Introduction
A massive outbreak of
the Respiratory syncytial virus is occurring everywhere. It is regarded as the primary cause of newborn pneumonia and bronchiolitis. In addition, it causes pneumonia in the elderly and patients with chronic cardio-pulmonary disorders, as well as otitis media in older children.[
1] One single-stranded, negatively polarized fragment of RNA and an enveloped virus with a helical nucleocapsid make up its characteristics. In a virion,
RNA polymerase is present. Its surface spikes solely contain a
fusion protein, unlike other
Paramyxoviruses. It is
hemagglutinin-free. The serotypes are two.[
2] Transmission happens when infected hands come into close contact with the nose or mouth or when respiratory droplets come into contact with the hands.[
3] Unlike many other cold viruses, which re-enter the community every few years,
RSV causes respiratory illness epidemics every winter. It affects almost everyone by the age of three and is present everywhere.[
4] Infection in babies largely affects the lower respiratory system; there is no systemic dissemination.[
5] Immune response most likely plays a role in pathogenesis.[
6]
RSV also contributes to respiratory infection epidemics in hospitalized babies.[
7] Enzyme immunoassay (
rapid antigen test), which finds
RSV antigens in respiratory secretions, is used in the laboratory to make a diagnosis. Cell culture isolation is also an option. Giant cells with several nuclei can be seen with an electronic microscope. Following that, immunofluorescence is used to confirm the
RSV infection's identification. When making a diagnosis of a baby, serology is useless.[
8] For critically unwell newborns, aerosolized
ribavirin is used as a treatment.[
9] Passive immunization with the monoclonal antibody
palivizumab[
10] or the injection of immune globulins from previously infected infants who were able to overcome this obstacle are two methods of prevention.[
11] In the nursery for new babies, nosocomial outbreaks may be avoided by hand washing and glove use.[
12]
RSV frequently leads to reinfection throughout life despite having a modest degree of antigenic change.[
13] Despite more than 50 years of study, there is still no licensed safe and effective
RSV vaccine.[
14] In children under the age of five,
RSV is thought to be the cause of roughly
22% of severe respiratory tract infections.[
15] The current study's objective was to develop lipid nanoparticles of
the mRNA vaccination of the
fusion spike protein to combat the fatal
RSV that affects newborns worldwide.
Patient and Methods
Ethical Statement
For the current investigation, all applicable national, institutional, and/or international rules for using both people and animals were postdated. By the recommendations of the Weather-all report, the local government, the Ethical Committee for Human and Animal Handling at Cairo University (ECAHCU), and the Pharmacy Faculty at, University of Cairo, Egypt, all approved all procedures used in the study, including those involving people and animals, with approval number P-13-2-2021. The number of volunteers and the suffering of the animals used in the study were both minimized.
The type of the study:
Screening experimental examination.
Source of animal models:
One hundred male transgenic mice, weighing between 40 and 50 g, were bought from Cairo University's College of pharmacy's Pharmacology and toxicology department and given the go-ahead for legalization. Human lung cells were injected into these animals.
Inclusion criteria for animal models:
Adult male mice weighing 40–50 gm; are susceptible to RSV infection, including transgenic mice. A lung human cell line was used to humanize transgenic mice, boosting viral protein expression and inducing potent humoral and cell-mediated immunity. Human lung epithelial BEAS-2B cells, which displayed features of mesenchymal stem cells, were bought from Accegen, USA. After receiving an intranasal dosage of the respiratory syncytial virus that was infectious at a dose of 170-200 viral units, transgenic mice that were adult males weighing 40–50 g were able to develop lower respiratory illnesses like pneumonia. Three to five days following the beginning of the incubation phase, symptoms started to manifest. During the current investigation, the fatal dosage surpassed 600 virus units.
Exclusion criteria:
Young mice; pregnant female mice and male mice weighing less than 40 gm.
Collection of the samples:
100 blood samples were collected from Respiratory syncytial virus infected infants, children and elderly in different locations in Egypt.
Material:
All chemical and biological components were sourced from Algomhoria Pharmaceutical Company in Cairo, Egypt, and Alnasr Pharmaceutical Company in Abo zabal Alkhanka, Qalyobia, Egypt. Riboblock RNase inhibitor[ 40 U/µl] with catalog number EO0384 was obtained from ThermoFisher Scientific, USA. T7 RNA Polymerase[ 20 U/µl] with catalog number EP0112 was purchased from ThermoFisher Scientific, USA. Pyrophosphatase, inorganic[ 0.1 U/µl] with catalog number EF0221 was purchased from ThermoFisher Scientific, USA. TrancriptAid T7 High Yield Transcription kit with catalog number K0441 was obtained from ThermoFisher Scientific, USA.
Date and of place study:
Between February 2021 and August 2023, this study was conducted at Cairo University's pharmacy faculty in Egypt.
Methods
The potential open reading frames of mRNA of surface spike fusion protein of RSV virus was identified by bioinformatics using NCBI website; mRNA of surface spike fusion protein was expressed then purified via organic extraction method. The purified mRNA was enclosed with lipid nano-particles bubbles synthesized using hot micro-emulsion technique. The vaccine delivery system was lipid nanoparticles with particle size 90 nanometre. Purified mRNA was administered intraperitoneally to 100 transgenic mice to test the immunogenicity in animal models. During the immunogenicity testing in preclinical trials animal testing and randomized human clinical trials stages 1/2, the pathogenic 2 valent Respiratory syncytial serotypes consisted of A, B serotypes.
Construction of mRNA transcripts of fusion spike protein of RSV:
[ DynabeadsTM mRNA purification kits with Catalog number: 61006 were obtained from Invitrogen Thermo Fisher Scientific, USA]
Procedure for in vitro mRNA transcription:
Using the following process, more than 10 µg of mRNA transcript could be produced from a 1 µg DNA template. The TranscriptAidTM T7 High Yield Transcription kit was utilized for high-yield transcription, producing up to 200 µg of mRNA. Thawed and combined frozen reagents under 300 rpm centrifugation for three minutes. Nucleotides and enzymes were kept cold. At room temperature, the reaction buffer was retained. At room temperature, the following combination for the reaction was created. The mRNA transcription reaction mixture was then incubated at 37 0C for 2 hours. 2 µl[ 2 U] of DNAase I, RNAase- free were added, mixed and incubated at 37 0C for 15 minutes in order to remove DNA template. The reaction was obstructed aside the addition of 2 µl 0.5 M EDTA at pH 8.0 and incubation at 65 0C for 10 minutes. mRNA was subjected to hydrolysis in the absence of a chelating agent such as EDTA.
Purification of mRNA transcripts:
This was done using the
liquid-liquid extraction method known as
AGPC, which stands for
Acid Guanidinium Thiocyanate-Phenol-Chloroform. Chloroform reagent solutions were manufactured and consisted of 96%
chloroform and 4%
Isoamyl alcohol.[
16]
Northern blot technique for the confirmation of the purification of mRNA transcripts coding for the fusion spike protein:
The sizes and amounts of the various mRNA transcripts for the 2 valent serotypes of RSV that code for the fusion spike protein formation were measured using the Northern blot method. Denaturing gel was originally employed in Northern blot to separate mRNA transcripts based on size. Then, with the same distribution as in the gel, mRNA was transferred into a nylon membrane. In order to hybridize the immobilized mRNA, a la-belled probe complementary to the target gene was added after the mRNA transcripts were fixed to the membrane. After that, the loosely bound probes were rinsed away. The solid membrane was then dried, made visible, and was subjected to examination with the probe precisely bound to the target mRNA transcripts. The Northern blot measured the quantities and seizes of the target mRNA transcripts.
Inclusion of mRNA vaccine with lipid nanoparticles:
Bubbles made of lipid nanoparticles were added. 45 mcg of dimethyl dioctadecyl ammonium bromide lipid( DDAB) were used to create these lipid nanoparticles. A Quaternary ammonium lipid called DDAB formed vesicles that contained mRNA transcripts when it complexed with mRNA to trigger innate immunity. The delivery system for the vaccine made of lipid nanoparticles included particles that were around 90 nm in size. It was accomplished throughout this investigation to create lipid nanoparticles with various cationic and solid lipid types and to assess their suitability as LNP-mRNA delivery systems. This was accomplished by using a hot micro-emulsion process to combine cationic lipids such stearylamine, DOMTA, or DDAB with solid lipids like Compritol 888 ATO[ C] or cetyl palmitate 15[ CP 15] to create a sequence of lipid nanoparticles. The created cationic solid nanoparticles systems( CSLNS) were examined using a Malvern NanoZS analyzer[ obtained from Biotechne, USA] to determine their particle size, size distribution, and zeta potential. Comparing and assessing the cytotoxicity of CSLNS systems was done using the Resazurin test. For the examination of the CSLNS systems, which effectively bound mRNA transcripts and shown minimal cytotoxicity, in-vitro cellular uptake [fluorescence microscopy] and gene silencing [Northern blot] experiments were used. On the other hand, high Performance size exclusion chromatography (HPSEC) was exploited to assess the particle configuration of mRNA transcripts. Later on loading on a CSLNS formulation, the transition temperature [Tm] of the mRNA transcripts was deliberated exploiting differential scanning fluorimetry (DSF).
Experimental preclinical trials [animal testing]:
In vitro vaccine immunogenicity testing on transgenic mice:
Transgenic mice have their genes changed by tissue culture and recombinant
DNA technologies. A transgenic animal has a gene of
DNA sequence (a trans-gene) incorporated into the cell's genome by human intervention. The vaccination was administered to 100 transgenic mice. 28 days separated the two dosages that were given to them. To boost immunity, the initial dosage was cut in half. For boosting viral protein expression and inducing potent humoral and cell-mediated immunity, transgenic mice were made human by lung human cell line [
Human lung epithelial BEAS-2B cells, which showed features of mesenchymal stem cells, bought from Accegen, USA]. After being inoculated with Respiratory syncytial virus at an infectious dosage of 170-200 viral units via an intranasal method of administration, transgenic mice, which were adult male mice weighing 40–50 g, might be triggered by lower respiratory illnesses such pneumonia.[
17]
Formulation:
lipid nanoparticle vaccination with mRNA. The dose form was an intramuscular injection of a sterile solution of the mRNA transcripts of the genes encoding the proteins promoting the synthesis of RSV fusion spike protein. Each 1 ml dose contained 5 mcg of the mRNA transcript for each cluster gene of RSV that codes for spike fusion protein of 2 valent RSV pathogenic serotypes, 45 mcg of the lipid dimethyl dioctadecyl ammonium bromide( DDAB), and 0.8 mg of aluminium hydroxide. Additionally, 0.923 mg of sodium dihydrogen phosphate dihydrate and 7 mg of sodium chloride were included in each dose.
By administering the pure LNP-mRNA vaccine via intraperitoneal route to 200 male transgenic mice weighing 45–50 gm, immunogenicity in animal models was assessed:
The effectiveness of vaccinations was evaluated using Active Protection testing. 100 transgenic mice were challenged with an increasing quantity of infectious microorganisms by intranasal delivery after receiving the vaccination under test. The infectious dosage of RSV delivered by intranasal delivery varied from 170 to 200 virus units. To assess the immunogenicity and efficacy of the vaccine, the lowest number of microorganisms required to cause 50% of transgenic mice to die ( LD 50%) was established and compared to LD 50% in non-vaccinated subjects. To conduct passive protection testing, 100 transgenic mice were given varying dosages of immunized subjects' serum intraperitoneally. The mice were subsequently challenged with 180 to 200 viral units of the infectious agent via an intranasal route. The greatest serum dilution that could protect 50% of the animals (i.e., ED 50%) was chosen as a benchmark for the vaccine's effectiveness.
ELISA:
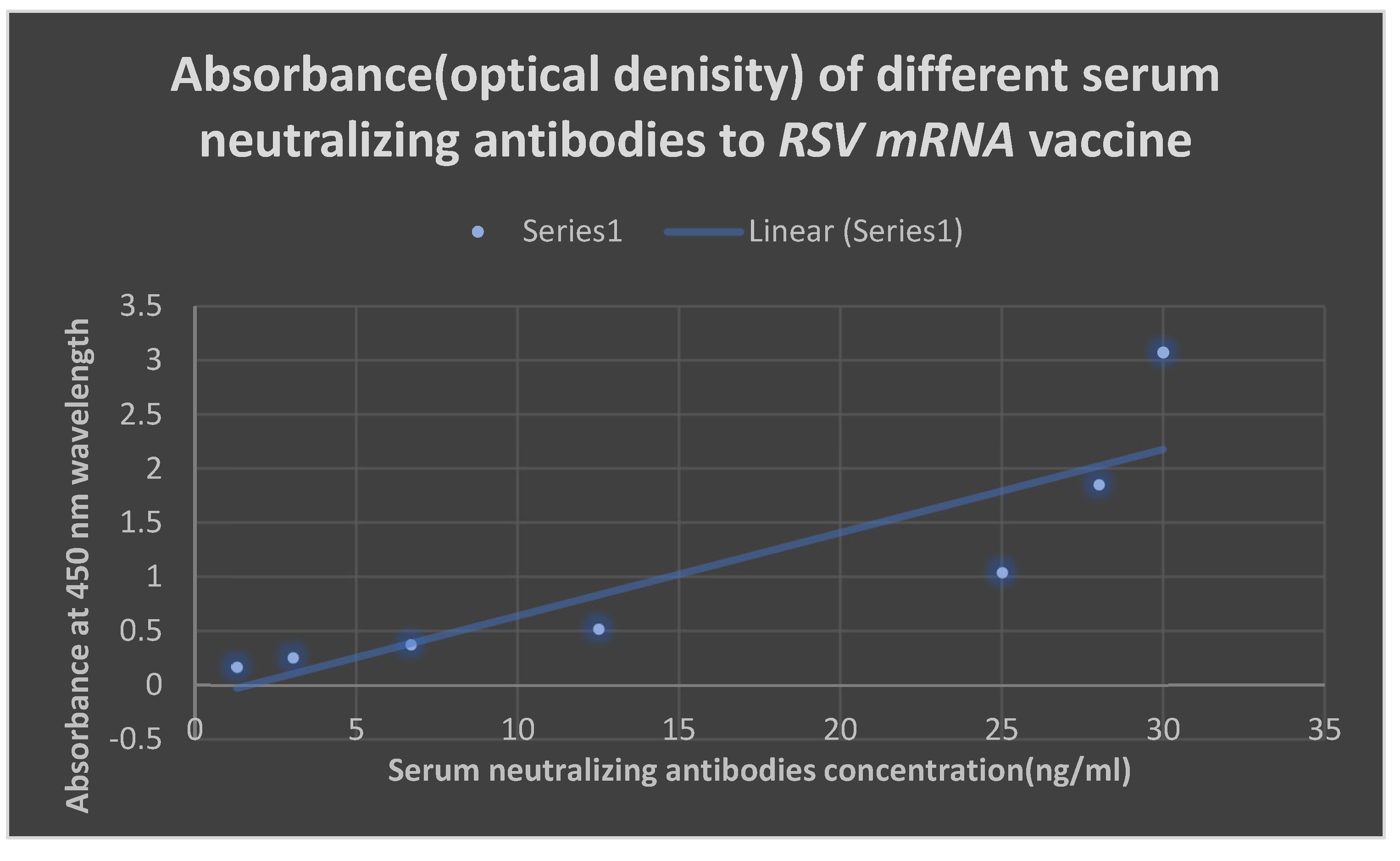
ELISA for detection of neutralizing antibodies to the test vaccine:
[ Invitrogen coated and instant ELISA kits with product number 37581 obtained from Thermo Fisher scientific company, USA]. Each well received 10 µl of the antigen suspension by passive adsorption, which was then given an hour to incubate. Bovine serum albumin served to inhibit the extra binding sites. Three PBS-T washes were applied to the plates to remove unattached molecules. Biotinylated IgG was incubated for 15 minutes after being mixed with 50 µl of horseradish peroxidase (HRP) in each well. The wells were once again washed with PBS-T to remove any unattached molecules. The enzyme and then the antigen could be identified after each well received 50 µl of chromophore substrate( TMB) for 15 minutes.
Flow cytometry:
Flow cytometry for detection of CD+4 and CD+8 T lymphocytes:
The CD+4 and CD+8 T cells that were specific to the mRNA gene cluster vaccination were seen and examined using an Invitrogen Attune Cytpix flow cytometer (obtained from the USA). The patient's cells were marked in this experiment using a monoclonal antibody. The CD4 protein, which is used to count T helper cells, is one of the proteins that these antibodies were created against. Fluorescent dyes like rhodamine and fluorescein were used to label monoclonal antibodies. Individual cells came into contact with the laser beam and lit up. A fluorescence-activated cell Sorter( FACS) was used to measure the fluorescence.
Randomized human clinical trials phases 1/2:
Vaccine immunogenicity evaluation was carried out through human randomized clinical trials phases 1/2: Three groups of human participants were employed in the current experiment. 100 people were split among each group: Group 1 (the negative control group) received an intramuscular injection of the placebo.
Injections of the standard 2-valent RSV vaccine were given intramuscularly to Group 2 (the positive control group).
Group 3 (the test group) received an intramuscular injection of the RSV test LNP-mRNA vaccine. The three groups were exposed to graded amounts of the infectious 2 serotypes of RSV and after two weeks to enhance the production of protective neutralizing antibodies( this was ethical and approved to determine the effectiveness of the test vaccination). Following a 21-day period, intradermal booster doses were administered to the three groups. The degree of protection provided by the test vaccination was evaluated over a three-year period. In contrast to the protective cell-mediated immunity, which was assessed using a flow cytometry method, the protective antibodies were identified using an enzyme-linked immunosorbent assay( ELISA).
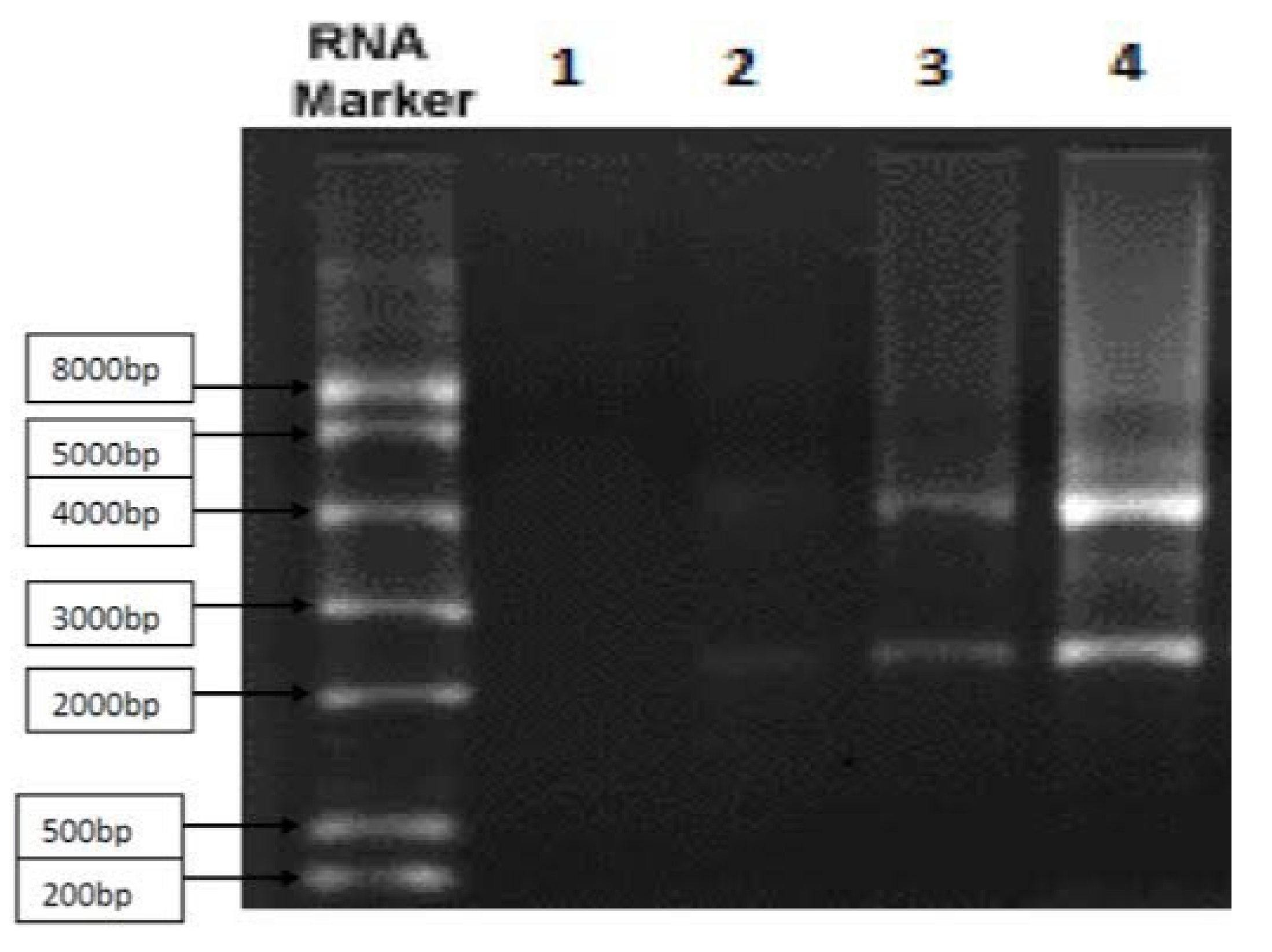
Figure 1.
By using the Northern blot technique, it depicts the messenger RNA of gene cluster that helps synthesizes RSV fusion spike protein. Approximately 84% of the sample was pure.
Figure 1.
By using the Northern blot technique, it depicts the messenger RNA of gene cluster that helps synthesizes RSV fusion spike protein. Approximately 84% of the sample was pure.
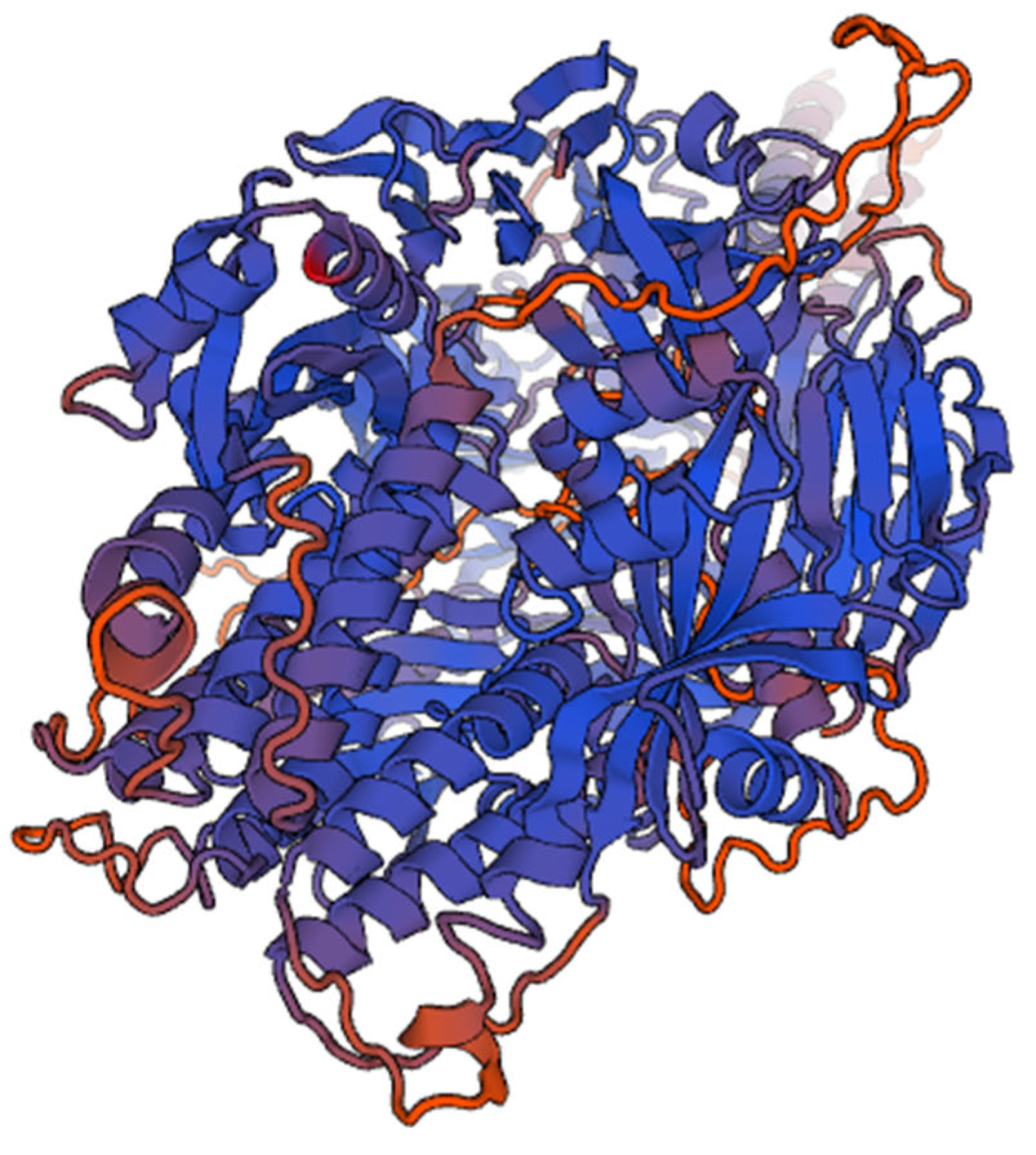
Figure 2.
It shows 3D structure of Human Respiratory virus fusion protein.
Figure 2.
It shows 3D structure of Human Respiratory virus fusion protein.
Disscussion
To prevent children from developing life-threatening pneumonia and bronchiolitis, the current study involved the creation of an LNP-mRNA RSV vaccine. In children and newborns across the world, Respiratory syncytial virus( RSV) is the leading cause of viral lower respiratory tract disease( LRI). RSV also significantly worsens LRI in the elderly and patients with immune system deficiencies. Preventing major LRI linked to RSV is the aim of the RSV vaccine. The need to immunize very young infants, who may not respond well to vaccination, the existence of two antigenically distinct RSV groups, A and B, and the history of disease enhancement following administration of a formalin-inactivated vaccine are a few of the barriers to the development of effective RSV vaccines.
Only
the pure F protein (PFP) subunit vaccines and live attenuated vaccines have recently undergone clinical trials, even though vector delivery methods, synthetic peptide, and immune-stimulating complex vaccines have all been tested in animal models. While live cold-passaged, temperature-sensitive
RSV vaccinations( also known as
cpts vaccines) will likely be helpful in early infants,
PFP-2 looks to be a beneficial vaccine for the elderly and for children with underlying pulmonary illnesses who are
RSV-seropositive. According to a 1998 research by Dudas RA et al.[
18] In a phase 2a research, healthy people (18 to 50 years of age) were randomly allocated in a 1:1 ratio to receive either a single intramuscular injection of
the bivalent prefusion F (RSVpreF) vaccine or a placebo.
The RSV A Memphis 37b challenge virus was administered intravenously to subjects about 28 days after injection, and they had a 12-day observation period. The following were the per-protocol predetermined primary endpoints:
The area under the curve (AUC) for the
RSV viral load in nasal-wash samples measured employing
reverse-transcriptase-quantitative polymerase-chain reaction (RT-qPCR) from day 2 after challenge to discharge, the total symptom score from day 1 to discharge, and
the area under the curve( AUC) for
the RSV viral load in nasal-wash samples from day 1 to discharge were all confirmed by
RT-qPCR. Also, immunogenicity and safety were evaluated. For symptomatic
RSV infection determined by any detectable viral RNA on at least 2 consecutive days after individuals received the challenge virus vaccination, vaccine effectiveness of 86.7% (95%
CI, 53.8 to 96.5) was reported. The median
AUC for
the RSV viral load (hours×log10 copies per milliliter) as measured by
RT-qPCR assay was 0.0 (interquartile range, 0.0 to 19.0) in the vaccine group and 96.7 (interquartile range, 0.0 to 675.3) in the placebo group. The geometric mean factor increase from baseline in
RSV A–neutralizing titers 28 days after injection was 20.5 (95%
CI, 16.6 to 25.3) in the vaccine group and 1.1 (95%
CI, 0.9 to 1.3) in the placebo group. More local injection-site pain was noted in the vaccine group than in the placebo group. No serious adverse events were observed in either group.[
19] The rapid creation of a live-attenuated
RSV vaccine might significantly lessen the burden of
RSV illness worldwide. The Ruth A. Karron et al., 2021 research provided evidence for this.[
20] According to
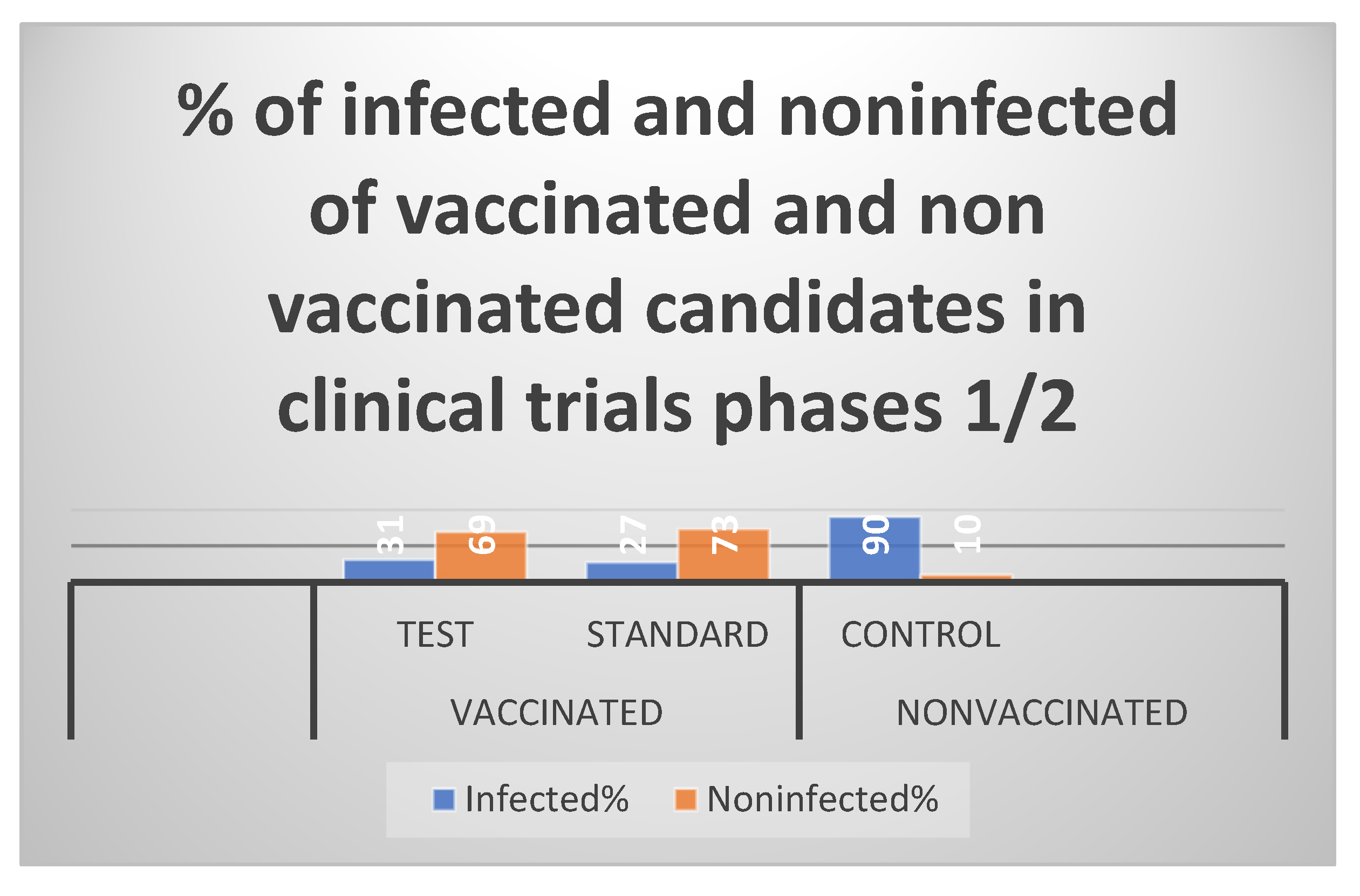
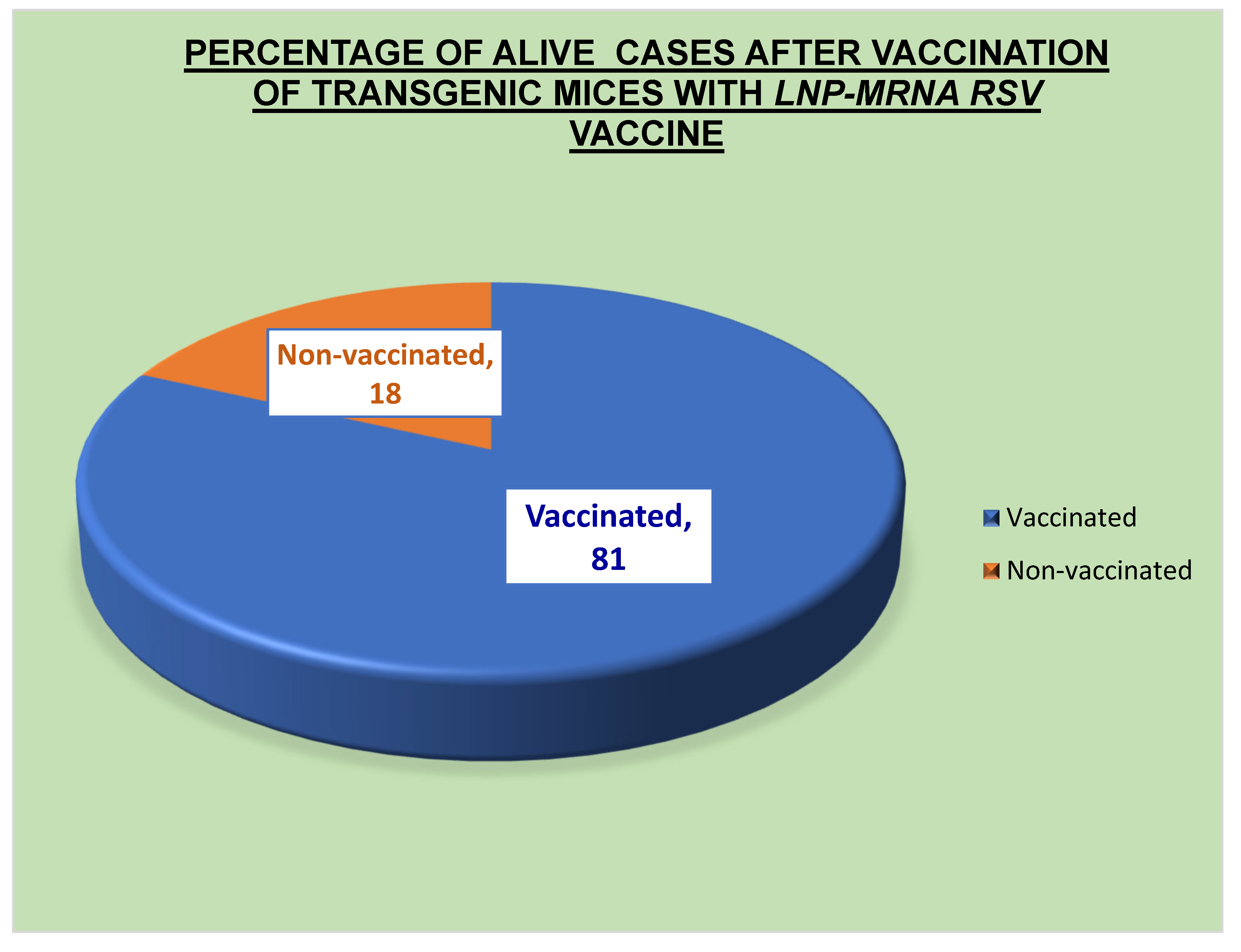
Table 3 and Table 5, the current vaccine had an effectiveness rate of 69% throughout phases 1 and 2 of clinical studies and 81% during preclinical testing. The primary bodily defense system that stopped the virus was humoral immunity. The primary
RSV viral surface spike fusion protein-neutralizing antibodies in blood were
IgM2 and
IgG3. Due to the virus' antigen not being acquired during a natural infection, few
IgA antibodies were generated against it. The current vaccination only slightly stimulated cell-mediated immunity against
RSV viral infection.
Advantages: No return to virulence was feasible; however, the immunogenicity was improved due to an increase in the number of antibodies that neutralize the fusion spike protein of
RSV as well as a modest improvement in cell-mediated immunity. Cons: The vaccine virus couldn't be excreted and transmitted to contacts who were not immune, which prevented the development of herd immunity against
RSV viral infection. Its duration of action was also shorter than that of the live attenuated vaccine, and it needed to be kept at a temperature of
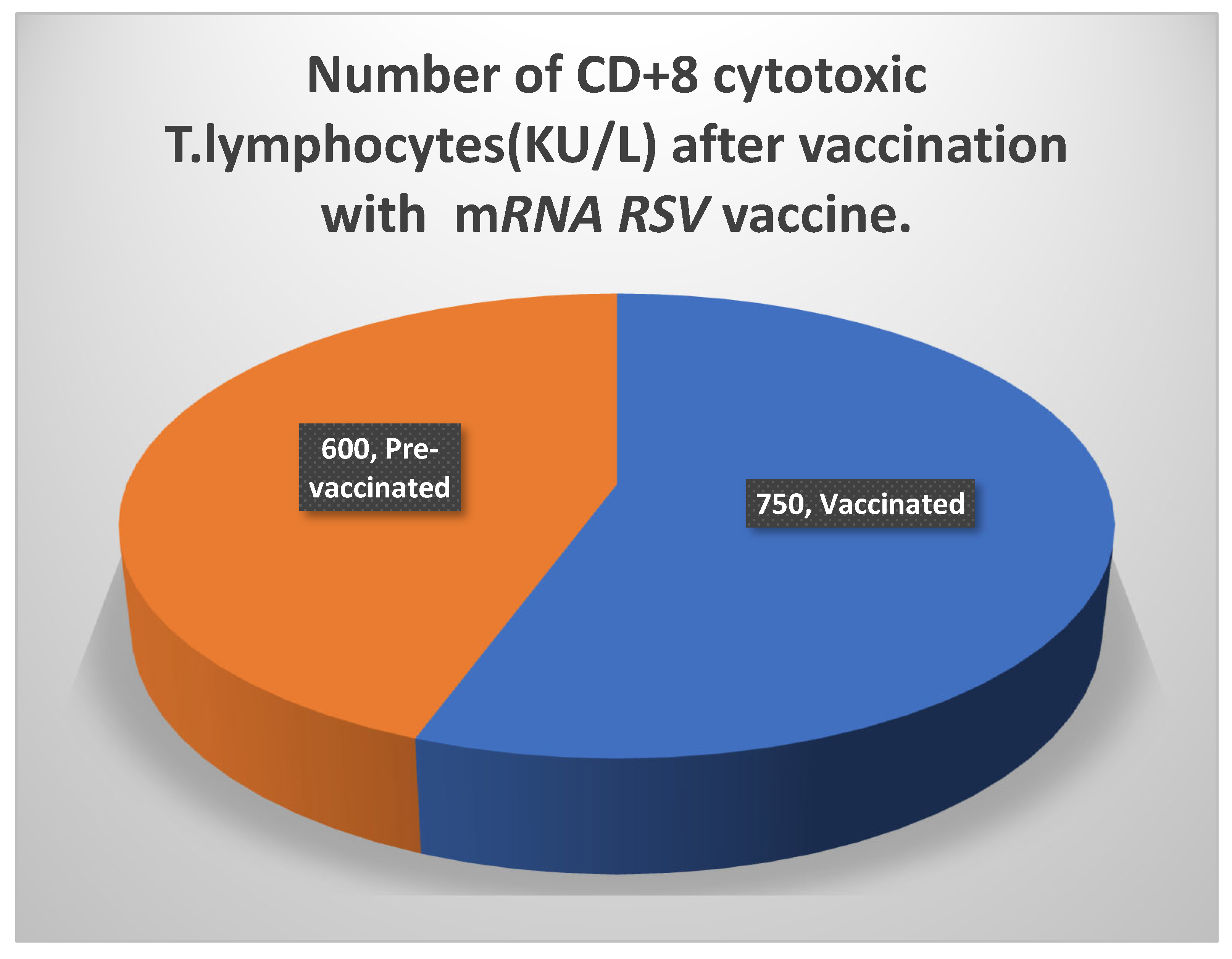
-70 0C in a refrigerator to prevent contamination and spoilage. The reason for the enhanced immunogenicity, as seen in Table 6, was a rise in both categories of T lymphocyte numbers. While there were more than 1000 KU/ L of
CD4+ T lymphocytes, there were also more than
740 KU/ L of
CD8+ T cells. On the other hand, there was a considerable rise in the titers of the potent neutralizing antibodies against proteins aiding the attachment of
RSV to the host human lung cells following vaccination with
the mRNA 2 valent RSV vaccine in randomized human clinical trials stages 1/2. In the addition of 0.5 mg of aluminum hydroxide as an adjuvant, the immunogenicity increased from 69% to 71%. Infants who are allergic to any of the substances in the test vaccination should not get it. Simple analgesics like paracetamol and ketoprofen were successful in treating a brief duration of moderate fever as well as a minor amount of pain at the site of intramuscular and intradermal injections. According to reports, the killed
RSV vaccination used in earlier experiments caused hypersensitive responses by forming antigen-antibody complexes as a result of the creation of non-protective antibodies.
21 There was no antibody-dependent enhancement in the current
LNP-mRNA RSV vaccine, which prevents the development of protective antibodies. Comparing the present vaccination to the standards specified in the Yulia E et al, 2022 research, it showed only little promise in
the LNP formulation.[
21]
The recommended dosage regimen for infants in the current study was intramuscular or intradermal administration of two consecutive doses of
the LNP-mRNA RSV vaccine spaced by 28 days, followed by the administration of a booster dose every year to prevent the development of lower respiratory infections, which are fatal to infants, especially those older than 6 months who lost the protective neutralizing antibodies transmitted to them from their mothers through the placenta. Weight loss has been documented for several
RSV vaccinations that are now being evaluated in clinical trials;[
22] however, in the current investigation, which includes the establishment of an
LNP-mRNA RSV vaccine, this condition only sometimes occurred throughout clinical trial phases 1/2. During the first two clinical trial stages of the current investigation, the babies who were susceptible to the unusual weight loss syndrome could be controlled by providing them with a balanced diet.
RSV fusion protein's bioinformatics research revealed that it causes cells to merge, resulting in multi-nucleated large cells known as syncytia. A total of 39 different
RSV vaccines have been developed. Only 19 of them advanced to the clinical trial phase, and there is still no approved
RSV vaccine.[
23]