L.; Darling, A.; Hohna, S.; Larget, B.; Liu, L.; Suchard, M.A.; Huelsenbeck, J.P. MrBayes 3.2: efficient Bayesian phylogenetic inference and model choice across a large model space. Syst Biol 2012, 61, 539–542. [Google Scholar] [CrossRef] [PubMed]
- Kozlov, A.M.; Darriba, D.; Flouri, T.; Morel, B.; Stamatakis, A. RAxML-NG: A fast, scalable, and user-friendly tool for maximum likelihood phylogenetic inference. Bioinformatics 2019, 35, 4453–4455. [Google Scholar] [CrossRef] [PubMed]
- Stöver, B.C.; Müller, K.F. TreeGraph 2: Combining and visualizing evidence from different phylogenetic analyses. BMC Bioinformatics 2010, 11, 7. [Google Scholar] [CrossRef] [PubMed]
- Berger, S.A.; Krompass, D.; Stamatakis, A. Performance, accuracy, and web server for evolutionary placement of short sequence reads under maximum likelihood. Syst Biol 2011, 60, 291–302. [Google Scholar] [CrossRef]
- Czech, L.; Barbera, P.; Stamatakis, A. Genesis and Gappa: processing, analyzing and visualizing phylogenetic (placement) data. Bioinformatics 2020, 36, 3263–3265. [Google Scholar] [CrossRef] [PubMed]
- Malicka, M.; Magurno, F.; Piotrowska-Seget, Z. Phenol and Polyaromatic Hydrocarbons are Stronger Drivers Than Host Plant Species in Shaping the Arbuscular Mycorrhizal Fungal Component of the Mycorrhizosphere. Int J Mol Sci 2022, 23, 12585. [Google Scholar] [CrossRef] [PubMed]
- Błaszkowski, J.; Chwat, G.; Góralska, A.; Bobrowska-Chwat, A. Glomus tetrastratosum, a new species of arbuscular mycorrhizal fungi (Glomeromycota). MycoScience 2015, 56, 280–286. [Google Scholar] [CrossRef]
- Dalpé, Y.; Plenchette, C.; Frenot, Y.; Gloaguen, J.C.; Strullu, D.G. Glomus kerguelense, a new Glomales species from sub-Antarctic. Mycotaxon 2002, 84, 51–60. [Google Scholar]
- Rodriguez, A.; Clapp, J.P.; Robinson, L.; Dodd, J.C. Studies on the diversity of the distinct phylogenetic lineage encompassing Glomus claroideum and Glomus etunicatum. Mycorrhiza 2005, 15, 33–46. [Google Scholar] [CrossRef]
- VanKuren, N.W.; den Bakker, H.C.; Morton, J.B.; Pawlowska, T.E. Ribosomal RNA gene diversity, effective population size, and evolutionary longevity in asexual Glomeromycota. Evolution 2013, 67, 207–224. [Google Scholar] [CrossRef]
- Sperschneider, J.; Yildirir, G.; Rizzi, Y.S.; Malar, M.C.; Nicol, AM.; Sorwar, E.; Villeneuve-Laroche, M.; Chen, E.C.H.; Iwasaki, W.; Brauer, E.K.; Bosnich, W.; Gutjahr, C.; Corradi, N. Arbuscular mycorrhizal fungi heterokaryons have two nuclear populations with distinct roles in host–plant interactions. Nat Microbiol 2023, 8, 2142–2153. [Google Scholar] [CrossRef] [PubMed]
- Yildirir, G.; Sperschneider, J.; Malar, M.C.; Chen, E.C.H.; Iwasaki, W.; Cornell, C.; Corradi, N. Long reads and Hi-C sequencing illuminate the two-compartment genome of the model arbuscular mycorrhizal symbiont Rhizophagus irregularis. New Phytol 2022, 233, 1097–1107. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




