Submitted:
08 April 2024
Posted:
09 April 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Preparation PFA-I (Control)
- Retention Time Impact: We observed that irrespective of the acetic acid quantity used, the retention time during adding the primary acid significantly impacted the resulting molecular weight (Mw). Thus, exploring a wider range of acetic acid quantities would not have yielded meaningful impacts on the Mw.
- Viscometer Constraints: In-situ monitoring of the reaction within a viscometer was deemed infeasible due to potential instrument damage. Therefore, we opted for the pragmatic approach of physically observing the viscosity increase to determine the optimal neutralisation point and halt the reaction progression.
2.2.2. Preparation of PFA-II (Primary)
2.3. Characterization of Resins
2.3.1. Size Exclusion Chromatography
2.3.2. Fourier Transform Infra-Red Spectroscopy (FTIR)
2.3.3. Thermogravimetric Analysis (TGA)
2.3.4. Differential Scanning Calorimetry (DSC)
2.3.5. Scanning Electron Microscopy (SEM)
2.3.6. 1. H, 13C, and DOSY Nuclear Magnetic Resonance (NMR)
2.3.7. Matrix-Assisted Laser Desorption/Ionization Time-of-Flight Spectrometry (MALDI-TOF)
3. Results and Discussion
3.1. Molecular Weight Determination
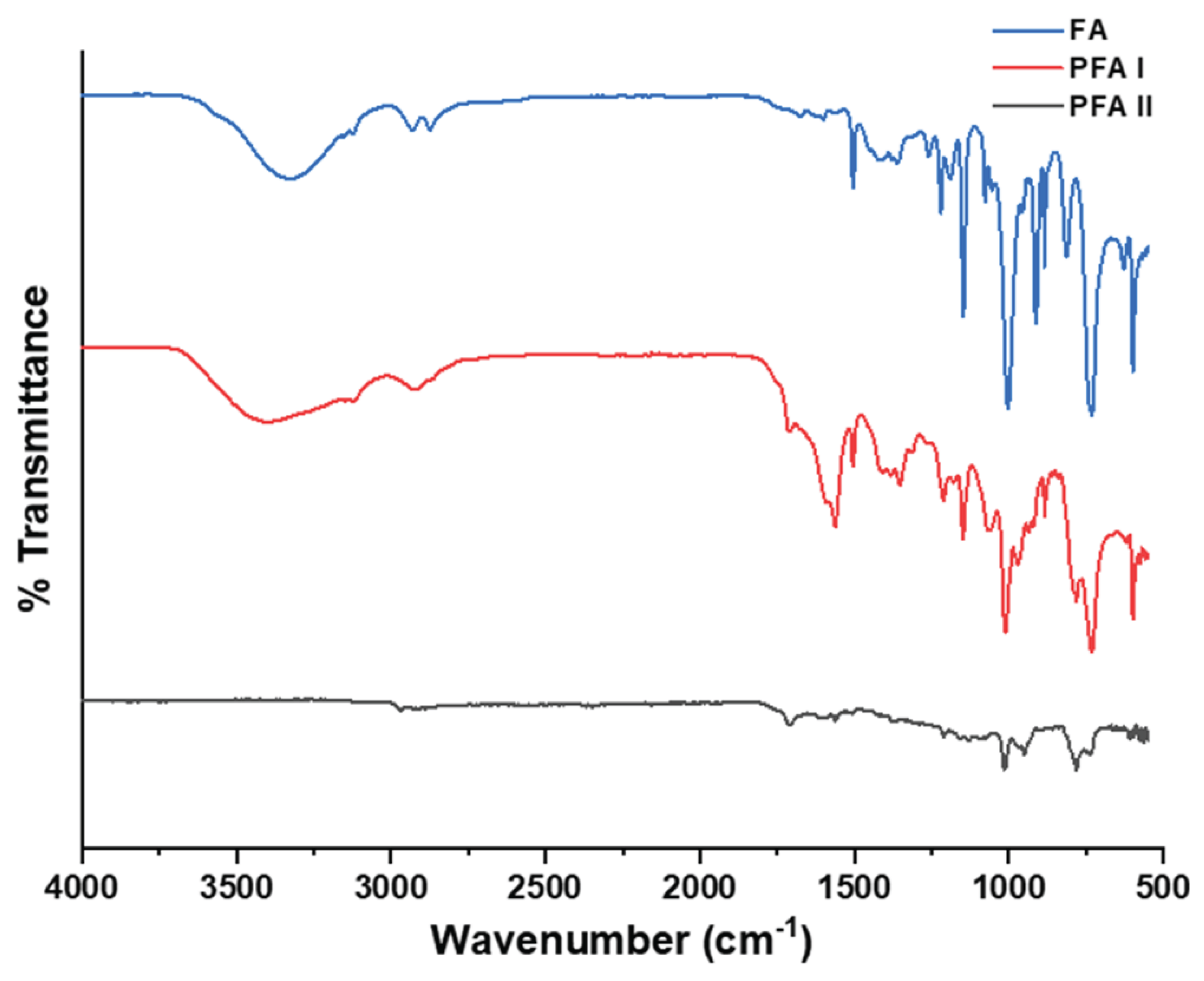
3.2. FTIR Spectroscopy Analysis
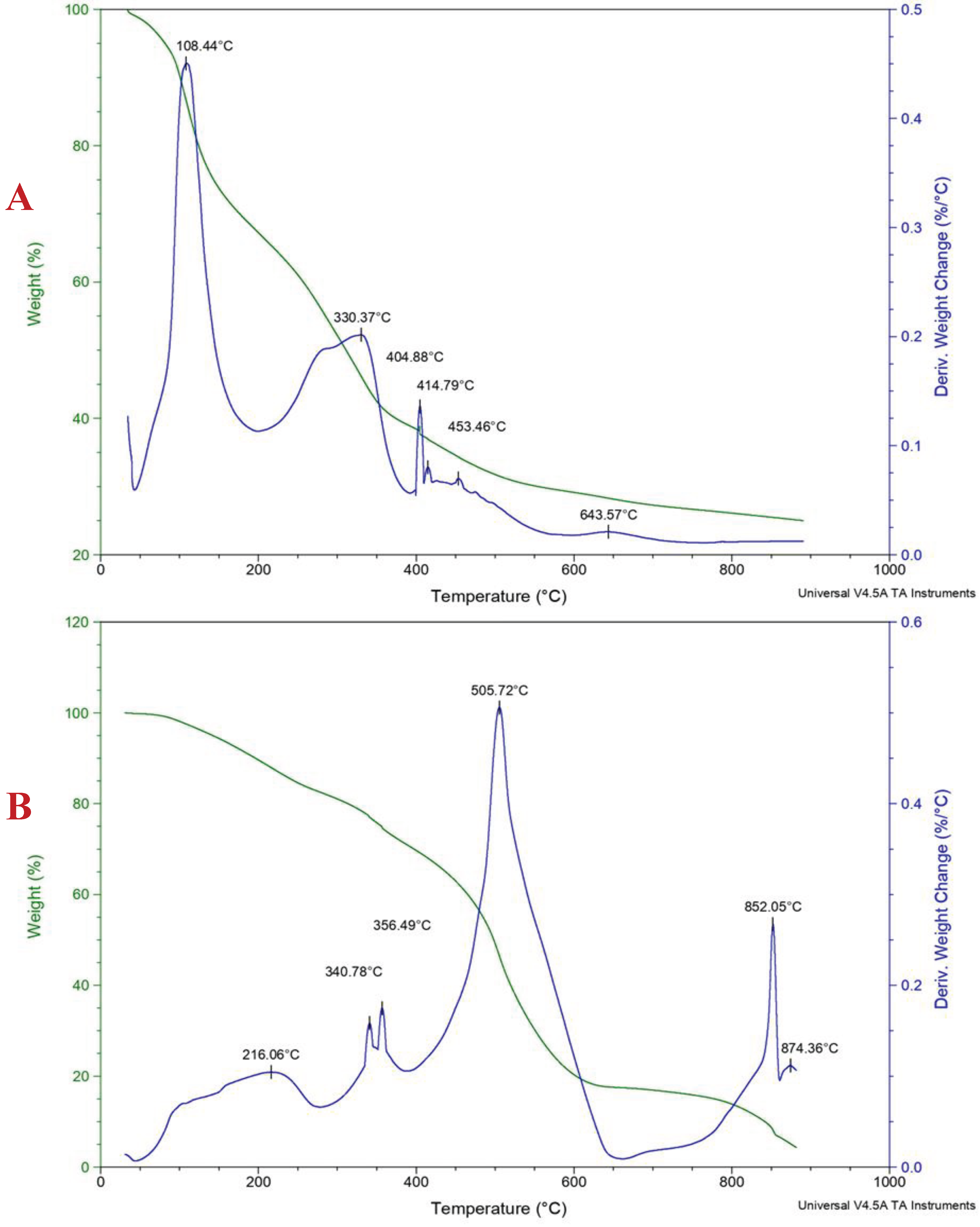
3.3. Thermal Degradation Characteristics
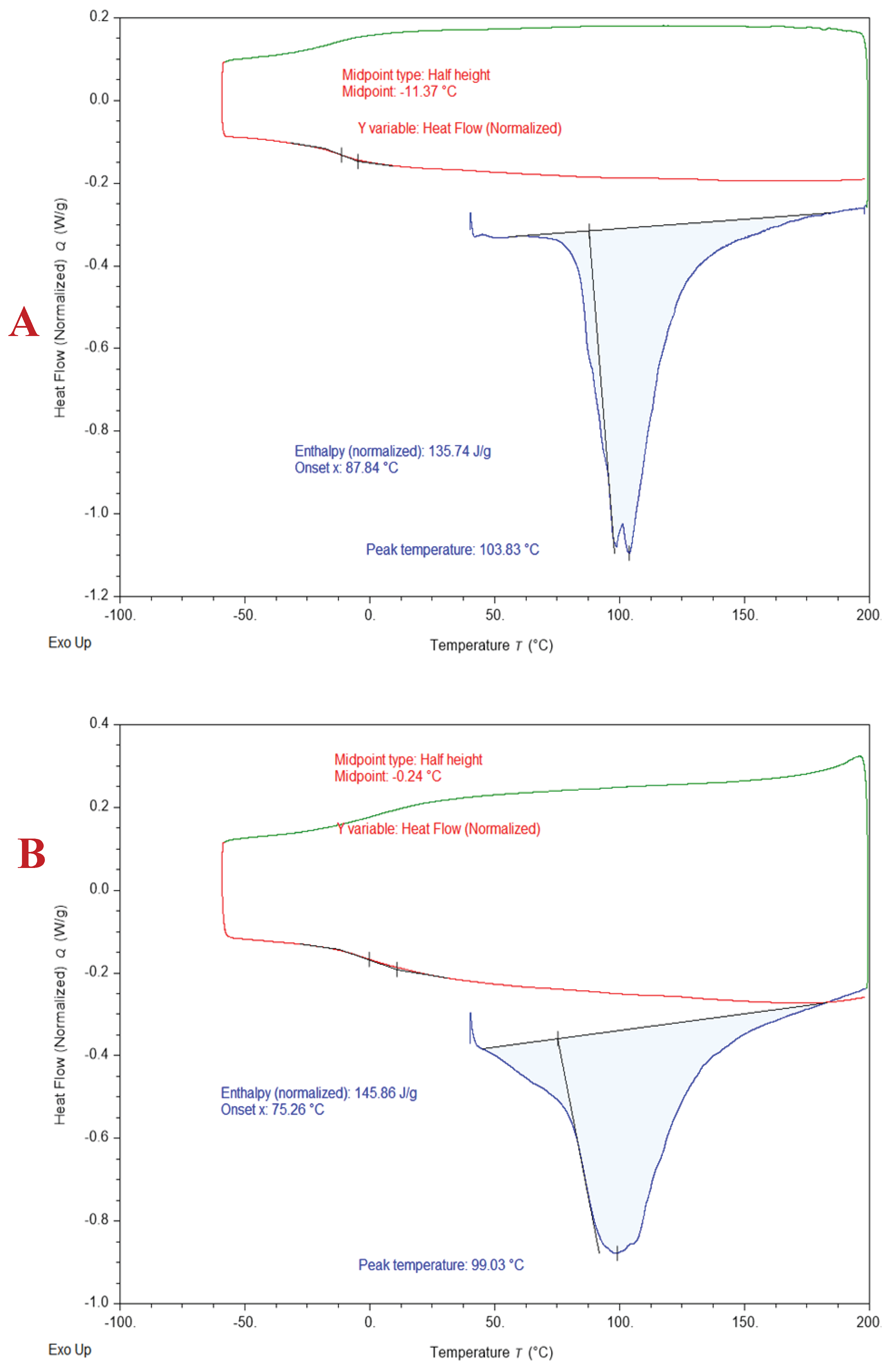
3.4. DSC Analysis
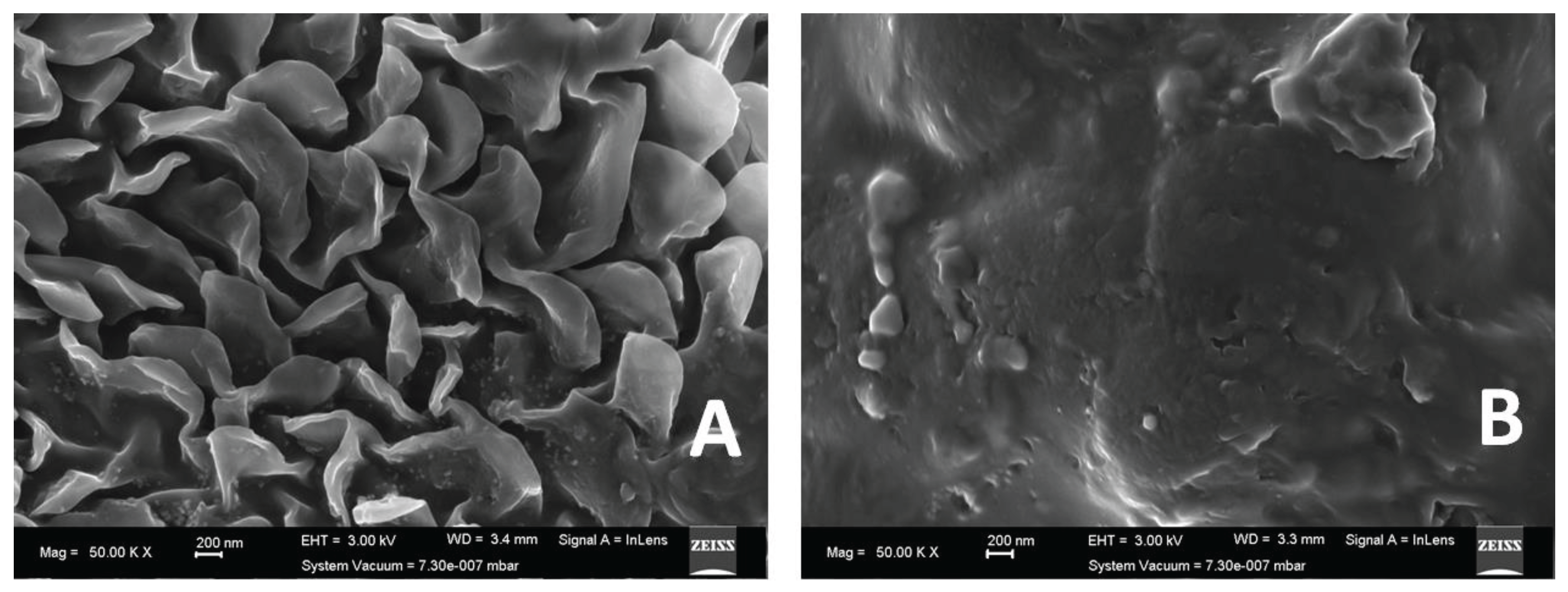
3.5. SEM Analysis
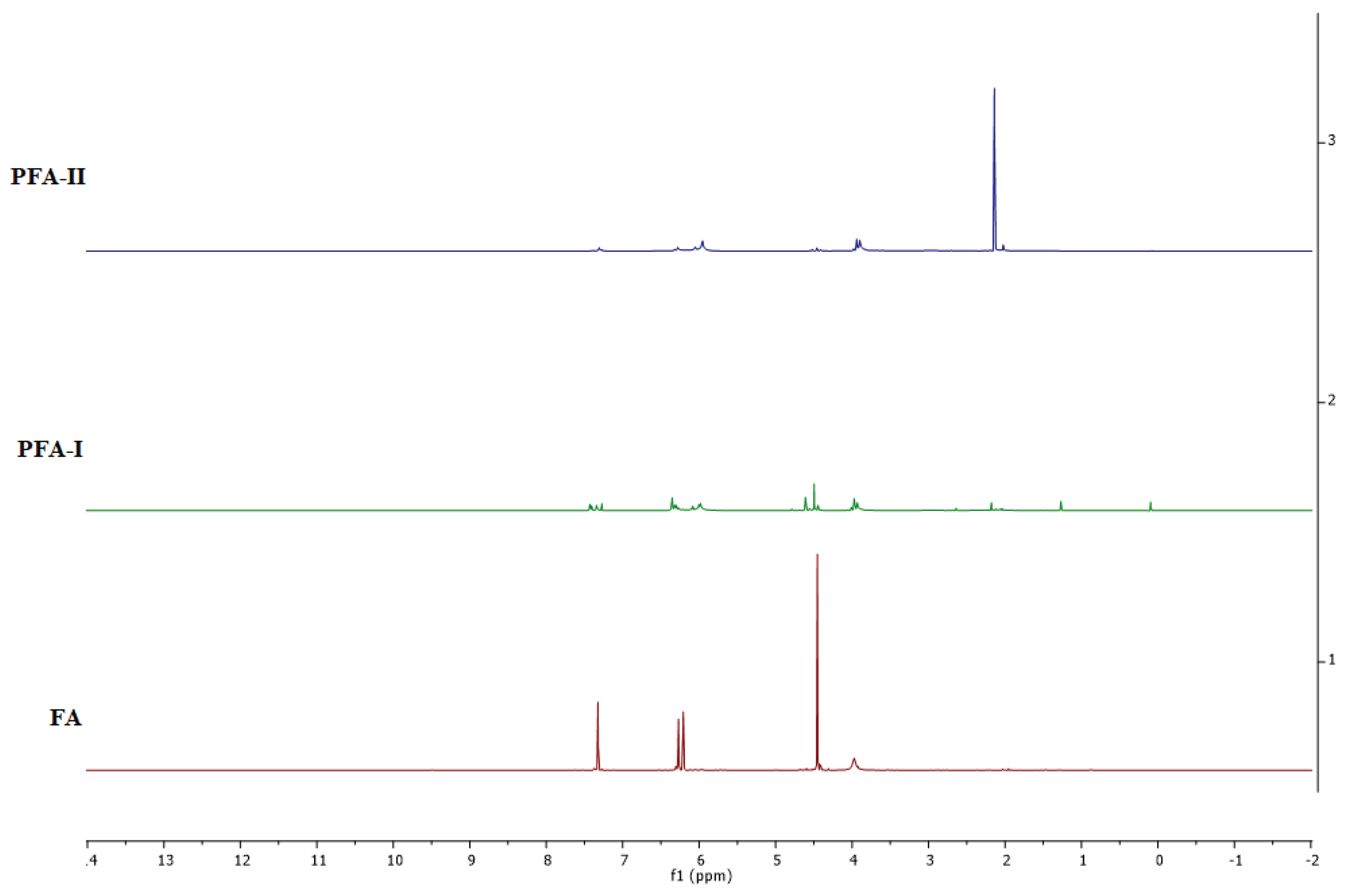
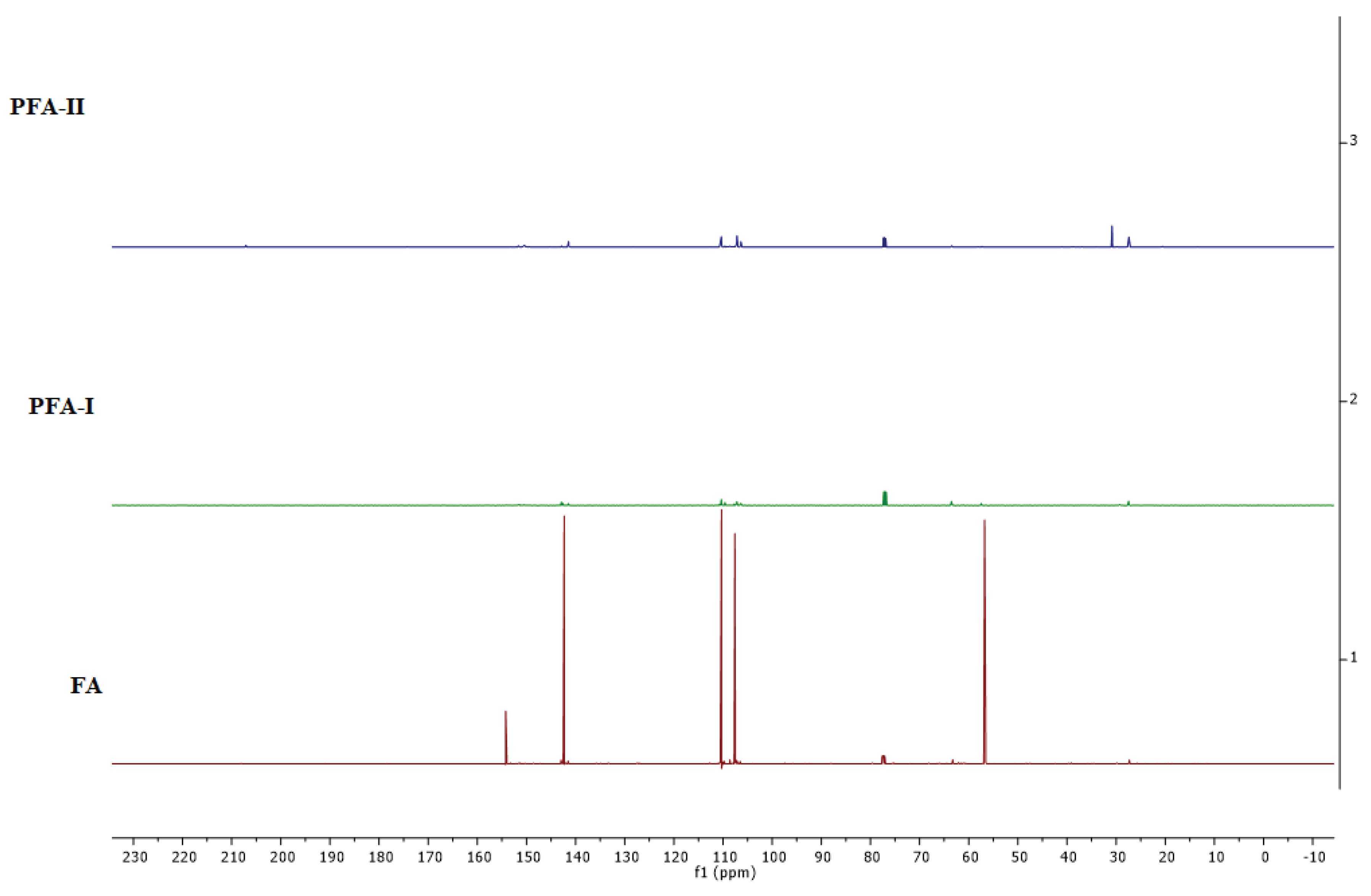
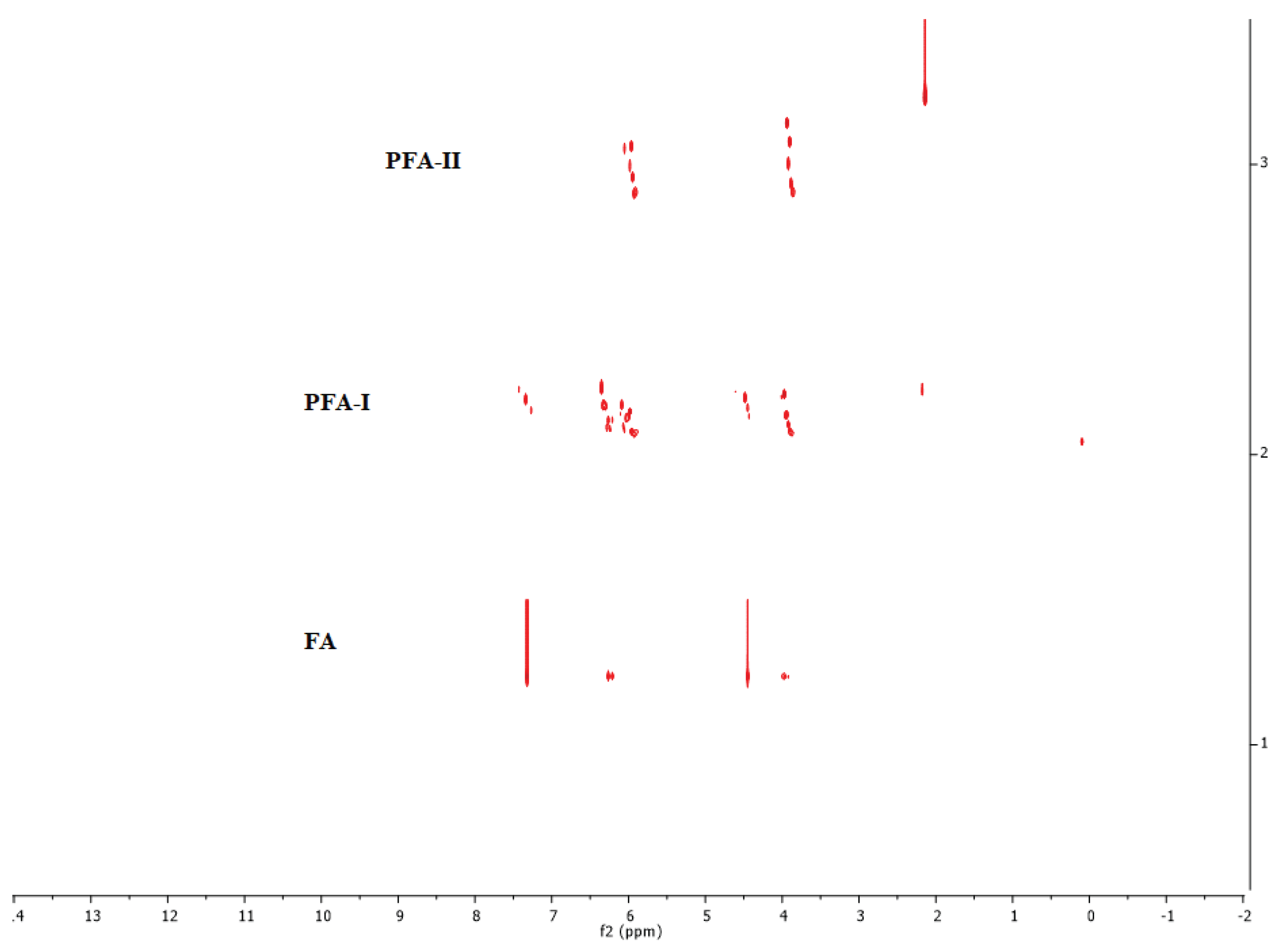
3.6. NMR Analysis
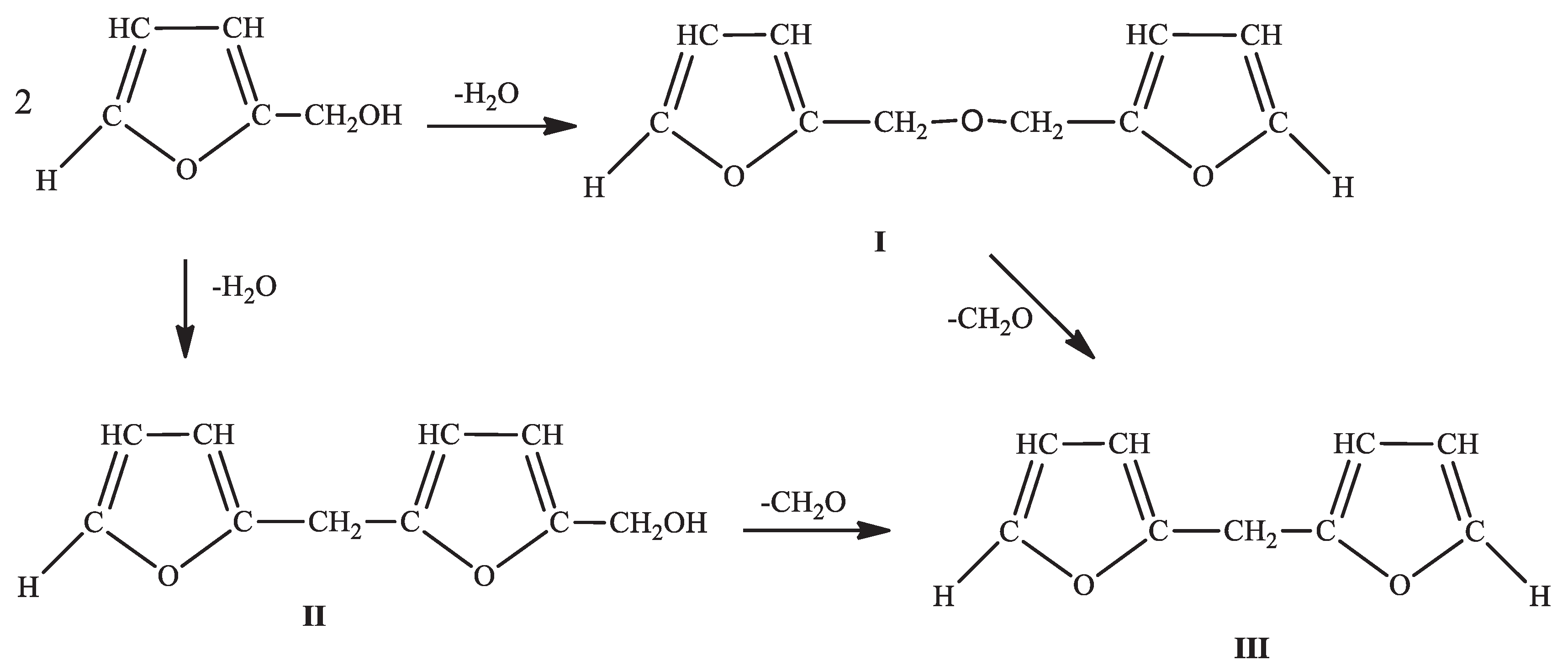
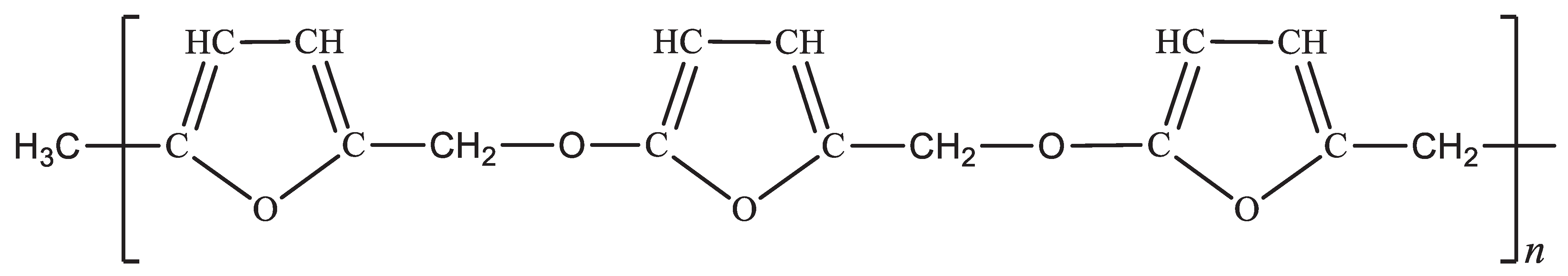
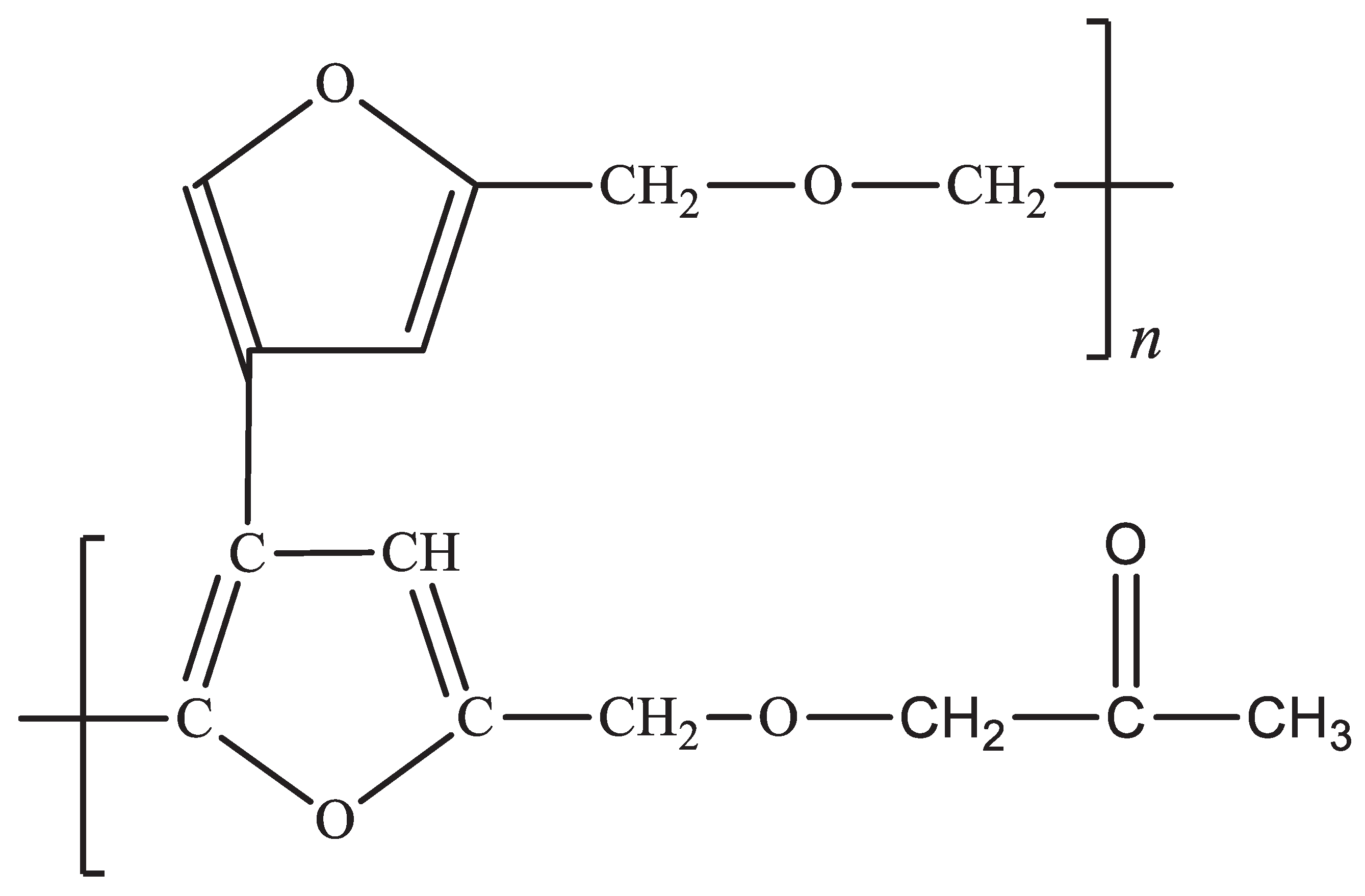
4. Structural Elucidation
5. Conclusion
6. Prospect
Supplementary Materials
Author Contributions
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflict of Interests
References
- Dunlop, A. P.; Peters, F. N. The Nature of Furfuryl Alcohol. Ind Eng Chem 1942, 34, 814–817. [Google Scholar] [CrossRef]
- Dunlop, A. P.; Peters, F. N. The Furans; Reinhold Publishing Corporation: United States, 1953. [Google Scholar]
- Conley, R. T.; Metil, I. An Investigation of the Structure of Furfuryl Alcohol Polycondensates with Infrared Spectroscopy. J Appl Polym Sci 1963, 7, 37–52. [Google Scholar] [CrossRef]
- Iroegbu, A. O.; Hlangothi, S. P. Effects of the Type of Catalyst on the Polymerisation Mechanism of Furfuryl Alcohol and Its Resultant Properties. Chemistry Africa 2018, 1, 187–197. [Google Scholar] [CrossRef]
- Hirasaki, T.; Meguro, T.; Wakihara, T.; Tatami, J.; Komeya, K. Effect of Ultrasonic Irradiation in the Polymerization of Furfuryl Alcohol, with Ethylene Glycol as a Pore-Forming Agent, on the Microstructure of the Derived Carbons. Carbon N Y 2008, 46, 1630. [Google Scholar] [CrossRef]
- Hoshi, K.; Akatsu, T.; Tanabe, Y.; Yasuda, E. Curing Properties of Furfuryl Alcohol Condensate with Carbonaceous Fine Particles under Ultrasonication. Ultrason Sonochem 2001, 8, 89–92. [Google Scholar] [CrossRef] [PubMed]
- Batista, P. dos S.; de Souza, M. F. Furfuryl Alcohol Polymerisation inside Capillaries. Synth Met 1999, 101, 635–636. [Google Scholar] [CrossRef]
- Barr, J. B.; Wallon, S. B. The Chemistry of Furfuryl Alcohol Resins. J Appl Polym Sci 1971, 15, 1079–1090. [Google Scholar] [CrossRef]
- Ünver, H.; Öktem, Z. Controlled Cationic Polymerization of Furfuryl Alcohol. Eur Polym J 2013, 49, 1023–1030. [Google Scholar] [CrossRef]
- Choura, M.; Belgacem, N. M.; Gandini, A. Acid-Catalyzed Polycondensation of Furfuryl Alcohol: Mechanisms of Chromophore Formation and Cross-Linking. Macromolecules 1996, 29, 3839–3850. [Google Scholar] [CrossRef]
- Rathi, A. K. A.; Chanda, M. Kinetics of Resinification of Furfuryl Alcohol in Aqueous Solution. J Appl Polym Sci 1974, 18, 1541–1548. [Google Scholar] [CrossRef]
- Krishnan, T. A.; Chanda, M. Kinetics of Polymerisation of Furfuryl Alcohol in Aqueous Solution. Die Angewandte Makromolekulare Chemie 1975, 43, 145–156. [Google Scholar] [CrossRef]
- Wewerka, E. M. Study of the γ-Alumina Polymerization of Furfuryl Alcohol. J Polym Sci A1 1971, 9, 2703–2715. [Google Scholar] [CrossRef]
- Hronec, M.; Fulajtárová, K.; Soták, T. Kinetics of High Temperature Conversion of Furfuryl Alcohol in Water. Journal of Industrial and Engineering Chemistry 2014, 20, 650–655. [Google Scholar] [CrossRef]
- Conley, R. T.; Metil, I. An Investigation of the Structure of Furfuryl Alcohol Polycondensates with Infrared Spectroscopy. J Appl Polym Sci 1963, 7, 37–52. [Google Scholar] [CrossRef]
- Kim, T.; Jeong, J.; Rahman, M.; Zhu, E.; Mahajan, D. Characterizations of Furfuryl Alcohol Oligomer/Polymerization Catalyzed by Homogeneous and Heterogeneous Acid Catalysts. Korean Journal of Chemical Engineering 2014, 31, 2124–2129. [Google Scholar] [CrossRef]
- Wewerka, E. M. An Investigation of the Polymerization of Furfuryl Alcohol with Gel Permeation Chromatography. J Appl Polym Sci 1968, 12, 1671–1681. [Google Scholar] [CrossRef]
- Ruelens, W.; Koolen, G.; Gricourt, B.; Willem van Vuure, A.; Smet, M. Co-Polymerisation of Furfuryl Alcohol for Furan Resins with Increased Toughness. Mater Lett 2022, 328, 133025. [Google Scholar] [CrossRef]
- Reyhani, A.; Allison-Logan, S.; Ranji-Burachaloo, H.; McKenzie, T. G.; Bryant, G.; Qiao, G. G. Synthesis of Ultra-high Molecular Weight Polymers by Controlled Production of Initiating Radicals. J Polym Sci A Polym Chem 2019, 57, 1922–1930. [Google Scholar] [CrossRef]
- Bai, Y.; He, J.; Zhang, Y. Ultra-High-Molecular-Weight Polymers Produced by the Immortal Phosphine-Based Catalyst System. Angewandte Chemie 2018, 130, 17476–17480. [Google Scholar] [CrossRef]
- Charles, E. Carraher Jr. Carraher’s Polymer Chemistry, 10th ed.; Taylor & Francis Group, LLC: Boca Raton, FL, United States, 2018. [Google Scholar]
- Billmeyer, F. W. Textbook of Polymer Science, 3rd ed.; Wiley-VCH: New York, 1962. [Google Scholar]
- KIM, Y.; PARK, H.; LEE, Y. Gas Separation Properties of Carbon Molecular Sieve Membranes Derived from Polyimide/Polyvinylpyrrolidone Blends: Effect of the Molecular Weight of Polyvinylpyrrolidone. J Memb Sci 2005, 251, 159–167. [Google Scholar] [CrossRef]
- Tsai, J.-S.; Lin, C.-H. The Effect of Molecular Weight on the Cross Section and Properties of Polyacrylonitrile Precursor and Resulting Carbon Fiber. J Appl Polym Sci 1991, 42, 3045–3050. [Google Scholar] [CrossRef]
- Morris, E. A.; Weisenberger, M. C.; Bradley, S. B.; Abdallah, M. G.; Mecham, S. J.; Pisipati, P.; McGrath, J. E. Synthesis, Spinning, and Properties of Very High Molecular Weight Poly(Acrylonitrile-Co-Methyl Acrylate) for High Performance Precursors for Carbon Fiber. Polymer (Guildf) 2014, 55, 6471–6482. [Google Scholar] [CrossRef]
- Iroegbu, A. O. C.; Ray, S. S. Bamboos: From Bioresource to Sustainable Materials and Chemicals. Sustainability (Switzerland) 2021, 13, 12200. [Google Scholar] [CrossRef]
- Iroegbu, A. O. C.; Ray, S. S. Biorenewables: Properties and Functions in Materials Application. In ACS Symposium Series; Pathania, D., Singh, L., Eds.; ACS Symposium Series, 2022; Vol. 1, pp 129–161. [CrossRef]
- Schmitt, C. R. Polyfurfuryl Alcohol Resins. Polym Plast Technol Eng 1974, 3, 121–158. [Google Scholar] [CrossRef]
- Wewerka, E. M.; Loughran, E. D.; Walters, K. L. A Study of the Low Molecular Weight Components of Furfuryl Alcohol Polymers. J Appl Polym Sci 1971, 15, 1437–1451. [Google Scholar] [CrossRef]
- Brian, C. Smith. Fundamentals of Fourier Transform Infrared Spectroscopy; Taylor and Francis Group, LLC: Boca Raton, FL, USA, 2011. [Google Scholar]
- Tondi, G.; Cefarin, N.; Sepperer, T.; D’Amico, F.; Berger, R. J. F.; Musso, M.; Birarda, G.; Reyer, A.; Schnabel, T.; Vaccari, L. Understanding the Polymerization of Polyfurfuryl Alcohol: Ring Opening and Diels-Alder Reactions. Polymers (Basel) 2019, 11, 2126. [Google Scholar] [CrossRef]
- D’Amico, F.; Musso, M. E.; Berger, R. J. F.; Cefarin, N.; Birarda, G.; Tondi, G.; Bertoldo Menezes, D.; Reyer, A.; Scarabattoli, L.; Sepperer, T.; Schnabel, T.; Vaccari, L. Chemical Constitution of Polyfurfuryl Alcohol Investigated by FTIR and Resonant Raman Spectroscopy. Spectrochim Acta A Mol Biomol Spectrosc 2021, 262, 120090. [Google Scholar] [CrossRef] [PubMed]
- Schmitt, C. R. Polyfurfuryl Alcohol Resins. Polym Plast Technol Eng 1974, 3, 121–158. [Google Scholar] [CrossRef]
- Wang, Z.; Lu, Z.; Huang, X.; Xue, R.; Chen, L. Chemical and Crystalline Structure Characterizations of Polyfurfuryl Alcohol Pyrolyzed at 600 °C. Carbon N Y 1998, 36, 51–59. [Google Scholar] [CrossRef]
- Guigo, N.; Mija, A.; Zavaglia, R.; Vincent, L.; Sbirrazzuoli, N. New Insights on the Thermal Degradation Pathways of Neat Poly(Furfuryl Alcohol) and Poly(Furfuryl Alcohol)/SiO2 Hybrid Materials. Polym Degrad Stab 2009, 94, 908–913. [Google Scholar] [CrossRef]
- Ahmad, E. E. M.; Luyt, A. S.; Djoković, V. Thermal and Dynamic Mechanical Properties of Bio-Based Poly(Furfuryl Alcohol)/Sisal Whiskers Nanocomposites. Polymer Bulletin 2013, 70, 1265–1276. [Google Scholar] [CrossRef]
- Chuang, I. S.; Gary E., Maciel; George, E. Myers. Carbon-13 NMR Study of Curing in Furfuryl Alcohol Resins. Macromolecules 1984, 17, 1087–1090. [Google Scholar] [CrossRef]
- Barr, J. B.; Wallon, S. B. The Chemistry of Furfuryl Alcohol Resins. J Appl Polym Sci 1971, 15, 1079–1090. [Google Scholar] [CrossRef]
- D’Amico, F.; Musso, M. E.; Berger, R. J. F.; Cefarin, N.; Birarda, G.; Tondi, G.; Bertoldo Menezes, D.; Reyer, A.; Scarabattoli, L.; Sepperer, T.; Schnabel, T.; Vaccari, L. Chemical Constitution of Polyfurfuryl Alcohol Investigated by FTIR and Resonant Raman Spectroscopy. Spectrochim Acta A Mol Biomol Spectrosc 2021, 262, 120090. [Google Scholar] [CrossRef]
- Gandini, A.; M. Lacerda, T. Furan Polymers: State of the Art and Perspectives. Macromol Mater Eng 2022, 307, 2100902. [Google Scholar] [CrossRef]
- Iroegbu, A. O. C.; Ray, S. S. On the Chemistry of Furfuryl Alcohol Polymerization: A Review. Journal of Polymer Science 2023. [Google Scholar] [CrossRef]
- Iroegbu, A. O.; Hlangothi, S. P. Furfuryl Alcohol a Versatile, Eco-Sustainable Compound in Perspective. Chemistry Africa 2019, 2, 223–239. [Google Scholar] [CrossRef]

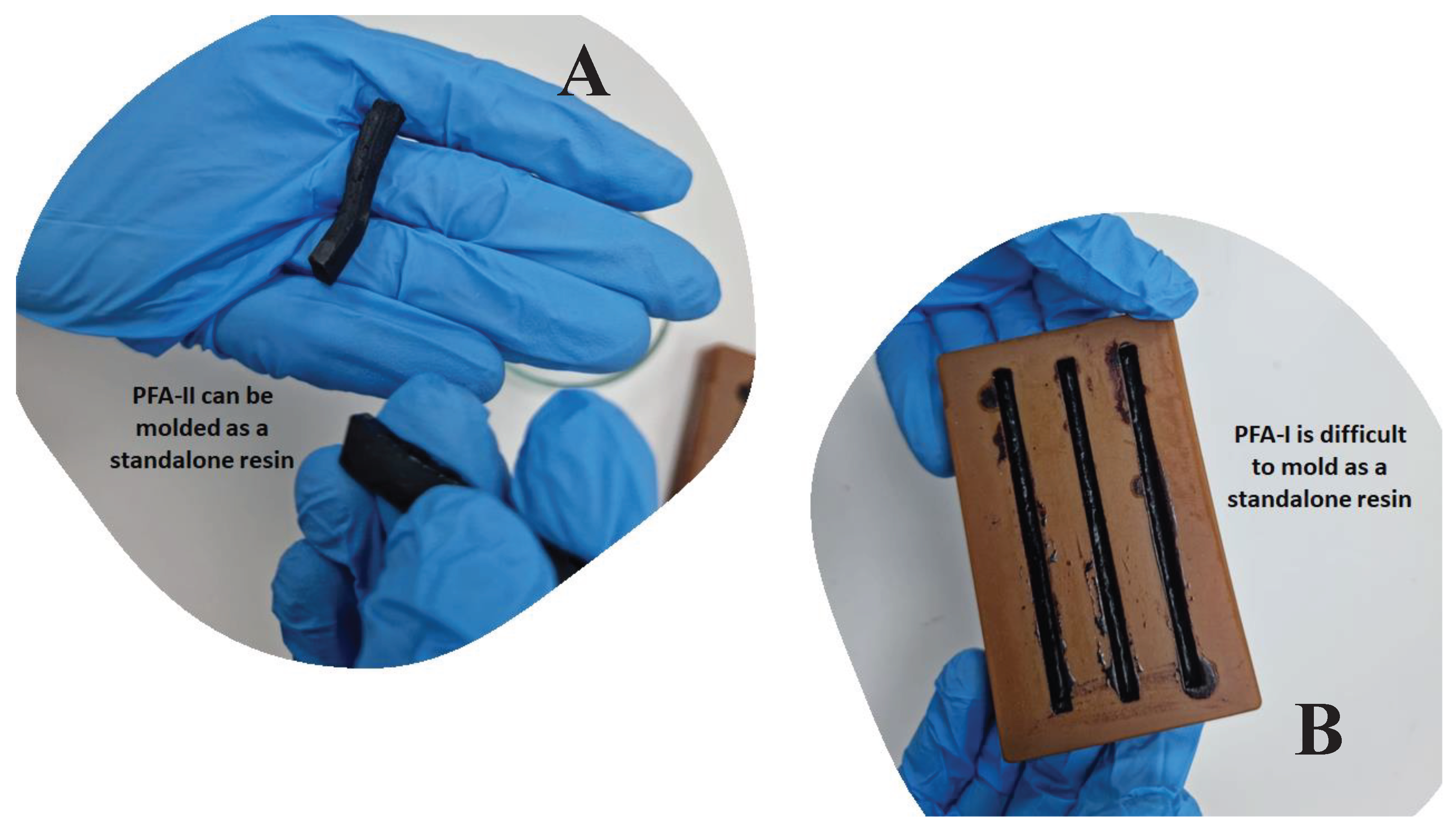
| Samples | ||
|---|---|---|
| PFA-I | PFA-II | |
| Reaction condition | Refluxed without acetic acid for 72 hours | Refluxed with Acetic Acid for 72 hours |
| Mn (g/mol) | 1 917 | 1 783 |
| Mw (g/mol) | 26 494 | 118 959 |
| Polydispersity Index (PDI) | 13 822 | 66 729 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).