1. Introduction
The obesity is a heterogeneous condition characterized by excessive accumulation of fat in various fat depots. Under conditions of excess energy (nutrients), its excess accumulates in white adipocytes of subcutaneous adipose tissue (SAT), becoming both a reserve that can be used up in conditions of energy deficiency and protection against cooling. Meanwhile, in the conditions of modern life, these functions of SAT have ceased to be relevant for most people, and since the consumption of fat reserves from the depot practically does not occur due to the absence of energy deficiency, its reserves become excessive, exceeding the depositing capacity of cells [
1,
2]. According to the World Health Organization, overweight and obesity are the fifth leading cause of death in the world. The leading cause of death in humans from obesity is associated with an increased risk of a number of concominant chronic diseases, such as type 2 diabetes, insulin resistance, cardiovascular disease, non-alcoholic fatty liver disease, and especially hyperlipidemia [
3,
4].
The people with hyperlipidemia have more than twice increased risk of developing cardiovascular disease (including myocardial infarction and stroke) than the people with normal cholesterol levels [
5]. Hyperlipidemia is an imbalance in the blood levels of cholesterol, including low-density lipoprotein cholesterol (LDL-C) and high-density lipoprotein cholesterol (HDL-C). Other forms of hyperlipidemia include hypertriglyceridemia, as well as mixed hyperlipidemia, in which both cholesterol and triglyceride levels are elevated [
5,
6]. Under the excess intake of cholesterol with food, its production by the liver is reduced, and the excess is excreted by the intestines. If this process is disrupted, the level of cholesterol in the blood increases pathologically, what leads to hypercholesterolemia development. The implementation of cholesterol homeostasis largely depends on the amount and spectrum of steroids, as well as other lipids included in food, the intensity of endogenous cholesterol synthesis, its absorption from the digestive tract, destruction and transformation into other compounds by tissue and microbial enzymes, relationships with bile acids, the intensity of their enterohepatic circulation, the amount of excretion in feces, hormonal status and other factors. High fat diets, especially rich in saturated fatty acids and refined carbohydrates, fried meats, high-cholesterol foods, and sugary drinks significantly increase cholesterol levels [
7]. Clinical and experimental studies showed that high-fat (HFD) and/or high cholesterol diet can cause non-alcoholic fatty liver disease in both obese and non-obese people [
8,
9].
Some of the ways to prevent obesity and its complications are changing eating habits, adequate nutrition, using functional foods enriched with biologically active substances, and increasing physical activity. The results of preclinical in vivo studies on experimental animal models play an important role for an objective assessment of the effectiveness of functional products enriched with biologically active food substances from the standpoint of evidence-based medicine. The quality of scientific research is largely determined by the methodology that the researcher uses. The more difficult the studied problem is, the higher the importance of the methodological correctness of the work performed.
The following protocols are used to model in vivo hyperlipidemia: the addition of 1 or 2% cholesterol, as well as 1-2% cholesterol + 0.5% cholic acid to the animal diet without or with a change in the fat component. However, the main limitation of these studies is the fact that a hypercholesterolemic diet is usually used as a positive control [
10]. However, there is not much information about the effect of the hypercholesterolemic diet itself on the animals. The aim of our study was a comprehensive comparative assessment of the effect of consumption of 1% and 2% cholesterol with and without regular physical activity on mature male Wistar rats, and the search for valid biomarkers characterizing disturbances in the functional state of the animal’s body, lipid and cholesterol metabolism, liver condition, as well as the intestinal microbiota of the animals.
2. Results
We conducted preliminary testing of animals in an Open Field to divide them according to individual behavioral differences. This approach can increase the degree of verifiability and reliability of the results. The general condition of all animals in appearance, fur quality, food and water consumption and behavior during daily inspection was satisfactory.
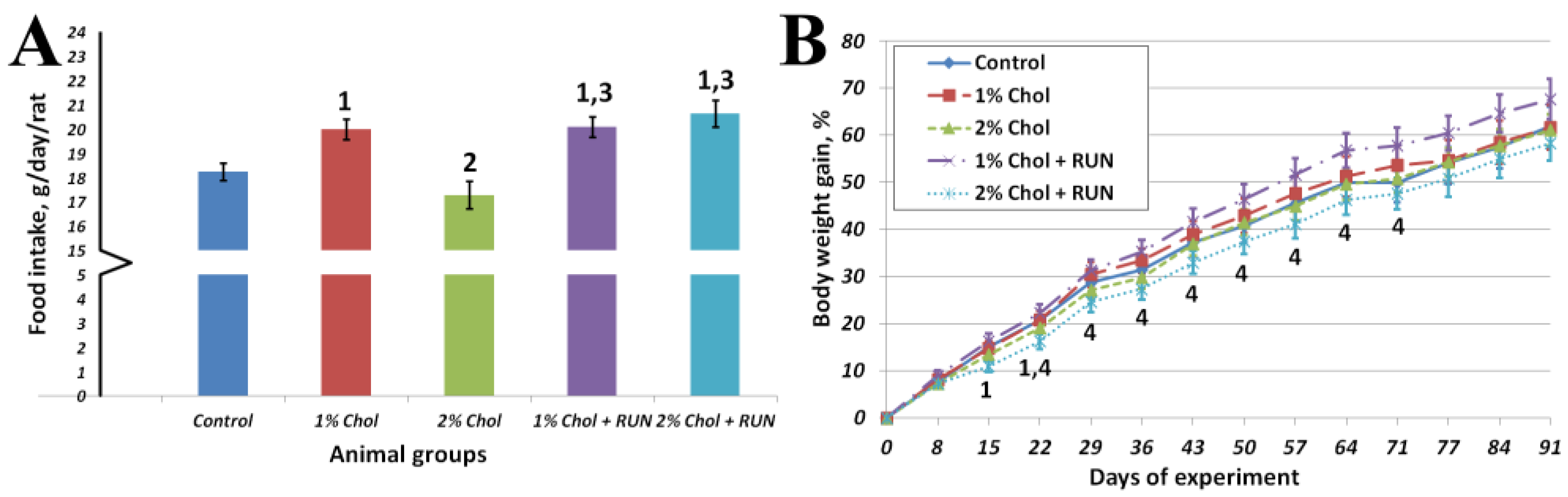
Figure 1 shows the average cumulative feed consumption of animals for the entire experiment and the dynamics of body weight gain of animals throughout the experiment.
Animals of group 1% Chol consumed significantly more food compared to animals of the Control group and animals of group 2% Chol. Animals of groups 1% Chol + RUN and 2% Chol + RUN, exposed to running exercise, consumed significantly more food compared to groups Control and 2% Chol.
From the 14th day of the experiment the body weight gain of 2% Chol + RUN animals was significantly lower compared to group 1% Chol + RUN. The absolute body weight of all animals did not differ significantly throughout the experiment and was 465 ± 10; 470±19; 464±18; 484±13 and 458±13 grams on day 94, respectively. The increase in body weight at the end of the experiment also did not differ significantly between the groups.
We detected no significant differences in the glucose level of all animals (5.5±0.2; 5.1±0.3; 5.4±0.2; 5.2±0.2 and 5. 3±0.2 mmol/l, respectively) on the 75th day of the experiment.
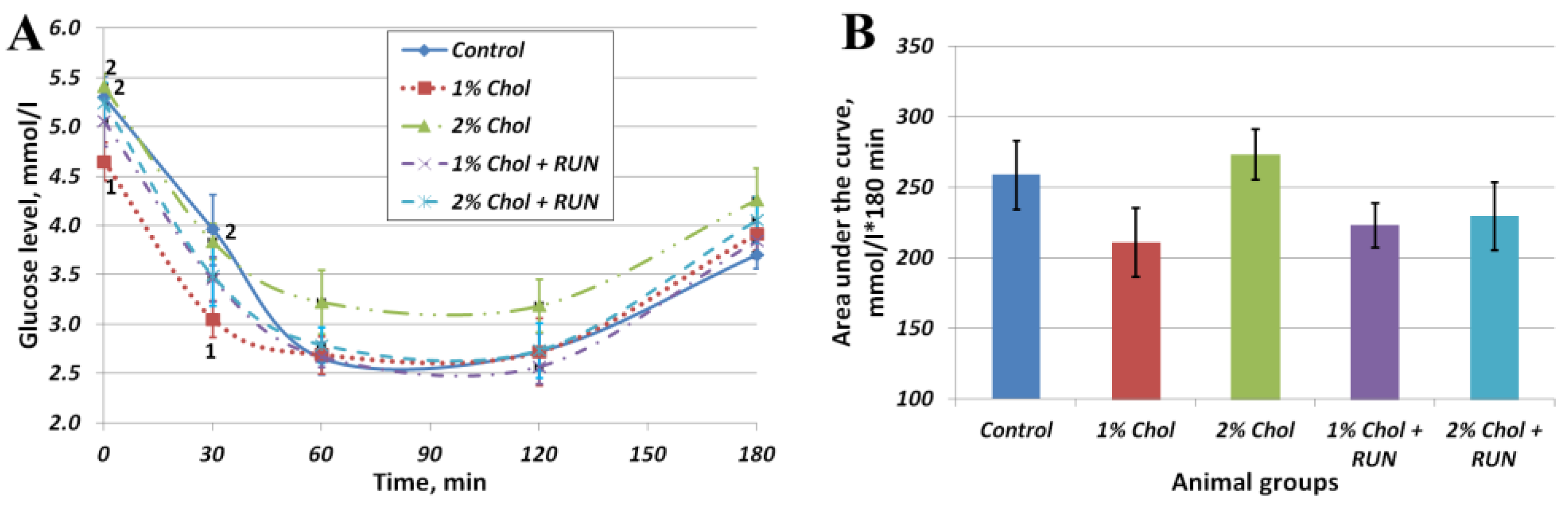
Figure 2 shows the results of the insulin resistance test.
The obtained values of area under the curve (AUC) indicators (Fig. 2 B) indicate the absence of the development of insulin resistance in rats of all experimental groups on the background of consumption of exogenous insulin compared with rats of the Control group. At the same time, in animals of groups 1% Chol, 1% Chol + RUN and 2% Chol + RUN, the AUC indicator was slightly lower (at the level of a trend) than in animals of groups Control and 2% Chol.
Figure 2A reflects the changes in the blood glucose level of animals after insulin administration over time. The glucose level of 2% Chol animals decreased less than in other animals, which may indicate the beginning of the development of insulin resistance in these animals.
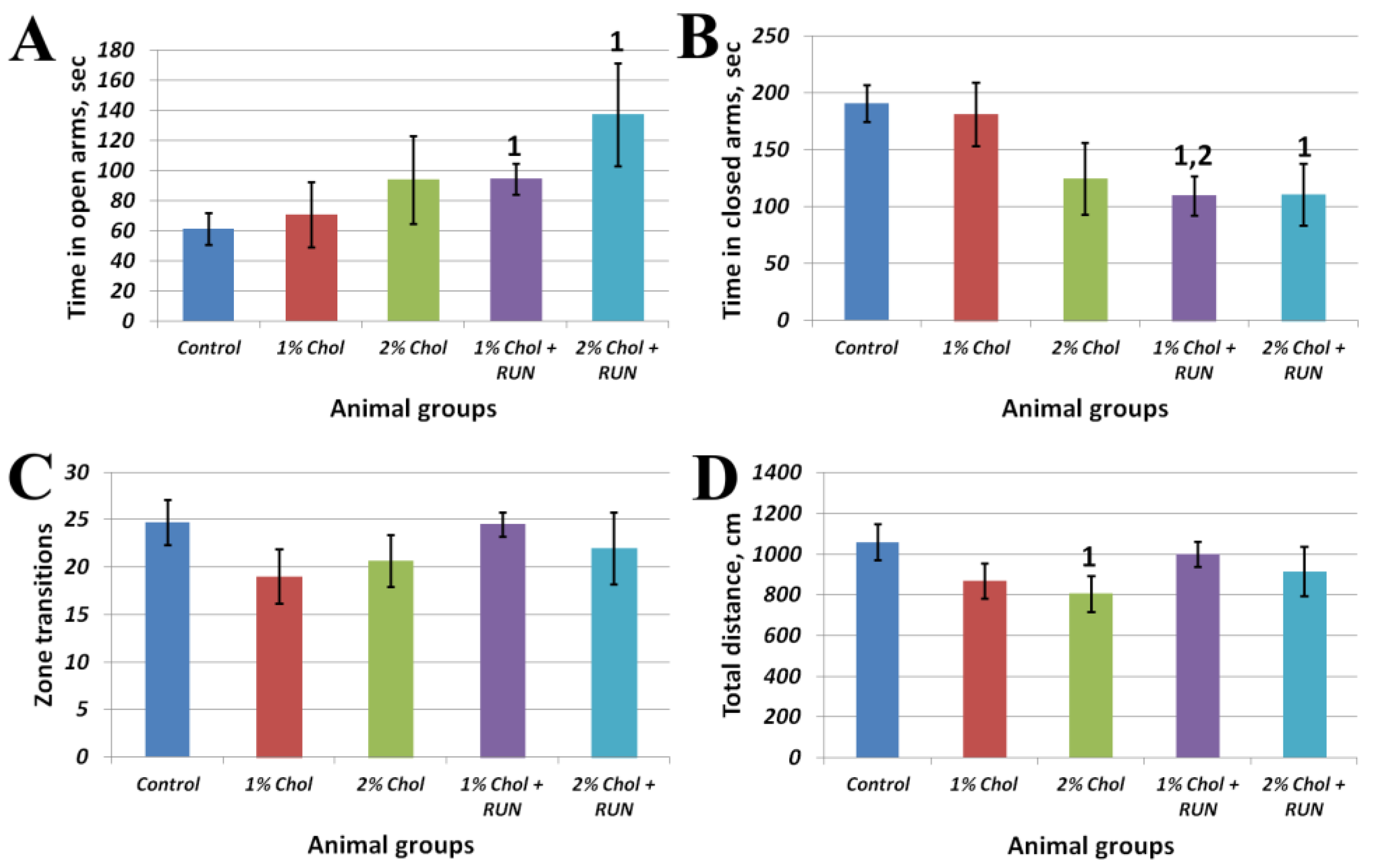
Figure 3 shows the results of the Elevated Plus Maze test.
Animals of groups 1% Chol + RUN and 2% Chol + RUN, exposed to daily physical activity, spent significantly more time in the open arms of the maze and significantly less time in the closed arms of the maze compared to animals in the Control group. The obtained result indicates the influence of running loads against the background of a high-cholesterol diet on the anxiety component, characterized by the time spent in the open and closed arms of the maze, without changing motor activity (Fig. 3 C,D).
Table 1 presents the results of studying the body composition of experimental animals.
The body fat mass of animals in groups 1% Chol, 1% Chol + RUN and 2% Chol + RUN) significantly decreased on the 33rd day of the experiment compared to the beginning of the experiment, remained almost unchanged until the end of the experiment and was significantly lower both in comparison with control animals and in comparison with animals of group 2% Chol.
On the 82nd day of the experiment, the body composition of animals in group 2% Chol, changed significantly compared to intact animals in group Control: the percentage of fat in these animals became significantly higher, while the percentage of lean body mass and free water decreased significantly.
Table 2 presents the results of a general blood analysis of experimental animals.
The significant decrease in the absolute values of leukocytes, lymphocytes, monocytes and granulocytes in the blood, as well as the average volume of erythrocytes, can be noted in animals of the experimental group 2% Chol compared to animals of group 1% Chol. The animals in this group showed a decrease in the average erythrocyte volume, average hemoglobin content in the erythrocyte and hematocrit compared to animals in the Сontrol group.
Only a significant decrease in hematocrit was detected in animals of groups 1% Chol and 1% Chol + RUN, compared to the Control group.
Table 3 and
Table 4 and
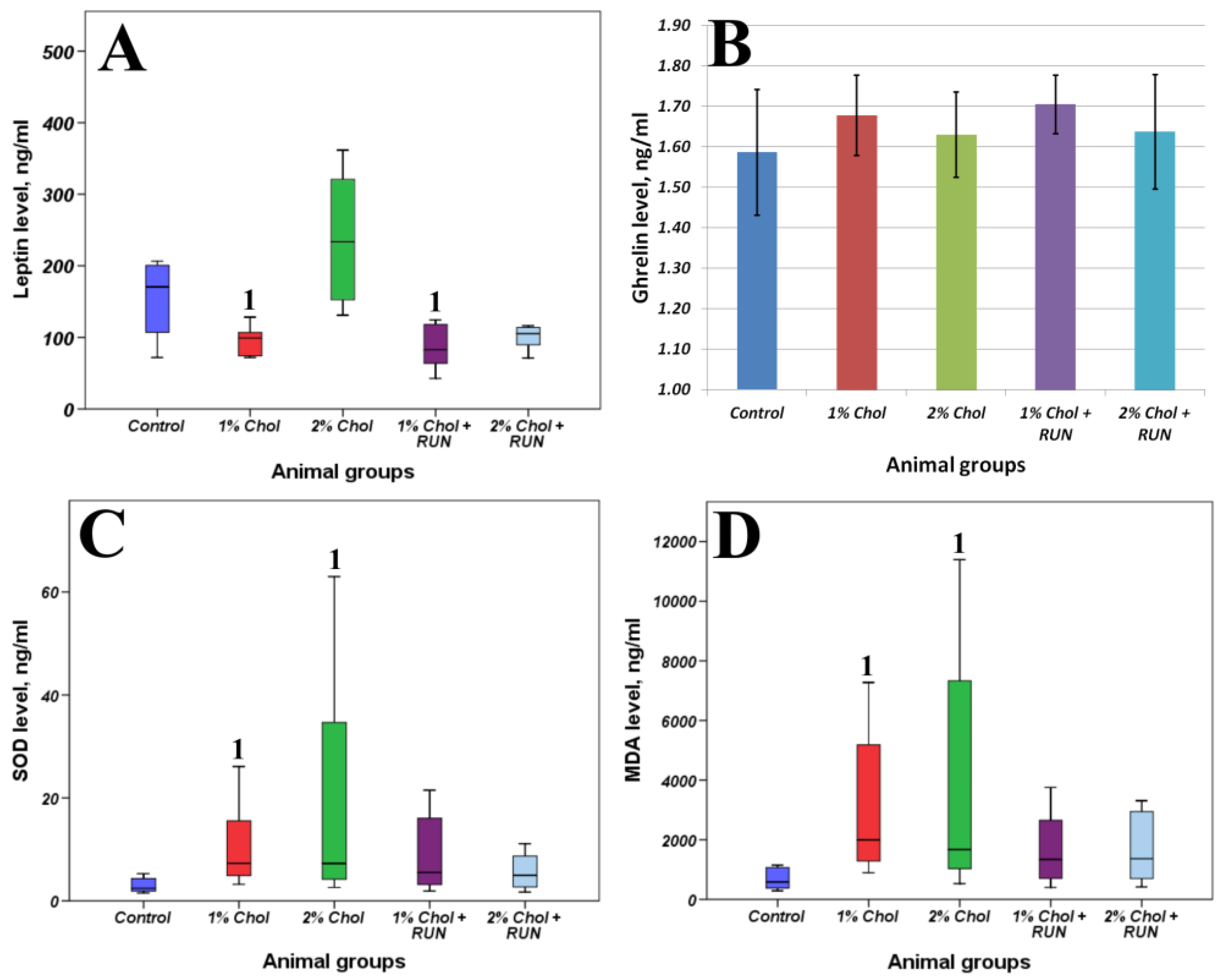
Figure 4 present the results of determining the general biochemical analysis of animal blood serum, the levels of SOD, MDA, leptin and ghrelin in the blood serum of animals, as well as indicators of lipid metabolism in the liver of animals.
A significant increase in the levels of low-density lipoproteins (LDL) was noted in the blood of animals of group 1% Chol along with the decrease in the levels of high-density lipoproteins (HDL) compared to the Control group. Also the level of total bilirubin was significantly reduced in these animals.
Significantly higher levels of globulins and albumin were noted in the blood of animals of group 2% Chol compared to the Control group. Consumption of 2% cholesterol in the diet led to a significant increase in the blood levels of cholesterol and LDL of animals compared to animals of the Control group without changes in HDL levels. Animals consuming 2% cholesterol showed a significant increase in the levels of total bilirubin, AST and ALT compared to the Control group.
The increase in the LDL level against the background of the decrease in HDL level was noted in the blood of animals of group 1% Chol + RUN compared to animals of the Control group. The level of total bilirubin in these animals was significantly reduced, and the level of AST was significantly increased compared to the Control group.
Significantly higher levels of globulins and albumin were noted in the blood of animals of group 2% Chol + RUN, similarly to group 2% Chol. Cholesterol and LDL levels were also increased compared to Control. A significantly lower level of bilirubin was revealed in comparison with animals of the Control group. Additionally, an increase in creatinine and alkaline phosphatase was detected in these animals.
Our study did not reveal a deficiency of detectable minerals in the blood serum of animals. Only a significant increase in the level of calcium in the blood of animals of 2% Chol and 2% Chol + RUN groups compared to animals in the control group was shown.
All animals that received exogenous cholesterol with food showed a significant increase in both absolute and relative liver weight. The significantly higher accumulation of fat (more than 2 times) and cholesterol (more than 5 times) in the liver of these animals was shown compared with animals of Control group. The accumulation of triglycerides was not significant only for group 1% Chol + RUN, which received a low dose of cholesterol against the background of physical exercise. There was a tendency towards a decrease in liver triglycerides level against the background of physical exercise.
The animals of groups 1% Chol and 2% Chol receiving cholesterol had a significant increase in SOD content with a significant increase in the accumulation of MDA in the blood serum compared to animals of the Control group. We observed compensation for these parameters in animals of groups 1% Chol + RUN and 2% Chol + RUN, which received cholesterol against the background of running exercise: neither the SOD level nor the MDA level differed significantly from the control.
We did not detect any significant changes in the serum ghrelin level. At the same time, a significant decrease in leptin levels was shown in animals of 1% Chol and 1%Chol + RUN groups. On the contrary we see an increase in serum leptin in the animals of group 2% Chol, which may indicate the development of resistance to this hormone. The animals of group 2% Chol + RUN showed a trend towards a decrease in blood leptin, which indicates that physical exercise can prevent the development of leptin resistance.
Intestinal microbiota population levels are presented in
Table 5. The results obtained indicate the effect of both doses of cholesterol on the intestinal microbiota.
A significant increase in the content of Escherichia coli was detected in animals of groups 1% Chol, 2% Chol and 1% Chol + RUN compared to Control animals, while the changes for group 2% Chol + RUN were characterized as trend (p < 0.1).
There was a decrease in the levels of Faecalibacterium prausnitzii against the background of cholesterol consumption, and this was statistically significant for animals of groups 1% Chol, 2% Chol and 2% Chol + RUN, however for animals of group 1% Chol + RUN a trend towards similar changes was revealed.
Also, animals of groups 1% Chol, 2% Chol and 2% Chol + RUN had signs of anaerobic imbalance, namely, an increase in the ratio of Bacteroides spp. and F. prausnitzii. At the same time, the indicated ratio in group 1% Chol + RUN was significantly different from the values in group 1% Chol, but did not differ from the values in the Control group.
In addition, cholesterol consumption led to an increase in the content of lactobacilli: in animals of the 1% Chol group there was a tendency to increase (p<0.1), and for the remaining groups of animals this increase was significant.
In animals of group 1% Chol, an increase in the content of Blautia spp., and Ruminococcus spp., belonging to the Clostridia class, was detected and in the others experimental groups there was a tendency (p<0.1) to an increase in the content of Blautia spp. and a slight increase in the frequency of occurrence of Ruminococcus spp.
There was a decrease in the level of bifidobacteria in animals of groups 1% Chol + RUN and 2% Chol + RUN compared to the Control, and animals in group 2% Chol showed a tendency (p<0.01) to a decrease in this protective population.
3. Discussion
As a part of broad efforts to combat non-communicable diseases including nutrition-dependent, the main targets are to identify the principal risk factors, to find ways to prevent, to treat and to manage the disease. The results of in vivo preclinical studies using experimental animal models undoubtedly play an important role. The aim of our study was to find the simplest, valid and, most importantly, reproducible model that can adequately reflect the course of the disease to further ensure its correction.
When assessing the validity and the possibility of further successful use of any model, one should take into account that the extreme effects applied on the animal lead to the development of reversible changes, but do not cause the development of severe pathological disorders. To ensure successful correction of the modeled disease, including nutrition and physical activity factors, which are the most accessible and economically acceptable forms of behavior, we chose a model with unchanged proportions of the main energy sources in the diet. The administration of exogenous cholesterol in two dosages, most often found in the literature (1 and 2%) was considered as the factor of malnutrition. Additionally, we assessed the influence of the moderate physical activity on the formation of changes.
The main limitation of our model is the lack of changes in body weight and body weight gain of animals, what corresponds to literature data [
11]. In [
12], adult Sprague–Dawley rats (n = 8) were fed a normal diet containing 1% cholesterol and 0.5% cholic acid for 4 weeks, and there were no changes in body weight, growth, or food intake. Male Sprague Dawley rats (n = 15) received a standard diet supplemented with 1% cholesterol and 0.3% sodium cholate for 16 weeks; no significant differences in body weight were shown [
13]. The similar study found no weight gain over 16 weeks [
14]. However, in our study, using the impendansometry method, we found that when consuming 2% cholesterol, there was a significant accumulation of fat in the rats` body, which was accompanied by a loss of lean mass. Thus, although we do not see a difference in weight and growth between animals, the process of obesity development can be considered as developing.
There is also a modification of the hypercholesterolemia model where cholesterol is introduced into a high-fat or high-carbohydrate diet. The study [
15] on growing male Wistar rats also showed that consumption of a high-fat diet (13% fat) with the addition of 1% cholesterol led to an increase in body weight in rats (n=5) after 30 days of the experiment. A high-fat diet (60 kcal fat), supplemented with 1.25% cholesterol and 0.5% cholic acid, caused an increase in body weight gain in female SD rats (n=6) only at 8 weeks of feeding [
11].
Our own studies [
16] also showed an increase in body weight in male Wistar rats fed a high-fat high-carbohydrate diet supplemented with 2% cholesterol for 90 days. This modeling method is limited by the severe and possibly irreversible disorders developing in the animal’s body due to the strong exogenous cholesterol load, which are expressed primarily by the extreme accumulation of fat, cholesterol and triglycerides in the liver. There are concerns that further successful correction of these disorders will be impossible, especially using nutritional approaches.
Changing only the fat or carbohydrate component in the animal's diet does not always lead to excessive weight gain, requires a long period of exposure and is difficult to reproduce, which corresponds to the high adaptive ability of animals of this species [
17,
18].
The main biomarkers of hyperlipidemia development in vivo are total cholesterol, LDL and HDL cholesterol, and triglycerides [
19].
In our study, animals consuming a normal diet supplemented with 1% cholesterol had a statistically significant decrease in serum HDL and an increase in serum LDL compared to intact animals. The levels of total cholesterol and triglycerides did not change. Supplementation with 2% of cholesterol led to an increase in total cholesterol along with an increase in LDL. The running exercise did not have any beneficial effect on the lipid profile. The work [
13] also revealed only a significant increase in total cholesterol along with LDL growth, without changes in serum triglyceride levels in animals receiving 1% cholesterol for 16 weeks. In the study [
14], a significant increase in total cholesterol was detected only by the 16th week of feeding. There were no changes in blood triglycerides.
The total blood bilirubin is a promising biomarker, from the point of view of its influence on the processes of inhibition of LDL oxidation [
20]. It is known that oxidative modification of human low-density lipoproteins (LDL) is involved in the formation of plaques in blood vessels, thereby significantly increasing the risks of developing cardiovascular diseases and type 2 diabetes [
21,
22]. The introduction of cholesterol into the diet of animals, regardless of the dose and the presence/absence of training, led to a significant decrease in the level of total bilirubin.
Non-alcoholic fatty liver disease is the most common chronic liver disease and is often caused by excessive consumption of fatty foods and foods containing high concentrations of cholesterol, and is mainly treated through lifestyle changes [
23]. Regular exercise or physical activity may help reduce fat deposition in the liver by increasing energy expenditure, improving skeletal lipid oxidation, and reducing body weight [
24]. When studying the hepatoprotective effects of various drugs, the content of AST and ALT in the blood serum is usually assessed [
25]. In our case, only the introduction of 2% cholesterol led to a significant increase in both indicators. During running training, ALT levels decreased to normal control levels.
We have shown a significant increase in indicators characterizing changes in the liver: an increase in liver weight, extreme accumulation of fat, triglycerides and cholesterol against the background of consumption of both 1 and 2% cholesterol. No dose-dependent effect was detected. Running excercise against the background of a small dose of cholesterol led to a decrease in the liver triglycerides level to the level of the control group; when consuming a high dose of cholesterol, a tendency towards a decrease in this indicator can also be noted. [
26] showed that intense exercise had a greater effect on liver vacuolation density and lipid reduction in male Wistar rats fed a high-fat diet than moderate exercise. Serum and liver triglyceride and total cholesterol levels also demonstrated different sensitivity to exercise intensity.
Oxidative stress and lipid peroxidation are involved in the pathogenesis of many diseases, including atherosclerosis, coronary heart disease, diabetic angiopathy and NAFLD [
27]. An increased content of free fatty acids may cause liver dysfunction, since fatty acids are chemically active and stimulate free radical oxidation of biomolecules. The resulting reactive oxygen species (ROS) can damage the biological membranes of liver cells. Reactive oxygen species attack polyunsaturated fatty acids and initiate lipid peroxidation within the cell, leading to the formation of aldehyde byproducts such as MDA [
28]. Normally, this process is physiologically balanced due to the activity of endogenous antioxidant systems, which are able to increase activity in response to an increase in pro-oxidant effects.
Consumption of 1% and 2% cholesterol led to a statistically significant accumulation of MDA and SOD in the blood serum of animals. The observed increase in SOD concentration may reflect the mobilization of the antioxidant defense component in response to the development of oxidative stress in the body, reflected by the accumulation of MDA. SOD is an inducible enzyme, i.e. its synthesis increases if peroxidation is activated in cells. Accordingly, when exogenous cholesterol is consumed in both doses, there is no depletion of the antioxidant defense system. Running exercise led to a decrease in both indicators to the level of control animals.
In our study, we obtained a significant increase in animals` appetite receiving 1% cholesterol, as well as both doses of cholesterol against the background of running exercise. According to the results of a bioimpedance analysis, a decrease in fat mass in the body composition was registered in animals of these groups. On the contrary, only animals receiving 2% cholesterol had an increase in appetite and, accordingly, an increase in the accumulation of body fat (significant compared to the control by the 82nd day of the experiment). The results obtained are consistent with changes in the serum leptin level: in animals receiving 1% cholesterol without exercise, a significant decrease in leptin was shown; in animals receiving 2% cholesterol against the background of a running exercise, no significant differences were shown with the control, although its concentration was lower at the trend level. The highest leptin levels were recorded in animals receiving 2% cholesterol.
The hormone leptin, produced by adipocytes, controls body weight, food intake and energy expenditure. Its secretion is proportional to the amount of adipose tissue present in the body, which gives an idea of the current level of fat in the body [
29]. Leptin levels increase with increasing fat mass, thereby suppressing food intake, whereas loss of fat mass leads to a decrease in leptin levels and a subsequent increase in food intake. This mechanism maintains homeostatic control of adipose tissue mass within a relatively narrow range, thereby serving an important evolutionary function [
30]. Accordingly, serum leptin, as a marker of the obesity development, increases (at a trend level) only with the introduction of a larger dose of cholesterol into the diet. A similar result was shown when determining insulin resistance: an increase, also at a trend level, was noted only in animals receiving 2% cholesterol. Running exercise levels out both the increase in insulin resistance and the increase in leptin level to the level of control animals.
A picture of the dysbiotic changes against the background of cholesterol consumption was revealed, consisting of a decrease in the levels of
F. prausnitzii and an increase in the ratio of
Bacteroides spp./
F. prausnitzii due to increased levels of
Escherichia coli.
F. prausnitzii is a significant protective population of microbiota that produces short-chain fatty acids, in particular butyrate, and exhibits anti-inflammatory properties, and when their levels decrease, inflammatory processes develop [
31,
32]. At the same time, the predominance of bacteria of the genus
Bacteroides over
F. prausnitzii is considered as a dysbiotic disorder of the intestinal microbiota of a pro-inflammatory nature [
33]. Despite the fact that, in general, the levels of gram-negative
E. coli in the experimental groups did not reach too high values, an increase in the content of this population against the background of a decrease in protective populations (
F. prausnitzii,
Bifidobacterium) compared to the control group is a negative factor. The outer wall of gram-negative microorganisms contains lipopolysaccharides (LPS). When the barrier function of the intestinal epithelium is impaired, an increase in LPS levels in the blood plasma can occur (so-called metabolic endotoxemia), which, in turn, leads to nonspecific systemic inflammation [
34]. Physical activity only against the background of a low dose of cholesterol prevented the development of anaerobic imbalance (the ratio of
Bacteroides spp. and
F. prausnitzii) and prevented a pronounced decrease in
F. prausnitzii. However, the levels of this population still did not reach the values of the control group. The increase in lactobacilli levels compared to controls in cholesterol-fed rats may be due to the fact that many members of the
Lactobacillaceae family participate in the enterohepatic circulation and are characterized by high bile salt hydrolase activity [
35,
36,
37]. It is known that taking probiotic lactobacilli leads to a decrease in serum LDL levels in rats [
15,
38,
39] and in humans [
40,
41,
42,
43,
44]. Consumption of a high dose of cholesterol (2%) along with or without running exercise caused changes in the microbiota similar to the lower dose of cholesterol (1%). The absence of significant differences between these groups probably indicates the absence of a dose-dependent effect. With an excess of cholesterol in the diet, dysbiotic changes develop in the intestinal microbiota of rats, characteristic of the so-called “Western” type of microbiota with a predominance of
Bacteroides, a decrease in
F. prausnitzii and bifidobacteria, as well as an increase in
Escherichia. The additional energy consumption in the form of physical activity does not lead to complete normalization of microbiota.
4. Materials and Methods
4.1. Experimental Animals
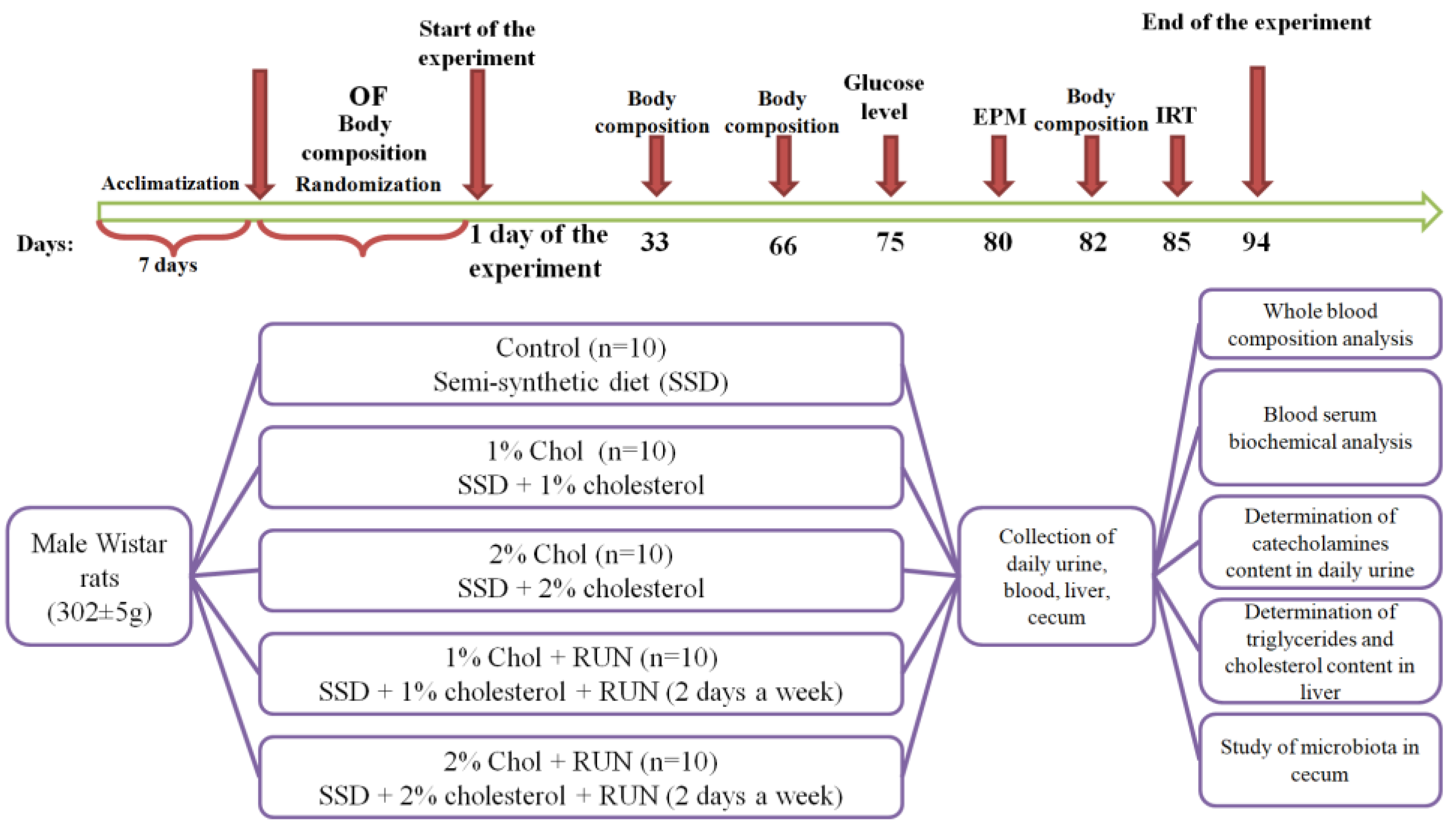
The experiment was carried out for 94 days on 50 growing male Wistar rats with an initial body weight of 250±5g (age 7 weeks). The animals were obtained from the laboratory animal nursery of the Stolbovaya branch of the Federal State Budgetary Institution of Science "Scientific Center for Biomedical Technologies of the Federal Medical and Biological Agency." Animal studies were carried out in accordance with the requirements set out in the National Standards of the Russian Federation GOST 33647-2015 “Principles of Good Laboratory Practice” and GOST 33216-2014 “Guide to the care and maintenance of laboratory animals. Rules for keeping and caring for laboratory rodents and rabbits” and approved by the Local Ethics Committee of the Federal State Budgetary Institution “Federal Research Center for Nutrition and Biotechnology” (Protocol No. 11 of December 15, 2021). Animals were kept under controlled environmental conditions (temperature 20-24°C, relative humidity 30-60%, 12 hour light cycle).
4.2. Experimental Design
To preliminary divide the animals into groups, the Open Field test was used. Testing was carried out before the start of the experiment after the animals were acclimatized for seven days. In the Open Field test, an animal is placed in an unfamiliar open space from which it cannot escape. An unfamiliar environment triggers a complex set of behavioral reactions that reflect anxiety and the desire to explore new territory, i.e. the animal's behavior in the OF is determined by the ratio of defensive and exploratory tendencies. Testing was carried out under standard lighting conditions for 3 minutes (180 sec). During testing, the following behavioral indicators were recorded: the number of zones transitions, time spent in each zone, distance traveled. The movement of animals across the field was recorded using the Smart 3.0.04 software (Panlab, Spain).
The experimental design is presented in
Figure 5.
The animals were randomly divided into 5 groups according to body weight and OF test results: Control (n=10), 1% Chol (n=10), 2% Chol (n=10), 1% Chol + RUN (n=10) and 2% Chol + RUN (n=10) (
Table 6).
Animals of all groups received a standard semi-synthetic diet [
45] and drinking water ad libitum during the whole experiment.
Food consumption was monitored three times a week, and animals were weighed once a week. Cholesterol was additionally added in the amount of 1g/100g to the diet of animals in groups 1% Chol and 1% Chol + RUN. Cholesterol in the amount of 2g/100g was additionally added to the diet of animals in groups 2% Chol and 2% Chol + RUN.
Rats of experimental groups 1% Chol + RUN and 2% Chol + RUN were subjected to regular (2 days a week) physical activity on a Treadmill (Panlab, Spain), equipped with 5 running belts (which allows 5 animals to be tested simultaneously) with an adjustable speed (0-150 cm/s) and slope (from –25° to 25°). The speed of the belt was gradually increased from 20 to 30 cm/s according to the scheme presented in
Table 7, the running time was 10 minutes, the slope of the track was 0 degrees. The shock strength in the shock zone of the track was set to 0.4 mA.
Animals are forced to run by electrical shock using an electrode placed at the lower end of the track (the shock strength can be set from 0 to 2 mA). Recorded parameters are: distance traveled, number of electric shocks received, total time of shocks.
The body composition of rats was studied before the start of the experiment (day 0) and on days 33, 66 and 82 by magnetic resonance relaxometry using an EchoMRI-1100 analyzer (EchoMRI LLC, USA). This device is a quantitative magnetic resonance system that allows you to measure in less than 1 minute the total fat and lean body mass, the amount of free water and the total volume of water in the animal's body without anesthesia or euthanasia.
To assess the degree of anxiety and exploratory activity of the animals, on the 80th day of the experiment, an Elevated Plus Maze test was performed. The movement of rats through the maze was recorded using the Smart 3.0.04 video system (Panlab Harvard Apparatus, Spain). Testing of animals was carried out during periods of their minimum daily activity (from 10.00 to 15.00). The time spent in the open and closed arms of the maze, the number of zone transitions and the distance traveled were recorded.
On the 75th day of the experiment, fasting glucose levels were measured in blood taken from the tail vein using an electrochemical glucometer (OneTouchSelect, USA).
When conducting an insulin resistance test on the 85th day of the experiment, animals of all experimental groups were administered insulin intraperitoneally at a dose of 0.25 U/kg. Blood glucose levels were measured before the administration of insulin solution (0 point) and after 30, 60, 120 and 180 minutes. Curves of the dependence of glucose levels on time after insulin administration were plotted, and the value of the area under the curve (AUC mmol/l * 180 min) was determined.
24 hours before end of the experiment, the animals were placed in exchange cages to collect 24-hour urine.
On day 94, rats of all groups (deprived by starvation for 12 hours) were decapitated under light anesthesia and a postmortem examination was performed. The cecum was isolated to study the microbiota. Cecal samples were stored at –70°C. 1 ml of blood was collected into tubes with K2EDTA for hematological analysis, the rest of the blood collected after decapitation of the animal was incubated at a temperature of 2-8°C for 3 hours, centrifuged for 30 minutes at 3000 rpm at 4°C, and the resulting serum was stored at -20°C.
At the end of the experiment, the content of ghrelin, leptin, superoxide dismutase and MDA were determined in the blood serum of rats using competitive ELISA according to the manufacturer’s instructions (Elabscience, USA).
The content of indicators of protein metabolism (total protein, albumin, globulins, urea, creatinine), lipid metabolism (total cholesterol, HDL cholesterol, LDL cholesterol, triglycerides), carbohydrate metabolism (glucose), purine metabolism (uric acid), mineral metabolism (calcium, phosphorus, magnesium), functional state of the liver (total bilirubin, ALT, AST, alkaline phosphatase) in the blood serum of animals was determined on an automatic biochemical analyzer “Konelab 20i” (ThermoScientific, USA). Fat was extracted from the liver using the Folch method [
46]. The content of triglycerides and cholesterol in fat extracted from the liver was determined photometrically on an automatic biochemical analyzer Konelab 20i (ThermoScientific, USA).
A general hematological analysis of samples of collected blood was carried out using a veterinary hemoanalyzer Exigo H400 (Boule, Sweden), the following parameters were determined: the concentration of leukocytes, lymphocytes, monocytes, granulocytes, platelets, erythrocytes, the average erythrocyte volume, hemoglobin level, the average hemoglobin content in an erythrocyte, hematocrit, width distribution of red blood cells by volume.
The microbiota was studied by real-time PCR using the Colonoflor 16 Premium test system (Alfa Labs, Russia).
DNA extraction from the contents of the cecum (0.1 g) was carried out using the DNA-Sorb-S kit (FBUN Central Research Institute of Epidemiology) with additional homogenization of samples in 2 ml microtubes containing lysis buffer from the extraction kit and 0.8 g sterile glass beads with a diameter of 0.1 mm. Amplification and detection were carried out using a CFX96 Real Time System amplifier (Bio-Rad, USA), threshold cycle values (Cq) were calculated automatically by the CFX Manager software, the results were interpreted using the supplied Colonoflor software, the results were expressed as decimal logarithms of the number of genome-equivalent CFU/g. Additionally, the ratio of log levels of B. fragilis group/F. prausnitzii was calculated.
Statistical processing of the obtained results was carried out using the SPSS Statistics 20 (IBM) software. The sample was tested for normality using the Kolmogorov–Smirnov test at p=0.05. In the case of normal distribution, parametric research methods were used: ANOVA according to plan 41 (one-factor, four-level, balanced experiment). In case of rejection of the null hypothesis, the method of multiple comparison of means was used—Tukey's test (q-test). The mean (M) and standard error of the mean (SEM) were calculated; data are presented as M±SEM. If the sample did not correspond to a normal distribution, nonparametric goodness-of-fit tests were used: Kruskal–Wallis H test. If the null hypothesis was rejected, the multiple comparison method, Duncan's test (MRT), was used. The median (Me), lower (Q1) and upper (Q3) quartiles were determined. Data are presented as Me (Q1-Q3). Differences were considered statistically significant at p<0.05.
5. Conclusions
A feature of this study was the use of a hypercholesterolemia model in a subchronic experiment in comparison and in combination with forced running exercise. We found that the introduction of 2% cholesterol into the diet of animals has the greatest effect on the body of rats, without causing serious pathology or mortality, which is also confirmed by the successful correction of some indicators by running exercise. The data obtained may be of interest when modeling hyperlipidemia in rodents for the assessment of the hypolipidemic and hypocholesterolemic properties of both drugs and functional foods. This model can be effectively used to reproduce non-alcoholic fatty liver disease in vivo to find ways to correct it. The dysbiotic changes shown in the experiment, characteristic of a diet high in fats and simple sugars, can also be considered as a valid biomarker for possible dietary correction using functional foods. The combination of factors used, simulating poor nutrition, against the background of regular moderate physical activity showed, first of all, the prospects for using physical activity in the prevention and treatment of the disease.
Author Contributions
Conceptualization, Y.S.S.; methodology, S.N.Z.; software, N.A.P.; validation, Y.S.S. and Y.M.M.; formal analysis, A.I.K.; investigation, Y.S.S., N.A.P., Y.M.M., A.I.K. and S.N.Z.; data curation, Y.S.S.; writing—original draft preparation, N.A.P.; writing—review and editing, Y.S.S.; visualization, S.N.Z.; supervision, Y.S.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Russian Science Foundation, grant number 21-76-10049.
Institutional Review Board Statement
The study was approved by the Ethics Committee of the Federal Research Centre of Nutrition and Biotechnology (protocol code No.11 15 December 2021).
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Piché, M.-E.; Tchernof, A.; Després, J.-P. Obesity Phenotypes, Diabetes, and Cardiovascular Diseases. Circ. Res. 2020, 126 (11), 1477–1500. [CrossRef]
- Babenko, A. Yu.; Golikova, T. I. Obesity as a predictor of metabolic disorders and a target for personalized interventions. Rossiiskiy zhurnal personalizirovannoy meditsiny 2021, 1 (1), 59–94.
- Chai, Z.; Yan, Y.; Zan, S.; Meng, X.; Zhang, F. Probiotic-fermented blueberry pomace alleviates obesity and hyperlipidemia in high-fat diet C57BL/6J mice. Food Res. Int. 2022, 157, 111396. [CrossRef]
- Perdomo, C. M.; Cohen, R. V; Sumithran, P.; Clément, K.; Frühbeck, G. Contemporary medical, device, and surgical therapies for obesity in adults. Lancet 2023, 401 (10382), 1116–1130. [CrossRef]
- Karr, S. Epidemiology and management of hyperlipidemia. Am. J. Manag. Care 2017, 23 (9 Suppl), S139–S148.
- Santos-Baez, L. S.; Ginsberg, H. N. Hypertriglyceridemia—Causes, Significance, and Approaches to Therapy. Front. Endocrinol. (Lausanne). 2020, 11. [CrossRef]
- Gao, M.; Jebb, S. A.; Aveyard, P.; Ambrosini, G. L.; Perez-Cornago, A.; Carter, J.; Sun, X.; Piernas, C. Associations between dietary patterns and the incidence of total and fatal cardiovascular disease and all-cause mortality in 116,806 individuals from the UK Biobank: a prospective cohort study. BMC Med. 2021, 19 (1), 83. [CrossRef]
- AlFaris, N. A.; Alshammari, G. M.; AlTamimi, J. Z.; AlMousa, L. A.; AlKehayez, N. M.; Aljabryn, D. H.; Alagal, R. I.; Yahya, M. A. The protective effect of shrimp cooked in different methods on high-cholesterol- induced fatty liver in rats. Saudi J. Biol. Sci. 2021, 28 (1), 170–182. [CrossRef]
- Tirosh, O. Hypoxic Signaling and Cholesterol Lipotoxicity in Fatty Liver Disease Progression. Oxid. Med. Cell. Longev. 2018, 2018, 1–15. [CrossRef]
- Cunha, L. F.; Ongaratto, M. A.; Endres, M.; Barschak, A. G. Modelling hypercholesterolaemia in rats using high cholesterol diet. Int. J. Exp. Pathol. 2021, 102 (2), 74–79. [CrossRef]
- Saigo, Y.; Sasase, T.; Tohma, M.; Uno, K.; Shinozaki, Y.; Maekawa, T.; Sano, R.; Miyajima, K.; Ohta, T. High-Cholesterol Diet in Combination With Hydroxypropyl-β Cyclodextrin Induces NASH-Like Disorders in the Liver of Rats. Physiol. Res. 2023, 72 (3). [CrossRef]
- Jang, S.; Lee, M.-S.; Kang, S.-A.; Kim, C.-T.; Kim, Y. Portulaca oleracea L. Extract Regulates Hepatic Cholesterol Metabolism via the AMPK/MicroRNA-33/34a Pathway in Rats Fed a High-Cholesterol Diet. Nutrients 2022, 14 (16), 3330. [CrossRef]
- Liu, L.; Zhang, M.; Jiang, F.; Luo, D.; Liu, S.; Su, Y.; Guan, Q.; Yu, C. High cholesterol diet–induced testicular dysfunction in rats. Hormones 2023, 22 (4), 685–694. [CrossRef]
- Yu, C.; Jiang, F.; Zhang, M.; Luo, D.; Shao, S.; Zhao, J.; Gao, L.; Zuo, C.; Guan, Q. HC diet inhibited testosterone synthesis by activating endoplasmic reticulum stress in testicular Leydig cells. J. Cell. Mol. Med. 2019, 23 (5), 3140–3150. [CrossRef]
- Zafar, H.; Ain, N. ul; Alshammari, A.; Alghamdi, S.; Raja, H.; Ali, A.; Siddique, A.; Tahir, S. D.; Akbar, S.; Arif, M.; Alharbi, M.; Rahman, A. Lacticaseibacillus rhamnosus FM9 and Limosilactobacillus fermentum Y57 Are as Effective as Statins at Improving Blood Lipid Profile in High Cholesterol, High-Fat Diet Model in Male Wistar Rats. Nutrients 2022, 14 (8), 1654. [CrossRef]
- Biryulina, N.A.; Sidorova, Yu.S.; Petrov, N.A.; Guseva, G.V.; Mazo, V.K. The effect of semi-synthetic and high-fat high-carbohydrate diets containing cholesterol on lipid and cholesterol metabolism of male Wistar rats. Bulletin of Experimental Biology and Medicine 2023, 175(4), 451–456. [CrossRef]
- Kovacs, P.; Hajnal, A. Short-term high-fat diet consumption increases body weight and body adiposity and alters brain stem taste information processing in rats. Chem Senses 2022 47, bjac020. [CrossRef]
- Moreno-Fernández, S.; Garcés-Rimón, M.; Vera, G.; Astier, J.; Landrier, J.F.; Miguel, M.; High Fat/High Glucose Diet Induces Metabolic Syndrome in an Experimental Rat Model. Nutrients 2018 10 (10), 1502. [CrossRef]
- Pathak, R.; Kumar, A.; Palfrey, H. A.; Stone, K. P.; Raju, N. R.; Gettys, T. W.; Murthy, S. N. Prolonged effects of DPP-4 inhibitors on steato-hepatitic changes in Sprague–Dawley rats fed a high-cholesterol diet. Inflamm. Res. 2022, 71 (5–6), 711–722. [CrossRef]
- Oda, E. A decrease in total bilirubin predicted hyper-LDL cholesterolemia in a health screening population. Atherosclerosis 2014, 235 (2), 334–338. [CrossRef]
- Su, Q.; Chen, H.; Du, S.; Dai, Y.; Chen, C.; He (何天敏), T.; Feng, R.; Tao, T.; Hu, Z.; Zhao, H.; Guo, P.; Ye, W. Association Between Serum Bilirubin, Lipid Levels, and Prevalence of Femoral and Carotid Atherosclerosis: A Population-Based Cross-Sectional Study. Arterioscler. Thromb. Vasc. Biol. 2023, 43 (1), 136–145. [CrossRef]
- Li, X.-H.; Lin, H.-Y.; Guan, L.-Y.; Peng, H.; Wen, M.-M.; Cao, Y.-Q.; Jiang, X.-Y.; Wang, Y.-B. Direct Bilirubin Levels and Risk of Metabolic Syndrome in Healthy Chinese Men. Biomed Res. Int. 2017, 2017, 1–8. [CrossRef]
- Wu, I.-T.; Yeh, W.-J.; Huang, W.-C.; Yang, H.-Y. Very low-carbohydrate diet with higher protein ratio improves lipid metabolism and inflammation in rats with diet-induced nonalcoholic fatty liver disease. J. Nutr. Biochem. 2024, 126, 109583. [CrossRef]
- Spassiani, N. A.; Kuk, J. L. Exercise and the fatty liver. Appl. Physiol. Nutr. Metab. 2008, 33 (4), 802–807. [CrossRef]
- Botezelli, J. D.; Mora, R. F.; Dalia, R. A.; Moura, L. P.; Cambri, L. T.; Ghezzi, A. C.; Voltarelli, F. A.; Mello, M. A. Exercise counteracts fatty liver disease in rats fed on fructose-rich diet. Lipids Health Dis. 2010, 9 (1), 116. [CrossRef]
- Gu, X.; Ma, X.; Mo, L.; Wang, Q. The Role of Exercise Intensity on Fatty Liver in Rats. Chin. J. Physiol. 2022, 65 (6), 301–310. [CrossRef]
- Pan, M.; Song, Y.; Xu, J.; Gan, H. Melatonin ameliorates nonalcoholic fatty liver induced by high-fat diet in rats. J. Pineal Res. 2006, 41 (1), 79–84. [CrossRef]
- Qi, J.; Kim, J.-W.; Zhou, Z.; Lim, C.-W.; Kim, B. Ferroptosis Affects the Progression of Nonalcoholic Steatohepatitis via the Modulation of Lipid Peroxidation–Mediated Cell Death in Mice. Am. J. Pathol. 2020, 190 (1), 68–81. [CrossRef]
- Münzberg, H.; Morrison, C. D. Structure, production and signaling of leptin. Metabolism 2015, 64 (1), 13–23. [CrossRef]
- Martelli, D.; Brooks, V. L. Leptin Increases: Physiological Roles in the Control of Sympathetic Nerve Activity, Energy Balance, and the Hypothalamic–Pituitary–Thyroid Axis. Int. J. Mol. Sci. 2023, 24 (3), 2684. [CrossRef]
- Ferreira-Halder, C. V.; Faria, A. V. de S.; Andrade, S. S. Action and function of Faecalibacterium prausnitzii in health and disease. Best Pract. Res. Clin. Gastroenterol. 2017, 31 (6), 643–648. [CrossRef]
- Leylabadlo, H. E.; Ghotaslou, R.; Feizabadi, M. M.; Farajnia, S.; Moaddab, S. Y.; Ganbarov, K.; Khodadadi, E.; Tanomand, A.; Sheykhsaran, E.; Yousefi, B.; Kafil, H. S. The critical role of Faecalibacterium prausnitzii in human health: An overview. Microb. Pathog. 2020, 149, 104344. [CrossRef]
- Sitkin, S. I.; Vakhitov, T. Y.; Tkachenko, E. I.; Oreshko, L. S.; Zhigalova, T. N.; Radchenko, V. G.; Seliverstov, P. V.; Avalueva, E. B.; Suvorova, M. A.; Komlichenko, E. V. Gut microbiota in ulcerative colitis and celiac disease. Exp. Clin. Gastroenterol. 2017, No 1, 8–30.
- Mohammad, S.; Thiemermann, C. Role of Metabolic Endotoxemia in Systemic Inflammation and Potential Interventions. Front. Immunol. 2021, 11. [CrossRef]
- Khare, A.; Gaur, S. Cholesterol-Lowering Effects of Lactobacillus Species. Curr. Microbiol. 2020, 77 (4), 638–644. [CrossRef]
- O’Flaherty, S.; Briner Crawley, A.; Theriot, C. M.; Barrangou, R. The Lactobacillus Bile Salt Hydrolase Repertoire Reveals Niche-Specific Adaptation. mSphere 2018, 3 (3). [CrossRef]
- Frappier, M.; Auclair, J.; Bouasker, S.; Gunaratnam, S.; Diarra, C.; Millette, M. Screening and Characterization of Some Lactobacillaceae for Detection of Cholesterol-Lowering Activities. Probiotics Antimicrob. Proteins 2022, 14 (5), 873–883. [CrossRef]
- de Araújo Henriques Ferreira, G.; Magnani, M.; Cabral, L.; Brandão, L. R.; Noronha, M. F.; de Campos Cruz, J.; de Souza, E. L.; de Brito Alves, J. L. Potentially Probiotic Limosilactobacillus fermentum Fruit-Derived Strains Alleviate Cardiometabolic Disorders and Gut Microbiota Impairment in Male Rats Fed a High-Fat Diet. Probiotics Antimicrob. Proteins 2022, 14 (2), 349–359. [CrossRef]
- Li, X.; Xiao, Y.; Huang, Y.; Song, L.; Li, M.; Ren, Z. Lactobacillus gasseri RW2014 Ameliorates Hyperlipidemia by Modulating Bile Acid Metabolism and Gut Microbiota Composition in Rats. Nutrients 2022, 14 (23), 4945. [CrossRef]
- Kumar, M.; Nagpal, R.; Kumar, R.; Hemalatha, R.; Verma, V.; Kumar, A.; Chakraborty, C.; Singh, B.; Marotta, F.; Jain, S.; Yadav, H. Cholesterol-Lowering Probiotics as Potential Biotherapeutics for Metabolic Diseases. Exp. Diabetes Res. 2012, 2012, 1–14. [CrossRef]
- DiRienzo, D. B. Effect of probiotics on biomarkers of cardiovascular disease: implications for heart-healthy diets. Nutr. Rev. 2014, 72 (1), 18–29. [CrossRef]
- Tonucci, L. B.; Olbrich dos Santos, K. M.; Licursi de Oliveira, L.; Rocha Ribeiro, S. M.; Duarte Martino, H. S. Clinical application of probiotics in type 2 diabetes mellitus: A randomized, double-blind, placebo-controlled study. Clin. Nutr. 2017, 36 (1), 85–92. [CrossRef]
- Choi, B. S.-Y.; Brunelle, L.; Pilon, G.; Cautela, B. G.; Tompkins, T. A.; Drapeau, V.; Marette, A.; Tremblay, A. Lacticaseibacillus rhamnosus HA-114 improves eating behaviors and mood-related factors in adults with overweight during weight loss: a randomized controlled trial. Nutr. Neurosci. 2023, 26 (7), 667–679. [CrossRef]
- Huang, W.; Wang, G.; Xia, Y.; Xiong, Z.; Ai, L. Bile salt hydrolase-overexpressing Lactobacillus strains can improve hepatic lipid accumulation in vitro in an NAFLD cell model. Food Nutr. Res. 2020, 64. [CrossRef]
- Reeves, P. G. Components of the AIN-93 Diets as Improvements in the AIN-76A Diet. J. Nutr. 1997, 127 (5), 838S-841S. [CrossRef]
- Omar, A. M.; Zhang, Q. Evaluation of Lipid Extraction Protocols for Untargeted Analysis of Mouse Tissue Lipidome. Metabolites 2023, 13 (9), 1002. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).