Submitted:
09 April 2024
Posted:
11 April 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
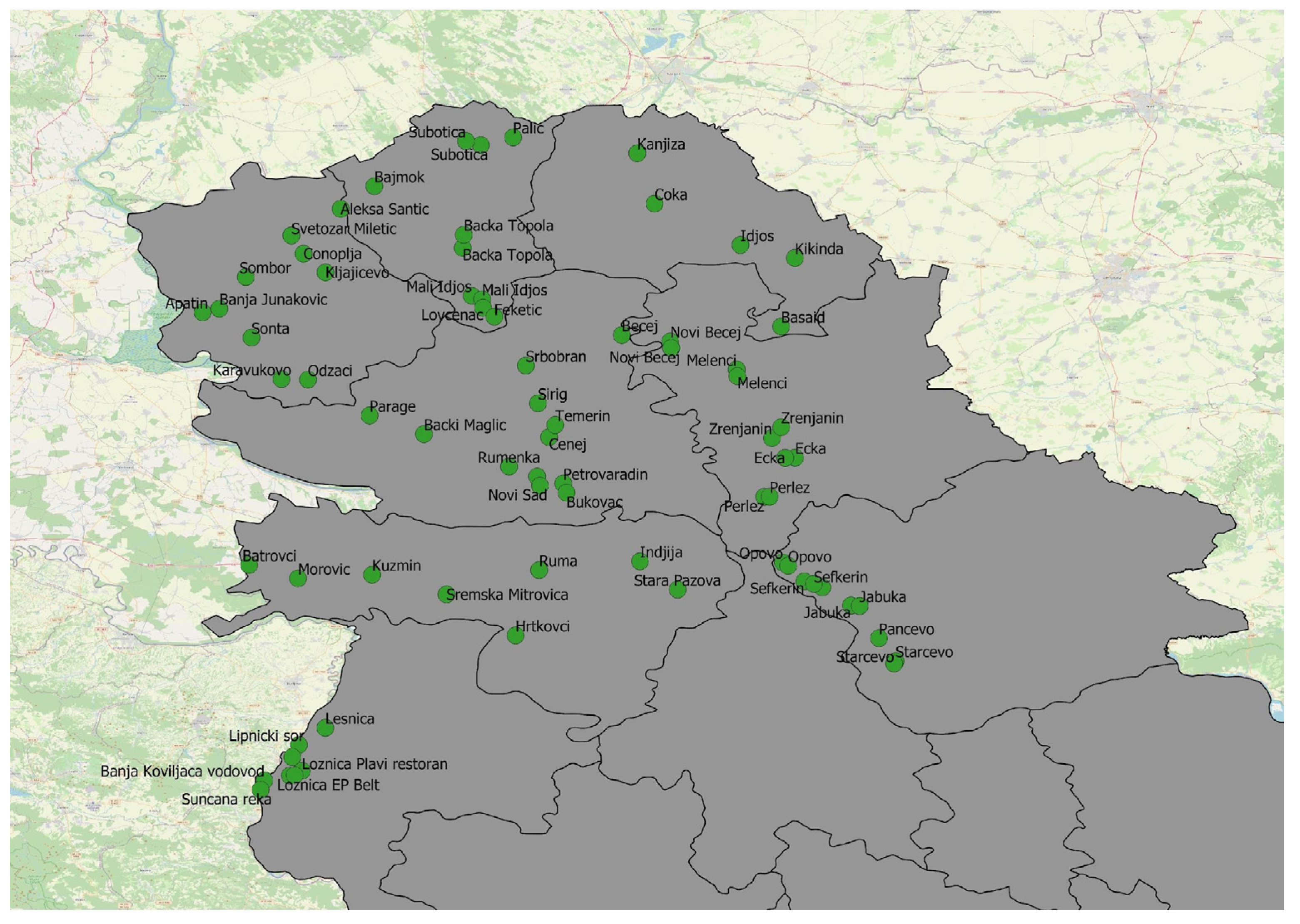
2.1. Mosquito Sampling and Vector Identification
2.2. DNA extraction
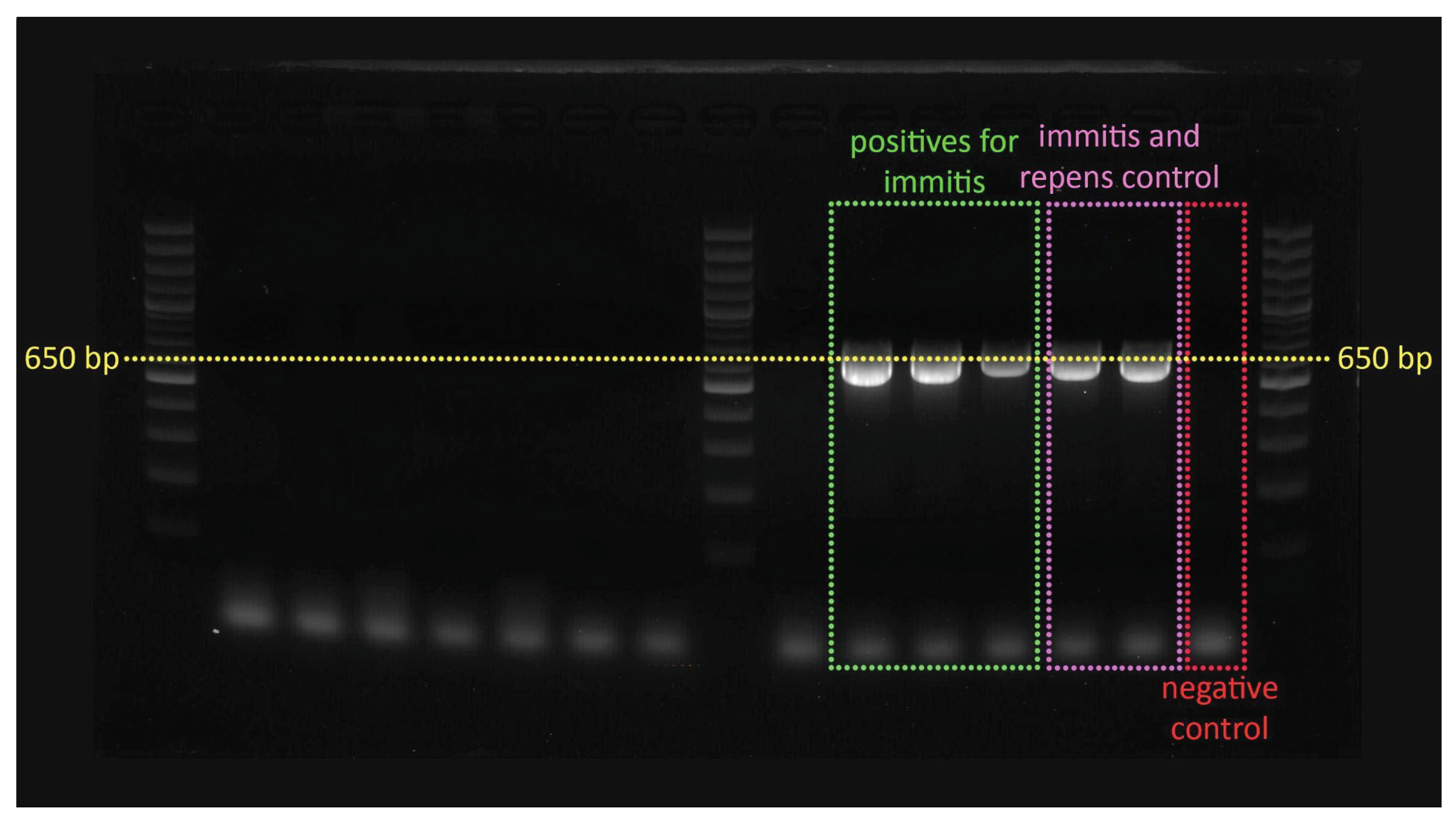
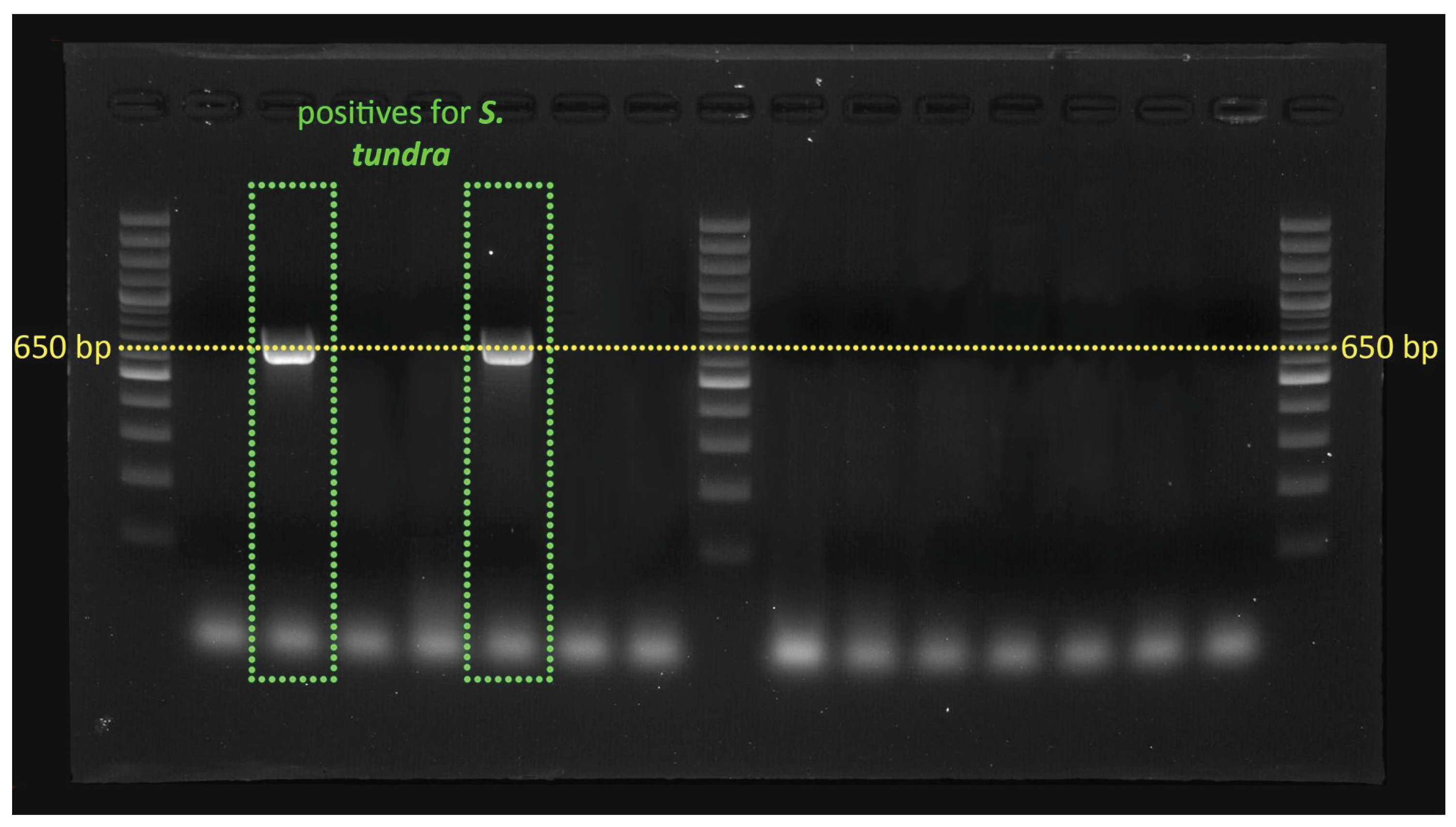
2.3. Identification of Dirofilaria sp.
2.4. Identification of Blood Meal Host
3. Results
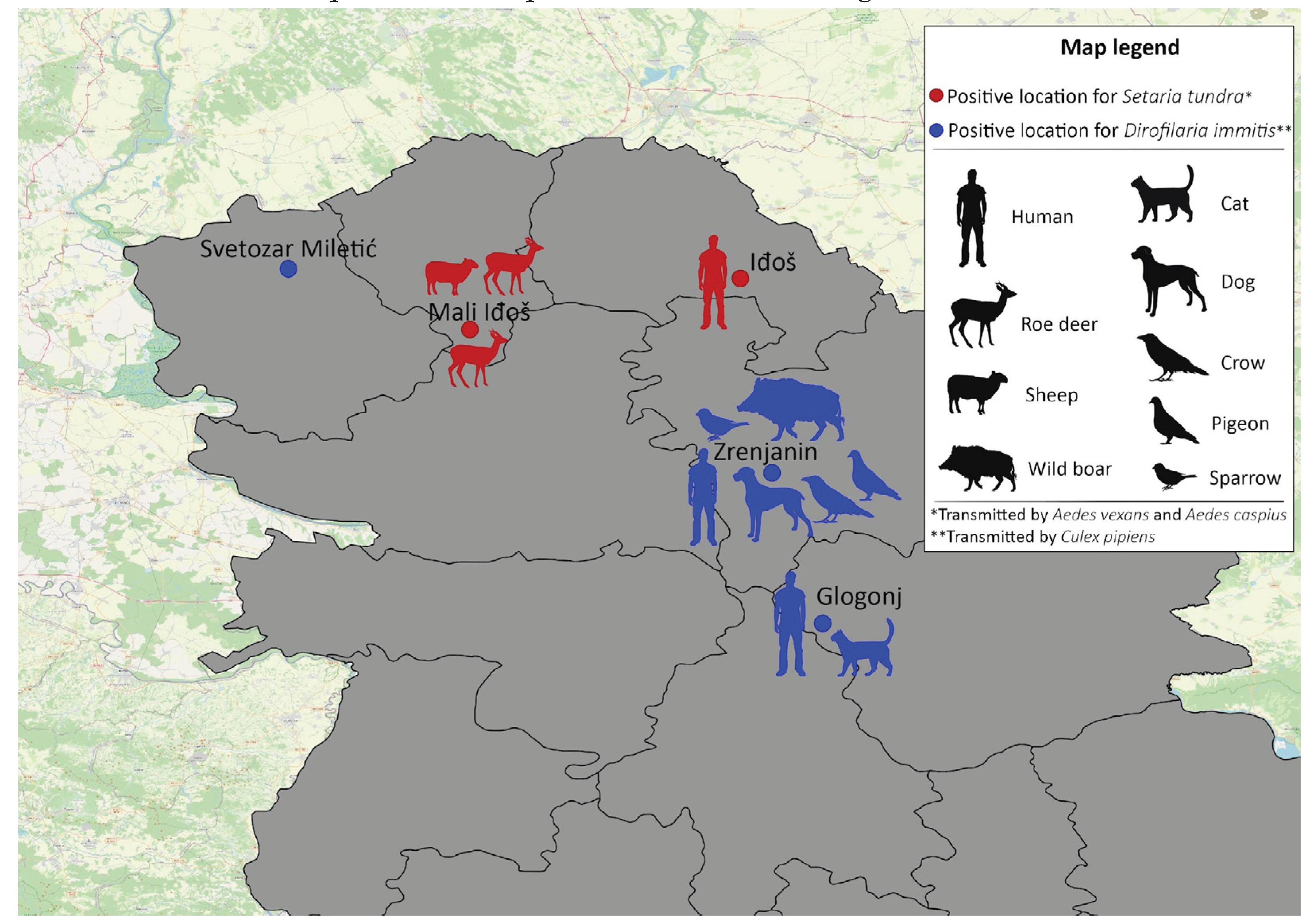
3.1. Presence of Dirofilaria immitis and Setaria tundra in Mosquitoes
3.2. Blood Meal Host Detection
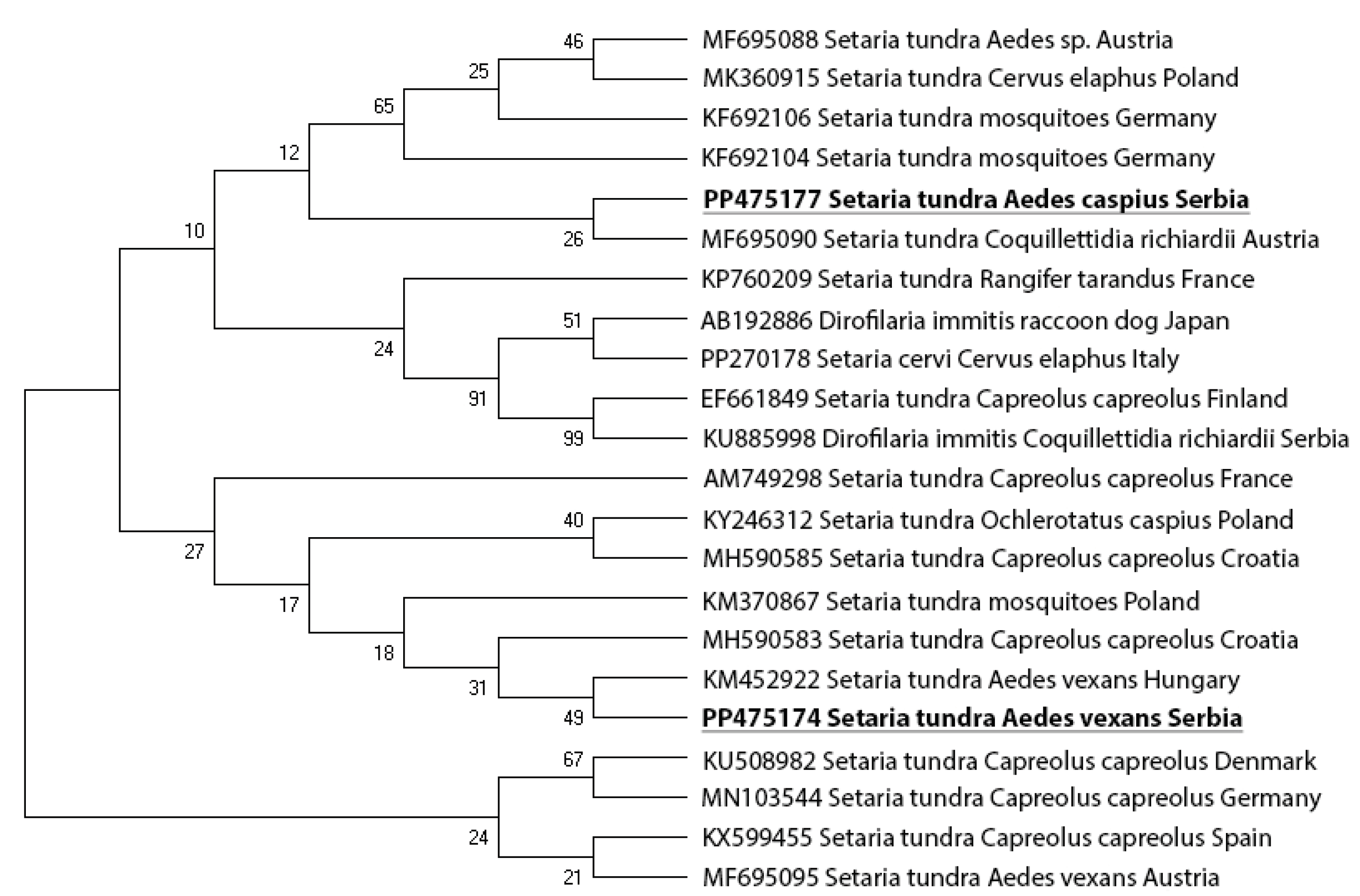
3.3. Phylogenetic analysis of Setaria tundra
4. Discussion
5. Conclusion
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
References
- R. Morchón, J. A. Montoya-Alonso, I. Rodríguez-Escolar, and E. Carretón, “What Has Happened to Heartworm Disease in Europe in the Last 10 Years?,” Pathogens, vol. 11, no. 9, p. 1042, Sep. 2022. [CrossRef]
- J. A. Montoya-Alonso et al., “First epidemiological report of feline heartworm infection in the Barcelona metropolitan area (Spain),” Parasit Vectors, vol. 7, no. 1, p. 506, Dec. 2014. [CrossRef]
- W. Tarello, “Clinical Aspects of Dermatitis Associated with Dirofilaria repens in Pets: A Review of 100 Canine and 31 Feline Cases (1990–2010) and a Report of a New Clinic Case Imported from Italy to Dubai,” J Parasitol Res, vol. 2011, pp. 1–7, 2011. [CrossRef]
- D. Traversa, A. Di Cesare, and G. Conboy, “Canine and feline cardiopulmonary parasitic nematodes in Europe: emerging and underestimated,” Parasit Vectors, vol. 3, no. 1, p. 62, 2010. [CrossRef]
- C. Genchi and L. H. Kramer, “The prevalence of Dirofilaria immitis and D. repens in the Old World,” Vet Parasitol, vol. 280, p. 108995, Apr. 2020. [CrossRef]
- Sadighian, “Helminth Parasites of Stray Dogs and Jackals in Shahsavar Area, Caspian Region, Iran,” J Parasitol, vol. 55, no. 2, p. 372, Apr. 1969. [CrossRef]
- J. W. McCall, C. Genchi, L. H. Kramer, J. Guerrero, and L. Venco, “Chapter 4 Heartworm Disease in Animals and Humans,” 2008, pp. 193–285. [CrossRef]
- D. Ćirović et al., “First records of Dirofilaria repens in wild canids from the region of Central Balkan,” Acta Vet Hung, vol. 62, no. 4, pp. 481–488, Dec. 2014. [CrossRef]
- M. Ionică et al., “Role of golden jackals (Canis aureus) as natural reservoirs of Dirofilaria spp. in Romania,” Parasit Vectors, vol. 9, no. 1, p. 240, Dec. 2016. [CrossRef]
- R. Morchón, E. Carretón, J. González-Miguel, and I. Mellado-Hernández, “Heartworm Disease (Dirofilaria immitis) and Their Vectors in Europe – New Distribution Trends,” Front Physiol, vol. 3, 2012. [CrossRef]
- D. Otranto et al., “Vector-borne helminths of dogs and humans in Europe,” Parasit Vectors, vol. 6, no. 1, p. 16, Dec. 2013. [CrossRef]
- F. Rivasi, R. Boldorini, P. Criante, M. Leutner, and S. Pampiglione, “Detection of Dirofilaria (Nochtiella) repens DNA by polymerase chain reaction in embedded paraffin tissues from two human pulmonary locations,” APMIS, vol. 114, no. 7–8, pp. 566–573, Aug. 2006. [CrossRef]
- F. Simón et al., “Human and Animal Dirofilariasis: the Emergence of a Zoonotic Mosaic,” Clin Microbiol Rev, vol. 25, no. 3, pp. 507–544, Jul. 2012. [CrossRef]
- B. K. Saha et al., “Human Pulmonary Dirofilariasis: A Review for the Clinicians,” Am J Med Sci, vol. 363, no. 1, pp. 11–17, Jan. 2022. [CrossRef]
- T. Sako et al., “Human pulmonary dirofilariasis presenting as a small nodule with a cavity.,” J Med Invest, vol. 47, no. 3–4, pp. 161–3, Aug. 2000.
- Muro, C. Genchi, M. Cordero, and F. Simón, “Human Dirofilariasis in the European Union,” Parasitology Today, vol. 15, no. 9, pp. 386–389, Sep. 1999. [CrossRef]
- S. Savić et al., “Seroepidemiological Study of Canine and Human Dirofilariasis in the Endemic Region of Northern Serbia,” Front Vet Sci, vol. 7, Sep. 2020. [CrossRef]
- L. Zumaquero, F. Simón, E. Carretón, I. Hernández, C. Sandoval, and R. Morchón, “Prevalence of canine and human dirofilariosis in Puebla, Mexico,” Vet Parasitol, vol. 282, p. 109098, Jun. 2020. [CrossRef]
- P. A. Ferrari, A. Grisolia, S. Reale, R. Liotta, A. Mularoni, and A. Bertani, “A rare case of human pulmonary dirofilariasis with nodules mimicking malignancy: approach to diagnosis and treatment,” J Cardiothorac Surg, vol. 13, no. 1, p. 65, Dec. 2018. [CrossRef]
- J. M. Medlock, I. Barrass, E. Kerrod, M. A. Taylor, and S. Leach, “Analysis of Climatic Predictions for Extrinsic Incubation of Dirofilaria in The United Kingdom,” Vector-Borne and Zoonotic Diseases, vol. 7, no. 1, pp. 4–14, Mar. 2007. [CrossRef]
- J. Fortin and J. Slocombe, “Temperature requirements for the development of Dirofilaria immitis in Aedes triseriatus and Ae. vexans.,” Mosq News, vol. 41, no. 4, pp. 625–633, 1981.
- Bruce., M., Christensen, and A. Hollander, “Effect of temperature on vector-parasite relationships of Aedes trivittatus and Dirofilaria immitis.,” Proceedings of Helminthology Society, vol. 45, pp. 115–119, 1978.
- T. J. Jankowski and W. E. Bickley, “The Mosquitoes, Aedes canadensis and A. vexans as Potential Vectors of Dirofilaria immitis in Maryland 1,” Ann Entomol Soc Am, vol. 69, no. 5, pp. 781–783, Sep. 1976. [CrossRef]
- F. W. Kutz and R. C. Dobson, “Effects of Temperature on the Development of Dirofilaria immitis (Leidy) in Anopheles quadrimaculatus Say1 and on Vector Mortality Resulting from this Development2,3,” Ann Entomol Soc Am, vol. 67, no. 3, pp. 325–331, May 1974. [CrossRef]
- G. Cancrini et al., “Natural vectors of dirofilariasis in rural and urban areas of the Tuscan region, central Italy.,” J Med Entomol, vol. 43, no. 3, pp. 574–9, May 2006. [CrossRef]
- S. Dimitrijević, A. Tasić, S. Tasić, V. Adamović, T. Ilić, and N. Miladinović-Tasić, “Filariosis in dogs in Serbia,” in Dirofilaria immitis and D. repens in dog and cat and human infections, Mappe parassitologiche, vol. 8, C. Genchi, L. Rinaldi, and G. Cringoli, Eds., 2007, p. 201.
- Tasić, L. Rossi, S. Tasić, N. Miladinović-Tasić, T. Ilić, and S. Dimitrijević, “Survey of canine dirofilariasis in Vojvodina, Serbia,” Parasitol Res, vol. 103, no. 6, pp. 1297–1302, Nov. 2008. [CrossRef]
- Tasić et al., “Canine Dirofilaria Infections in Two Uninvestigated Areas of Serbia: Epidemiological and Genetic Aspects,” Vector-Borne and Zoonotic Diseases, vol. 12, no. 12, pp. 1031–1035, Dec. 2012. [CrossRef]
- Tasić, S. Tasić, N. Miladinović-Tasić, D. Zdravković, and J. Đorđević, “Prevalence of Dirofilaria repens-cause of zoonosis in dogs,” Acta Fac Med Naiss, vol. 24, no. 2, pp. 71–74, 2007.
- Penezić, S. Selaković, I. Pavlović, and D. Ćirović, “First findings and prevalence of adult heartworms (Dirofilaria immitis) in wild carnivores from Serbia,” Parasitol Res, vol. 113, no. 9, pp. 3281–3285, Sep. 2014. [CrossRef]
- P. Gavrilović, G. Blitva-Robertson, J. Özvegy, F. Kiskároly, and Z. Becskei, “Case Report of dirofilariasis in grey wolf in Serbia.,” Acta Parasitol, vol. 60, no. 1, pp. 175–8, Mar. 2014. [CrossRef]
- Potkonjak et al., “Molecular survey of Dirofilaria species in stray dogs, red foxes and golden jackals from Vojvodina, Serbia,” Comp Immunol Microbiol Infect Dis, vol. 68, p. 101409, Feb. 2020. [CrossRef]
- K. Kurucz et al., “First molecular identification of Dirofilaria spp. (Onchocercidae) in mosquitoes from Serbia,” Parasitol Res, vol. 115, no. 8, pp. 3257–3260, Aug. 2016. [CrossRef]
- N. Becker et al., Mosquitoes, 3rd ed. Cham: Springer International Publishing, 2020. [CrossRef]
- M. Casiraghi, T. J. C. Anderson, C. Bandi, C. Bazzocchi, and C. Genchi, “A phylogenetic analysis of filarial nematodes: comparison with the phylogeny of Wolbachia endosymbionts,” Parasitology, vol. 122, no. 1, pp. 93–103, Jan. 2001. [CrossRef]
- M. Casiraghi et al., “Mapping the presence of Wolbachia pipientis on the phylogeny of filarial nematodes: evidence for symbiont loss during evolution,” Int J Parasitol, vol. 34, no. 2, pp. 191–203, Feb. 2004. [CrossRef]
- S. Gabrielli et al., “Molecular Identification of New Cases of Human Dirofilariosis (Dirofilaria repens) in Italy,” Pathogens, vol. 10, no. 2, p. 251, Feb. 2021. [CrossRef]
- S. Tasić-Otašević et al., “Molecular Survey of Dirofilaria and Leishmania Species in Dogs from Central Balkan,” Animals, vol. 12, no. 7, p. 911, Apr. 2022. [CrossRef]
- S. Boessenkool et al., “Blocking human contaminant DNA during PCR allows amplification of rare mammal species from sedimentary ancient DNA,” Mol Ecol, vol. 21, no. 8, pp. 1806–1815, Apr. 2012. [CrossRef]
- K. Tamura, G. Stecher, and S. Kumar, “MEGA11: Molecular Evolutionary Genetics Analysis Version 11,” Mol Biol Evol, vol. 38, no. 7, pp. 3022–3027, Jun. 2021. [CrossRef]
- . Morchón et al., “Haplotype H1 of Culex pipiens Implicated as Natural Vector of Dirofilaria immitis in an Endemic Area of Western Spain,” Vector-Borne and Zoonotic Diseases, vol. 7, no. 4, pp. 653–658, Dec. 2007. [CrossRef]
- \Yildirim, A. Inci, O. Duzlu, Z. Biskin, A. Ica, and I. Sahin, “Aedes vexans and Culex pipiens as the potential vectors of Dirofilaria immitis in Central Turkey,” Vet Parasitol, vol. 178, no. 1–2, pp. 143–147, May 2011. [CrossRef]
- M. Santa-Ana, M. Khadem, and R. Capela, “Natural Infection of Culex theileri (Diptera: Culicidae) with Dirofilaria immitis (Nematoda: Filarioidea) on Madeira Island, Portugal,” J Med Entomol, vol. 43, no. 1, pp. 104–106, Jan. 2006. [CrossRef]
- R. Morchón et al., “Molecular Characterization of Culex Theileri from Canary Islands, Spain, a Potential Vector of Dirofilaria Immitis,” J Clin Exp Pathol, vol. 01, no. S3, pp. 1–6, 2011. [CrossRef]
- Z. Bışkın, O. Düzlü, A. Yildirim, and A. Incı, “The molecular diagnosis of Dirofilaria immitis in vector mosquitoes in Felahiye district of Kayseri.,” Turkiye Parazitol Derg, vol. 34, no. 3, pp. 200–5, 2010.
- G. Cancrini, M. Pietrobelli, A. F. Frangipane di Regalbono, M. P. Tampieri, and A. della Torre, “Development of Dirofilaria and Setaria nematodes in Aedes albopictus.,” Parassitologia, vol. 37, no. 2–3, pp. 141–5, Dec. 1995.
- G. Cancrini, A. Frangipane di Regalbono, I. Ricci, C. Tessarin, S. Gabrielli, and M. Pietrobelli, “Aedes albopictus is a natural vector of Dirofilaria immitis in Italy,” Vet Parasitol, vol. 118, no. 3–4, pp. 195–202, Dec. 2003. [CrossRef]
- D. Petrić et al., “West Nile virus ‘circulation’ in Vojvodina, Serbia: Mosquito, bird, horse and human surveillance.,” Mol Cell Probes, vol. 31, pp. 28–36, Feb. 2017. [CrossRef]
- G. Capelli et al., “Recent advances on Dirofilaria repens in dogs and humans in Europe,” Parasit Vectors, vol. 11, no. 1, p. 663, Dec. 2018. [CrossRef]
- M. Krstić et al., “An appraisal of canine and human cases reveals an endemic status of dirofilariosis in parts of Serbia,” Mol Cell Probes, vol. 31, pp. 37–41, Feb. 2017. [CrossRef]
- L. Spasojević-Kosić, V. Lalošević, D. Lalošević, S. Simin, I. Vasić, and L. Kuruca, “Prevalence of Dirofilariosis in pet dogs in Novi Sad,” Contemporary Agriculture, vol. 61, pp. 247–254, 2012, Accessed: Mar. 20, 2024. [Online]. Available: https://www.researchgate.net/publication/259962443_Prevalence_of_Dirofilariosis_in_pet_dogs_in_Novi_Sad.
- L. Spasojević Kosić, V. Lalošević, S. Simin, and L. Kuruca, “Dirofilariosis and angiostrongilosis in pet and hunting dogs in Novi Sad, Vojvodina, Serbia,” Archives of Veterinary Medicine, vol. 9, no. 2, pp. 53–62, Mar. 2017. [CrossRef]
- J. J. Gawor, “The prevalence and abundance of internal parasites in working horses autopsied in Poland,” Vet Parasitol, vol. 58, no. 1–2, pp. 99–108, May 1995. [CrossRef]
- M. T. Manfredi, G. Piccolo, C. Fraquelli, and F. Perco, “Elmintofauna del cervo nel Parco Nazionale dello Stelvio,” J Mt Ecol, vol. 7, pp. 245–249, 2003.
- W. Demiaszkiewicz, J. Lachowicz, and G. Karbowiak, “Wzrost zarażenia żubrów w Puszczy Białowieskiej nicieniami Setaria labiatopapillosa,” Wiadomoœci Parazytologiczne, vol. 53, no. 4, pp. 335–338, 2007.
- W. Demiaszkiewicz, I. Kuligowska, A. M. Pyziel, and J. Lachowicz, “First cases of nematodes Setaria tundra invasion in elk (Alces alces) in Poland,” Med Weter, vol. 71, no. 8, pp. 510–512, 2015.
- Z. A. Raevskaya, “Setaria and their pathogenic significance,” Trudy Gosudarstvennogo Instituta Eksperimental’noi Veterinarii, vol. 5, pp. 1–58, 1928.
- G. Oloś, J. Nowakowska, and R. Welc-Falęciak, “Setaria tundra, what do we know, what is still to be discovered?,” Ann Parasitol, vol. 67, no. 1, pp. 1–10, 2021. [CrossRef]
- E. Kutzer and H. K. Hinaidy, “Die Parasiten der wildlebenden Wiederkäuer Österreichs,” Z Parasitenk , vol. 32, pp. 354–368, 1969.
- E. Savonen, “The implementation of domestic reindeer meat inspection,” Suomen Eläinlääkärilehti, vol. 84, pp. 651–653, 1978.
- Rehbinder, D. Christensson, and V. Glatthard, “Parasitic Granulomas in Swedish Forest Reindeer (Rangifer Tarandus),” in Wildlife Diseases, Boston, MA: Springer US, 1976, pp. 597–607. [CrossRef]
- T. T. Poppe, “Parasitic diseases recorded in reindeer during meat inspection in Kautokeino (Norway) in autumn and winter,” Norsk Veterinaertidsskrift, vol. 89, pp. 791–795, 1977.
- Y. Yanchev, “The helminth fauna of roe deer (Capreolus capreolus) in Bulgaria. 3. Material on helminth fauna in roe deer (Capreolus capreolus L.) in the mountains of southern Bulgaria,” Izvestiya naTsentralnata Khelmintologichna Laboratoriya, vol. 16, pp. 205–220, 1973.
- J. R. Andrews, B. Hörning, and A. Wandeler, “Endoparasites of roe deer (Capreolus capreolus L.) from Switzerland with special reference to hosts from the Emmental region of Canton Berne,” Revue Suisse de Zoologie, Annales de la Societe Zoologique Suisse et du Museum d’Histoire Naturelle de Geneve, vol. 81, p. 13, 1974.
- K. Büttner, “Untersuchungen zur Parasitierung des Rehwildes bei steigendem Jagddruck,” Zeitschrift für Jagdwissenschaft, vol. 24, pp. 139–155, 1978.
- G. Favia, “Molecular assay for the identification of Setaria tundra,” Vet Parasitol, vol. 117, no. 1–2, pp. 139–145, Nov. 2003. [CrossRef]
- E. Ferri et al., “Integrated taxonomy: traditional approach and DNA barcoding for the identification of filarioid worms and related parasites (Nematoda),” Front Zool, vol. 6, no. 1, p. 1, Dec. 2009. [CrossRef]
- H. L. Enemark, A. Oksanen, M. Chriél, J. le Fèvre Harslund, I. D. Woolsey, and M. N. S. Al-Sabi, “Detection and molecular characterization of the mosquito-borne filarial nematode Setaria tundra in Danish roe deer (Capreolus capreolus),” Int J Parasitol Parasites Wildl, vol. 6, no. 1, pp. 16–21, Apr. 2017. [CrossRef]
- M. Bednarski, T. Piasecki, M. Bednarska, and Z. Soltysiak, “Invasion of Setaria tundra in roedeer (Capreolus capreolus) - case report,” Acta Scientiarum Polonorum. Medicina Veterinaria, vol. 3, no. 09, pp. 21–25, 2010, Accessed: Mar. 20, 2024. [Online]. Available: https://www.infona.pl//resource/bwmeta1.element.agro-efb22ab5-15e9-47fa-9f42-6ad3af19da0f.
- Zittra et al., “Screening blood-fed mosquitoes for the diagnosis of filarioid helminths and avian malaria,” Parasit Vectors, vol. 8, no. 1, pp. 1–6, Dec. 2015. [CrossRef]
- S. Angelone-Alasaad et al., “First report of Setaria tundra in roe deer (Capreolus capreolus) from the Iberian Peninsula inferred from molecular data: epidemiological implications,” Parasit Vectors, vol. 9, no. 1, p. 521, Dec. 2016. [CrossRef]
- J. Čurlík, D. Konjević, M. Bujanić, Ž. Sabol, F. Martinković, and M. Sindičić, “The first description of Setaria tundra (Issaitshikoff & Rajewskaya, 1928) in roe deer from Croatia,” Helminthologia, vol. 56, no. 3, pp. 252–255, Sep. 2019. [CrossRef]
- J. Čurlík et al., “The first report of Setaria tundra (Issaitshikoff & Rajewskaya, 1928) in Slovakia by using of molecular methods,” Vet Res Commun, vol. 47, no. 4, pp. 2247–2251, Dec. 2023. [CrossRef]
- G. Oloś, J. Nowakowska, S. Rojewska, and R. Welc-Falęciak, “New findings of Setaria tundra and Setaria cervi in the red deer (Cervus elaphus) in Poland,” Parasitology, vol. 146, no. 10, pp. 1333–1337, Sep. 2019. [CrossRef]
- S. Laaksonen, J. Kuusela, S. Nikander, M. Nylund, and A. Oksanen, “Outbreak of parasitic peritonitis in reindeer in Finland,” Veterinary Record, vol. 160, no. 24, pp. 835–841, Jun. 2007. [CrossRef]
- S. Laaksonen, M. Solismaa, R. Kortet, J. Kuusela, and A. Oksanen, “Vectors and transmission dynamics for Setaria tundra (Filarioidea; Onchocercidae), a parasite of reindeer in Finland,” Parasit Vectors, vol. 2, no. 1, p. 3, 2009. [CrossRef]
- J. Kowal, S. Kornaś, P. Nosal, M. Basiaga, and M. Lesiak, “Setaria tundra in roe deer (Capreolus capreolus)--new findings in Poland.,” Ann Parasitol, vol. 59, no. 4, pp. 179–82, 2013.
- K. Tomczuk et al., “Internal parasites in roe deer of the Lubartów Forest Division in postmortem studies,” Med Weter, vol. 73, no. 11, pp. 726–730, 2017. [CrossRef]
- S. Laaksonen and A. Oksanen, “Status and review of the vector-borne nematode Setaria tundra in Finnish cervids,” A Journal Devoted to the Biology and Management of Moose, vol. 45, pp. 81–84, 2009, Accessed: Mar. 20, 2024. [Online]. Available: https://www.researchgate.net/publication/277218607_Status_and_review_of_the_vector-borne_nematode_Setaria_tundra_in_Finnish_cervids.
- T. Bazargani et al., “Cerebrospinal Nematodiasis of Cattle, Sheep and Goats in Iran,” Iran J Parasitol, vol. 3, no. 1, pp. 16–20, 2008, Accessed: Mar. 20, 2024. [Online]. Available: https://www.researchgate.net/publication/228483310_Cerebrospinal_Nematodiasis_of_Cattle_Sheep_and_Goats_in_Iran.
- G. Sharhuu and T. Sharkhuu, “The helminth fauna of wild and domestic ruminants in Mongolia-a review,” Eur J Wildl Res, Aug. 2004. [CrossRef]
- S. T. B. Sundar and P. E. D’Souza, “Morphological characterization of Setaria worms collected from cattle,” Journal of Parasitic Diseases, vol. 39, no. 3, pp. 572–576, Sep. 2015. [CrossRef]
- Kuchboev, O. Amirov, R. R. Karimova, and M. Asakawa, “Nematodes in the digestive tracts of domestic ruminants in Uzbekistan,” Jpn J Vet Parasitol, vol. 15, no. 2, pp. 124–128, 2016, Accessed: Mar. 20, 2024. [Online]. Available: https://www.researchgate.net/publication/313399954_Kuchboev_et_al_Jpn_J_Vet_Parasitol_Vol_15_No_2_2016.
- V. Lavadinovic, Z. Popovic, D. Beukovic, and K. Cokoski, “Wild boar management (Sus scrofa L.) in the Republic of Serbia,” Glasnik Sumarskog fakulteta, no. 121, pp. 47–60, 2020. [CrossRef]
- V. Milićević et al., “Cross-sectional serosurvey of selected infectious diseases in wild ruminants in Serbia,” Res Vet Sci, vol. 170, p. 105183, Apr. 2024. [CrossRef]
- S. Lanková et al., “Setaria cervi (Filarioidea, Onchocercidae) undressing in ungulates: altered morphology of developmental stages, their molecular detection and complete sequence cox 1 gene,” Parasitology, vol. 148, no. 5, pp. 598–611, Apr. 2021. [CrossRef]
- S. Nechybová et al., “Long-term occurrence of Trichuris species in wild ruminants in the Czech Republic,” Parasitol Res, vol. 117, no. 6, pp. 1699–1708, Jun. 2018. [CrossRef]
- R. C. Anderson, Nematode parasites of Vertebrates, their development and transmission, 2nd ed. New York: CABI Publishing, 2000. Accessed: Mar. 20, 2024. [Online]. Available: https://books.google.rs/books?hl=en&lr=&id=lEERbfsvP1EC&oi=fnd&pg=PR9&dq=The+superfamily+Filarioidea.+Nematode+parasites+of+Vertebrates,+their+development+and+transmission&ots=uWqQLNtQfb&sig=JfyKt0sUeJcCXIuApfj9SlKDUJU&redir_esc=y#v=onepage&q=The%20superfamily%20Filarioidea.%20Nematode%20parasites%20of%20Vertebrates%2C%20their%20development%20and%20transmission&f=false.
- Genchi, L. Rinaldi, M. Mortarino, M. Genchi, and G. Cringoli, “Climate and Dirofilaria infection in Europe,” Vet Parasitol, vol. 163, no. 4, pp. 286–292, Aug. 2009. [CrossRef]
- S. Laaksonen et al., “Climate Change Promotes the Emergence of Serious Disease Outbreaks of Filarioid Nematodes,” Ecohealth, vol. 7, no. 1, pp. 7–13, Aug. 2010. [CrossRef]
- K. Rydzanicz, E. Lonc, A. Masny, and E. Golab, “Detection of Setaria tundra microfilariae in mosquito populations from irrigated fields in Wroclaw (Poland,” Ann Parasitol, vol. 62, no. 132, 2016, Accessed: Mar. 20, 2024. [Online]. Available: https://annals-parasitology.eu/archive_2001_2022/2016-62-Suppl-S4_132.pdf.
- Masny, W. Rożej-Bielicka, and E. Gołąb, “Setaria tundra invasive larvae in a mosquito vector in Poland,” Annales of Parasitology, vol. 59, p. 178, 2013, Accessed: Mar. 20, 2024. [Online]. Available: yadda.icm.edu.p.
- G. Kemenesi et al., “Circulation of Dirofilaria repens, Setaria tundra, and Onchocercidae species in Hungary during the period 2011–2013,” Vet Parasitol, vol. 214, no. 1–2, pp. 108–113, Nov. 2015. [CrossRef]
- Czajka, N. Becker, S. Poppert, H. Jöst, J. Schmidt-Chanasit, and A. Krüger, “Molecular detection of Setaria tundra (Nematoda: Filarioidea) and an unidentified filarial species in mosquitoes in Germany,” Parasit Vectors, vol. 5, no. 1, p. 14, Dec. 2012. [CrossRef]
- M. Kronefeld, H. Kampen, R. Sassnau, and D. Werner, “Molecular detection of Dirofilaria immitis, Dirofilaria repens and Setaria tundra in mosquitoes from Germany,” Parasit Vectors, vol. 7, no. 1, p. 30, Dec. 2014. [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




