Submitted:
07 April 2024
Posted:
08 April 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
- Input data: AlphaFold takes the primary sequence of amino acids that make up a protein as input. This sequence is derived from the genetic code.
- Homology detection: AlphaFold starts by comparing the target protein sequence to a large database of known protein structures to identify proteins with similar sequences. This process is known as homology detection. If similar structures are found, they can provide valuable clues about the structure of the target protein.
- Multiple sequence alignment (MSA): After identifying similar sequences, AlphaFold aligns them with the target sequence to create a multiple sequence alignment (MSA). This step helps identify conserved regions in the protein sequence, which are likely to correspond to important structural elements.
- Structure prediction: Using the MSA and other relevant data, AlphaFold employs deep learning techniques, particularly deep neural networks, to predict the 3D structure of the target protein. The neural network is trained on a diverse set of protein structures to learn the complex relationships between amino acid sequences and their corresponding 3D structures.
- Model refinement: AlphaFold then refines the initial predicted structures through iterative optimization techniques to improve accuracy and consistency. This refinement process helps correct any errors and inconsistencies in the predicted structure.
- Output: The final output of AlphaFold is a predicted 3D model of the protein’s structure, including the positions of individual atoms. This model provides valuable insights into the protein’s function, interactions with other molecules, and potential implications for drug discovery and other applications [54,55,56,57,58,59,60].
- Accuracy for large proteins and complexes [69,70,71]: while current algorithms perform reasonably well for small to medium-sized proteins, accurately predicting the structures of large proteins or protein complexes remains challenging. Improvements are needed to capture the intricacies of these larger systems, including domain-domain interactions and conformational changes.
- Conformational flexibility [53,72,73]: Proteins exhibit conformational flexibility, adopting multiple states with different structural conformations. Current algorithms often struggle to accurately predict these flexible regions or transitions between states. Enhancements in sampling techniques and modeling approaches are needed to better capture and predict protein flexibility.
- Computational Efficiency [74,75]: High-resolution protein structure prediction often requires substantial computational resources and time. Improvements in algorithm efficiency and scalability are necessary to enable broader application and faster turnaround times for protein structure prediction tasks [76,77,78,79,80,81,82,83].
2. Motivation
3. Materials and Methods
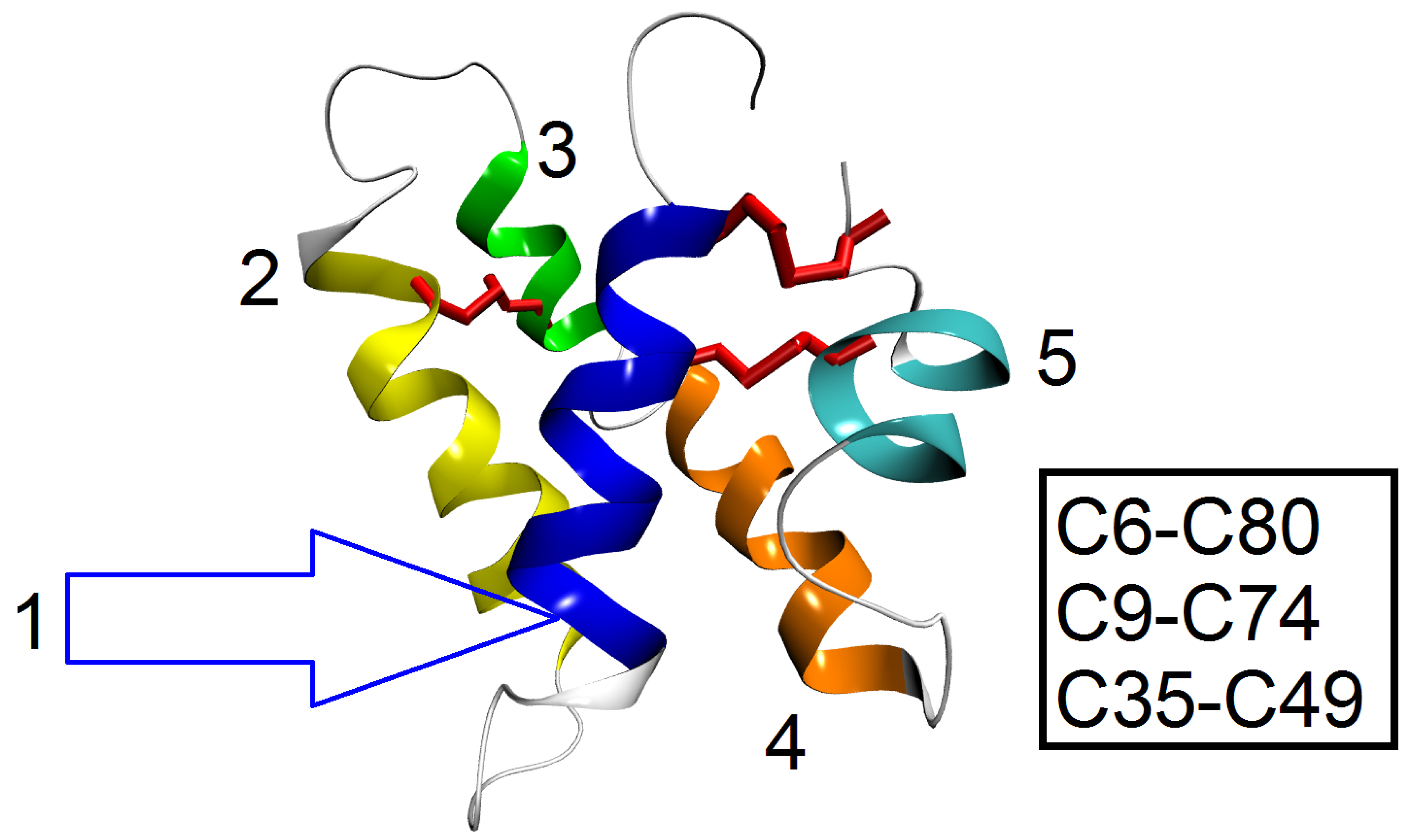
3.1. Redefining Protein Backbone Structure with , and : Caenopore-5 as an Example
3.2. Redefining Protein Backbone Structure with , and : How?
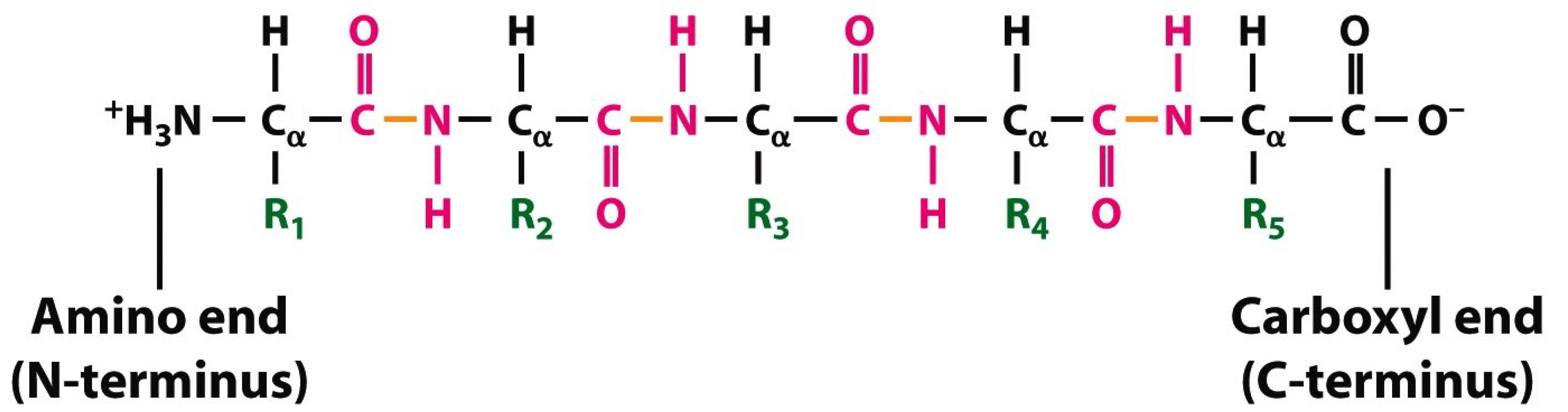
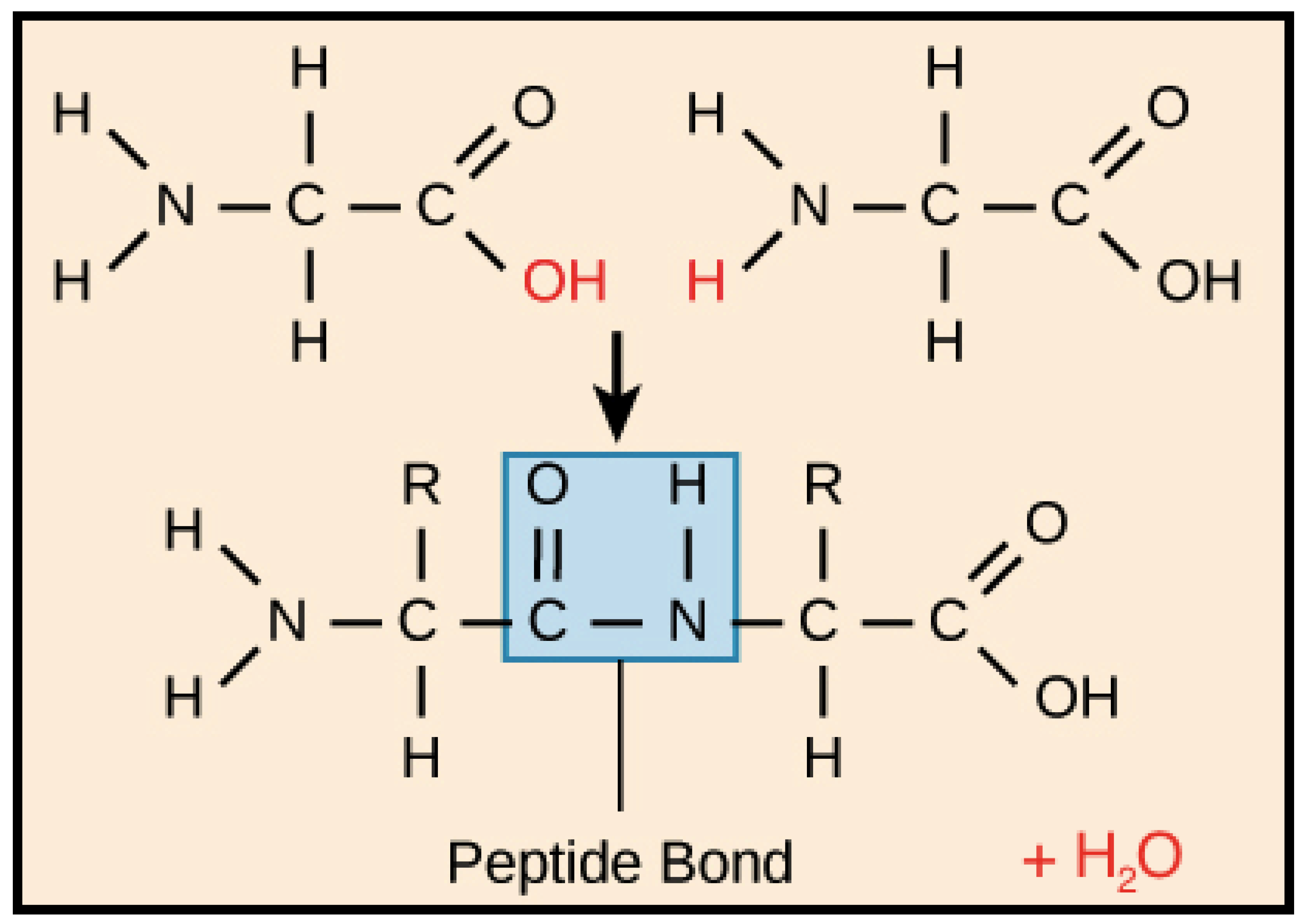
- the covalent bond between N-terminal amide nitrogen atom (Atom_initial in the IASCS coordinate system) of the first residue of the short peptide with five amino acids (Figure 4) and three N-terminal amide hydrogen atoms (Atom_finals in the IASCS coordinate system) of the backbone of the first residue of the short peptide with five amino acids (Figure 4);
- the covalent bond between N-terminal amide nitrogen atom (Atom_initial in the IASCS coordinate system) of the first residue of the short peptide with five amino acids (Figure 4) and carbon α(Cα, Atom_final in the IASCS coordinate system) of the backbone of the first residue of the short peptide with five amino acids (Figure 4);
- the covalent bond between carbon α(Cα, Atom_initial in the IASCS coordinate system) of the first residue of the short peptide with five amino acids (Figure 4) and hydrogen α(Hα, Atom_final in the IASCS coordinate system) of the backbone of the first residue of the short peptide with five amino acids (Figure 4);
- the covalent bond between carbon α(Cα, Atom_initial in the IASCS coordinate system) of the first residue of the short peptide with five amino acids (Figure 4) and carbonyl carbon (Atom_final in the IASCS coordinate system) of the backbone of the first residue of the short peptide with five amino acids (Figure 4);
- the covalent bond between carbonyl carbon (Atom_initial in the IASCS coordinate system) of the first residue of the short peptide with five amino acids (Figure 4) and carbonyl oxygen (the double-bonded oxygen atom, Atom_final in the IASCS coordinate system) of the backbone of the first residue of the short peptide with five amino acids (Figure 4);
- the covalent bond between carbonyl carbon (Atom_initial in the IASCS coordinate system) of the first residue of the short peptide with five amino acids (Figure 4) and the amide nitrogen atom (Atom_final in the IASCS coordinate system) of the second residue of the short peptide with five amino acids (Figure 4);
- the covalent bond between the amide nitrogen atom (Atom_initial in the IASCS coordinate system) of the second residue of the short peptide with five amino acids (Figure 4) and the amide hydrogen atom (Atom_final in the IASCS coordinate system) of the backbone of the second residue of the short peptide with five amino acids (Figure 4);
- the covalent bond between N-terminal amide nitrogen atom (Atom_initial in the IASCS coordinate system) of the second residue of the short peptide with five amino acids (Figure 4) and carbon α(Cα, Atom_final in the IASCS coordinate system) of the backbone of the second residue of the short peptide with five amino acids (Figure 4);
- the covalent bond between carbon α(Cα, Atom_initial in the IASCS coordinate system) of the second residue of the short peptide with five amino acids (Figure 4) and hydrogen α(Hα, Atom_final in the IASCS coordinate system) of the backbone of the second residue of the short peptide with five amino acids (Figure 4);
- the covalent bond between carbon α(Cα, Atom_initial in the IASCS coordinate system) of the second residue of the short peptide with five amino acids (Figure 4) and carbonyl carbon (Atom_final in the IASCS coordinate system) of the backbone of the second residue of the short peptide with five amino acids (Figure 4);
- the covalent bond between carbonyl carbon (Atom_initial in the IASCS coordinate system) of the second residue of the short peptide with five amino acids (Figure 4) and carbonyl oxygen (the double-bonded oxygen atom, Atom_final in the IASCS coordinate system) of the backbone of the second residue of the short peptide with five amino acids (Figure 4);
- the covalent bond between carbonyl carbon (Atom_initial in the IASCS coordinate system) of the second residue of the short peptide with five amino acids (Figure 4) and the amide nitrogen atom (Atom_final in the IASCS coordinate system) of the third residue of the short peptide with five amino acids (Figure 4);
- the covalent bond between the amide nitrogen atom (Atom_initial in the IASCS coordinate system) of the third residue of the short peptide with five amino acids (Figure 4) and the amide hydrogen atom (Atom_final in the IASCS coordinate system) of the backbone of the third residue of the short peptide with five amino acids (Figure 4);
- the covalent bond between N-terminal amide nitrogen atom (Atom_initial in the IASCS coordinate system) of the third residue of the short peptide with five amino acids (Figure 4) and carbon α(Cα, Atom_final in the IASCS coordinate system) of the backbone of the third residue of the short peptide with five amino acids (Figure 4);
- the covalent bond between carbon α(Cα, Atom_initial in the IASCS coordinate system) of the third residue of the short peptide with five amino acids (Figure 4) and hydrogen α(Hα, Atom_final in the IASCS coordinate system) of the backbone of the third residue of the short peptide with five amino acids (Figure 4);
- the covalent bond between carbon α(Cα, Atom_initial in the IASCS coordinate system) of the third residue of the short peptide with five amino acids (Figure 4) and carbonyl carbon (Atom_final in the IASCS coordinate system) of the backbone of the third residue of the short peptide with five amino acids (Figure 4);
- the covalent bond between carbonyl carbon (Atom_initial in the IASCS coordinate system) of the third residue of the short peptide with five amino acids (Figure 4) and carbonyl oxygen (the double-bonded oxygen atom, Atom_final in the IASCS coordinate system) of the backbone of the third residue of the short peptide with five amino acids (Figure 4);
- the covalent bond between carbonyl carbon (Atom_initial in the IASCS coordinate system) of the third residue of the short peptide with five amino acids (Figure 4) and the amide nitrogen atom (Atom_final in the IASCS coordinate system) of the fourth residue of the short peptide with five amino acids (Figure 4);
- the covalent bond between the amide nitrogen atom (Atom_initial in the IASCS coordinate system) of the fourth residue of the short peptide with five amino acids (Figure 4) and the amide hydrogen atom (Atom_final in the IASCS coordinate system) of the backbone of the fourth residue of the short peptide with five amino acids (Figure 4);
- the covalent bond between N-terminal amide nitrogen atom (Atom_initial in the IASCS coordinate system) of the fourth residue of the short peptide with five amino acids (Figure 4) and carbon α(Cα, Atom_final in the IASCS coordinate system) of the backbone of the fourth residue of the short peptide with five amino acids (Figure 4);
- the covalent bond between carbon α(Cα, Atom_initial in the IASCS coordinate system) of the fourth residue of the short peptide with five amino acids (Figure 4) and hydrogen α(Hα, Atom_final in the IASCS coordinate system) of the backbone of the fourth residue of the short peptide with five amino acids (Figure 4);
- the covalent bond between carbon α(Cα, Atom_initial in the IASCS coordinate system) of the fourth residue of the short peptide with five amino acids (Figure 4) and carbonyl carbon (Atom_final in the IASCS coordinate system) of the backbone of the fourth residue of the short peptide with five amino acids (Figure 4);
- the covalent bond between carbonyl carbon (Atom_initial in the IASCS coordinate system) of the fourth residue of the short peptide with five amino acids (Figure 4) and carbonyl oxygen (the double-bonded oxygen atom, Atom_final in the IASCS coordinate system) of the backbone of the fourth residue of the short peptide with five amino acids (Figure 4);
- the covalent bond between carbonyl carbon (Atom_initial in the IASCS coordinate system) of the fourth residue of the short peptide with five amino acids (Figure 4) and the amide nitrogen atom (Atom_final in the IASCS coordinate system) of the fifth residue of the short peptide with five amino acids (Figure 4);
- the covalent bond between the amide nitrogen atom (Atom_initial in the IASCS coordinate system) of the fifth residue of the short peptide with five amino acids (Figure 4) and the amide hydrogen atom (Atom_final in the IASCS coordinate system) of the backbone of the fifth residue of the short peptide with five amino acids (Figure 4);
- the covalent bond between N-terminal amide nitrogen atom (Atom_initial in the IASCS coordinate system) of the fifth residue of the short peptide with five amino acids (Figure 4) and carbon α(Cα, Atom_final in the IASCS coordinate system) of the backbone of the fifth residue of the short peptide with five amino acids (Figure 4);
- the covalent bond between carbon α(Cα, Atom_initial in the IASCS coordinate system) of the fifth residue of the short peptide with five amino acids (Figure 4) and hydrogen α(Hα, Atom_final in the IASCS coordinate system) of the backbone of the fifth residue of the short peptide with five amino acids (Figure 4);
- the covalent bond between carbon α(Cα, Atom_initial in the IASCS coordinate system) of the fifth residue of the short peptide with five amino acids (Figure 4) and carbonyl carbon (Atom_final in the IASCS coordinate system) of the backbone of the fifth residue of the short peptide with five amino acids (Figure 4);
- the covalent double bond between carbonyl carbon (Atom_initial in the IASCS coordinate system) of the fifth residue of the short peptide with five amino acids (Figure 4) and carbonyl oxygen (the double-bonded oxygen atom, Atom_final in the IASCS coordinate system) of the backbone of the fifth residue of the short peptide with five amino acids (Figure 4);
- the covalent single bond between carbonyl carbon (Atom_initial in the IASCS coordinate system) of the fifth residue of the short peptide with five amino acids (Figure 4) and the carboxyl carboxyl hydroxyl oxygen atom (Atom_final in the IASCS coordinate system) of the fifth residue of the short peptide with five amino acids (Figure 4);
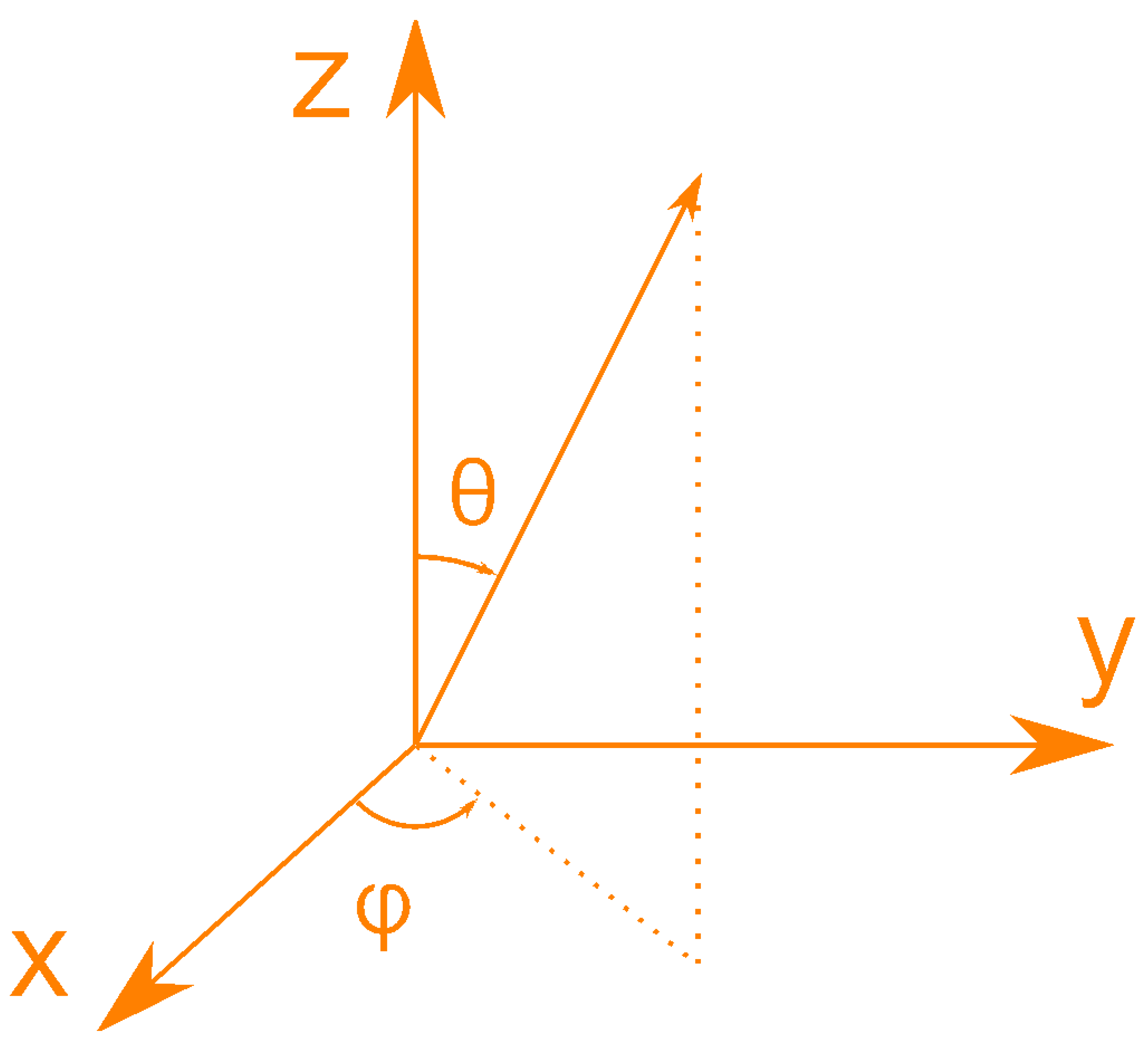
- CCS requires three parameters (X, Y and Z) for all atoms to construct a protein backbone structural model.
- GSCS requires three parameters (R, and ) for all atoms to construct a protein structural model, because GSCS takes the centroid of the protein molecule as origin, where R is used to signify the distance between the centroid (an imaginary point) and any atom of the protein.
-
IASCS requires two parameters ( and ) to construct a protein structural model, because
- (a)
- IASCS takes into account the fact that protein backbone structure essentially is a network of covalently bonded atoms.
- (b)
- (c)
- Neither CCS nor GSCS take into account the inter-atomic covalent bonding network of protein backbone structures.
4. Results
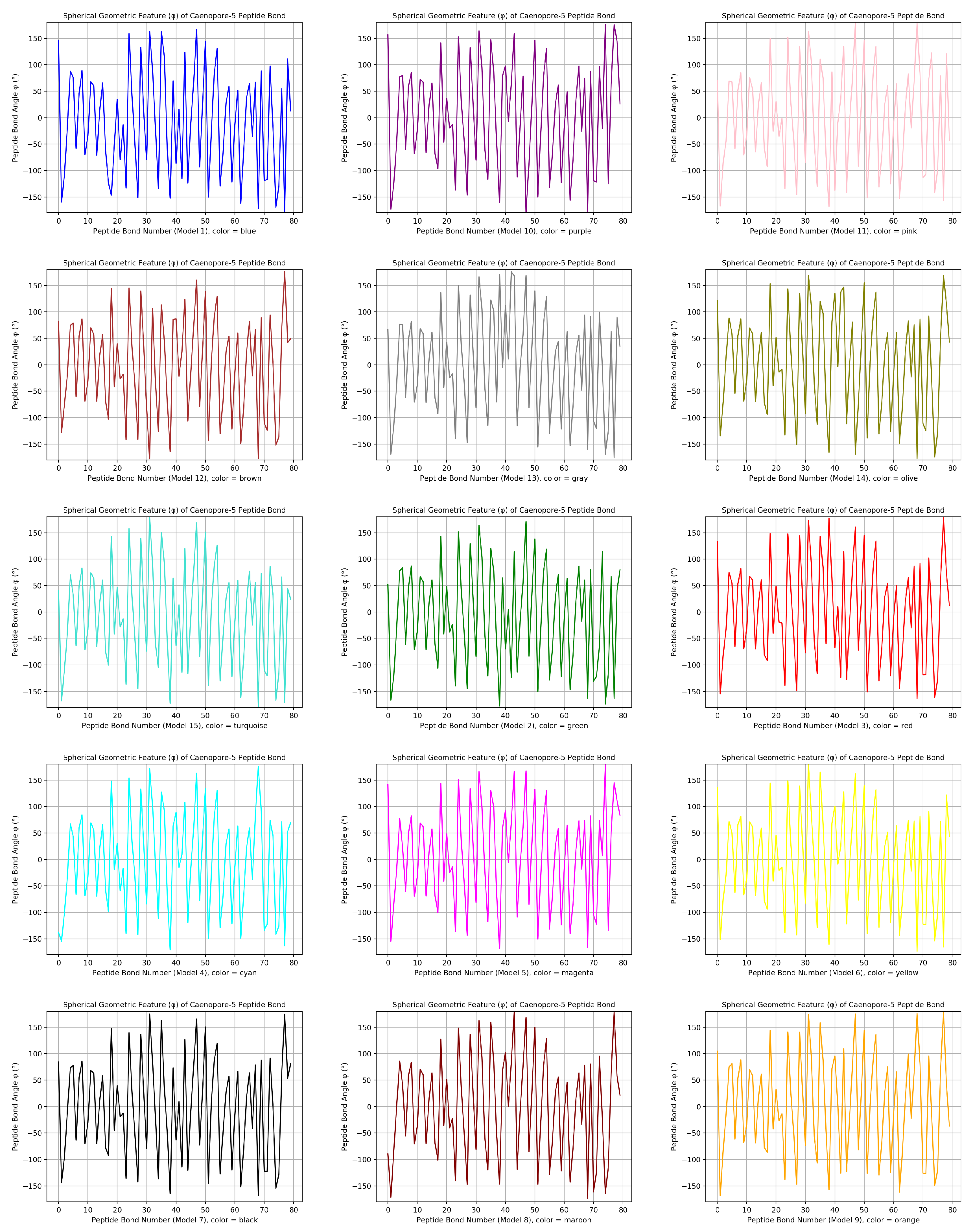
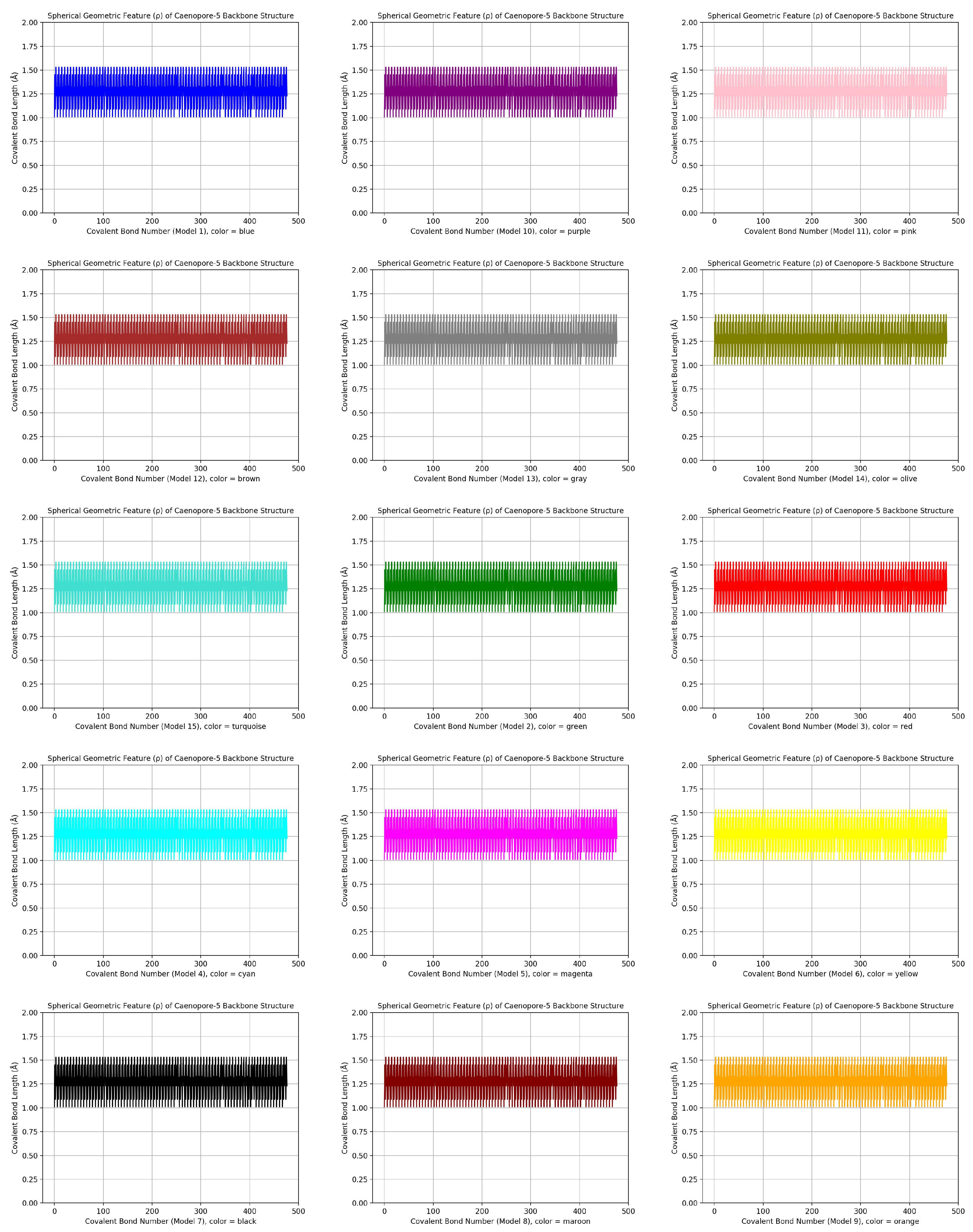
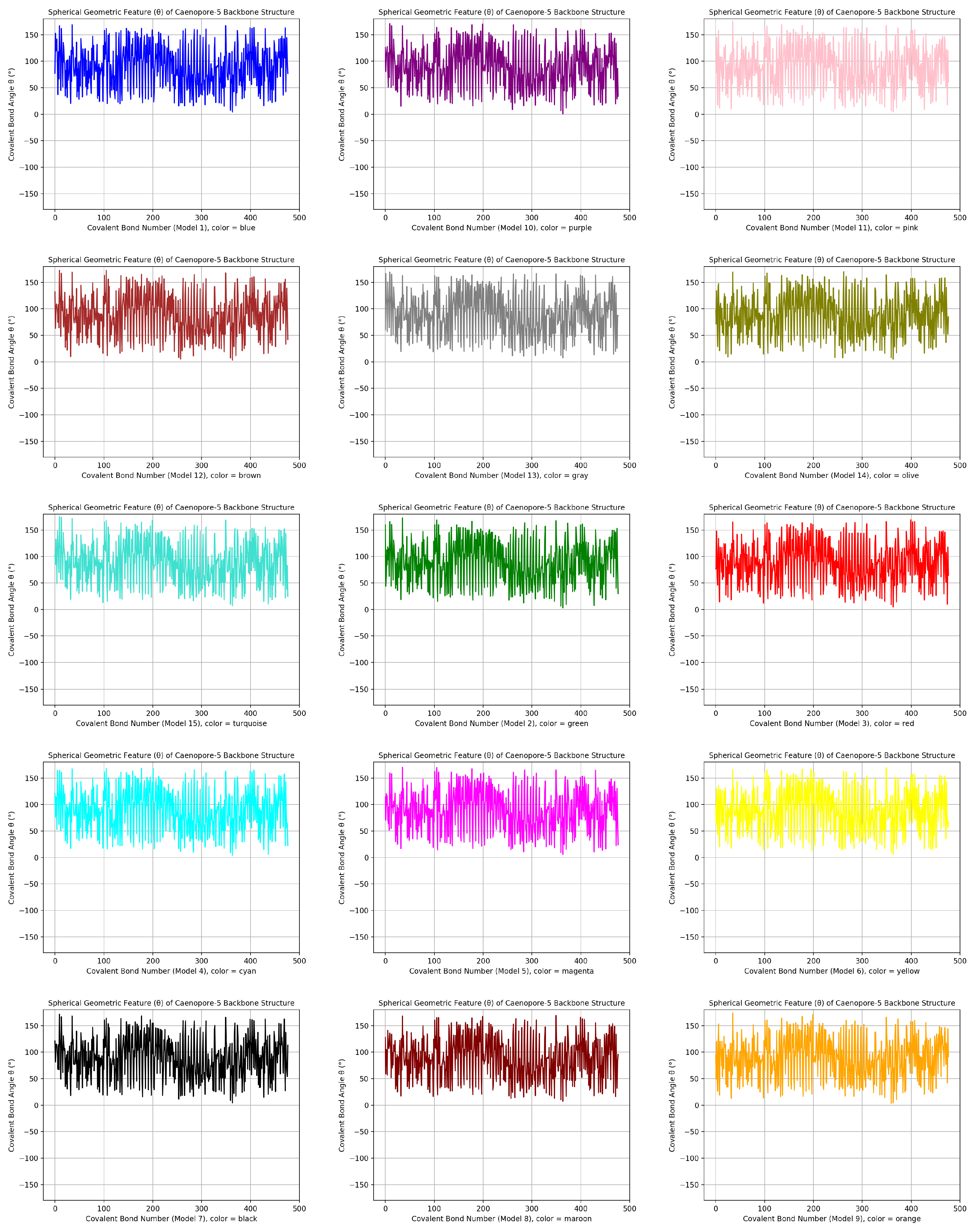
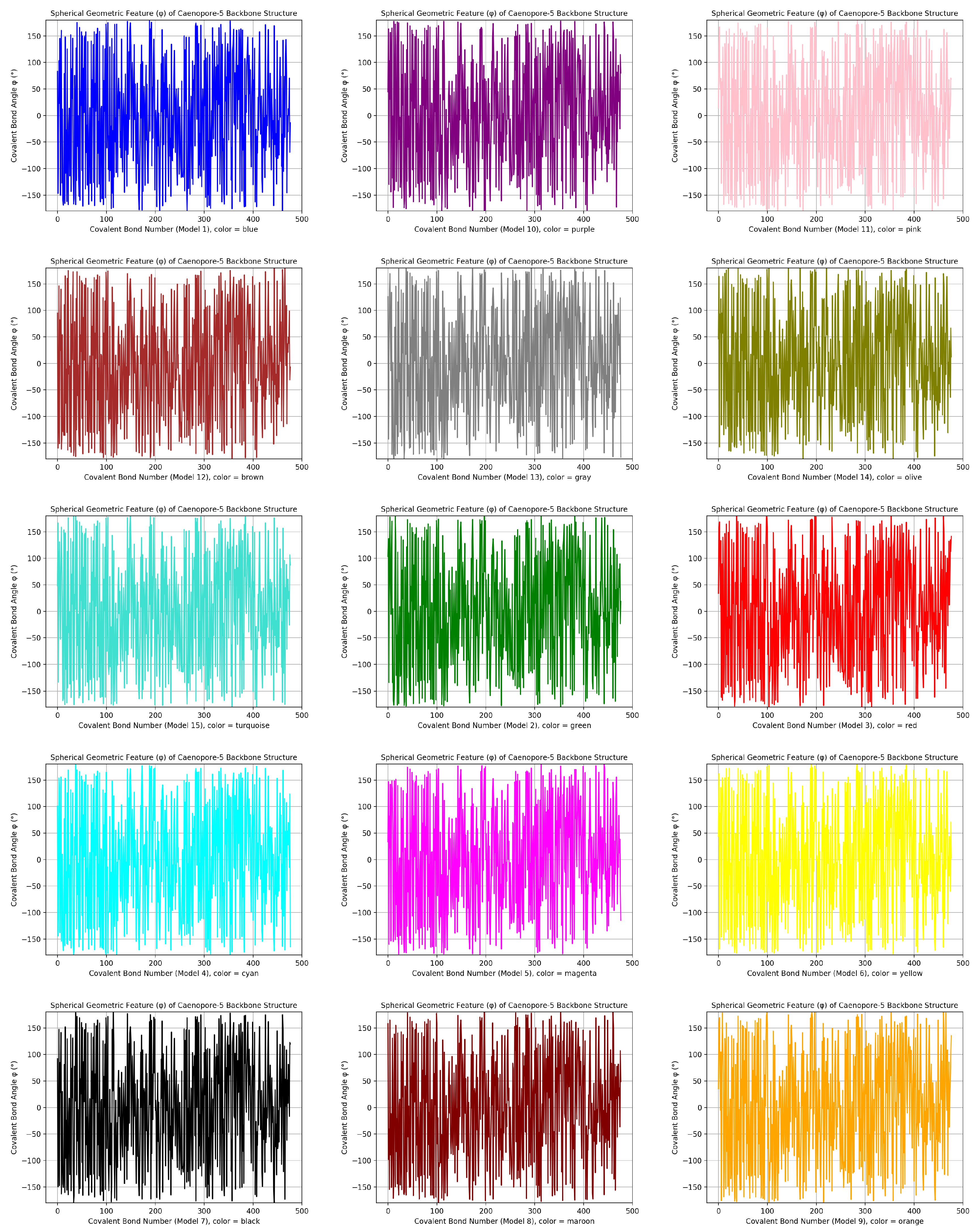
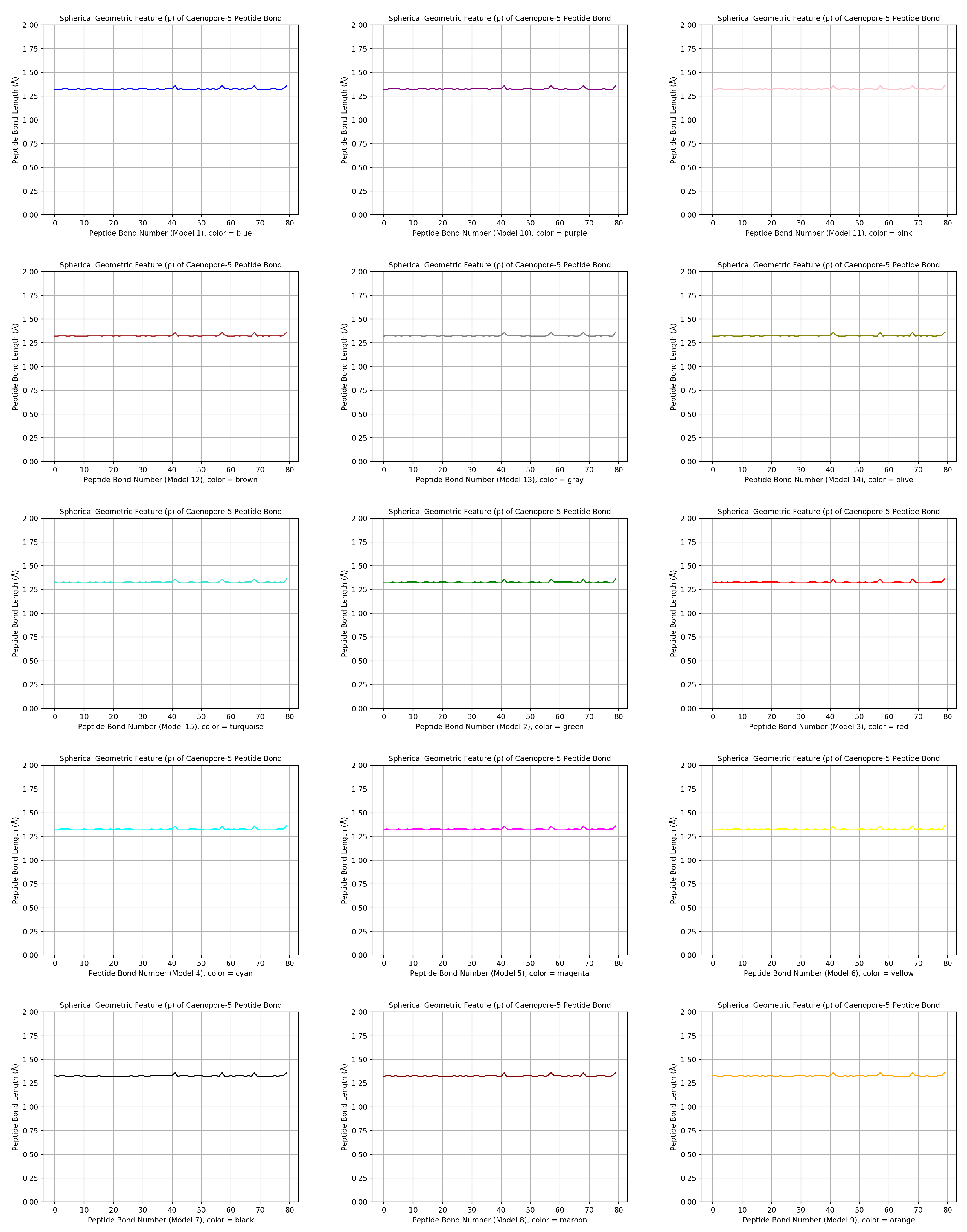
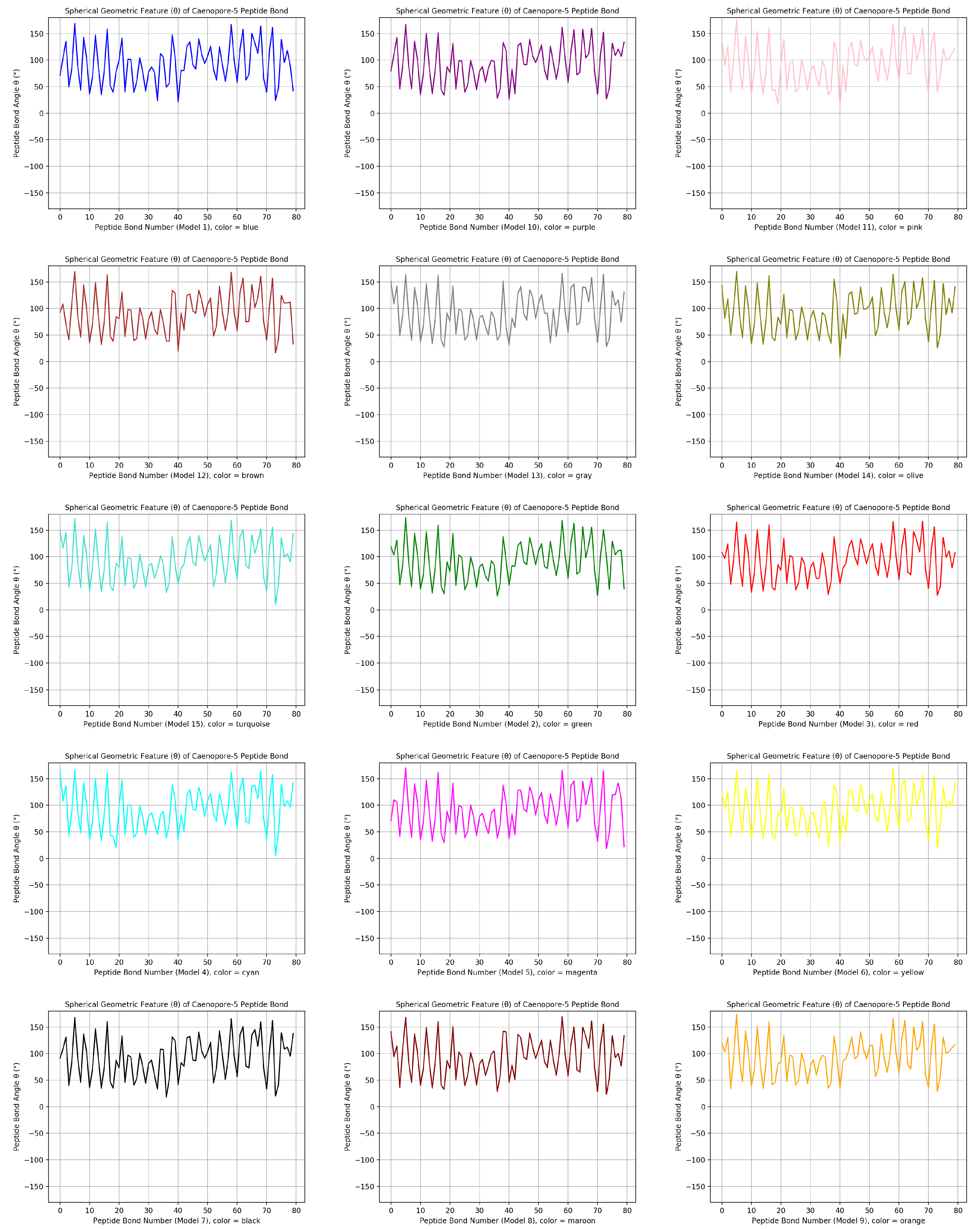
- the distribution pattern of the polar angle (also known as the zenith angle, ) of 477 covalent bonds of the backbone of Caenopore-5 [95,96,98] as reversibly extracted from the 15 NMR structural models of the three-dimensional NMR ensemble of Caenopore-5 (PDB ID: 2JSA) [95,96,98], as shown by Figure 8;
- the reversible nature of the spherical geometric conversion ensures that no information is lost during the geometric coordinate transformation process, preserving the fidelity of the original protein backbone structure and ensuring that it is able to be reconstructed using the extracted IASCS features (local , and values). This feature is crucial for maintaining prediction accuracy and enabling further analysis of the extracted structural features for its future application in protein structure prediction [13,14,15,16,17,18,101].
- the compact representation offered by spherical coordinates with Caenopore-5 as an example enhances computational efficiency, making our method suitable for large-scale (for both PDB and AlphaFoldDB) protein structure prediction tasks for the entire protein molecular space [47,102,103,104,105,106,107,108,109].
5. Conclusion
6. Discussion
Ethical statement:
Declaration of generative AI and AI-assisted technologies in the writing process
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- van Keulen, S.C.; Bonvin, A.M.J.J. Improving the quality of co-evolution intermolecular contact prediction with DisVis. Proteins: Structure, Function, and Bioinformatics 2023, 91, 1407–1416. [Google Scholar] [CrossRef] [PubMed]
- Hess, R.; Faessler, J.; Yun, D.; Saleh, D.; Grosch, J.H.; Schwab, T.; Hubbuch, J. Antibody sequence-based prediction of pH gradient elution in multimodal chromatography. Journal of Chromatography A 2023, 1711, 464437. [Google Scholar] [CrossRef] [PubMed]
- Tesei, G.; Trolle, A.I.; Jonsson, N.; Betz, J.; Knudsen, F.E.; Pesce, F.; Johansson, K.E.; Lindorff-Larsen, K. Conformational ensembles of the human intrinsically disordered proteome. Nature 2024, 626, 897–904. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Du, H.; Zhao, S.; Chen, Z.; Li, Y.; Xu, K.; Liu, B.; Cheng, X.; Wen, W.; Li, G.; Chen, G.; Zhao, Z.; Qiu, G.; Liu, P.; Zhang, T.J.; Wu, Z.; Wu, N. SIGMA leverages protein structural information to predict the pathogenicity of missense variants. Cell Reports Methods 2024, 4, 100687. [Google Scholar] [CrossRef] [PubMed]
- El Badaoui, L.; Barr, A.J. Analysis of Receptor-Type Protein Tyrosine Phosphatase Extracellular Regions with Insights from AlphaFold. International Journal of Molecular Sciences 2024, 25, 820. [Google Scholar] [CrossRef] [PubMed]
- Meller, A.; De Oliveira, S.; Davtyan, A.; Abramyan, T.; Bowman, G.R.; van den Bedem, H. Discovery of a cryptic pocket in the AI-predicted structure of PPM1D phosphatase explains the binding site and potency of its allosteric inhibitors. Frontiers in Molecular Biosciences 2023, 10. [Google Scholar] [CrossRef] [PubMed]
- Huber, S.T.; Jakobi, A.J. Structural biology of microbial gas vesicles: historical milestones and current knowledge. Biochemical Society Transactions 2024, 52, 205–215. [Google Scholar] [CrossRef] [PubMed]
- Gordon, C.H.; Hendrix, E.; He, Y.; Walker, M.C. AlphaFold Accurately Predicts the Structure of Ribosomally Synthesized and Post-Translationally Modified Peptide Biosynthetic Enzymes. Biomolecules 2023, 13, 1243. [Google Scholar] [CrossRef] [PubMed]
- Huang, B.; Kong, L.; Wang, C.; Ju, F.; Zhang, Q.; Zhu, J.; Gong, T.; Zhang, H.; Yu, C.; Zheng, W.M.; Bu, D. Protein Structure Prediction: Challenges, Advances, and the Shift of Research Paradigms. Genomics, Proteomics & Bioinformatics 2023, 21, 913–925. [Google Scholar] [CrossRef]
- Koehler Leman, J.; Künze, G. Recent Advances in NMR Protein Structure Prediction with ROSETTA. International Journal of Molecular Sciences 2023, 24, 7835. [Google Scholar] [CrossRef]
- Rachitskii, P.; Kruglov, I.; Finkelstein, A.V.; Oganov, A.R. Protein structure prediction using the evolutionary algorithm USPEX. Proteins: Structure, Function, and Bioinformatics 2023, 91, 933–943. [Google Scholar] [CrossRef] [PubMed]
- Wuyun, Q.; Chen, Y.; Shen, Y.; Cao, Y.; Hu, G.; Cui, W.; Gao, J.; Zheng, W. Recent Progress of Protein Tertiary Structure Prediction. Molecules 2024, 29, 832. [Google Scholar] [CrossRef] [PubMed]
- Hameduh, T.; Mokry, M.; Miller, A.D.; Heger, Z.; Haddad, Y. Solvent Accessibility Promotes Rotamer Errors during Protein Modeling with Major Side-Chain Prediction Programs. Journal of Chemical Information and Modeling 2023, 63, 4405–4422. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.; Guo, Z.; Cheng, J. Atomic protein structure refinement using all-atom graph representations and SE(3)-equivariant graph transformer. Bioinformatics 2023, 39. [Google Scholar] [CrossRef] [PubMed]
- Schafer, J.W.; Porter, L.L. Evolutionary selection of proteins with two folds. Nature Communications 2023, 14. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Gao, S.; Lei, Z.; Xiong, R.; Cheng, J. Pareto Dominance Archive and Coordinated Selection Strategy-Based Many-Objective Optimizer for Protein Structure Prediction. IEEE/ACM Transactions on Computational Biology and Bioinformatics 2023, 20, 2328–2340. [Google Scholar] [CrossRef] [PubMed]
- Du, K.; Huang, H. Development of anti-PD-L1 antibody based on structure prediction of AlphaFold2. Frontiers in Immunology 2023, 14. [Google Scholar] [CrossRef] [PubMed]
- Mutz, P.; Resch, W.; Faure, G.; Senkevich, T.G.; Koonin, E.V.; Moss, B. Exaptation of Inactivated Host Enzymes for Structural Roles in Orthopoxviruses and Novel Folds of Virus Proteins Revealed by Protein Structure Modeling. mBio 2023, 14. [Google Scholar] [CrossRef] [PubMed]
- Biasini, M.; Bienert, S.; Waterhouse, A.; Arnold, K.; Studer, G.; Schmidt, T.; Kiefer, F.; Cassarino, T.G.; Bertoni, M.; Bordoli, L.; Schwede, T. SWISS-MODEL: modelling protein tertiary and quaternary structure using evolutionary information. Nucleic Acids Research 2014, 42, W252–W258. [Google Scholar] [CrossRef] [PubMed]
- Webb, B.; Sali, A. Protein Structure Modeling with MODELLER. In Methods in Molecular Biology; Springer US, 2020; pp. 239–255.
- Jumper, J.; Evans, R.; Pritzel, A.; Green, T.; figurnov, M.; Ronneberger, O.; Tunyasuvunakool, K.; Bates, R.; Žídek, A.; Potapenko, A.; Bridgland, A.; Meyer, C.; Kohl, S.A.A.; Ballard, A.J.; Cowie, A.; Romera-Paredes, B.; Nikolov, S.; Jain, R.; Adler, J.; Back, T.; Petersen, S.; Reiman, D.; Clancy, E.; Zielinski, M.; Steinegger, M.; Pacholska, M.; Berghammer, T.; Bodenstein, S.; Silver, D.; Vinyals, O.; Senior, A.W.; Kavukcuoglu, K.; Kohli, P.; Hassabis, D. Highly accurate protein structure prediction with AlphaFold. Nature 2021, 596, 583–589. [Google Scholar] [CrossRef] [PubMed]
- AlQuraishi, M. Machine learning in protein structure prediction. Current Opinion in Chemical Biology 2021, 65, 1–8. [Google Scholar] [CrossRef] [PubMed]
- AlQuraishi, M. AlphaFold at CASP13. Bioinformatics 2019, 35, 4862–4865. [Google Scholar] [CrossRef] [PubMed]
- Djulbegovic, M.; Taylor Gonzalez, D.J.; Antonietti, M.; Uversky, V.N.; Shields, C.L.; Karp, C.L. Intrinsic disorder may drive the interaction of PROS1 and MERTK in uveal melanoma. International Journal of Biological Macromolecules 2023, 250, 126027. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Gong, W.; Zhou, T.; Sun, X.; Chen, L.; Zhou, W.; Li, C. emPDBA: protein-DNA binding affinity prediction by combining features from binding partners and interface learned with ensemble regression model. Briefings in Bioinformatics 2023, 24. [Google Scholar] [CrossRef] [PubMed]
- Choudhury, J.; Yonezawa, K.; Anu, A.; Shimizu, N.; Chaudhuri, B. SAXS/WAXS data of conformationally flexible ribose binding protein. Data in Brief 2024, 52, 109932. [Google Scholar] [CrossRef]
- Qi, H.; Yu, T.; Yu, W.; Liu, C. Drug–target affinity prediction with extended graph learning-convolutional networks. BMC Bioinformatics 2024, 25. [Google Scholar] [CrossRef] [PubMed]
- Reggiano, G.; Lugmayr, W.; Farrell, D.; Marlovits, T.C.; DiMaio, F. Residue-level error detection in cryoelectron microscopy models. Structure 2023, 31, 860–869.e4. [Google Scholar] [CrossRef] [PubMed]
- Ravnik, V.; Jukič, M.; Bren, U. Identifying Metal Binding Sites in Proteins Using Homologous Structures, the MADE Approach. Journal of Chemical Information and Modeling 2023, 63, 5204–5219. [Google Scholar] [CrossRef] [PubMed]
- Szikszai, M.; Magnus, M.; Sanghi, S.; Kadyan, S.; Bouatta, N.; Rivas, E. RNA3DB: A structurally-dissimilar dataset split for training and benchmarking deep learning models for RNA structure prediction 2024. [CrossRef]
- Garcia-Pardo, J.; Badaczewska-Dawid, A.E.; Pintado-Grima, C.; Iglesias, V.; Kuriata, A.; Kmiecik, S.; Ventura, S. A3DyDB: exploring structural aggregation propensities in the yeast proteome. Microbial Cell Factories 2023, 22. [Google Scholar] [CrossRef] [PubMed]
- Sassanarakkit, S.; Peerapen, P.; Thongboonkerd, V. OxaBIND: A tool for identifying oxalate-binding domain(s)/motif(s) in protein(s). International Journal of Biological Macromolecules 2023, 243, 125275. [Google Scholar] [CrossRef]
- Kumar, A.; Kaynak, B.T.; Dorman, K.S.; Doruker, P.; Jernigan, R.L. Predicting allosteric pockets in protein biological assemblages. Bioinformatics 2023, 39. [Google Scholar] [CrossRef] [PubMed]
- Kamga Youmbi, F.I.; Kengne Tchendji, V.; Tayou Djamegni, C. P-FARFAR2: A multithreaded greedy approach to sampling low-energy RNA structures in Rosetta FARFAR2. Computational Biology and Chemistry 2023, 104, 107878. [Google Scholar] [CrossRef] [PubMed]
- Kurniawan, J.; Ishida, T. Comparing Supervised Learning and Rigorous Approach for Predicting Protein Stability upon Point Mutations in Difficult Targets. Journal of Chemical Information and Modeling 2023, 63, 6778–6788. [Google Scholar] [CrossRef] [PubMed]
- Nikam, R.; Yugandhar, K.; Gromiha, M.M. Deep learning-based method for predicting and classifying the binding affinity of protein-protein complexes. Biochimica et Biophysica Acta (BBA) - Proteins and Proteomics 2023, 1871, 140948. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.; Wang, Y.; Ou, G. Utilizing the scale-invariant feature transform algorithm to align distance matrices facilitates systematic protein structure comparison. Bioinformatics 2024, 40. [Google Scholar] [CrossRef] [PubMed]
- Hatano, Y.; Ishihara, T.; Onodera, O. Accuracy of a machine learning method based on structural and locational information from AlphaFold2 for predicting the pathogenicity of TARDBP and FUS gene variants in ALS. BMC Bioinformatics 2023, 24. [Google Scholar] [CrossRef] [PubMed]
- Manfredi, M.; Savojardo, C.; Martelli, P.L.; Casadio, R. ISPRED-SEQ: Deep Neural Networks and Embeddings for Predicting Interaction Sites in Protein Sequences. Journal of Molecular Biology 2023, 435, 167963. [Google Scholar] [CrossRef]
- Lichtinger, S.M.; Biggin, P.C. Tackling Hysteresis in Conformational Sampling: How to Be Forgetful with MEMENTO. Journal of Chemical Theory and Computation 2023, 19, 3705–3720. [Google Scholar] [CrossRef] [PubMed]
- Schweke, H.; Pacesa, M.; Levin, T.; Goverde, C.A.; Kumar, P.; Duhoo, Y.; Dornfeld, L.J.; Dubreuil, B.; Georgeon, S.; Ovchinnikov, S.; Woolfson, D.N.; Correia, B.E.; Dey, S.; Levy, E.D. An atlas of protein homo-oligomerization across domains of life. Cell 2024, 187, 999–1010.e15. [Google Scholar] [CrossRef] [PubMed]
- King, J.E.; Koes, D.R. Interpreting forces as deep learning gradients improves quality of predicted protein structures. Biophysical Journal 2023. [Google Scholar] [CrossRef] [PubMed]
- Boadu, F.; Cao, H.; Cheng, J. Combining protein sequences and structures with transformers and equivariant graph neural networks to predict protein function. Bioinformatics 2023, 39, i318–i325. [Google Scholar] [CrossRef] [PubMed]
- Brixi, G.; Ye, T.; Hong, L.; Wang, T.; Monticello, C.; Lopez-Barbosa, N.; Vincoff, S.; Yudistyra, V.; Zhao, L.; Haarer, E.; Chen, T.; Pertsemlidis, S.; Palepu, K.; Bhat, S.; Christopher, J.; Li, X.; Liu, T.; Zhang, S.; Petersen, L.; DeLisa, M.P.; Chatterjee, P. SaLT&PepPr is an interface-predicting language model for designing peptide-guided protein degraders. Communications Biology 2023, 6. [Google Scholar] [CrossRef]
- Mulvaney, T.; Kretsch, R.C.; Elliott, L.; Beton, J.G.; Kryshtafovych, A.; Rigden, D.J.; Das, R.; Topf, M. CASP15 cryo-EM protein and RNA targets: Refinement and analysis using experimental maps. Proteins: Structure, Function, and Bioinformatics 2023, 91, 1935–1951. [Google Scholar] [CrossRef]
- Edmunds, N.S.; Alharbi, S.M.A.; Genc, A.G.; Adiyaman, R.; McGuffin, L.J. Estimation of model accuracy in CASP15 using the ModFOLDdock server. Proteins: Structure, Function, and Bioinformatics 2023, 91, 1871–1878. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Zhang, C.; Omenn, G.S.; O’Meara, M.J.; Welch, J.D. Predicting the Structural Impact of Human Alternative Splicing 2023. [CrossRef]
- Maloney, A.; Joseph, S. Validating the EMCV IRES Secondary Structure with Structure–Function Analysis. Biochemistry 2023, 63, 107–115. [Google Scholar] [CrossRef]
- Shanker, S.; Sanner, M.F. Predicting Protein–Peptide Interactions: Benchmarking Deep Learning Techniques and a Comparison with Focused Docking. Journal of Chemical Information and Modeling 2023, 63, 3158–3170. [Google Scholar] [CrossRef] [PubMed]
- Han, S.R.; Park, M.; Kosaraju, S.; Lee, J.; Lee, H.; Lee, J.H.; Oh, T.J.; Kang, M. Evidential deep learning for trustworthy prediction of enzyme commission number. Briefings in Bioinformatics 2023, 25. [Google Scholar] [CrossRef]
- Nikam, R.; Yugandhar, K.; Gromiha, M.M. DeepBSRPred: deep learning-based binding site residue prediction for proteins. Amino Acids 2022, 55, 1305–1316. [Google Scholar] [CrossRef] [PubMed]
- Mikhaylov, V.; Brambley, C.A.; Keller, G.L.; Arbuiso, A.G.; Weiss, L.I.; Baker, B.M.; Levine, A.J. Accurate modeling of peptide-MHC structures with AlphaFold. Structure 2024, 32, 228–241. [Google Scholar] [CrossRef]
- Meller, A.; Kelly, D.; Smith, L.G.; Bowman, G.R. Toward physics-based precision medicine: Exploiting protein dynamics to design new therapeutics and interpret variants. Protein Science 2024, 33. [Google Scholar] [CrossRef] [PubMed]
- Li, W. Structural and Functional Consequences of the SMA-Linked Missense Mutations of the Survival Motor Neuron Protein: A Brief Update. In Novel Aspects on Motor Neuron Disease [Working Title]; IntechOpen, 2019.
- Li, W. Inter-Molecular Electrostatic Interactions Stabilizing the Structure of the PD-1/PD-L1 Axis: A Structural Evolutionary Perspective 2020.
- Li, W. Calcium Channel Trafficking Blocker Gabapentin Bound to the α-2-δ-1 Subunit of Voltage-Gated Calcium Channel: A Computational Structural Investigation 2020.
- Li, W. Structural Identification of the Electrostatic Hot Spots for Severe Acute Respiratory Syndrome Coronavirus Spike Protein to Be Complexed with Its Receptor ACE2 and Its Neutralizing Antibodies 2020.
- Li, W. Strengthening Semaglutide-GLP-1R Binding Affinity via a Val27-Arg28 Exchange in the Peptide Backbone of Semaglutide: A Computational Structural Approach. Journal of Computational Biophysics and Chemistry 2021, 20, 495–499. [Google Scholar] [CrossRef]
- Li, W. Delving deep into the structural aspects of a furin cleavage site inserted into the spike protein of SARS-CoV-2: A structural biophysical perspective. Biophysical Chemistry 2020, 264, 106420. [Google Scholar] [CrossRef]
- Li, W. Delving Deep into the Structural Aspects of the BPro28-BLys29 Exchange in Insulin Lispro: A Structural Biophysical Lesson 2020.
- Jänes, J.; Beltrao, P. Deep learning for protein structure prediction and design—progress and applications. Molecular Systems Biology 2024, 20, 162–169. [Google Scholar] [CrossRef]
- Kim, Y.; Kwon, J. AttSec: protein secondary structure prediction by capturing local patterns from attention map. BMC Bioinformatics 2023, 24. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Li, S.; Meng, K.; Sun, S. Machine Learning for Sequence and Structure-Based Protein–Ligand Interaction Prediction. Journal of Chemical Information and Modeling 2024, 64, 1456–1472. [Google Scholar] [CrossRef] [PubMed]
- Ooka, K.; Arai, M. Accurate prediction of protein folding mechanisms by simple structure-based statistical mechanical models. Nature Communications 2023, 14. [Google Scholar] [CrossRef] [PubMed]
- Peng, C.X.; Liang, F.; Xia, Y.H.; Zhao, K.L.; Hou, M.H.; Zhang, G.J. Recent Advances and Challenges in Protein Structure Prediction. Journal of Chemical Information and Modeling 2023, 64, 76–95. [Google Scholar] [CrossRef] [PubMed]
- Leemann, M.; Sagasta, A.; Eberhardt, J.; Schwede, T.; Robin, X.; Durairaj, J. Automated benchmarking of combined protein structure and ligand conformation prediction. Proteins: Structure, Function, and Bioinformatics 2023, 91, 1912–1924. [Google Scholar] [CrossRef] [PubMed]
- Versini, R.; Sritharan, S.; Aykac Fas, B.; Tubiana, T.; Aimeur, S.Z.; Henri, J.; Erard, M.; Nüsse, O.; Andreani, J.; Baaden, M.; Fuchs, P.; Galochkina, T.; Chatzigoulas, A.; Cournia, Z.; Santuz, H.; Sacquin-Mora, S.; Taly, A. A Perspective on the Prospective Use of AI in Protein Structure Prediction. Journal of Chemical Information and Modeling 2023, 64, 26–41. [Google Scholar] [CrossRef] [PubMed]
- Jiao, P.; Wang, B.; Wang, X.; Liu, B.; Wang, Y.; Li, J. Struct2GO: protein function prediction based on graph pooling algorithm and AlphaFold2 structure information. Bioinformatics 2023, 39. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Yan, Z.; Li, Z.; Qian, X.; Lu, S.; Dong, M.; Zhou, Q.; Yan, N. Structure of the voltage-gated calcium channel CaV1.1 at 3.6 Å resolution. Nature 2016, 537, 191–196. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Huang, G.; Wu, J.; Wu, Q.; Gao, S.; Yan, Z.; Lei, J.; Yan, N. Molecular Basis for Ligand Modulation of a Mammalian Voltage-Gated Ca2+ Channel. Cell 2019, 177, 1495–1506.e12. [Google Scholar] [CrossRef] [PubMed]
- Catterall, W.A. Structure and Regulation of Voltage-Gated Ca2+ Channels. Annual Review of Cell and Developmental Biology 2000, 16, 521–555. [Google Scholar] [CrossRef] [PubMed]
- Farmer, J.; Kanwal, F.; Nikulsin, N.; Tsilimigras, M.; Jacobs, D. Statistical Measures to Quantify Similarity between Molecular Dynamics Simulation Trajectories. Entropy 2017, 19, 646. [Google Scholar] [CrossRef]
- Zheng, L.; Shi, S.; Sun, X.; Lu, M.; Liao, Y.; Zhu, S.; Zhang, H.; Pan, Z.; Fang, P.; Zeng, Z.; Li, H.; Li, Z.; Xue, W.; Zhu, F. MoDAFold: a strategy for predicting the structure of missense mutant protein based on AlphaFold2 and molecular dynamics. Briefings in Bioinformatics 2024, 25. [Google Scholar] [CrossRef] [PubMed]
- Verdicchio, M.; Teijeiro Barjas, C. , Introduction to High-Performance Computing. In High Performance Computing for Drug Discovery and Biomedicine; Springer US, 2023; p. 15–29. [CrossRef]
- Heifetz, A. , Accelerating COVID-19 Drug Discovery with High-Performance Computing. In High Performance Computing for Drug Discovery and Biomedicine; Springer US, 2023; p. 405–411. [CrossRef]
- Jing, X.; Wu, F.; Luo, X.; Xu, J. Single-sequence protein structure prediction by integrating protein language models. Proceedings of the National Academy of Sciences 2024, 121. [Google Scholar] [CrossRef] [PubMed]
- Moussad, B.; Roche, R.; Bhattacharya, D. The transformative power of transformers in protein structure prediction. Proceedings of the National Academy of Sciences 2023, 120. [Google Scholar] [CrossRef] [PubMed]
- Fang, Y.; Jiang, Y.; Wei, L.; Ma, Q.; Ren, Z.; Yuan, Q.; Wei, D.Q. DeepProSite: structure-aware protein binding site prediction using ESMFold and pretrained language model. Bioinformatics 2023, 39. [Google Scholar] [CrossRef] [PubMed]
- Harini, K.; Sekijima, M.; Gromiha, M.M. PRA-Pred: Structure-based prediction of protein-RNA binding affinity. International Journal of Biological Macromolecules 2024, 259, 129490. [Google Scholar] [CrossRef]
- Oda, T. Improving protein structure prediction with extended sequence similarity searches and deep-learning-based refinement in CASP15. Proteins: Structure, Function, and Bioinformatics 2023, 91, 1712–1723. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Duan, R.; Zou, X. Template-guided method for protein–ligand complex structure prediction: Application to CASP15 protein–ligand studies. Proteins: Structure, Function, and Bioinformatics 2023, 91, 1829–1836. [Google Scholar] [CrossRef] [PubMed]
- Cen, L.P.; Ng, T.K.; Ji, J.; Lin, J.W.; Yao, Y.; Yang, R.; Dong, G.; Cao, Y.; Chen, C.; Yao, S.Q.; Wang, W.Y.; Huang, Z.; Qiu, K.; Pang, C.P.; Liu, Q.; Zhang, M. Artificial Intelligence-based database for prediction of protein structure and their alterations in ocular diseases. Database 2023, 2023. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, A.; Mondal, R.; Lahiri, T.; Chaurasiya, D.; Pal, M.K. TemPred: A Novel Protein Template Search Engine to Improve Protein Structure Prediction. IEEE/ACM Transactions on Computational Biology and Bioinformatics 2023, 20, 2112–2121. [Google Scholar] [CrossRef] [PubMed]
- Crystallography: Protein Data Bank. Nature New Biology 1971, 233, 223–223. [CrossRef]
- Berman, H.; Henrick, K.; Nakamura, H. Announcing the worldwide Protein Data Bank. Nature Structural & Molecular Biology 2003, 10, 980–980. [Google Scholar]
- Bonvin, A.M.J.J. 50 years of PDB: a catalyst in structural biology. Nature Methods 2021, 18, 448–449. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Cui, F.; Jernigan, R.; Wu, Z. PIDD: database for Protein Inter-atomic Distance Distributions. Nucleic Acids Research 2007, 35, D202–D207. [Google Scholar] [CrossRef] [PubMed]
- Li, W. Visualising the Experimentally Uncharted Territories of Membrane Protein Structures inside Protein Data Bank 2020.
- Li, W. A Local Spherical Coordinate System Approach to Protein 3D Structure Description 2020.
- Li, W. Two Achilles’ Heels of the Ebolavirus Glycoprotein? 2020.
- Broz, M.; Jukič, M.; Bren, U. Naive Prediction of Protein Backbone Phi and Psi Dihedral Angles Using Deep Learning. Molecules 2023, 28, 7046. [Google Scholar] [CrossRef] [PubMed]
- Batsanov, S. Calculation of van der Waals radii of atoms from bond distances. Journal of Molecular Structure: THEOCHEM 1999, 468, 151–159. [Google Scholar] [CrossRef]
- Caine, B.A.; Bronzato, M.; Popelier, P.L.A. Experiment stands corrected: accurate prediction of the aqueous pKa values of sulfonamide drugs using equilibrium bond lengths. Chemical Science 2019, 10, 6368–6381. [Google Scholar] [CrossRef] [PubMed]
- Li, W. NMR-Observed Atomic Bond Length Stability Supports a Dimensionality Shift in Protein Main Chain 3D Structure Description and Representation. Current Research Bioorganic Organic Chemistry 2018. [Google Scholar]
- Mysliwy, J.; Dingley, A.J.; Stanisak, M.; Jung, S.; Lorenzen, I.; Roeder, T.; Leippe, M.; Grötzinger, J. Caenopore-5: The three-dimensional structure of an antimicrobial protein from Caenorhabditis elegans. Dev. Comp. Immunol. 2010, 34, 323–330. [Google Scholar] [CrossRef]
- Roeder, T.; Stanisak, M.; Gelhaus, C.; Bruchhaus, I.; Grötzinger, J.; Leippe, M. Caenopores are antimicrobial peptides in the nematode Caenorhabditis elegans instrumental in nutrition and immunity. Dev. Comp. Immunol. 2010, 34, 203–209. [Google Scholar] [CrossRef] [PubMed]
- Li, W. Gravity-driven pH adjustment for site-specific protein pKa measurement by solution-state NMR. Measurement Science and Technology 2017, 28, 127002. [Google Scholar] [CrossRef]
- Li, W. Characterising the interaction between caenopore-5 and model membranes by NMR spectroscopy and molecular dynamics simulations. PhD thesis, University of Auckland, 2016.
- Humphrey, W.; Dalke, A.; Schulten, K. VMD: visual molecular dynamics. Journal of Molecular Graphics 1996, 14, 33–38. [Google Scholar] [CrossRef] [PubMed]
- Reyes, V.M. Representation of protein 3D structures in spherical (ρ, ϕ, θ) coordinates and two of its potential applications. Interdisciplinary Sciences: Computational Life Sciences 2011, 3. [Google Scholar]
- Plonski, A.P.; Reed, S.M. Assessing protein homology models with docking reproducibility. Journal of Molecular Graphics and Modelling 2023, 121, 108430. [Google Scholar] [CrossRef]
- Li, W.; Vottevor, G. Towards a Truly General Intermolecular Binding Affinity Calculator for Drug Discovery and Design 2023. [CrossRef]
- Lategan, F.A.; Schreiber, C.; Patterton, H.G. SeqPredNN: a neural network that generates protein sequences that fold into specified tertiary structures. BMC Bioinformatics 2023, 24. [Google Scholar] [CrossRef] [PubMed]
- Chu, L.; Ruffolo, J.A.; Harmalkar, A.; Gray, J.J. Flexible protein–protein docking with a multitrack iterative transformer. Protein Science 2024, 33. [Google Scholar] [CrossRef] [PubMed]
- Matveev, E.V.; Safronov, V.V.; Ponomarev, G.V.; Kazanov, M.D. Predicting Structural Susceptibility of Proteins to Proteolytic Processing. International Journal of Molecular Sciences 2023, 24, 10761. [Google Scholar] [CrossRef] [PubMed]
- Udupa, A.; Kotha, S.R.; Staller, M.V. Commonly asked questions about transcriptional activation domains. Current Opinion in Structural Biology 2024, 84, 102732. [Google Scholar] [CrossRef] [PubMed]
- Badaczewska-Dawid, A.; Wróblewski, K.; Kurcinski, M.; Kmiecik, S. Structure prediction of linear and cyclic peptides using CABS-flex. Briefings in Bioinformatics 2024, 25. [Google Scholar] [CrossRef] [PubMed]
- Guillon, C.; Robert, X.; Gouet, P. “It’s Only a Model”: When Protein Structure Predictions Need Experimental Validation, the Case of the HTLV-1 Tax Protein. Pathogens 2024, 13, 241. [Google Scholar] [CrossRef] [PubMed]
- Waksman, T.; Astin, E.; Fisher, S.R.; Hunter, W.N.; Bos, J.I.B. Computational Prediction of Structure, Function, and Interaction of Myzus persicae (Green Peach Aphid) Salivary Effector Proteins. Molecular Plant-Microbe Interactions® 2024, 37, 338–346. [Google Scholar] [CrossRef] [PubMed]
- Li, W. How do SMA-linked mutations of SMN1 lead to structural/functional deficiency of the SMA protein? PLOS ONE 2017, 12, e0178519. [Google Scholar] [CrossRef] [PubMed]
- Segura, J.; Rose, Y.; Bi, C.; Duarte, J.; Burley, S.K.; Bittrich, S. RCSB Protein Data Bank: visualizing groups of experimentally determined PDB structures alongside computed structure models of proteins. Frontiers in Bioinformatics 2023, 3. [Google Scholar] [CrossRef]
- Gupta, K. In silico structural and functional characterization of hypothetical proteins from Monkeypox virus. Journal of Genetic Engineering and Biotechnology 2023, 21, 46. [Google Scholar] [CrossRef]
- Sawa, T.; Moriwaki, Y.; Jiang, H.; Murase, K.; Takayama, S.; Shimizu, K.; Terada, T. Comprehensive computational analysis of the SRK–SP11 molecular interaction underlying self-incompatibility in Brassicaceae using improved structure prediction for cysteine-rich proteins. Computational and Structural Biotechnology Journal 2023, 21, 5228–5239. [Google Scholar] [CrossRef] [PubMed]
- Kosugi, T.; Ohue, M. Design of Cyclic Peptides Targeting Protein–Protein Interactions Using AlphaFold. International Journal of Molecular Sciences 2023, 24, 13257. [Google Scholar] [CrossRef]
- Ertelt, M.; Mulligan, V.K.; Maguire, J.B.; Lyskov, S.; Moretti, R.; Schiffner, T.; Meiler, J.; Schoeder, C.T. Combining machine learning with structure-based protein design to predict and engineer post-translational modifications of proteins. PLOS Computational Biology 2024, 20, e1011939. [Google Scholar] [CrossRef]
- Li, S.; Tian, T.; Zhang, Z.; Zou, Z.; Zhao, D.; Zeng, J. PocketAnchor: Learning structure-based pocket representations for protein-ligand interaction prediction. Cell Systems 2023, 14, 692–705. [Google Scholar] [CrossRef] [PubMed]
- Davidson, R.B.; Coletti, M.; Gao, M.; Piatkowski, B.; Sreedasyam, A.; Quadir, F.; Weston, D.J.; Schmutz, J.; Cheng, J.; Skolnick, J.; Parks, J.M.; Sedova, A. Predicted structural proteome of Sphagnum divinum and proteome-scale annotation. Bioinformatics 2023, 39. [Google Scholar] [CrossRef] [PubMed]
- Guzmán-Vega, F.J.; González-Álvarez, A.C.; Peña-Guerra, K.A.; Cardona-Londoño, K.J.; Arold, S.T. Leveraging AI Advances and Online Tools for Structure-Based Variant Analysis. Current Protocols 2023, 3. [Google Scholar] [CrossRef] [PubMed]
- Li, W. Structurally Observed Electrostatic Features of the COVID-19 Coronavirus-Related Experimental Structures inside Protein Data Bank: A Brief Update 2020.
- Li, W. Extracting the Interfacial Electrostatic Features from Experimentally Determined Antigen and/or Antibody-Related Structures inside Protein Data Bank for Machine Learning-Based Antibody Design 2020.
- Jeppesen, M.; André, I. Accurate prediction of protein assembly structure by combining AlphaFold and symmetrical docking. Nature Communications 2023, 14. [Google Scholar] [CrossRef] [PubMed]
- Varadi, M.; Tsenkov, M.; Velankar, S. Challenges in bridging the gap between protein structure prediction and functional interpretation. Proteins: Structure, Function, and Bioinformatics. [CrossRef]
- Xia, Y.; Zhao, K.; Liu, D.; Zhou, X.; Zhang, G. Multi-domain and complex protein structure prediction using inter-domain interactions from deep learning. Communications Biology 2023, 6. [Google Scholar] [CrossRef]
- Camponeschi, C.; Righino, B.; Pirolli, D.; Semeraro, A.; Ria, F.; De Rosa, M.C. Prediction of CD44 Structure by Deep Learning-Based Protein Modeling. Biomolecules 2023, 13, 1047. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.W.; Won, J.H.; Jeon, S.; Choo, Y.; Yeon, Y.; Oh, J.S.; Kim, M.; Kim, S.; Joung, I.; Jang, C.; Lee, S.J.; Kim, T.H.; Jin, K.H.; Song, G.; Kim, E.S.; Yoo, J.; Paek, E.; Noh, Y.K.; Joo, K. DeepFold: enhancing protein structure prediction through optimized loss functions, improved template features, and re-optimized energy function. Bioinformatics 2023, 39. [Google Scholar] [CrossRef] [PubMed]
- Kryshtafovych, A.; Schwede, T.; Topf, M.; Fidelis, K.; Moult, J. Critical assessment of methods of protein structure prediction (CASP)—Round XV. Proteins: Structure, Function, and Bioinformatics 2023, 91, 1539–1549. [Google Scholar] [CrossRef] [PubMed]
- Li, W. Towards a General Intermolecular Binding Affinity Calculator 2022. [CrossRef]
- Baek, M.; DiMaio, F.; Anishchenko, I.; Dauparas, J.; Ovchinnikov, S.; Lee, G.R.; Wang, J.; Cong, Q.; Kinch, L.N.; Schaeffer, R.D.; Millán, C.; Park, H.; Adams, C.; Glassman, C.R.; DeGiovanni, A.; Pereira, J.H.; Rodrigues, A.V.; van Dijk, A.A.; Ebrecht, A.C.; Opperman, D.J.; Sagmeister, T.; Buhlheller, C.; Pavkov-Keller, T.; Rathinaswamy, M.K.; Dalwadi, U.; Yip, C.K.; Burke, J.E.; Garcia, K.C.; Grishin, N.V.; Adams, P.D.; Read, R.J.; Baker, D. Accurate prediction of protein structures and interactions using a three-track neural network. Science 2021, 373, 871–876. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Anishchenko, I.; Park, H.; Peng, Z.; Ovchinnikov, S.; Baker, D. Improved protein structure prediction using predicted interresidue orientations. Proceedings of the National Academy of Sciences 2020, 117, 1496–1503. [Google Scholar] [CrossRef] [PubMed]
- AlQuraishi, M. A watershed moment for protein structure prediction. Nature 2020, 577, 627–628. [Google Scholar] [CrossRef] [PubMed]
- Senior, A.W.; Evans, R.; Jumper, J.; Kirkpatrick, J.; Sifre, L.; Green, T.; Qin, C.; Žídek, A.; Nelson, A.W.R.; Bridgland, A.; Penedones, H.; Petersen, S.; Simonyan, K.; Crossan, S.; Kohli, P.; Jones, D.T.; Silver, D.; Kavukcuoglu, K.; Hassabis, D. Improved protein structure prediction using potentials from deep learning. Nature 2020, 577, 706–710. [Google Scholar] [CrossRef] [PubMed]
- Hey, T.; Butler, K.; Jackson, S.; Thiyagalingam, J. Machine learning and big scientific data. Philosophical Transactions of the Royal Society A: Mathematical, Physical and Engineering Sciences 2020, 378, 20190054. [Google Scholar] [CrossRef] [PubMed]
- Senior, A.W.; Evans, R.; Jumper, J.; Kirkpatrick, J.; Sifre, L.; Green, T.; Qin, C.; Žídek, A.; Nelson, A.W.R.; Bridgland, A.; Penedones, H.; Petersen, S.; Simonyan, K.; Crossan, S.; Kohli, P.; Jones, D.T.; Silver, D.; Kavukcuoglu, K.; Hassabis, D. Protein structure prediction using multiple deep neural networks in the 13th Critical Assessment of Protein Structure Prediction (CASP13). Proteins: Structure, Function, and Bioinformatics 2019, 87, 1141–1148. [Google Scholar] [CrossRef] [PubMed]
- Pinheiro, F.; Santos, J.; Ventura, S. AlphaFold and the amyloid landscape. Journal of Molecular Biology 2021, 167059. [Google Scholar] [CrossRef] [PubMed]
- Ruff, K.M.; Pappu, R.V. AlphaFold and Implications for Intrinsically Disordered Proteins. Journal of Molecular Biology 2021, 167208. [Google Scholar] [CrossRef] [PubMed]
- Higgins, M.K. Can We AlphaFold Our Way Out of the Next Pandemic? Journal of Molecular Biology 2021, 167093. [Google Scholar] [CrossRef] [PubMed]
- Fersht, A.R. AlphaFold: A Personal Perspective on the Impact of Machine Learning. Journal of Molecular Biology 2021, 167088. [Google Scholar] [CrossRef] [PubMed]
- Zweckstetter, M. NMR hawk-eyed view of AlphaFold2 structures. Protein Science 2021. [Google Scholar] [CrossRef] [PubMed]
- Strodel, B. Energy Landscapes of Protein Aggregation and Conformation Switching in Intrinsically Disordered Proteins. Journal of Molecular Biology 2021, 167182. [Google Scholar] [CrossRef] [PubMed]
- Ayoub, R.; Lee, Y. Protein structure search to support the development of protein structure prediction methods. Proteins: Structure, Function, and Bioinformatics 2021, 89, 648–658. [Google Scholar] [CrossRef] [PubMed]
- Tunyasuvunakool, K.; Adler, J.; Wu, Z.; Green, T.; Zielinski, M.; Žídek, A.; Bridgland, A.; Cowie, A.; Meyer, C.; Laydon, A.; Velankar, S.; Kleywegt, G.J.; Bateman, A.; Evans, R.; Pritzel, A.; figurnov, M.; Ronneberger, O.; Bates, R.; Kohl, S.A.A.; Potapenko, A.; Ballard, A.J.; Romera-Paredes, B.; Nikolov, S.; Jain, R.; Clancy, E.; Reiman, D.; Petersen, S.; Senior, A.W.; Kavukcuoglu, K.; Birney, E.; Kohli, P.; Jumper, J.; Hassabis, D. Highly accurate protein structure prediction for the human proteome. Nature 2021, 596, 590–596. [Google Scholar] [CrossRef] [PubMed]
- Simpkin, A.J.; filomeno Sánchez Rodríguez.; Mesdaghi, S.; Kryshtafovych, A. Rigden, D.J. Evaluation of model refinement in CASP14. Proteins, Structure, Function, and Bioinformatics 2021.
- Diwan, G.D.; Gonzalez-Sanchez, J.C.; Apic, G.; Russell, R.B. Next Generation Protein Structure Predictions and Genetic Variant Interpretation. Journal of Molecular Biology 2021, 167180. [Google Scholar] [CrossRef] [PubMed]
- Abriata, L.A.; Peraro, M.D. State-of-the-art web services for de novo protein structure prediction. Briefings in Bioinformatics 2020, 22. [Google Scholar]
- Billings, W.M.; Morris, C.J.; Corte, D.D. The whole is greater than its parts: ensembling improves protein contact prediction. Scientific Reports 2021, 11. [Google Scholar] [CrossRef] [PubMed]
- Layer, G.; Reichelt, J.; Jahn, D.; Heinz, D.W. Structure and function of enzymes in heme biosynthesis. Protein Science 2010, 19, 1137–1161. [Google Scholar] [CrossRef] [PubMed]
- McBride, J.M.; Polev, K.; Abdirasulov, A.; Reinharz, V.; Grzybowski, B.A.; Tlusty, T. AlphaFold2 Can Predict Single-Mutation Effects. Physical Review Letters 2023, 131. [Google Scholar] [CrossRef] [PubMed]
- Karelina, M.; Noh, J.J.; Dror, R.O. How accurately can one predict drug binding modes using AlphaFold models? eLife 2023, 12. [Google Scholar] [CrossRef]
- Jambrich, M.A.; Tusnady, G.E.; Dobson, L. How AlphaFold2 shaped the structural coverage of the human transmembrane proteome. Scientific Reports 2023, 13. [Google Scholar] [CrossRef] [PubMed]
- Perlinska, A.P.; Niemyska, W.H.; Gren, B.A.; Bukowicki, M.; Nowakowski, S.; Rubach, P.; Sulkowska, J.I. AlphaFold predicts novel human proteins with knots. Protein Science 2023, 32. [Google Scholar] [CrossRef] [PubMed]
- Manalastas-Cantos, K.; Adoni, K.R.; Pfeifer, M.; Märtens, B.; Grünewald, K.; Thalassinos, K.; Topf, M. Modeling Flexible Protein Structure With AlphaFold2 and Crosslinking Mass Spectrometry. Molecular & Cellular Proteomics 2024, 23, 100724. [Google Scholar] [CrossRef]
- Li, W. Half-a-century Burial of ρ, θ and φ in PDB 2021. [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).