1. Introduction
Cutaneous mycoses are highly prevalent, affecting up to 20-30% of the general population [
1] Among these, onychomycosis accounts for up to 30% of cases and is associated with various predisposing factors such as occupation, social class, climate, underlying diseases (diabetes, peripheral vascular disease, immunodeficiency, psoriasis), age, and genetic factors such as HLA. Although it is usually a mild infection without systemic involvement, it often leads to dermatological consultations due to aesthetic alterations and sometimes functional limitations in physical and occupational activities [
2,
3,
4].
Dermatophytes are the most common cause of onychomycosis, with Trichophyton rubrum being the most common etiological agent, responsible for more than half of cases. Yeasts, mainly Candida spp., account for up to 20% of cases [
1,
5]. Additionally, there is a third group of pathogens causing onychomycosis, non-dermatophytic filamentous fungi, including species such as Aspergillus spp., Fusarium spp., Scopulariopsis brevicaulis, and Onychocola canadensis, comprising around 10% of onychomycosis cases. Despite their lower frequency, managing these fungi is complex due to their survival in non-keratinized tissues, preference for immunocompromised patients, and high resistance of several species (Scopulariopsis brevicaulis, Fusarium oxysporum, Neoscytalidium hyalinum) to commonly used antifungals, especially terbinafine [
6,
7].
The cornerstone of onychomycosis treatment is oral antifungals, as topical agents have limited ability to penetrate the nail plate, restricting their use as adjuncts or for superficial infections. However, despite the widespread use of systemic antifungals, therapeutic failure rates can reach 30% in some series. Moreover, due to the need for prolonged treatments lasting on average 6 to 12 weeks, the likelihood of adverse effects such as hepatotoxicity or drug interactions is high, often precluding elderly patients, those with liver disease, or those on multiple medications from this treatment [
8,
9,
10,
11].
According to current guidelines and standard clinical practice, oral terbinafine is the preferred antifungal for onychomycosis treatment [
12]. Its main mechanism of action is the inhibition of squalene oxidase; however, it also induces oxidative damage in both fungi and epithelial cells. Resistance to this drug was previously rare, and routine sensitivity studies were not performed. However, in recent years, an increasing number of cases of resistance to this drug in common dermatophytic fungi have been reported. It is important to note that yeasts such as Candida spp. and especially non-dermatophytic filamentous fungi generally have a high rate of resistance to terbinafine [
13,
14,
15].
Therefore, due to the growing difficulty in treating cutaneous mycoses with antifungals, studies exploring other treatment modalities have been conducted. Physical therapies have emerged as the primary therapeutic alternative to antifungals. Photodynamic therapy (PDT), laser, and high-energy pulsed light are increasingly used in the treatment of cutaneous mycoses, especially onychomycosis, due to their antimicrobial properties. Irradiation with visible wavelengths, both blue (400 - 480 nm) and red (600 - 750 nm), can increase the production of reactive oxygen species, exerting an antifungal effect by directly damaging dermatophytes and stimulating the immune system. However, their main limitation lies in the difficulty of penetration of the photosensitizer and light into the nail plate. To address this, standard PDT protocols have been adapted for this new location, using prolonged occlusions or hydrocolloid dressings to improve the penetration of the photosensitizer, increasing the intensity of the administered light, or using keratolytics to reduce the thickness of the nail plate [
16,
17,
18].
Given terbinafine's ability to induce the accumulation of reactive oxygen species, combining PDT or other physical therapies with antifungal treatments could generate a synergistic effect, improving therapeutic response, allowing lower doses of antifungals to reduce toxicity and adverse effects, possibly enabling treatment of previously ineligible patients [
19,
20,
21,
22].
2. Results
2.1. Cell Viability
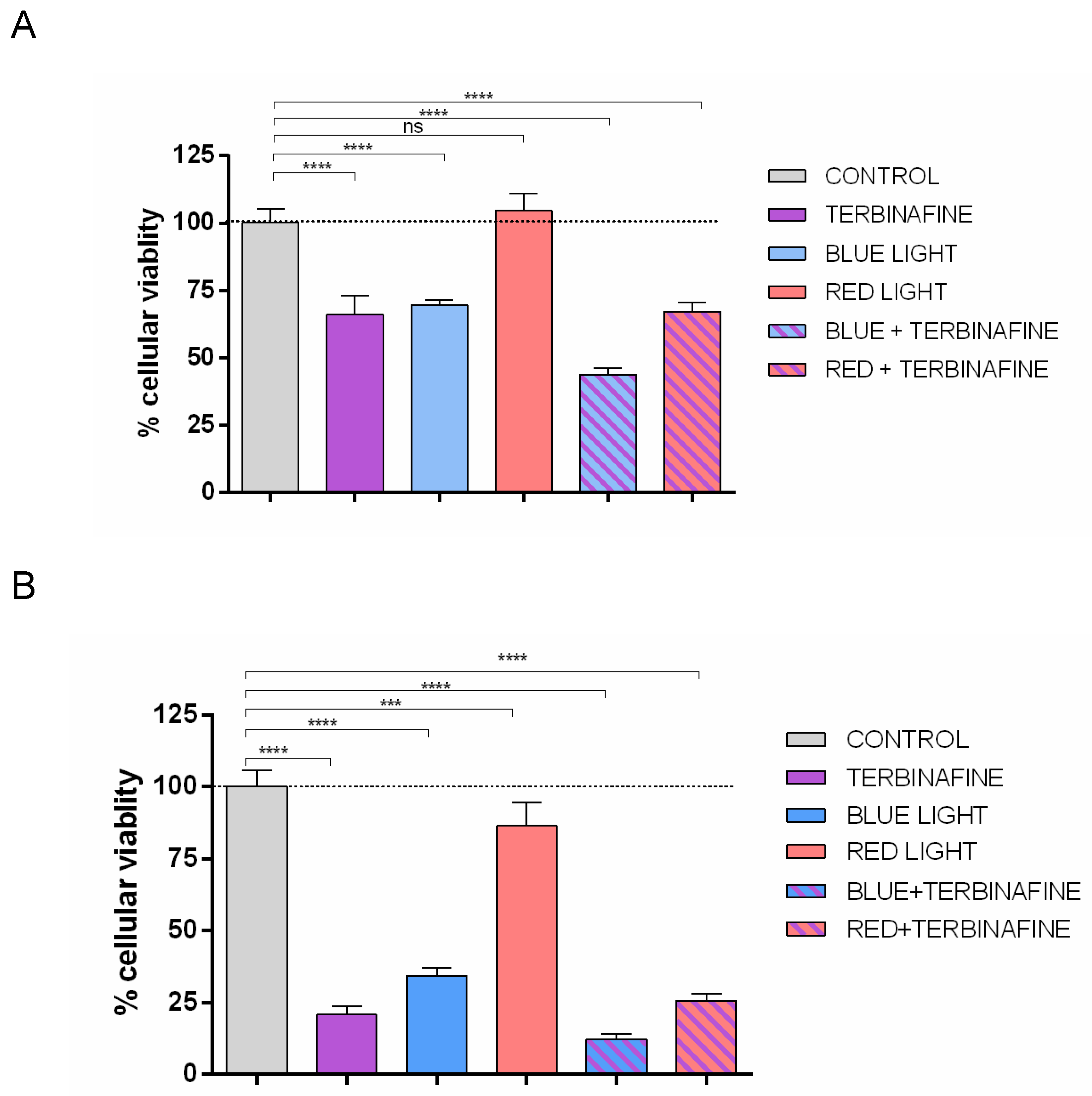
The addition of terbinafine to non-exposed light cultures induced a 33.66% ± 6.70% (p<0.0001) reduction in HaCat cell viability in the XTT assay, over control (non – light exposed and terbinafine-free cultures)
Exposure to blue light also triggered a reduction of 30.42% ± 1.9% (p<0.001) over control, in HaCat viability. Similarly, in terbinafine and blue light exposed cultures, viability was reduced 56.18 % ± 2.25% (p<0.0001) over control.
Red light did not show changes on cell proliferation. The group treated with red light increased 4.76 % ± 6.22 % his cell proliferation compared to controls, without statistically significant differences (p > 0.05). The combination of red light and terbinafine resulted in a 32.89% ± 3.60% decrease in cellular viability compared to controls (p<0.0001); however, differences compared to the group treated solely with terbinafine were not statistically significant (
Figure 1A).
On the other hand, XTT assay showed that in cultures with restriction of fetal bovine serum, the addition of terbinafine to non-light exposed cells induced a 79.18% ± 2.87% (p<0.0001) reduction in HaCat viability over control.
The exposure to blue light in nutrient-restricted conditions also decreased over control a 65.69% ± 2.65% (p<0.0001) in cell viability. The combination of terbinafine and blue light reduced cell viability by up to 87.73% ± 1.98% (p<0.0001) over controls in the XTT assay.
In nutrient-restricted conditions, the group treated with red light exhibited a decrease of 13.75% ± 8.4, (p<0.001) in cell proliferation compared to controls. The combination of red light and terbinafine resulted in a 74.34% ± 2.44%, (p<0.0001) decrease in cellular viability compared to controls Comparing the group treated solely with terbinafine and the group treated with red light and terbinafine, no statistically significant differences were found (
Figure 1B).
2.2. Reactive Oxygen Species (ROS) Production
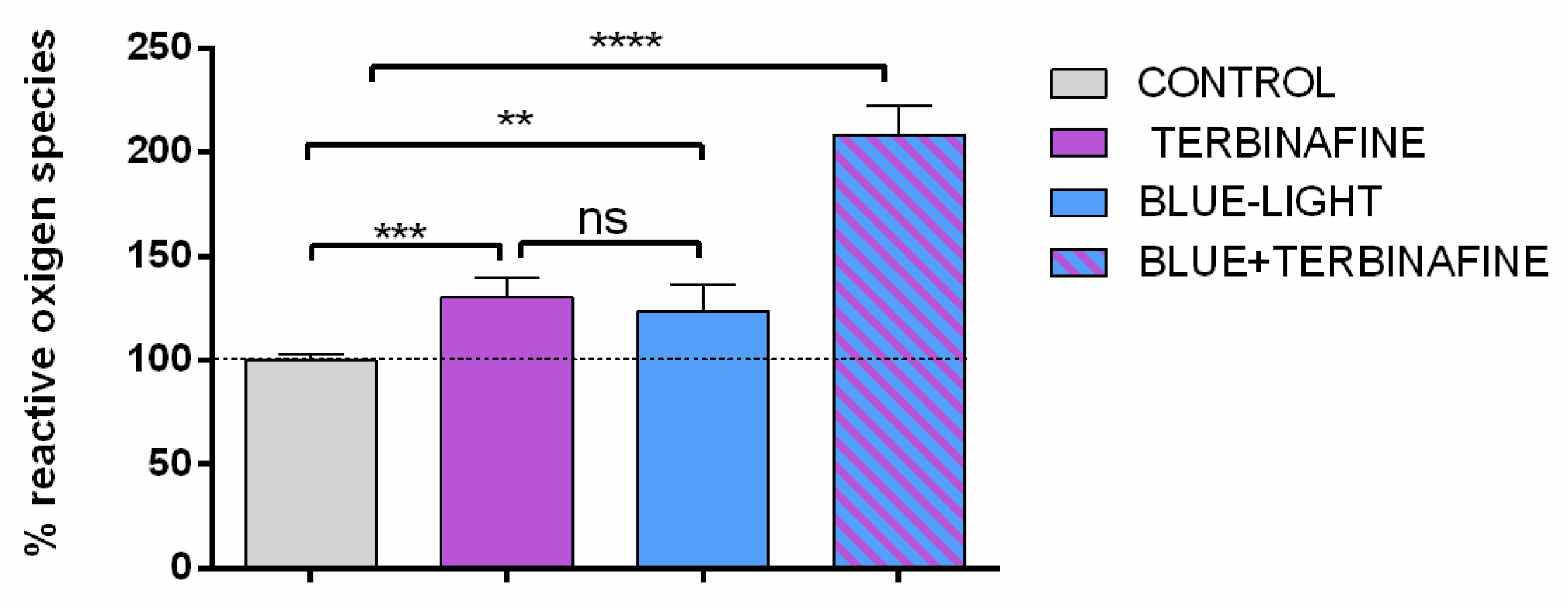
Because the effect of red light on cell viability was not relevant, the study was continued only with exposure to blue light. To determinate the ROS production induced by blue light, quantitative immunofluorescence assays were performed to assess the amount of fluorescent dye dichlorofluorescein, as a measure of the level of ROS. The quantification of ROS showed an increase of 30% ± 9,2% (p<0.001) when non- light exposed HaCat cultures were incubated with terbinafine. Also, cultures exposed to blue light showed an increment of 23% ± 12,5% (p<0.001) in ROS production in absence of terbinafine, and an intense increase of 108% (SD 13,4% p<0.001) in combining blue light and terbinafine cultures (
Figure 2).
2.3. Antioxidant Enzymes Superoxide Dismutase (SOD), Glutation Peroxidase (GPx) and Catalase Expression
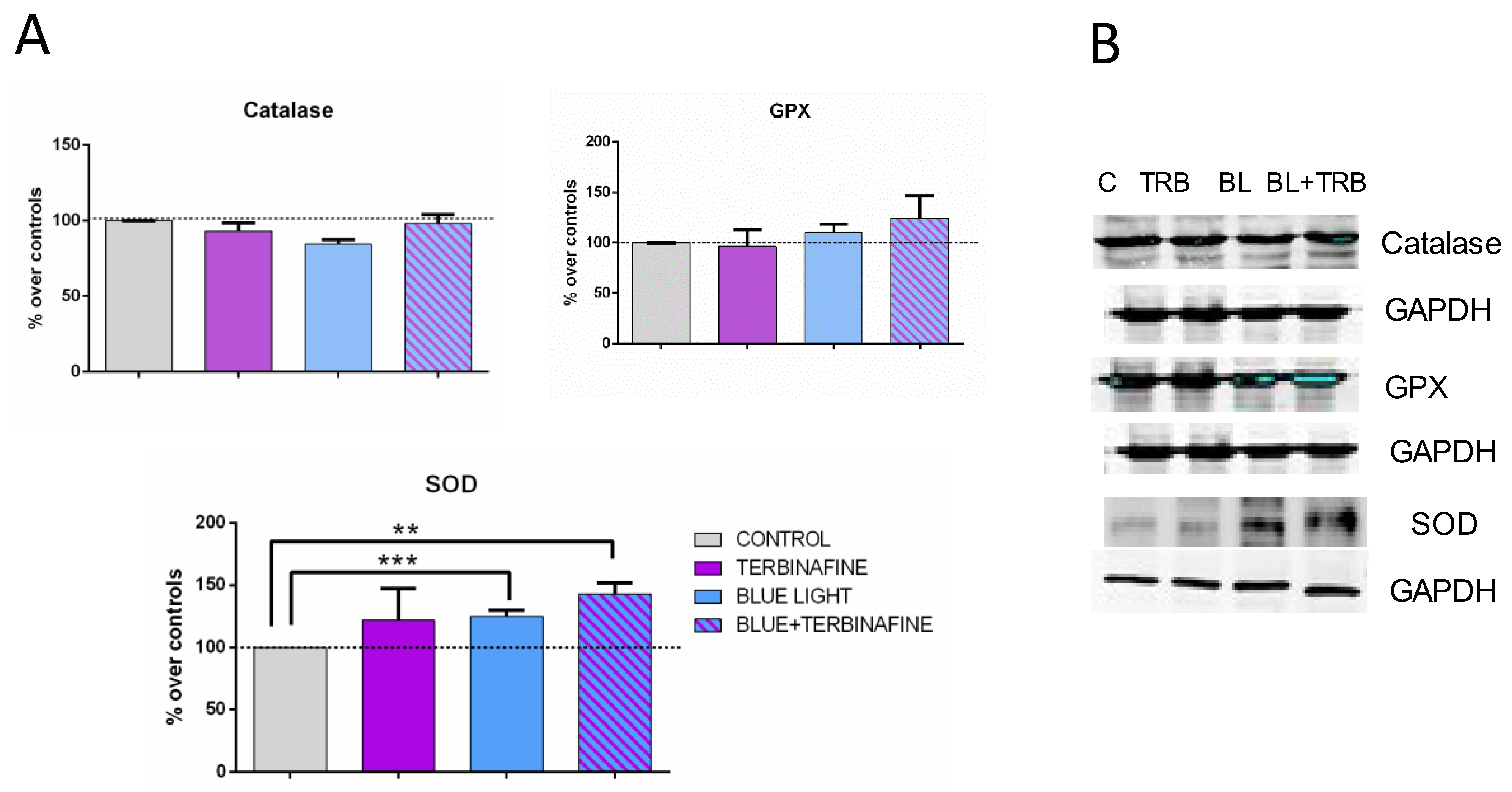
To establish the implication of antioxidant pathways in the response to Terbinafine and/or blue light treatment, the expression of SOD, GSH-Px, and catalase was analyzed. The results did not show significant changes in the overall analysis of all experimental groups (ANOVA). However, a significant increase in SOD enzyme expression was observed when comparing the control group with samples exposed to blue light alone in the absence (24.88 ± 10.79%; p < 0.001***) or presence of terbinafine treatment independently (43.15 ± 19.23%; p < 0.01**). No statistically significant changes were observed in the expression of GPx or catalase (
Figure 3).
2.4. Caspase 3 Expression
The antioxidant system comprises both antioxidant enzymes and non-enzymatic antioxidants capable of preventing oxidative damage in human skin [
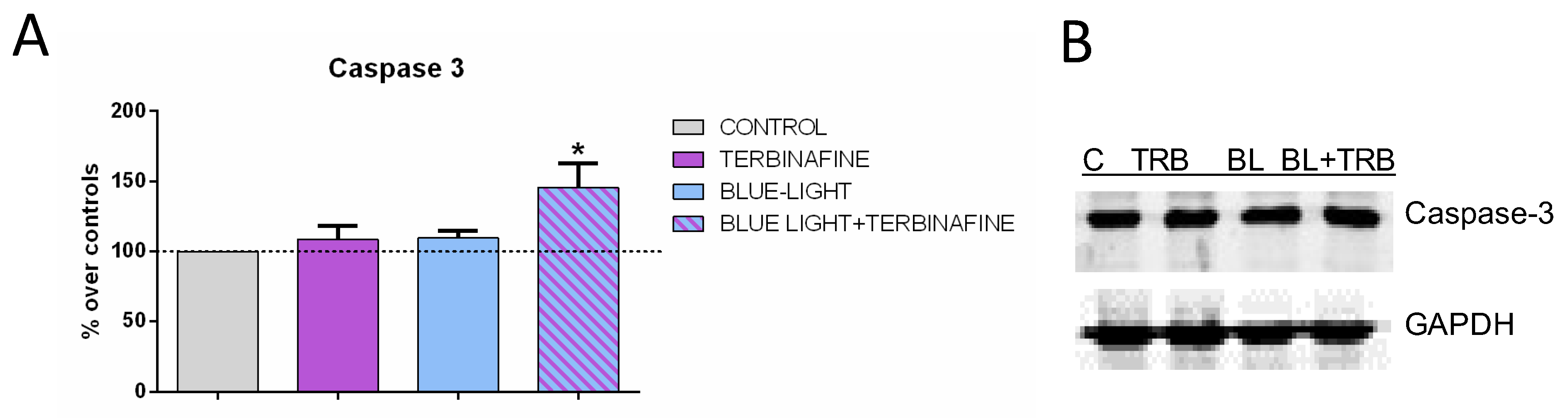
23]. Therefore, the expression of relevant proteins in the antioxidative defense, such as caspase 3, was also analyzed. Immunoblot analysis of caspase 3 expression revealed a significant increase in the expression of this protein (24.44 ± 31.6%; p < 0.05*) when cells were treated with light and terbinafine. No changes were observed when samples were treated with light or terbinafine alone (
Figure 4).
3. Discussion
The use of physical therapies in the treatment of onychomycosis has increased in recent years due to the rising therapeutic failures with oral antifungal agents. The growing number of publications in recent years reveals promising results, demonstrating the efficacy and safety of these therapies [
24,
25,
26,
27,
28]. However, there is a significant difficulty in reaching firm conclusions due to the significant heterogeneity among studies. A recent systematic review and meta-analysis of PDT use in dermatophytosis suggest its effectiveness, with cure rates ranging from 17% to 80% [
16,
18]. Variations in efficacy depend on the photosensitizer used, the number of sessions, and concurrent treatments were detected. Similarly, systematic reviews and meta-analyses evaluating the efficacy of pulsed dye laser, Nd:YAG, and CO
2 in onychomycosis have reported similar results to PDT, achieving cure rates of 63% to 74% [
29,
30]. On the other hand, evidence on the combined use of PDT with other treatments is scarce, mainly limited to small series of patients receiving heterogeneous treatments. Additionally, there are no experimental studies examining the molecular effect of combining these treatments with antifungals, limiting the evidence to isolated case reports [
31,
32,
33].
The results of our study demonstrate that blue light and terbinafine decrease the viability of keratinocyte cultures. Furthermore, the combination of blue light therapy and oral terbinafine increases the cytotoxic effect of both therapies. This cytotoxicity is likely due to the significant increase in reactive oxygen species production in the cultures and is supported by the results showing an increase in caspase 3 expression when treatment combines blue light and terbinafine. This synergistic effect is intensified under in vitro conditions of nutrient restriction simulating onychomycosis, thus mimicking the limited nutrient and oxygen supply due to inadequate acral circulation characteristic of this pathology. Thus, cell viability results correlate with reactive oxygen species quantification. It has been suggested that reactive oxygen species generation is the main fungicidal mechanism of oxidative agents. Reactive oxygen species can react with polyunsaturated fatty acids in cell membranes, nucleotides, and sulfhydryl bonds in proteins, and have been associated with tissue damage in fungal infections [
34]. The production, regulation, and response of reactive oxygen species are a central axis of host-pathogen interaction. Both hosts and fungi produce reactive oxygen species, utilize conserved mechanisms to metabolize these radicals, and leverage them in their local environment to mediate defense mechanisms [
21,
35].
Oxidative stress reflects an imbalance between the systemic manifestation of free radical formation and antioxidant defense capacity. Superoxide dismutase (SOD) and glutathione peroxidase (GPx) enzymes act as ROS detoxifiers. To prevent skin damage caused by excessive ROS levels and regulate epidermal homeostasis, skin cells activate their endogenous defense system and exert their antioxidant function. The maintenance of homeostasis is largely provided by a series of enzymes such as SOD, GPX and catalase. The results of our experiments showed that the administration of terbinafine and blue light balanced intracellular oxidative stress in HaCat cells by increasing SOD levels. This effect was also observed in cells treated only with blue light. A similar effect has been described in lesions caused by the inoculation of yeast cells into the back pad of BALB/c mice and treated with HeNe laser [
36]. SOD is responsible for catalyzing the superoxide radical dismutation reaction, converting the superoxide radical (O
2-) into oxygen and hydrogen peroxide (O
2- + O
2- + 2H+ → O
2 + H
2O
2), a potent antifungal [
37,
38]. Such a reaction may play an important role in onychomycosis for two reasons: A high concentration of superoxide radical (O
2-) can delay wound healing due to the formation of the hydroxyl radical, which has a cytotoxic effect [
36,
39,
40,
41,
42]. The second reason is that increased hydrogen peroxide (H
2O
2) generation plays an important role in eliminating pathogenic microorganisms [
40,
43,
44]. Thus, a reduced concentration of superoxide (O
2-) may lead to accelerated wound healing and increased release of hydrogen peroxide (H
2O
2), which could accelerate fungal elimination [
36,
39,
40,
41,
42]
The role of red light in our assays seems somewhat controversial. Thus, under standard culture conditions, red light apparently does not affect cell viability or reactive oxygen species production, although the group with the combined red light and terbinafine treatment decreased viability. However, given the absence of the red light effect alone, any decrease observed in cell viability is solely attributable to terbinafine. Under nutrient-restrictive conditions, red light also slightly decreased cell viability compared to control. This effect is likely due to nutrient-restricted cultures being more sensitive to external stimuli such as red light than under normal culture conditions, where it would not affect viability. Moreover, when both chemical and light treatments were applied together in these SFB-depleted cultures, the combined effect was much greater than that observed in cultures grown under standard conditions and exposed to both treatments.
The synergistic effect described in our study is supported by previous evidence showing the ability of various antifungals to induce oxidative stress and the inherent mechanism of action of PDT. Applying this synergistic effect in clinical practice could improve current treatments, reduce the dose and duration of antifungal therapy, and limit the cellular damage induced by terbinafine treatment on skin cells by being able, in the presence of blue light, to stimulate antioxidant defenses through SOD activation, reducing the long-term toxic effect of free radicals, reducing healing time, and accelerating fungal elimination. The main limitations of our study include the lack of in vivo studies comparing combined terbinafine and blue light treatment with other therapies, as well as the lack of in vitro microbiological studies confirming whether the increase in reactive oxygen species is also accompanied by an increase in the antifungal effect.
4. Materials and Methods
Cell culture: Human keratinocytes (HaCaT cell line, CLS Cell Lines Service, 300493 were seeded in medium composed of high-glucose D-MEM (Biowhittaker, Lonza, Verviers, Belgium) supplemented with 10% inactivated fetal bovine serum (Gibco, MA USA), 2 mM L-glutamine, and 100 U/ml penicillin, 100 U/ml streptomycin and 0.25 μg/ml of amphotericin B (Gibco) and maintained in a 5% CO2 atmosphere at a temperature of 37 ºC inside CO2 incubators (Thermo Fisher Scientific, Waltham, MA, USA). The cells were subcultured once a week and de culture medium replaced each 3 days.
For experiments, HaCaT were seeded in 24 multi well plates (Corning Costar TC-Treated Multiple Well Plates) and incubated with D-MEM supplemented with 10 % fetal bovine serum 2 mM L-glutamine, and 100 U/ml penicillin, 100 U/ml streptomycin and 0.25 μg/ml of amphotericin B. Furthermore, in a series of experiments HaCat cells were incubated in DMEM in the absence of serum (0% FBS) but in the presence of the rest of the culture medium components described above. The aim of this experiment was to study in vitro, the low nutrient input of nails affected by onycomycosis. Cells were growth for 2 days in a 5% CO2 atmosphere at a temperature of 37 ºC to provide adherence to the bottom of the plate. Only 12 wells were seeded to exposure with the light exposition device. Thirty minutes before the light exposition, culture medium was changed to a non-colored maintenance culture medium to avoid interference with light treatments and colorimetric measurements.
Terbinafine incubation: Terbinafine drug was added to a half of the wells, immediately before exposure to light. The terbinafine dosage used in this study was 1 µM (Terbinafine hydrochloride, Merck, 78628-80-5, Darmstadt, Germany). This dosage was the same used in other publications that previously exposed keratinocytes to this drug [
45,
46].
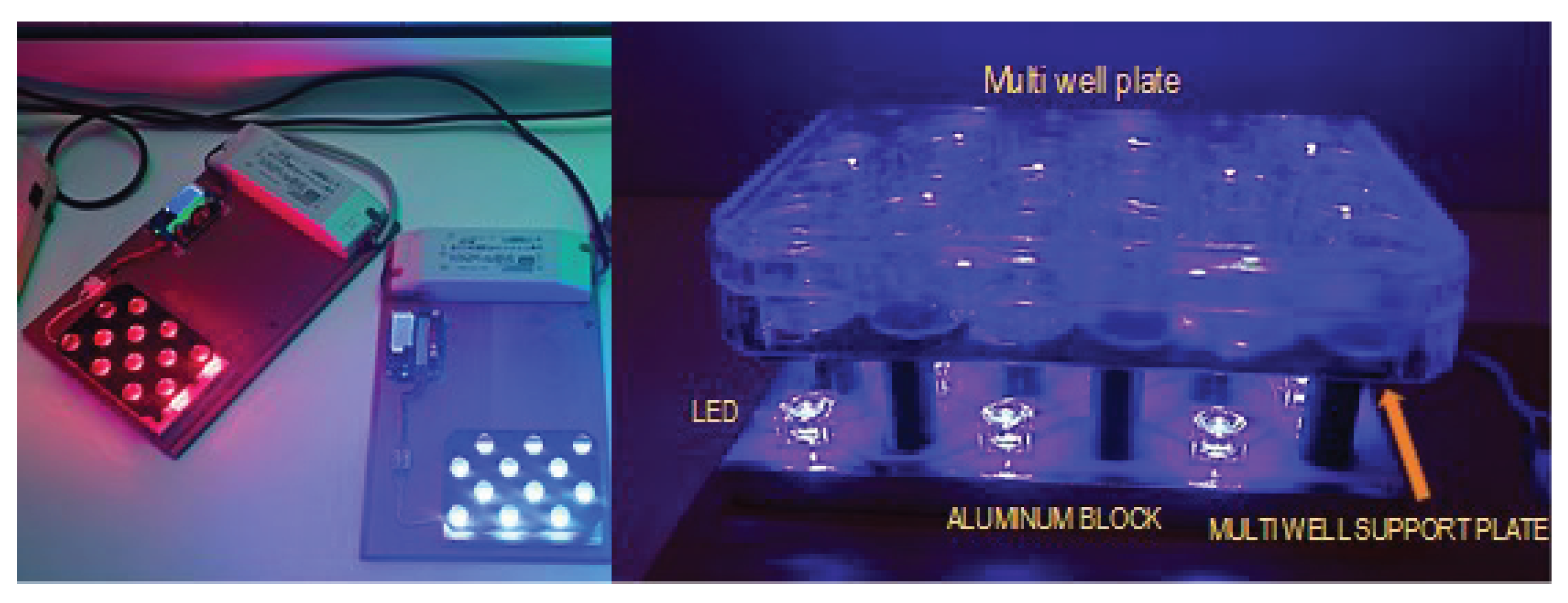
Blue or red light exposure: The light exposure was performed with an original 3D impression specific device that emits 448 nm blue light and 645 nm red light. The device was equipped with 12 blue LEDs (Lumileds LUXEON Rebel color Royal Blue 448 nm LXML-PR01-0500) and 12 red LEDs (Lumileds LUXEON Rebel Color Red 645 nm LXM2-PD01-005). Our exposure device allows controlled treatments and includes a focusing lens that ensures that the exposure of the culture is homogeneous (
Figure 5).
The light treatment consisted of two sessions of 52 mW/cm2 irradiance (Blue or red light) and 20 minutes duration applied every 24 hours on two consecutive days. The experiment was controlled by a multiwell plate cultured in identical conditions but in dark.
Cell proliferation assay: To determinate the effects of terbinafine and light exposition in HaCaT, proliferation and viability XTT assays (cell proliferation assay Roche - Sigma-Aldrich) were performed. XTT assays were carried out 24 h after the second light exposure.
Reactive oxygen species (ROS) assay: To quantify the ROS accumulation in the cultures due to terbinafine and light exposition, an immunofluorescence assay were performed. ROS assays were carried out immediately after second exposure to minimize their degradation. ROS production was assessed by quantification of the intracellular oxidative transformation of the oxidation-sensitive probe DCFH-DA into the fluorescent dye dichlorofluorescein (DCF). Then, HaCat cultures were incubated with the fluorescent probe 2′7-Dichloro dihydrofluorescein diacetate (5 μM DCFH-DA, Sigma-Aldrich) for in the dark at 37°C and 5% CO2 during 30 min. They were then read with a TECAN plate reader (TECAN SpectraFluor, Gödrig, Austria) at a λexc 490 nm, λemi 535 nm wavelength.
Inmunoblot for superoxide dismutase (SOD), glutation peroxidase (GPx), catalase and caspase 3: The immunoblot procedure has been described in detail elsewhere [
47,
48]. Briefly, the total cell lysate was obtained by lysing cells in RIPA buffer (Thermo Fisher Scientific) containing protease-phosphate inhibitor cocktail (Thermo Fisher Scientific). Protein content was determined by BCA protein assay kit (Pierce; Thermo Fisher Scientific). Equal protein volumes (50 μg) was separated in 10% sodium dodecyl sulphate-polyacrylamide gel and electrophoretically transferred to nitrocellulose membrane (Amersham, Buckinghamshire, UK). The membranes were incubated at 4°C overnight in polyclonal rabbit anti-Catalase (1:1000, Invitrogen) polyclonal rabbit anti-glutation peroxidase 2 antibody (1:1000, Abcam, Cambridge, UK), monoclonal rabbit Cleaved Caspase-3 antibody (1:1000; Cell Signaling, Danvers, MA, USA), and monoclonal rabbit anti-superoxide Dismutase 1 (Abcam, Ab 51254). Monoclonal mouse anti-GAPDH (1:1000, Santa Cruz Biotechnology, Heidelberg, Germany) were used as loading controls. The membranes were incubated for one hour at room temperature with anti-rabbit IgG conjugated to IRdye 800 CW (1:10000, LI-COR Biosciences, Nebraska, USA) and with anti-mouse IgG conjugated to IRdye 680 LT (1:15000, LI-COR Biosciences) or with anti-mouse IgG horseradish peroxidase conjugated antibody (1:10000 NA931; GE Heathcare, Hatfield, UK) and with anti-rabbit IgG horseradish peroxidase conjugated antibody (1:5000 NA934; GE Heathcare). For detection and visualization of the immunoreactive bands, the enhanced detection kit ECL (RPN2132; GE Healthcare; Cytiva). The membranes were scanned with a LI-COR Odyssey scanner (LI-COR Biosciences) or with a Bio-Rad imaging system (Hercules, CA, USA). The obtained bands were densitometry evaluated (PDI Quantity One 4.5.2 software, BioRad). At least 3 experimental replicates were conducted per protein. All values were normalized over the loading control.
Statistical analysis: At least three independent replicates were conducted per experiment. Data were normalized and expressed as means ± standard desviation (SD) of at least three independent experimental runs. One way ANOVA with Turkey post-test or two-tailed student’s t-test was applied using GraphPad Prism 6.01 software (GraphPad Software, San Diego, CA, USA) when comparing multiple or two experimental groups, respectively. Differences p < 0.05 were considered significant statistically.
Author Contributions
Conceptualization M.L.H.-B, L.A.P-G., M.F-G and M.A.M.P.; methodology, M.L.H.-B, L.A.P-G, E.T.-M, R.C-J-L and M.A.M.-P.; software, E.T.-M.; validation, M.L.H.-B..; formal analysis, M.L.H.-B and L.A.P-G.; investigation, M.L.H.-B., L.A.P-G, E.T.-M., M.A.M.-P, R.C-J-L. and M.F-G.; resources, M.L.H.-B, L.A.P-G and M.A.M.-P data curation M.L.H.-B. and E.T.-M.;.; writing—original draft preparation, M.L.H.-B.; writing—review and editing, M.L.H.-B., L.A.P-G , E.T.-M, M.A.M.-P. and M.F.-G.; visualization, M.L.H.-B., L.A.P-G , E.T.-M., M.F.-G., M.A.M.-P. R.C.J-L.; supervision, M.L.H.-B.; project administration, M.L.H.-B..; funding acquisition, M.L.H.-B and M.F-G. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Acknowledgments
We thank Cesar Úbeda and Dr. José Aguilera for their technical assistance in the development and calibration of the in vitro light exposure system.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Havlickova, B.; Czaika, V.A.; Friedrich, M. Epidemiological Trends in Skin Mycoses Worldwide. Mycoses 2008, 51 Suppl 4, 2–15. [Google Scholar] [CrossRef]
- García-Romero, M.T.; Granados, J.; Vega-Memije, M.E.; Arenas, R. Analysis of Genetic Polymorphism of the HLA-B and HLA-DR Loci in Patients with Dermatophytic Onychomycosis and in Their First-Degree Relatives. Actas Dermo-Sifiliográficas (English Edition) 2012, 103, 59–62. [Google Scholar] [CrossRef]
- Gupta, A.K.; Mays, R.R. The Impact of Onychomycosis on Quality of Life: A Systematic Review of the Available Literature. Skin Appendage Disord 2018, 4, 208–216. [Google Scholar] [CrossRef] [PubMed]
- Leeyaphan, C.; Chayangsu, O.; Bunyaratavej, S.; Kulthanan, K.; Bunyaratavej, S.; Pattanaprichakul, P. Onychomycosis: A Study of Self-Recognition by Patients and Quality of Life. Indian J Dermatol Venereol Leprol 2015, 81, 270. [Google Scholar] [CrossRef] [PubMed]
- Thomas, J.; Jacobson, G.A.; Narkowicz, C.K.; Peterson, G.M.; Burnet, H.; Sharpe, C. Toenail Onychomycosis: An Important Global Disease Burden. J Clin Pharm Ther 2010, 35, 497–519. [Google Scholar] [CrossRef] [PubMed]
- Gilaberte, Y.; Aspiroz, C.; Alejandre, M.C.; Andres-Ciriano, E.; Fortuño, B.; Charlez, L.; Revillo, M.J.; Hamblin, M.R.; Rezusta, A. Cutaneous Sporotrichosis Treated with Photodynamic Therapy: An in Vitro and in Vivo Study. Photomedicine and Laser Surgery 2014, 32, 54–57. [Google Scholar] [CrossRef]
- Robres P; Aspiroz C; Rezusta A; Gilaberte Y Actas Dermosifiliogr. pp. 795–805.
- Elewski, B.E. A Full “Cure” for Onychomycosis Is Not Always Possible. Archives of Dermatology 1999, 135, 852–853. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.K. Ciclopirox Nail Lacquer: A Brush with Onychomycosis. Cutis 2001, 68, 13–16. [Google Scholar]
- Marr, K.A.; Schlamm, H.T.; Herbrecht, R.; Rottinghaus, S.T.; Bow, E.J.; Cornely, O.A.; Heinz, W.J.; Jagannatha, S.; Koh, L.P.; Kontoyiannis, D.P.; et al. Combination Antifungal Therapy for Invasive Aspergillosis: A Randomized Trial. Ann Intern Med 2015, 162, 81–89. [Google Scholar] [CrossRef]
- Kyriakidis, I.; Tragiannidis, A.; Munchen, S.; Groll, A.H. Clinical Hepatotoxicity Associated with Antifungal Agents. Expert Opin Drug Saf 2017, 16, 149–165. [Google Scholar] [CrossRef]
- Ameen, M.; Lear, J.T.; Madan, V.; Mohd Mustapa, M.F.; Richardson, M.; Hughes, J.R.; Sahota, A.; Griffiths, M.; McDonagh, A.J.; Punjabi, S.; et al. British Association of Dermatologists’ Guidelines for the Management of Onychomycosis 2014. Br J Dermatol 2014, 171, 937–958. [Google Scholar] [CrossRef] [PubMed]
- Yamada, T.; Maeda, M.; Alshahni, M.M.; Tanaka, R.; Yaguchi, T.; Bontems, O.; Salamin, K.; Fratti, M.; Monod, M. Terbinafine Resistance of Trichophyton Clinical Isolates Caused by Specific Point Mutations in the Squalene Epoxidase Gene. Antimicrob Agents Chemother 2017, 61, e00115–17. [Google Scholar] [CrossRef] [PubMed]
- Saunte, D.M.L.; Hare, R.K.; Jørgensen, K.M.; Jørgensen, R.; Deleuran, M.; Zachariae, C.O.; Thomsen, S.F.; Bjørnskov-Halkier, L.; Kofoed, K.; Arendrup, M.C. Emerging Terbinafine Resistance in Trichophyton : Clinical Characteristics, Squalene Epoxidase Gene Mutations, and a Reliable EUCAST Method for Detection. Antimicrob Agents Chemother 2019, 63, e01126–19. [Google Scholar] [CrossRef]
- Singh, A.; Masih, A.; Khurana, A.; Singh, P.K.; Gupta, M.; Hagen, F.; Meis, J.F.; Chowdhary, A. High Terbinafine Resistance in Trichophyton Interdigitale Isolates in Delhi, India Harbouring Mutations in the Squalene Epoxidase Gene. Mycoses 2018, 61, 477–484. [Google Scholar] [CrossRef] [PubMed]
- Dong, Q.; Kang, Y.; Zhang, R. Treatment of Superficial Mycoses Using Photodynamic Therapy: A Systematic Review and Meta-Analysis. Photobiomodulation, Photomedicine, and Laser Surgery 2023, 41, 37–47. [Google Scholar] [CrossRef] [PubMed]
- Dai, T.; Fuchs, B.B.; Coleman, J.J.; Prates, R.A.; Astrakas, C.; St. Denis, T.G.; Ribeiro, M.S.; Mylonakis, E.; Hamblin, M.R.; Tegos, G.P. Concepts and Principles of Photodynamic Therapy as an Alternative Antifungal Discovery Platform. Front. Microbio. 2012, 3. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.J.; Jemec, G.B.E.; Arendrup, M.C.; Saunte, D.M.L. Photodynamic Therapy Treatment of Superficial Fungal Infections: A Systematic Review. Photodiagnosis and Photodynamic Therapy 2020, 31, 101774. [Google Scholar] [CrossRef]
- Lam, P.-L.; Wong, M.-M.; Hung, L.-K.; Yung, L.-H.; Tang, J.C.-O.; Lam, K.-H.; Chung, P.-Y.; Wong, W.-Y.; Ho, Y.-W.; Wong, R.S.-M.; et al. Miconazole and Terbinafine Induced Reactive Oxygen Species Accumulation and Topical Toxicity in Human Keratinocytes. Drug and Chemical Toxicology 2022, 45, 834–838. [Google Scholar] [CrossRef]
- Shekhova, E.; Kniemeyer, O.; Brakhage, A.A. Induction of Mitochondrial Reactive Oxygen Species Production by Itraconazole, Terbinafine, and Amphotericin B as a Mode of Action against Aspergillus Fumigatus. Antimicrob Agents Chemother 2017, 61, e00978–17. [Google Scholar] [CrossRef]
- Gonzalez-Jimenez, I.; Perlin, D.S.; Shor, E. Reactive Oxidant Species Induced by Antifungal Drugs: Identity, Origins, Functions, and Connection to Stress-Induced Cell Death. Front Cell Infect Microbiol 2023, 13, 1276406. [Google Scholar] [CrossRef]
- Lee, V.; Gober, M.D.; Bashir, H.; O’Day, C.; Blair, I.A.; Mesaros, C.; Weng, L.; Huang, A.; Chen, A.; Tang, R.; et al. Voriconazole Enhances UV-Induced DNA Damage by Inhibiting Catalase and Promoting Oxidative Stress. Exp Dermatol 2020, 29, 29–38. [Google Scholar] [CrossRef] [PubMed]
- Haida, Z.; Hakiman, M. A Comprehensive Review on the Determination of Enzymatic Assay and Nonenzymatic Antioxidant Activities. Food Sci Nutr 2019, 7, 1555–1563. [Google Scholar] [CrossRef] [PubMed]
- Sotiriou, E.; Panagiotidou, D.; Ioannides, D. 5-Aminolevulininic Acid Photodynamic Therapy Treatment for Tinea Cruris Caused by Trichophyton Rubrum : Report of 10 Cases. Acad Dermatol Venereol 2009, 23, 341–342. [Google Scholar] [CrossRef] [PubMed]
- Figueiredo Souza, L.W.; Souza, S.V.T.; Botelho, A.C.C. Randomized Controlled Trial Comparing Photodynamic Therapy Based on Methylene Blue Dye and Fluconazole for Toenail Onychomycosis: Trial Photodynamic Therapy Onychomycosis. Dermatol Ther 2014, 27, 43–47. [Google Scholar] [CrossRef] [PubMed]
- Tardivo, J.P.; Wainwright, M.; Baptista, M. Small Scale Trial of Photodynamic Treatment of Onychomycosis in São Paulo. Journal of Photochemistry and Photobiology B: Biology 2015, 150, 66–68. [Google Scholar] [CrossRef] [PubMed]
- Souza, L.W.F.; Souza, S.V.T.; Botelho, A.C.D.C. Distal and Lateral Toenail Onychomycosis Caused by Trichophyton Rubrum: Treatment with Photodynamic Therapy Based on Methylene Blue Dye. An. Bras. Dermatol. 2014, 89, 184–186. [Google Scholar] [CrossRef] [PubMed]
- Calzavara-Pinton, P.G.; Venturini, M.; Capezzera, R.; Sala, R.; Zane, C. Photodynamic Therapy of Interdigital Mycoses of the Feet with Topical Application of 5-aminolevulinic Acid. Photoderm Photoimm Photomed 2004, 20, 144–147. [Google Scholar] [CrossRef] [PubMed]
- Ma, W.; Si, C.; Kasyanju Carrero, L.M.; Liu, H.-F.; Yin, X.-F.; Liu, J.; Xu, Y.; Zhou, B. Laser Treatment for Onychomycosis: A Systematic Review and Meta-Analysis. Medicine 2019, 98, e17948. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.K.; Foley, K.A.; Versteeg, S.G. Lasers for Onychomycosis. J Cutan Med Surg 2017, 21, 114–116. [Google Scholar] [CrossRef]
- Navarro-Bielsa, A.; Gracia-Cazaña, T.; Robres, P.; Lopez, C.; Calvo-Priego, M.D.; Aspiroz, C.; Gilaberte, Y. Combination of Photodynamic Therapy and Oral Antifungals for the Treatment of Onychomycosis. Pharmaceuticals (Basel) 2022, 15, 722. [Google Scholar] [CrossRef]
- Lu, J.; Li, W.; Zheng, W.; Huang, R.; Wu, W. Successful Treatment of Kerion with Itraconazole and ALA-PDT: A Case Report. Photodiagnosis and Photodynamic Therapy 2019, 27, 385–387. [Google Scholar] [CrossRef] [PubMed]
- Ana Paula Da Silva; Fernanda M. Carbinatto; Vanderlei S. Bagnato; Natalia M. Inada A Promising Strategy for the Treatment of Onychomycosis with Curcumin and Photodynamic Therapy. JPP 2015, 3. [Google Scholar] [CrossRef]
- Juan, C.A.; Pérez De La Lastra, J.M.; Plou, F.J.; Pérez-Lebeña, E. The Chemistry of Reactive Oxygen Species (ROS) Revisited: Outlining Their Role in Biological Macromolecules (DNA, Lipids and Proteins) and Induced Pathologies. IJMS 2021, 22, 4642. [Google Scholar] [CrossRef] [PubMed]
- Paiva, C.N.; Bozza, M.T. Are Reactive Oxygen Species Always Detrimental to Pathogens? Antioxidants & Redox Signaling 2014, 20, 1000–1037. [Google Scholar] [CrossRef]
- Alves da Costa T; Francelin C; Di Gangi R; Nogueira Costa M.R.S; Thomé R; Paulino L.C; Verinaud L Trends in photochemistry and photobiology. 2013, pp. 63–75.
- Klebanov, G.I.; POltanov, E.A.; Vladimirov, I.A. [Effect of low intensity laser light in the red range on macrophage superoxide dismutase activity]. Biofizika 2003, 48, 462–473. [Google Scholar] [PubMed]
- Klebanov, G.I.; Poltanov, E.A.; Chichuk, T.V.; Osipov, A.N.; Vladimirov, Y.A. Changes in Superoxide Dismutase Activity and Peroxynitrite Content in Rat Peritoneal Macrophages Exposed to He-Ne Laser Radiation. Biochemistry (Mosc) 2005, 70, 1335–1340. [Google Scholar] [CrossRef] [PubMed]
- Phan, T.-T.; Wang, L.; See, P.; Grayer, R.J.; Chan, S.-Y.; Lee, S.T. Phenolic Compounds of Chromolaena Odorata Protect Cultured Skin Cells from Oxidative Damage: Implication for Cutaneous Wound Healing. Biological & Pharmaceutical Bulletin 2001, 24, 1373–1379. [Google Scholar] [CrossRef]
- Carmo, J.P.M.; Dias-Melicio, L.A.; Calvi, S.A.; Peraçoli, M.T.S.; Soares, A.M.V.C. TNF-α Activates Human Monocytes for Paracoccidioides Brasiliensis Killing by an H 2 O 2 -Dependent Mechanism. Med Mycol 2006, 44, 363–368. [Google Scholar] [CrossRef]
- Rodrigues, D.R.; Dias-Melicio, L.A.; Calvi, S.A.; Peraçoli, M.T.S.; Soares, A.M.V.C. Paracoccidioides Brasiliensis Killing by IFN-γ, TNF-α and GM-CSF Activated Human Neutrophils: Role for Oxygen Metabolites. Med Mycol 2007, 45, 27–33. [Google Scholar] [CrossRef]
- Kerkweg, U.; Petrat, F.; Korth, H.-G.; De Groot, H. DISRUPTION OF SKELETAL MYOCYTES INITIATES SUPEROXIDE RELEASE: CONTRIBUTION OF NAD(P)H OXIDASE. Shock 2007, 27, 552–558. [Google Scholar] [CrossRef]
- Gonzalez, A.; De Gregori, W.; Velez, D.; Restrepo, A.; Cano, L.E. Nitric Oxide Participation in the Fungicidal Mechanism of Gamma Interferon-Activated Murine Macrophages against Paracoccidioides Brasiliensis Conidia. Infect Immun 2000, 68, 2546–2552. [Google Scholar] [CrossRef] [PubMed]
- Ducrocq, C.; Blanchard, B.; Pignatelli, B.; Ohshima, H. Peroxynitrite: An Endogenous Oxidizing and Nitrating Agent. CMLS, Cell. Mol. Life Sci. 1999, 55, 1068. [Google Scholar] [CrossRef] [PubMed]
- Hau, C.S.; Kanda, N.; Watanabe, S. Suppressive Effects of Antimycotics on Thymic Stromal Lymphopoietin Production in Human Keratinocytes. J Dermatol Sci 2013, 71, 174–183. [Google Scholar] [CrossRef] [PubMed]
- Kanda, N.; Kano, R.; Ishikawa, T.; Watanabe, S. The Antimycotic Drugs Itraconazole and Terbinafine Hydrochloride Induce the Production of Human β-Defensin-3 in Human Keratinocytes. Immunobiology 2011, 216, 497–504. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Bule, M.L.; Trillo, M.A.; Cid, M.A.; Leal, J.; Ubeda, A. In Vitro Exposure to 0.57-MHz Electric Currents Exerts Cytostatic Effects in HepG2 Human Hepatocarcinoma Cells. Int J Oncol 2007, 30, 583–592. [Google Scholar] [CrossRef]
- Hernández-Bule, M.L.; Martínez-Botas, J.; Trillo, M.Á.; Paíno, C.L.; Úbeda, A. Antiadipogenic Effects of Subthermal Electric Stimulation at 448 kHz on Differentiating Human Mesenchymal Stem Cells. Mol Med Rep 2016, 13, 3895–3903. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).