Introduction (A brief review)
Aggressive mature T-cell lymphomas (TCL) comprise peripheral T-cell lymphoma, not otherwise specified (PTCL-NOS), angioimmunoblastic T-cell lymphoma (AITL), and anaplastic large T-cell lymphoma (ALCL). TCL is commonly treated with the CHOP regimen, but these tumors are often refractory to this treatment, and the 5-year survival rate of these patients is approximately 30%[
1]. While a comprehensive analysis of many genes involved in treatment resistance of malignant hematologic tumors has been performed [
2], no such comprehensive analyses of functional proteins have been performed, although there are numerous isolated reports.
In the present study, we selected 22 functional proteins that are considered as relevant to treatment resistance based on previous reports on resistance to treatment, and performed a retrospective analysis of these prognostic factors. The study subjects were 16 patients with TCL who had received first-line CHOP therapy, including 7 patients with PTCL-NOS, 6 patients with AITL, and 3 patients with ALCL. Immunohistopathological staining was performed using control antibodies to identify the tumor cells. Then, we determined the tumor expressions of the 22 types of treatment-resistance-related proteins using the corresponding antibodies. As control factors, three prognostic factors (International Prognostic Index [IPI], Prognostic Index for peripheral T-cell lymphoma, unspecified [PTCL-U] [PIT], and non-remission [non-CR] and relapse within 1 year) were selected[
3,
4].
The 22 types of proteins associated with treatment resistance selected by us can be classified into the following categories (1) to (4):
(1) Microenvironmental components:
1) Endoplasmic reticulum (ER) stress proteins, which are non-immune cell components of the tumor microenvironment (n = 4)
2) Immune cell components in the tumor microenvironment (n = 3)
(2) Enzymes metabolizing anticancer drugs (CHOP) (n = 5)
(3) Anticancer drug (CHOP) efflux pumps (n = 3)
(4) Other functional proteins (n = 7)
For the purpose of diagnosis, the expressions of hematoxylin-eosin staining (HE), cluster of differentiation 2 (CD2), CD3, CD5, CD7, CD8, CD10, CD23, and CD30 were also examined.
Furthermore, we reviewed (5) previously reported prognostic factors, (6) previous reports on large B-cell lymphoma (LBCL), and (7) the present report on TCL.
The above items (1) to (7) are explained below.
(1) Microenvironmental components:
1) Non-immune cell components in the tumor microenvironment: ER stress proteins 1 to 4 (n = 4)
1. Glucose-regulated protein 94 (GRP94)[
5,
6]
2. Glucose-regulated protein 78 (GRP78)[
7]
3. Transforming growth factor β1 (TGFβ1)[
8]
4. Tumor necrosis factor α1 (TNFα1)[
9]
1. GRP94 and 2. GRP78 are molecules released into the extracellular space upon stress. They allow tumors to overcome various stressful conditions in the tumor microenvironment, such as hypoxia, hypoglycemia, dysregulation of homeostasis, altered cellular metabolism, and acidosis. 3. TGFβ1 plays an important role in promoting tumor progression; 4. TNFα1 inhibits tumor progression.
2) Immune cell components 1 to 3 in the tumor microenvironment (n = 3)
1. Programmed cell death 1 (PD-1) (CD279)
2. Programmed cell death-ligand 1 (PD-L1, CD274)
3. Programmed cell death-ligand 2 (PD-L2, CD273)
In non-Hodgkin's lymphoma, the expression of PD-1/PD-L1 on the cell surface is also important, and immune checkpoint inhibitor therapy is useful[
10]. PD-L2-positive DLBCL carries a poor prognosis[
11].
(2) Enzymes (1 to 5) metabolizing anticancer drugs (CHOP) (n = 5):
1. CYP3A4[
12], which degrades CHOP in PTCL patients, inactivates many anticancer drugs. It promotes inactivation of drugs inside tumors such as PTCL, causing a decrease in drug efficacy and possibly resulting in drug resistance. 2. CYP2B6[
13], which partially degrades CHOP, has been reported in acute leukemia. CYP2B6 is associated with an increased risk of transformation to acute lymphoblastic leukemia (ALL). As enzymes degrading CHO, 3. Aldo-keto reductase family 1 member C3 (AKR1C3)[
14] in T-cell acute lymphoblastic leukemia (T-ALL) as well as 4. AKR1B1[
15] and 5. AKR1B10[
16] in cancers have been reported. AKR1 is mainly present in the cytoplasm. AKR1 catalyzes the reduction of carbonyl groups to alcohols, converting them into a soluble form. AKR1 (CHO-metabolizing enzyme) also attenuates the efficacy of cyclophosphamide (C-metabolizing enzyme), adriamycin (hydroxy doxorubicin; H-metabolizing enzyme), and oncovin (O-metabolizing enzyme; vincristine).
Anticancer drugs efflux pumps 1 to 3 (n = 3):
1. Multidrug resistance protein 1 (MDR1)[
17] and 2. multidrug resistance-associated protein 1 (MRP1)[
18] in PTCL-NOS as well as 3. MRP4[
19] in cancers have been reported. MDR1and MRP1 are present in the plasma membrane and are hydroxy doxorubicin-oncovin (HO) efflux pumps. Overexpression of MDR1 and MRP1 results in tumor resistance to drugs.
Other functional proteins (1 to 7) (n = 7)
1. Thymidine phosphorylase (TP)[
20], which is involved in starvation resistance, angiogenesis, invasion, and metastasis, is expressed in DLBCL and other cancers. TP is said to be identical to platelet-derived endothelial cell growth factor (PDGF). The expression of TP in cancers and DLBCL is associated with a poor prognosis due to its anti-apoptotic and angiogenic effects. 2. p53[
21] mutations in PTCL and 3. MYC expression[
22] in NK/T-cell lymphoma make the disease refractory to treatment. In children with acute leukemia, FLT3 gene mutations affecting the expression of 4. equilibrative nucleoside transporter 1 (ENT-1)[
23], which mediates uptake of cylocide into leukemia cells, make the disease refractory to treatment. In malignant lymphoma and leukemia, chemotherapy including platinum-based therapy involves 5. GST[
24]. Concomitant development of 6. Fibrosis[
25] and high 7. Ki-67 expression[
26] can also lead to a poor prognosis.
(3) Previously reported prognostic factors (n = 3)
IPI[
27], PIT[
27], and non-remission and relapse within 1 year after remission in patients receiving CHOP-like regimens[
28] were used as controls.
(6) Previous report: LBCL Urayasu Classification[
29]
Using immunohistochemistry, we examined the expressions of 17 drug-resistance protein molecules reported previously that may be involved in the mechanism of resistance to R-CHOP-like treatment in 42 patients with newly diagnosed LBCL. Based on the results, we were able to stratify the LBCL patients them into four prognostic groups based on the histological prognostic index (HPI): [
1] Very good prognosis group: 5-year OS, 100%, negative tumor cell staining for both GRP94 and CYP3A4; [
2] Good prognosis group: 5-year OS, about 60%–80%, positive tumor cell staining for GRP94 and negative staining for CYP3A4, and either negative staining for AKR1C3 and MDR1 or MRP1 and P53; [
3] Poor: 5-year OS of about 10%–20%, positive tumor staining for GRP94 and negative staining for CYP3A4, plus positive staining for at least one of AKR1C3, MDR1, MRP1, and P53; [
4] Very poor: 1-year OS of 0%, positive tumor staining for both GRP94 and CYP3A4. Based on the above findings, we believe that the HPI (TCL Urayasu Classification) is a simple, useful, and innovative classification system for stratifying patients based on their resistance pattern to treatment, and could be expected to be applied to clinical practice in future.
(7) The present report: TCL Urayasu Classification
We examined tumor specimens from 16 patients with newly diagnosed mature TCL by immunohistochemistry, to determine the expressions of 22 drug resistance protein molecules previously reported as being potentially involved in the mechanism of resistance to CHOP-like treatments. Based on the results, we were able to stratify the patients into three prognostic groups with differing overall survivals (OS): [
1] Negative tumor staining for GRP94; [
2] Positive tumor staining for GRP94 and one or two other factors; and [
3] Positive tumor staining for GRP94 and at least three other factors. The TCL Urayasu prognostic classification a simple, useful, and innovative classification according to the expressions of treatment resistance factors for stratifying the prognosis of TCL patients from the viewpoint of the treatment resistance mechanism. It is expected to be applied to a stratified treatment approach of patients with TCL in the future.
We compared the results from (5) and (6), and summarized and discussed in
Figure 6. We propose to accumulate more cases and analyze data from patients with other hematological tumors in the future.
Discussion
In general, immunohistochemistry performed with an optical microscope is considered as being very useful to determine the results of staining while allowing the tumor cells to be visualized. In the present study on TCL, immunohistochemistry was performed using the corresponding antibodies to determine the expressions of 22 functional proteins [
1,
2,
3,
4,
5,
6,
7,
8,
9,
10,
11,
12,
13,
14,
15,
16,
17,
18,
19,
20,
21,
22,
23,
24,
25,
26,
27,
28] that were considered as being relevant, based on a review of the literature, from the point of view of resistance to treatment. The 16 patients with newly diagnosed TCL in the present study consisted of 7 patients with PTCL-NOS, 6 patients with AITL, and 3 patients with ALCL. As shown in
Figure 1B, the selected patients with ALCL had a worse prognosis than previously reported. Herein, we propose a new classification, namely the HPI (TLC-Urayasu Classification) for predicting the response to CHOP therapy in patients with newly diagnosed TCL. As shown in
Figures 4A to 4C, the patients could be divided into the following three groups according to the results of IHC for the treatment resistance proteins: Group 1 (relatively good prognosis): negative tumor staining for GRP94 (n = 6; median OS, 88 months; p < 0.01) (representative case: Case 1 in
Figure 5A); Group 2 (poor prognosis): positive tumor staining for GRP94 and also for one or two of the 6 other poor prognostic factors described in the text (n = 5; median OS, 25 months; p > 0.02) (representative case: Case 2 in
Figure 5B); Group 3 (absolutely poor prognosis): positive tumor staining for GRP94 and also for at least 3 of the 6 other poor prognostic factors (n = 5; median OS, 10 months; p < 0.01) (representative case: Case 3 in
Figure 5C). As shown in
Figures 4D to 4F, patients with ALCL (n = 3) and PTCL-NOS (n = 2) fell under Group3 (absolutely poor prognosis). Although the present study was limited to a small sample size, the TCL Urayasu Classification, which is based on the mechanism of treatment resistance of the tumors, is expected to contribute to stratified treatment, namely, selection of the appropriate treatment depending on the disease classification.
The results were compared with three conventional prognostic indicators (IPI, PIT, non-CR and relapse within 1 year).
Figures 1C and 1D show no significant differences in the IPI or PIT1 among the patients. However, as shown in
Figures 1E and 1F, 10 patients with non-CR or relapse within 1 year showed a significantly worse prognosis. As shown in
Figure 2B, the 10 patients with non-CR or relapse within 1 year were identical to the 10 patients who showed positive tumor staining for GRP94, and showed a significantly worse prognosis (median OS, 13.5 months; p < 0.01) (
Figure 2A and 2B). In other words, GRP94-positive patients are more likely to show non-CR or disease relapse within one year after remission, and therefore more likely to have a poor prognosis. Multivariate analysis identified GRP94 as a significant poor prognostic factor, with a predicted survival of patients showing tumor GRP94 of 87.5–68.2x (1 or 0) months (1: GRP94-positive; 0: GRP94-negative). In our previous report on the LBCL-Urayasu Classification for LBCL patients[
29], we proposed a considerably simplified formula, unlike the complicated formula that includes many factors: “Predicted survival time (month) = 5.77 _ GRP94 (0 or 1) + (24.85 _ [P53 or AKR1C3] [0 or 1]) + 86.39.” Thus, our findings suggest that in patients with TCL, tumor expression of GRP94 and other factors play a central role in the microenvironment, endowing tumors with the ability to adapt to adverse environmental conditions, thereby resulting in a poor prognosis. In addition to the microenvironmental component 1 (GRP94), a tumor suppressor protein (P53) and AKR1C3, which is an enzyme that inactivates CHO of CHOP, were also found to be important for the case of LBCL. Four additional microenvironmental components (PD-L1, TP, PD1, and GRP78) were found to be important for the case of TLC (
Figure 3). On the other hand, for LBCL, not only the enzyme inactivating CHOP (CYP3A4), but also HO efflux pumps (MDR1 and MRP1) were found to be important prognostic factors.
Figure 6 summarizes the relationship between the treatment resistance factors in the Urayasu Classification for TCL and LBCL. Aggressive mature TCL (PTCL-NOS, AITL, and ALCL) tumors have at least five types of tumor pro-survival components produced and released by the tumors themselves. This indicates that TCL tumors have a better ability to adapt to the microenvironment and survive than LBCL tumors, which produce only one factor (GRP94) to survive in the microenvironment. On the contrary, according to previously reported results[
29], LBCL tumors have a variety of at least four types of tumor chemotherapy inactivators that are produced and released by the tumors themselves. Therefore, as compared with TCL tumors, which produce only one type of enzyme, namely, AKR1C, LBCL tumors are considered as having a better ability to detoxify anticancer drugs through enzymatic degradation and promoting efflux pumping of the CHOP anticancer drugs from the tumor. These findings suggest that TCL tumors have an excellent ability to adapt to the microenvironment, and that LBCL tumors additionally have a superior ability to detoxify anticancer drugs. Furthermore, since p53 is expressed in both TCLs and LBCLs, both tumors are considered to develop mutations of TP53, a tumor suppressor gene. Furthermore, in both TCL and LBCL, GRP94, a pro-survival component, and AKR1C3, an inactivator of HO of CHOP chemotherapy, were commonly expressed in the tumor microenvironment. These results suggest that GRP94, AKR1C3, and P53, which are expressed in both TCL and LBCL, are important for the development of resistance to treatment in mature aggressive lymphomas.
Representative cases of the three groups classified according to the HPI (TLC Urayasu Classification) are presented. Case 1 shown in
Figure 5A showed negative tumor expression for GRP94 and therefore fell under Group 1 (relatively good prognosis group: n = 6; median OS, 88 months; p < 0.01). The diagnosis was stage IIIB PTCL-NOS (high-risk IPI) and survived for 114 months without relapse. She was classified at diagnosis as PIT-Group 1 with good prognosis, which was inconsistent with the categorization of “high-risk IPI” at diagnosis. Case 2 shown in
Figure 5B showed positive tumor staining for two factors (AKR1C3 and GRP78) and therefore fell under Group 2 (poor prognosis: n = 5; median OS, 25 months; p > 0.05). The diagnosis was stage ⅣB AITL (high-intermediate risk IPI, PIT-Group 3 as assessed at diagnosis). The patient died 25 months after the diagnosis. The IPI and PIT were also consistent with a poor prognosis. Case 3 shown in
Figure 5C showed positive tumor staining for GRP94 and 3 other factors (PD-L1, TP, and GRP78), satisfying the requirement of positivity for at least 3 of the 6 other poor prognostic factors described in the text, and fell under Group 3 (absolutely poor prognosis) (n = 5; median OS, 10 months; p < 0.01). The diagnosis was stage ⅡA ALCL (low-intermediate IPI [IPIe], PIT-Group 1 at diagnosis). The patient died after only about 2 months of survival. The IPI and PIT predicted a good prognosis in this case, but turned out to be inaccurate predictors of the very poor prognosis.
In regard to the Urayasu Classification for TCL shown in
Figure 6, the most significant factor, namely, GRP94, and the 6 additional poor prognostic factors (PD-L1, TP, AKR1C3, P53, PD-1, and GRP78) are discussed below 1) to 6), along with a review of the literature, including their potential clinical applications.
1) GRP94, GRP78, and TP: Molecules released into the extracellular space upon stress. They allow tumors to overcome various stressful conditions in the tumor microenvironment, such as hypoxia, hypoglycemia, dysregulation of homeostasis, altered cellular metabolism, and acidosis. As shown in
Figures 2A and 2B, GRP94 was identified as the most important factor as in the previous study on LBCL. From the aspect of treatment, pimitespib, a GRP94 inhibitor, has been used in the treatment of GISTs[
31], and is also expected to be used for the treatment of AT[
32]. GRP94 antibody therapy for other cancers is also being investigated[
33]. GRP78 is a know[n risk factor for high-risk ALL in children[
34]. In the future, therapy targeting GRP94 is expected to become available for TCL, as shown in the study on LBCL[
29], which could be expected to allow long-term complete remission of the disease.
2) PD-1/PD-L1: A cell surface immune-checkpoint molecule, which is important for immune checkpoint inhibitor therapy[
10]. PD-1 inhibitory therapy in classical Hodgkin lymphoma[
35], the importance of PD L1 in the diagnosis and treatment of B-cell malignant lymphoma[
36] have been reported. As shown in
Figure 2C, PD-L1-positive patients (n = 4) showed a median OS of 5.5 months (p < 0.01), reflecting a poor prognosis. This suggests that cell surface expression of PD-L1 in DLBCL allows the tumor to escape immune checkpoints[
36].
3) TP: It has been reported as being a poor prognostic factor in breast cancer patients[
37]. TCL patients with positive tumor staining for TP also showed a poor prognosis, falling under Group 3 of the TCL Urayasu Classification, and all of them (3 patients) had ALCL. The median OS of TP-positive patients was 6 months (p < 0.01). TP inhibitors have been suggested as being useful in the treatment of glioblastomas[
38].
4) AKR1C3: AKR1C3 inhibitors enhance the efficacy of chemotherapy in AML and T-ALL[
39].
5) P53: TP53 mutations identify high-risk events in patients with peripheral T-cell lymphomas receiving CHOP-based chemotherapy[
21]. Therefore, drugs tailored to specific types of p53 mutations are emerging, and p53-based immunotherapy approaches are being devised[
40].
6) PD-1: The immune checkpoint receptor PD-1 is recurrently inactivated in TCL, and is therefore said to be predictive of a poor prognosis[
41]. PD1/PDL1 checkpoint inhibitors in tumor immunotherapy have been developed as a promising combination therapy[
42]. However, PD-1 signaling suppresses the growth of TCL[
41]. Unlike IPI and TPI, HPI (TCL Urayasu Classification) is specialized for tumor resistance factors. It is an innovative classification for predicting the sensitivity to chemotherapy, even if the patient is very old and frail and has serious comorbidities. In addition, the classification is easy to use, and the results can be obtained within a short period of time.
In summary, we propose a novel prognostic classification index, namely, the new HPI (TCL Urayasu Classification) for predicting the prognosis in patients with TCL; the classification requires immunohistochemistry for at least 6 proteins (PD-L1, AKR1C3, P53, PD-1, GRP78, and TP) in addition to GRP94 at the time of immunohistopathological diagnosis of TCL. Serology for HTLV-1 and HIV antibodies is also recommended. In the future, we expect to obtain improved treatment outcomes in patients with first-episode TCL by selecting the appropriate treatment for patients with TCL according to the HPI, including use of the appropriate inhibitors and antibodies. We also propose to accumulate more cases and analyze other hematologic tumors and compile the results.
Among a large number of proteins reported in the literature, we selected 22 proteins and 3 prognostic factors that are considered relevant for this type of study. We conducted a comprehensive retrospective analysis of the expressions of these proteins by conducting immunohistochemical analysis on tissue sections prepared from patients with TCL (PTCL-NOS, AITL, and ALCL) using antibodies against the proteins. The proteins included: (1) four ER stress proteins, (2) five enzymes that can metabolize anticancer drugs, (3) three anticancer drug efflux pumps, (4) three immune check point molecules, and (5) six other proteins. Three existing prognostic factors were used as controls.
Figure 1.
Overall survival of TCL patients with and without the prognostic factors—Comparison of the Kaplan-Meier survival curves and disease/existing prognostic factors between 2 groups (log-rank test)—+: positive; -: negative. A. TCL overall survival (OS) (n = 16) The median OS after initial treatment with CHOP-like regimens was 33.5 months. B. ALCL, n = 3; AITL, n = 6; PTCL NOS, n = 7; p < 0.05 The median OS values indicated a poor prognosis in the ALCL patients in this study: the OS was about 8 months in the patients with ALCL, about 34 months in the patients with AITL, and about 72 months in the patients with PTCL-NOS. The prognosis of the patients with ALCL in this study was worse than previously reported. C. A conventional poor prognostic factor IPI high positive, n = 7; negative, n = 9; p > 0.05 No significant difference was observed in the IPI among the groups. D. No significant differences were observed in the distribution of the conventional poor prognostic factors in the high-risk PIT groups (Groups 2, 3 and 4: n = 4) as compared with the other groups (n = 12). No significant differences were observed in the PIT among the groups (p > 0.05). E. CR-positive and CR-negative patients (n = 8 in both groups). Those who showed CR had a significantly better prognosis (n = 8; median OS, about 72 months; p < 0.01). Those who did not achieve CR had a significantly worse prognosis (n = 8; median OS, about 10 months; p < 0.01). F. Non-CR plus relapse within 1 year- positive, n = 10; negative, n = 6, p < 0.01. Patients with non-CR or relapse within 1 year had a significantly worse prognosis (n = 10; median OS, 13.5 months; p < 0.01).
Figure 1.
Overall survival of TCL patients with and without the prognostic factors—Comparison of the Kaplan-Meier survival curves and disease/existing prognostic factors between 2 groups (log-rank test)—+: positive; -: negative. A. TCL overall survival (OS) (n = 16) The median OS after initial treatment with CHOP-like regimens was 33.5 months. B. ALCL, n = 3; AITL, n = 6; PTCL NOS, n = 7; p < 0.05 The median OS values indicated a poor prognosis in the ALCL patients in this study: the OS was about 8 months in the patients with ALCL, about 34 months in the patients with AITL, and about 72 months in the patients with PTCL-NOS. The prognosis of the patients with ALCL in this study was worse than previously reported. C. A conventional poor prognostic factor IPI high positive, n = 7; negative, n = 9; p > 0.05 No significant difference was observed in the IPI among the groups. D. No significant differences were observed in the distribution of the conventional poor prognostic factors in the high-risk PIT groups (Groups 2, 3 and 4: n = 4) as compared with the other groups (n = 12). No significant differences were observed in the PIT among the groups (p > 0.05). E. CR-positive and CR-negative patients (n = 8 in both groups). Those who showed CR had a significantly better prognosis (n = 8; median OS, about 72 months; p < 0.01). Those who did not achieve CR had a significantly worse prognosis (n = 8; median OS, about 10 months; p < 0.01). F. Non-CR plus relapse within 1 year- positive, n = 10; negative, n = 6, p < 0.01. Patients with non-CR or relapse within 1 year had a significantly worse prognosis (n = 10; median OS, 13.5 months; p < 0.01).
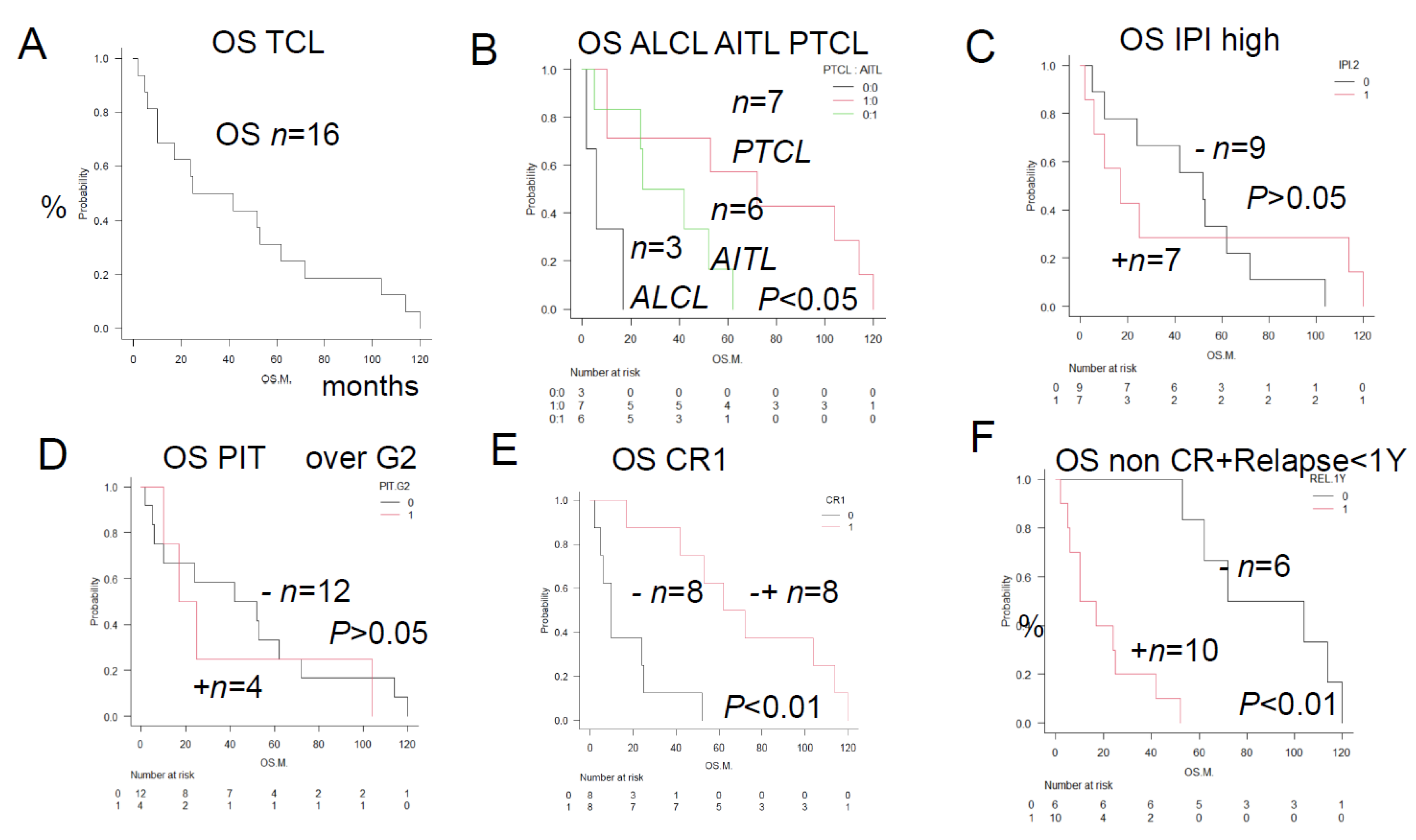
Figure 2.
Overall survival in TCL patients with and without the prognostic factors—Comparison of the Kaplan-Meier survival curves and prognostic factors, positive/negative immunostaining between Group 2 and Group 3 (log-rank test)—A and B show the results of comparisons among 3 groups, and C to F show the results of comparison between 2 groups. A GRP94+, CR1+, n = 2; GRP94+, CR-, n = 8; GRP94-, CR+, n = 6; P < 0.01. GRP94-positive patients showed a non-CR treatment response, resulting in a poor prognosis, whereas GRP94-negative patients showed CR, resulting in a good prognosis. B GRP94-positive patients with relapse (n = 10): Poor prognosis with the median OS of 13.5 months (p < 0.01). GRP94-negative patients without relapse (n = 6): Relatively good prognosis with a median OS of 102 months (p < 0.01). A total of 10 patients, consisting of 6 patients who showed a non-CR treatment response after the initial therapy and 4 patients who developed relapse within 1 year, were identical to the 10 patients showing positive tumor staining for GRP94. GRP94-positive patients showed a “non-CR” treatment response after the initial therapy or developed relapse within 1 year, resulting in a poor prognosis. In the tumor microenvironment, GRP94 expression is associated with cell survival against hypoxia, hypoglycemia, dysregulation of homeostasis, thereby promoting survival of the TCL cells and leading to a poor prognosis. In order to overcome various stressful conditions, such as altered cellular metabolism and acidosis, TCL cells survive and lead to a poor prognosis. C. PD-L1-positive patients (n = 4, median OS, 5.5 months, p < 0.01). PD-L1-negative patients (n = 12, median OS, 50 months, p < 0.01). In the immune microenvironment, PD-L1 plays an important role as a key component of the immune surveillance mechanism. PD-L1 positivity allows TCL cells to proliferate by escaping the surveillance mechanism, which results in a poor prognosis. D. AKR1C3-positive patients (n = 6, median OS, 10 months: p < 0.01). AKR1C3-negative patients (n = 10, median OS, 62 months; p < 0.01). AKR1C3 expression in TCL cells decreases the intracellular metabolism of HO of the CHOP regimen drugs, attenuating their cytotoxic activity against the TCL cells and making the disease refractory, which results in a poor prognosis. E. TP-positive patients (n = 3, median OS, 6 months; p < 0.01). TP-negative patients (n = 13, median OS, 56 months; p < 0.01). TP expression is mainly linked to antiapoptotic and angiogenic activities, leading to a poor prognosis. F. CYP2B6-positive patients (n = 4, median OS, 13.5 months; p < 0.05). CYP2B6-negative patients (n = 12, median OS, 46 months; p < 0.01). CYP2B6 expression is linked to an increased risk of development of PTCL, as seen in ALL.
Figure 2.
Overall survival in TCL patients with and without the prognostic factors—Comparison of the Kaplan-Meier survival curves and prognostic factors, positive/negative immunostaining between Group 2 and Group 3 (log-rank test)—A and B show the results of comparisons among 3 groups, and C to F show the results of comparison between 2 groups. A GRP94+, CR1+, n = 2; GRP94+, CR-, n = 8; GRP94-, CR+, n = 6; P < 0.01. GRP94-positive patients showed a non-CR treatment response, resulting in a poor prognosis, whereas GRP94-negative patients showed CR, resulting in a good prognosis. B GRP94-positive patients with relapse (n = 10): Poor prognosis with the median OS of 13.5 months (p < 0.01). GRP94-negative patients without relapse (n = 6): Relatively good prognosis with a median OS of 102 months (p < 0.01). A total of 10 patients, consisting of 6 patients who showed a non-CR treatment response after the initial therapy and 4 patients who developed relapse within 1 year, were identical to the 10 patients showing positive tumor staining for GRP94. GRP94-positive patients showed a “non-CR” treatment response after the initial therapy or developed relapse within 1 year, resulting in a poor prognosis. In the tumor microenvironment, GRP94 expression is associated with cell survival against hypoxia, hypoglycemia, dysregulation of homeostasis, thereby promoting survival of the TCL cells and leading to a poor prognosis. In order to overcome various stressful conditions, such as altered cellular metabolism and acidosis, TCL cells survive and lead to a poor prognosis. C. PD-L1-positive patients (n = 4, median OS, 5.5 months, p < 0.01). PD-L1-negative patients (n = 12, median OS, 50 months, p < 0.01). In the immune microenvironment, PD-L1 plays an important role as a key component of the immune surveillance mechanism. PD-L1 positivity allows TCL cells to proliferate by escaping the surveillance mechanism, which results in a poor prognosis. D. AKR1C3-positive patients (n = 6, median OS, 10 months: p < 0.01). AKR1C3-negative patients (n = 10, median OS, 62 months; p < 0.01). AKR1C3 expression in TCL cells decreases the intracellular metabolism of HO of the CHOP regimen drugs, attenuating their cytotoxic activity against the TCL cells and making the disease refractory, which results in a poor prognosis. E. TP-positive patients (n = 3, median OS, 6 months; p < 0.01). TP-negative patients (n = 13, median OS, 56 months; p < 0.01). TP expression is mainly linked to antiapoptotic and angiogenic activities, leading to a poor prognosis. F. CYP2B6-positive patients (n = 4, median OS, 13.5 months; p < 0.05). CYP2B6-negative patients (n = 12, median OS, 46 months; p < 0.01). CYP2B6 expression is linked to an increased risk of development of PTCL, as seen in ALL.
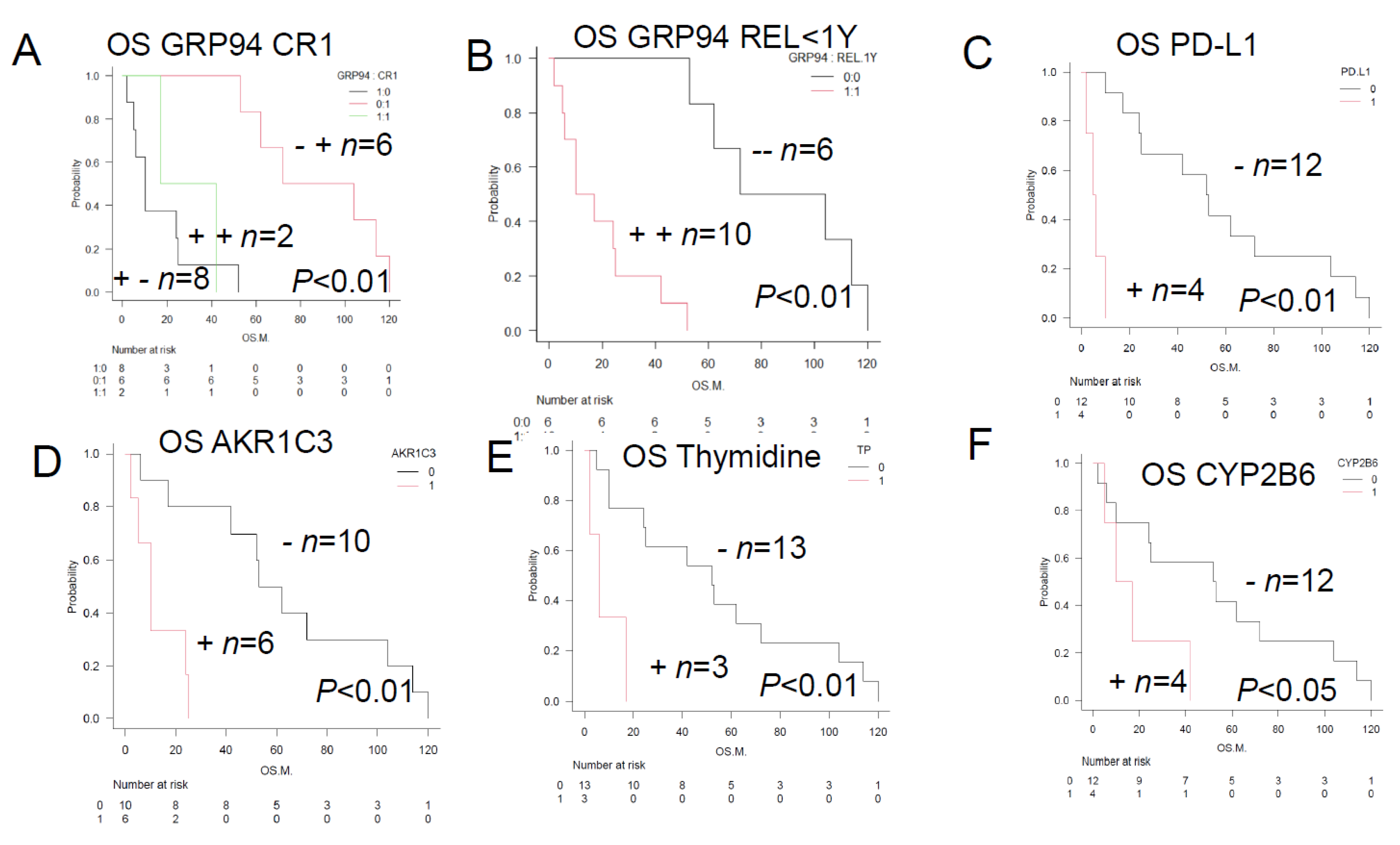
Figure 3.
Overall survival of TCL patients with and without the prognostic factors—Comparison of the Kaplan-Meier survival curves and positive/negative immunostainings among 3 groups (log-rank test)—A. Patients showing positive tumor staining for GRP94 and PD-L1 (n = 4; median OS, 8.5 months; p < 0.01). Patients showing positive tumor staining for GRP94, but negative staining for PD-L1 (n = 6; median OS, 24.5 months; p < 0.01). Patients with tumors showing negative staining for both GRP94 and PD-L1 (n = 6; median OS, 88 months; p < 0.01). Thus, expression of GRP94 on the tumor cell surface is associated with a poor prognosis. This is because GRP94 allows tumors to overcome various stressful conditions in the tumor microenvironment, such as hypoxia, hypoglycemia, dysregulation of homeostasis, altered cell metabolism, and acidosis. Expression of PD-L1 on the cell surface also leads to a poor prognosis. This is due to the activation of immune checkpoint escape mechanisms. B. Patients showing positive tumor staining for GRP94 and TP (n = 3; median OS of 6 months; p < 0.01). Patients showing positive tumor staining for GRP94, but negative staining for TP (n = 7; median OS of about 24 months; p < 0.01). Patients with tumors showing negative staining for both GRP94 and TP (n = 6; median OS, about 88 months; p < 0.01). TP expression is mainly linked to antiapoptotic and angiogenic activities, and is associated with a poor prognosis. C. Patients showing positive tumor staining for GRP94 and AKR1C3 (n = 6; median OS, about 10 months; p < 0.01). Patients showing positive tumor staining for GRP94, but negative staining for AKR1C3 (n = 4; median OS, about 29.5 months; p < 0.01). Patients with tumors showing negative staining for both GRP94 and AKR1C3 (n = 6; median OS, about 88 months; p < 0.01). AKR1C3 is an aldo-keto reductase, mainly present in the cytoplasm. It catalyzes the reduction of carbonyl groups to alcohols, converting them into a soluble form. It is an HO-metabolizing enzyme, which reduces the intracellular levels of adriamycin (hydroxy doxorubicin; H-metabolizing enzyme) and oncovin (O-metabolizing enzyme; vincristine). These activities lead to the development of resistance to CHOP therapy and a poor prognosis. D. Patients showing positive tumor staining for P53 and GRP94 (n = 2; median OS, about 11.5 months; p < 0.01). Patients with tumors showing negative staining for P53, but positive staining for GRP94 (n = 8; median OS, about 17 months; p < 0.01). Patients with tumors showing negative staining for both P53 and GRP94 (n = 6; median OS, about 88 months; p < 0.01). P53 mutations are often associated with increased expression levels of P53, and are associated with a poor prognosis. E. Patients with tumors showing negative staining for PD-1, but positive staining for GRP94 (n = 7; median OS, about 7 months; p < 0.01). Patients showing positive tumor staining for PD-1 and GRP94 (n = 3; median OS, about 42 months; p < 0.01). Patients showing negative staining for PD-1, but positive staining for GRP94 (n = 7; median OS, about 10 months; p < 0.01). Patients showing positive tumor staining for PD-1, but negative staining for GRP94 (n = 2; median OS, about 96 months; p < 0.01). Patients showing negative staining for both PD-1 and GRP94 (n = 3; median OS, about 104 months; p < 0.01). PD-1 signaling suppresses the growth of TCL. F. Patients showing positive tumor staining for GRP78 and GRP94 (n = 8; median OS, about 10 months; p < 0.01). Patients showing positive tumor staining for GRP78, but negative staining for GRP94 (n = 2; median OS, about 47 months; p < 0.01). Patients showing negative tumor staining for both GRP78 and GRP94 (n = 4; median OS, about 83 months; p < 0.01). Patients with tumors showing negative staining for GRP78, but positive staining for GRP94 (n = 2; median OS, about 96 months; p < 0.01). In regard to the non-immune cell components in the tumor microenvironment, patients showing positive tumor staining for both the ER stress proteins GRP78 and GRP94 show a poor prognosis.
Figure 3.
Overall survival of TCL patients with and without the prognostic factors—Comparison of the Kaplan-Meier survival curves and positive/negative immunostainings among 3 groups (log-rank test)—A. Patients showing positive tumor staining for GRP94 and PD-L1 (n = 4; median OS, 8.5 months; p < 0.01). Patients showing positive tumor staining for GRP94, but negative staining for PD-L1 (n = 6; median OS, 24.5 months; p < 0.01). Patients with tumors showing negative staining for both GRP94 and PD-L1 (n = 6; median OS, 88 months; p < 0.01). Thus, expression of GRP94 on the tumor cell surface is associated with a poor prognosis. This is because GRP94 allows tumors to overcome various stressful conditions in the tumor microenvironment, such as hypoxia, hypoglycemia, dysregulation of homeostasis, altered cell metabolism, and acidosis. Expression of PD-L1 on the cell surface also leads to a poor prognosis. This is due to the activation of immune checkpoint escape mechanisms. B. Patients showing positive tumor staining for GRP94 and TP (n = 3; median OS of 6 months; p < 0.01). Patients showing positive tumor staining for GRP94, but negative staining for TP (n = 7; median OS of about 24 months; p < 0.01). Patients with tumors showing negative staining for both GRP94 and TP (n = 6; median OS, about 88 months; p < 0.01). TP expression is mainly linked to antiapoptotic and angiogenic activities, and is associated with a poor prognosis. C. Patients showing positive tumor staining for GRP94 and AKR1C3 (n = 6; median OS, about 10 months; p < 0.01). Patients showing positive tumor staining for GRP94, but negative staining for AKR1C3 (n = 4; median OS, about 29.5 months; p < 0.01). Patients with tumors showing negative staining for both GRP94 and AKR1C3 (n = 6; median OS, about 88 months; p < 0.01). AKR1C3 is an aldo-keto reductase, mainly present in the cytoplasm. It catalyzes the reduction of carbonyl groups to alcohols, converting them into a soluble form. It is an HO-metabolizing enzyme, which reduces the intracellular levels of adriamycin (hydroxy doxorubicin; H-metabolizing enzyme) and oncovin (O-metabolizing enzyme; vincristine). These activities lead to the development of resistance to CHOP therapy and a poor prognosis. D. Patients showing positive tumor staining for P53 and GRP94 (n = 2; median OS, about 11.5 months; p < 0.01). Patients with tumors showing negative staining for P53, but positive staining for GRP94 (n = 8; median OS, about 17 months; p < 0.01). Patients with tumors showing negative staining for both P53 and GRP94 (n = 6; median OS, about 88 months; p < 0.01). P53 mutations are often associated with increased expression levels of P53, and are associated with a poor prognosis. E. Patients with tumors showing negative staining for PD-1, but positive staining for GRP94 (n = 7; median OS, about 7 months; p < 0.01). Patients showing positive tumor staining for PD-1 and GRP94 (n = 3; median OS, about 42 months; p < 0.01). Patients showing negative staining for PD-1, but positive staining for GRP94 (n = 7; median OS, about 10 months; p < 0.01). Patients showing positive tumor staining for PD-1, but negative staining for GRP94 (n = 2; median OS, about 96 months; p < 0.01). Patients showing negative staining for both PD-1 and GRP94 (n = 3; median OS, about 104 months; p < 0.01). PD-1 signaling suppresses the growth of TCL. F. Patients showing positive tumor staining for GRP78 and GRP94 (n = 8; median OS, about 10 months; p < 0.01). Patients showing positive tumor staining for GRP78, but negative staining for GRP94 (n = 2; median OS, about 47 months; p < 0.01). Patients showing negative tumor staining for both GRP78 and GRP94 (n = 4; median OS, about 83 months; p < 0.01). Patients with tumors showing negative staining for GRP78, but positive staining for GRP94 (n = 2; median OS, about 96 months; p < 0.01). In regard to the non-immune cell components in the tumor microenvironment, patients showing positive tumor staining for both the ER stress proteins GRP78 and GRP94 show a poor prognosis.
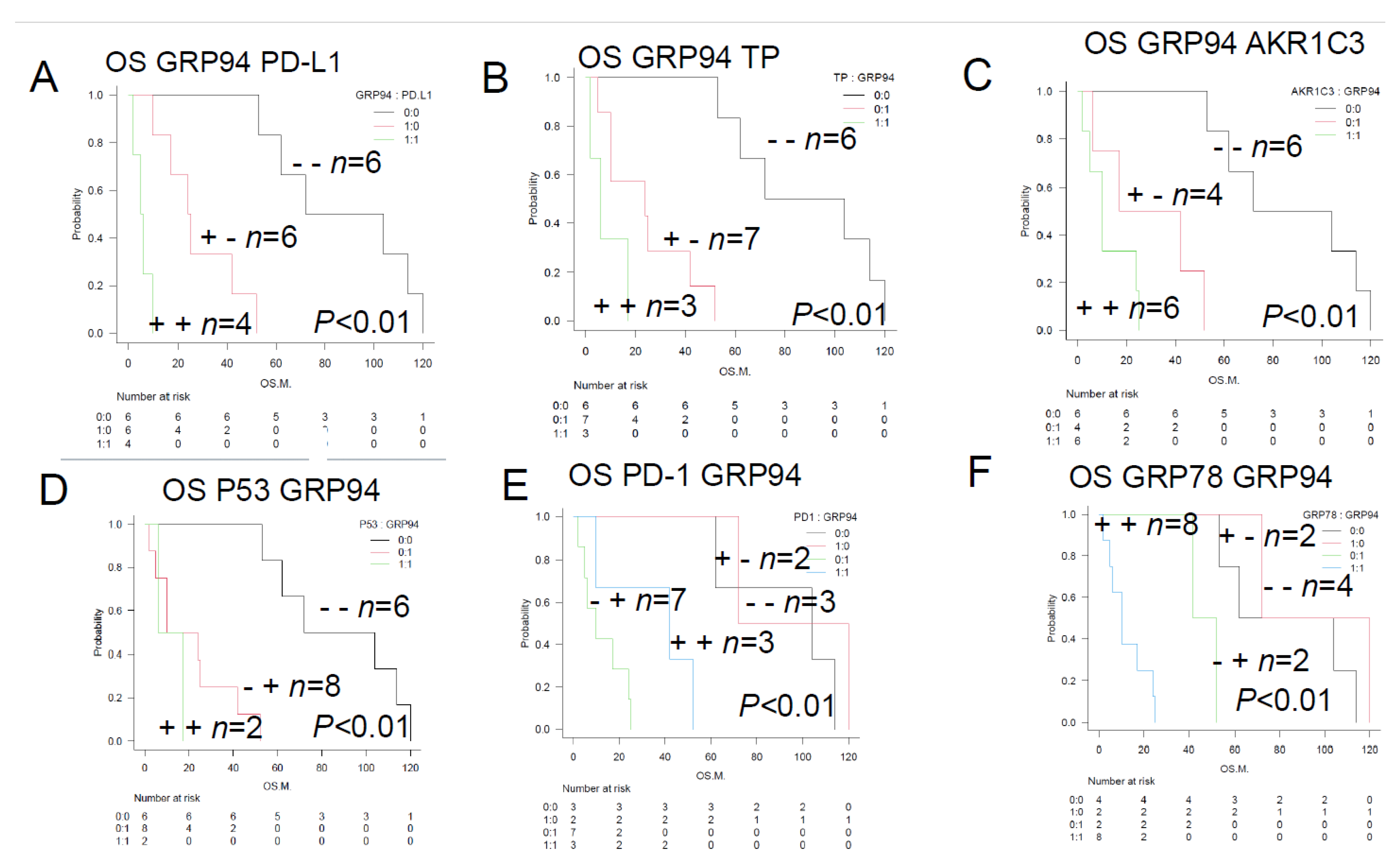
Figure 4.
Overall survival of TCL patients with and without the prognostic factors (TCL Urayasu classification)—Comparison of the Kaplan-Meier survival curves among 3 groups (log-rank test)—A. TCL Urayasu Group 1 (positive; n = 6; median OS, 68 months; p < 0.01).The median OS after the initial treatment with the CHOP-like regimens shown in
Figure 1A almost doubled from 33.5 months, which is a relatively good prognosis for patients with TCL. For Group 2 and Group 3 (negative; n = 10; median OS, 39.5 months; p < 0.01), the median OS was about 40% of 33.5 months in the overall patient population, indicating a relatively poor prognosis.B. TCL Urayasu Group 2 (positive; n = 5; median OS, 25 months; p > 0.05) and other groups (negative; n = 11; median OS, 53 months; p > 0.05). There was no significant difference in the OS between positive groups and the negative group (p > 0.05). The median OS in the overall patient population was about 75% of 33.5 months, indicating a relatively poor prognosis for Group 2. C. TCL Urayasu Group 3 (positive; n = 5; median OS, 10 months; p > 0.05) and other groups (negative; n = 11; median OS, 53 months; p < 0.01). The median OS in the overall patient population was about 30% of 33.5 months, indicating a poor prognosis for Group 3. In Group 1 and group 2 (negative; n = 11), the median OS was 53 months (p < 0.01), being about 1.6 times that (33.5 months) of the medial OS in the overall patients. This indicates a relatively good prognosis. D. TCL Urayasu Group 3 vs. PTCL-NOS (p < 0.01). Group 3 with PTCL-NOS (n = 2; median OS of about 10 months). Group 3 without PTCL-NOS (n = 3; median OS of about 8 months). Group 3 with either disease type had a poor prognosis. Group 1 or 2, with PTCL-NOS (n = 5; median OS of about 104 months) had a good prognosis. Group 3, without PTCL-NOS (n = 6; median OS of about 33.5 months). E. TCL Urayasu Group 3 vs. AITL (p < 0.01).Group 3 with AITL (n = 0).Group 3 without AITL (n = 5).The median OS was about 10 months in both groups.Group 1 or 2, with AITL (n = 6).The median OS was about 33.5 months, which was the same as that for the OS in all patients. Patients with AITL were classified into either Group 1 or 2, and their prognosis was intermediate.Group 1 or 2, without AITL (n = 5).The median OS was about 104 months.F. TCL Urayasu Group 3 vs. ALCL (p < 0.05).Group 3 with ALCL (n = 3).The median OS was about 6 months. All patients with ALCL were classified intoGroup 3, and thus had a poor prognosis.Group 3 without ALCL (n = 2).These patients had PTCL-NOS and had a median OS of about 10 months.Group 1 or 2, with ALCL (n = 0).Group 1 or 2, without ALCL (n = 11).The median OS was about 53 months.
Figure 4.
Overall survival of TCL patients with and without the prognostic factors (TCL Urayasu classification)—Comparison of the Kaplan-Meier survival curves among 3 groups (log-rank test)—A. TCL Urayasu Group 1 (positive; n = 6; median OS, 68 months; p < 0.01).The median OS after the initial treatment with the CHOP-like regimens shown in
Figure 1A almost doubled from 33.5 months, which is a relatively good prognosis for patients with TCL. For Group 2 and Group 3 (negative; n = 10; median OS, 39.5 months; p < 0.01), the median OS was about 40% of 33.5 months in the overall patient population, indicating a relatively poor prognosis.B. TCL Urayasu Group 2 (positive; n = 5; median OS, 25 months; p > 0.05) and other groups (negative; n = 11; median OS, 53 months; p > 0.05). There was no significant difference in the OS between positive groups and the negative group (p > 0.05). The median OS in the overall patient population was about 75% of 33.5 months, indicating a relatively poor prognosis for Group 2. C. TCL Urayasu Group 3 (positive; n = 5; median OS, 10 months; p > 0.05) and other groups (negative; n = 11; median OS, 53 months; p < 0.01). The median OS in the overall patient population was about 30% of 33.5 months, indicating a poor prognosis for Group 3. In Group 1 and group 2 (negative; n = 11), the median OS was 53 months (p < 0.01), being about 1.6 times that (33.5 months) of the medial OS in the overall patients. This indicates a relatively good prognosis. D. TCL Urayasu Group 3 vs. PTCL-NOS (p < 0.01). Group 3 with PTCL-NOS (n = 2; median OS of about 10 months). Group 3 without PTCL-NOS (n = 3; median OS of about 8 months). Group 3 with either disease type had a poor prognosis. Group 1 or 2, with PTCL-NOS (n = 5; median OS of about 104 months) had a good prognosis. Group 3, without PTCL-NOS (n = 6; median OS of about 33.5 months). E. TCL Urayasu Group 3 vs. AITL (p < 0.01).Group 3 with AITL (n = 0).Group 3 without AITL (n = 5).The median OS was about 10 months in both groups.Group 1 or 2, with AITL (n = 6).The median OS was about 33.5 months, which was the same as that for the OS in all patients. Patients with AITL were classified into either Group 1 or 2, and their prognosis was intermediate.Group 1 or 2, without AITL (n = 5).The median OS was about 104 months.F. TCL Urayasu Group 3 vs. ALCL (p < 0.05).Group 3 with ALCL (n = 3).The median OS was about 6 months. All patients with ALCL were classified intoGroup 3, and thus had a poor prognosis.Group 3 without ALCL (n = 2).These patients had PTCL-NOS and had a median OS of about 10 months.Group 1 or 2, with ALCL (n = 0).Group 1 or 2, without ALCL (n = 11).The median OS was about 53 months.
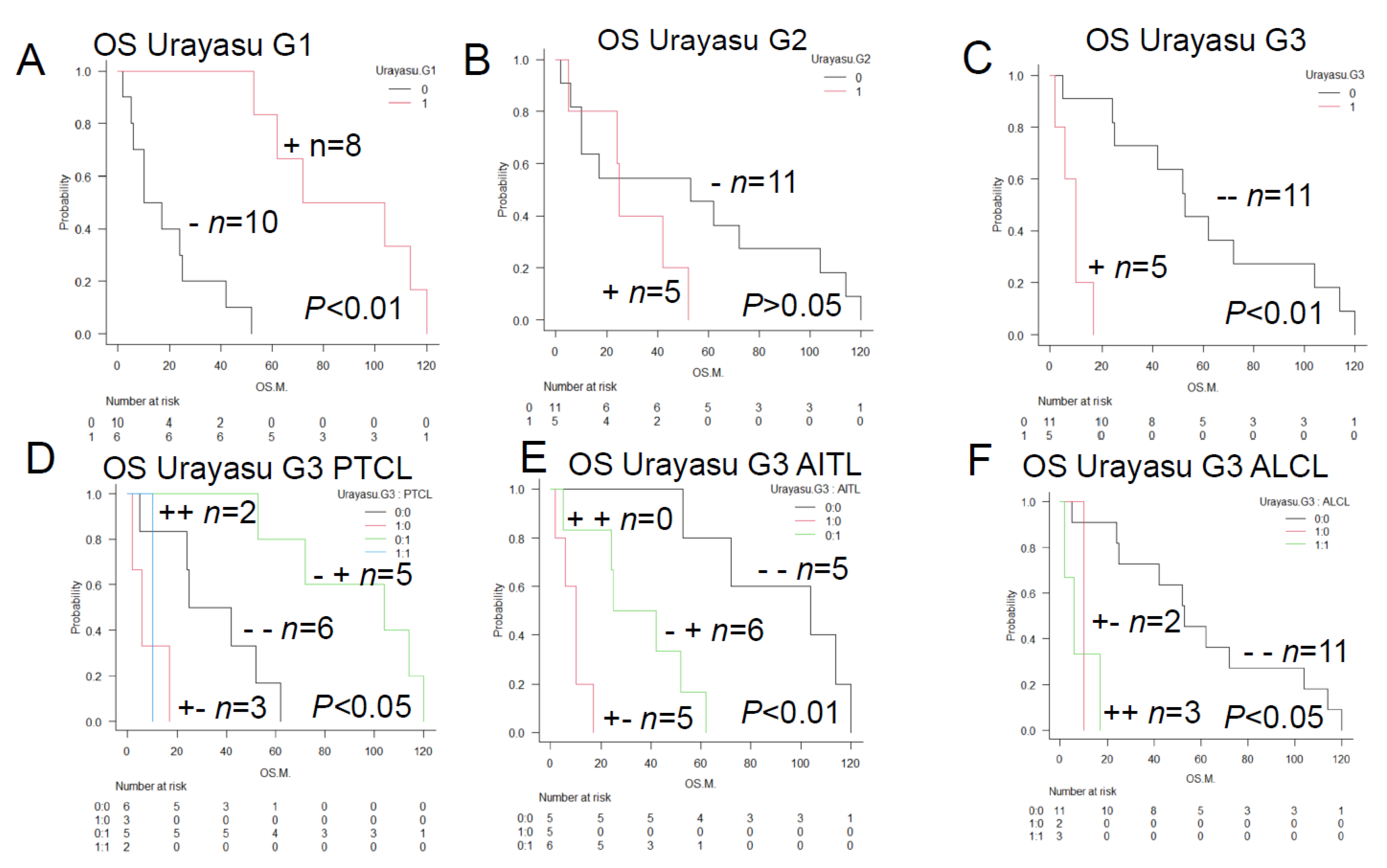
Figure 5.
Results of immunohistochemistry in 3 representative patients classified by the HPI (TCL Urayasu Classification). Red indicates positive staining, and black indicates negative staining. CR1 means first complete remission. HE stands for hematoxylin-eosin staining.
Figure 5A. Case 1: A 73-year-old woman diagnosed with stage IIIB PTCL-NOS (high-risk IPI, PIT-Group 1). The diagnosis of PTCL-NOS was made based on the presence of medium-to-large atypical cells that were detected by HE staining and showed positive staining for CD3 Her PTCL showed a high KI-67 index and showed negative staining for GRP94. She tested negative for any other tumor cells. Therefore, she was classified into TCL Urayasu Classification Group 1 (relatively good prognosis). In fact, she has remained in complete remission for a long period of time after 6 cycles of CHOP therapy, surviving for 114 months without relapse. The absence of treatment resistance factors may account for her good prognosis.
Figure 5B. HPI (TCL Urayasu classification) Group 2 (the poor prognosis group). Case 2: A 65-year-old woman diagnosed with stage IVB AITL (high-intermediate IPI, PIT-Group 3) that showed positive immunohistochemical staining for CD7 and a highly positive Ki-67 index. She did not achieve CR after CHOP therapy, developed resistance to CHOP treatment as well as to 5 types of salvage treatment regimens, and died after surviving for 25 months after the diagnosis. Her tumor showed positive staining for GRP94, AKR1C3, and AKR1C3. The failure to achieve CR could be attributable to a reduced treatment effect due to metabolism of HO chemotherapeutic agents by intracellular AKR1C3. In addition, GRP94 and GRP78 are both ER stress proteins expressed on the cell surface that allow tumors to overcome various stressful conditions in the tumor microenvironment, such as hypoxia, hypoglycemia, dysregulation of homeostasis, altered cell metabolism, and acidosis, which results in resistance to various treatment regimens.
Figure 5C. HPI (TCL Urayasu classification) Group 3 (the very poor prognosis group)Case 3: A 33-year-old woman diagnosed with stage IIA ALK-positive ALCL (low-intermediate IPI [IPIe], PIT-Group 1). ALK positivity is generally associated with a good prognosis. However, this patient developed resistance to both CHOP and ESHAP regimens, and died after only about 2 months of treatment. The diagnosis was ALCL. Her ALCL showed positive staining for GRP94 (Figure 8) and 3 (PD-L1, TP, and GRP78, shown in Figure 10, Figure 12, and Figure 20) of the other 6 poor prognostic factors (PD-L1, TP, AKR1C3, P53, PD1, and GRP78). In the tumor microenvironment, expression of PD-L1 on the cell surface blocks the immune checkpoint molecules, allowing the tumor to grow. TP is involved in starvation resistance, angiogenesis, invasion, and metastasis. GRP78 allows the tumors to overcome various stressful conditions, such as hypoxia, hypoglycemia, dysregulation of homeostasis, altered cell metabolism, and acidosis, which results in treatment resistance.
Figure 5.
Results of immunohistochemistry in 3 representative patients classified by the HPI (TCL Urayasu Classification). Red indicates positive staining, and black indicates negative staining. CR1 means first complete remission. HE stands for hematoxylin-eosin staining.
Figure 5A. Case 1: A 73-year-old woman diagnosed with stage IIIB PTCL-NOS (high-risk IPI, PIT-Group 1). The diagnosis of PTCL-NOS was made based on the presence of medium-to-large atypical cells that were detected by HE staining and showed positive staining for CD3 Her PTCL showed a high KI-67 index and showed negative staining for GRP94. She tested negative for any other tumor cells. Therefore, she was classified into TCL Urayasu Classification Group 1 (relatively good prognosis). In fact, she has remained in complete remission for a long period of time after 6 cycles of CHOP therapy, surviving for 114 months without relapse. The absence of treatment resistance factors may account for her good prognosis.
Figure 5B. HPI (TCL Urayasu classification) Group 2 (the poor prognosis group). Case 2: A 65-year-old woman diagnosed with stage IVB AITL (high-intermediate IPI, PIT-Group 3) that showed positive immunohistochemical staining for CD7 and a highly positive Ki-67 index. She did not achieve CR after CHOP therapy, developed resistance to CHOP treatment as well as to 5 types of salvage treatment regimens, and died after surviving for 25 months after the diagnosis. Her tumor showed positive staining for GRP94, AKR1C3, and AKR1C3. The failure to achieve CR could be attributable to a reduced treatment effect due to metabolism of HO chemotherapeutic agents by intracellular AKR1C3. In addition, GRP94 and GRP78 are both ER stress proteins expressed on the cell surface that allow tumors to overcome various stressful conditions in the tumor microenvironment, such as hypoxia, hypoglycemia, dysregulation of homeostasis, altered cell metabolism, and acidosis, which results in resistance to various treatment regimens.
Figure 5C. HPI (TCL Urayasu classification) Group 3 (the very poor prognosis group)Case 3: A 33-year-old woman diagnosed with stage IIA ALK-positive ALCL (low-intermediate IPI [IPIe], PIT-Group 1). ALK positivity is generally associated with a good prognosis. However, this patient developed resistance to both CHOP and ESHAP regimens, and died after only about 2 months of treatment. The diagnosis was ALCL. Her ALCL showed positive staining for GRP94 (Figure 8) and 3 (PD-L1, TP, and GRP78, shown in Figure 10, Figure 12, and Figure 20) of the other 6 poor prognostic factors (PD-L1, TP, AKR1C3, P53, PD1, and GRP78). In the tumor microenvironment, expression of PD-L1 on the cell surface blocks the immune checkpoint molecules, allowing the tumor to grow. TP is involved in starvation resistance, angiogenesis, invasion, and metastasis. GRP78 allows the tumors to overcome various stressful conditions, such as hypoxia, hypoglycemia, dysregulation of homeostasis, altered cell metabolism, and acidosis, which results in treatment resistance.
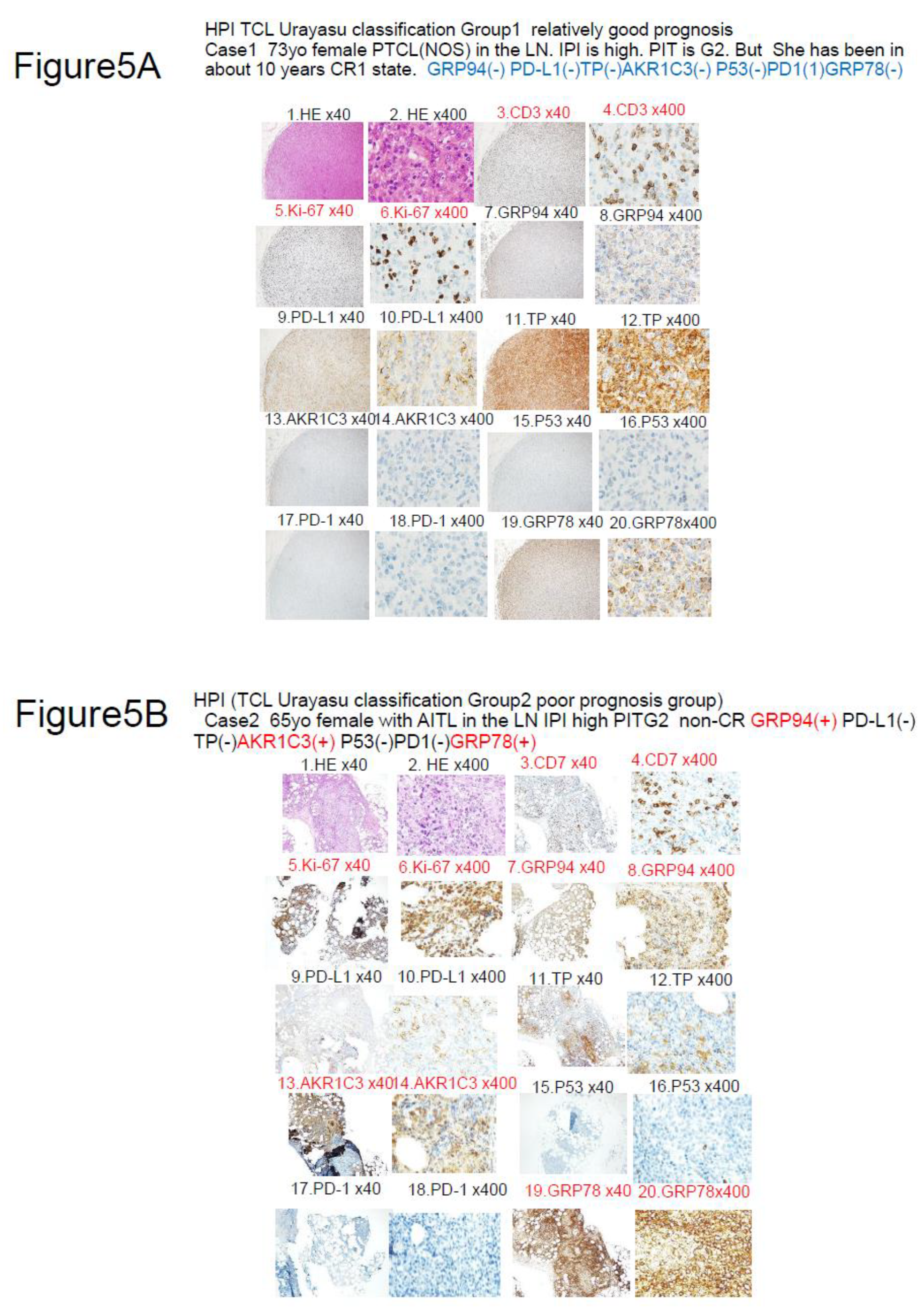
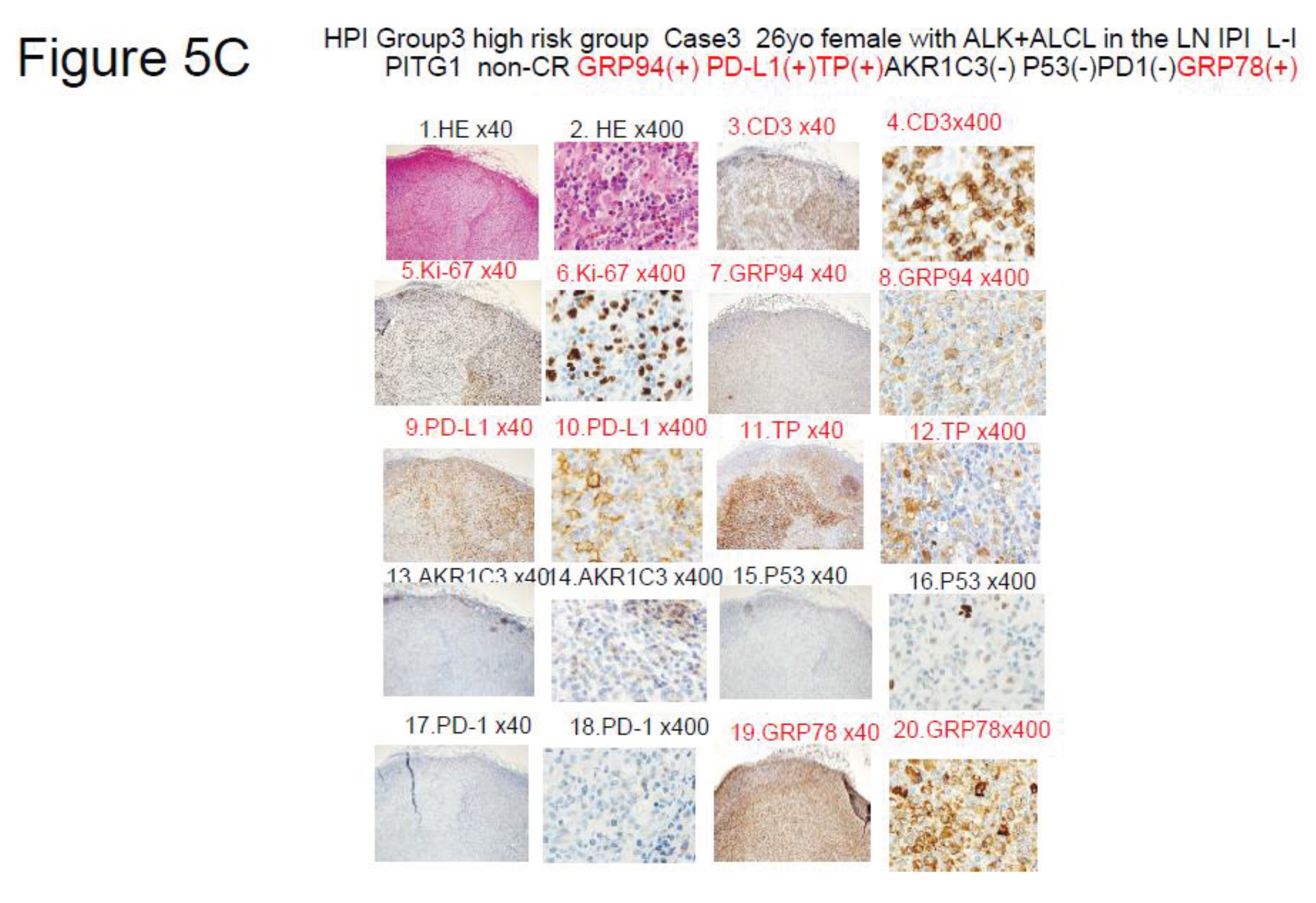
Figure 6.
Relationships between the prognostic factors in the TCL and LBCL Urayasu classifications. The relationships between the 7 factors involved in treatment resistance in the TCL Urayasu Classification and the 6 factors involved in treatment resistance in the LBCL Urayasu Classification are summarized in the following set diagram. Of the 7 TCL factors, the 4 factors unique to the TCL classification are the immune checkpoint molecules (PD-1 and PD-L1) serving as pro-survival components in the microenvironment, and TP and GRP78, both of which are involved in angiogenesis, invasion, and metastasis in order to overcome stressful conditions, such as hypoxia, hypoglycemia, and starvation resistance. On the other hand, the other 3 factors, which are common to both the TCL and LBCL classifications, are highly important, and consist of GRP94, AKR1C3 (an enzyme that inactivates the activity of chemotherapeutic agents by metabolizing HO), and P53 (product of a tumor suppressor gene). On the other hand, the 3 factors unique to the LBCL Urayasu classification are CYP3A4 (an enzyme that metabolizes and inactivates the activities of the CHOP regimen), MDR1, and MRP1, with both the latter being HO efflux pumps.
Figure 6.
Relationships between the prognostic factors in the TCL and LBCL Urayasu classifications. The relationships between the 7 factors involved in treatment resistance in the TCL Urayasu Classification and the 6 factors involved in treatment resistance in the LBCL Urayasu Classification are summarized in the following set diagram. Of the 7 TCL factors, the 4 factors unique to the TCL classification are the immune checkpoint molecules (PD-1 and PD-L1) serving as pro-survival components in the microenvironment, and TP and GRP78, both of which are involved in angiogenesis, invasion, and metastasis in order to overcome stressful conditions, such as hypoxia, hypoglycemia, and starvation resistance. On the other hand, the other 3 factors, which are common to both the TCL and LBCL classifications, are highly important, and consist of GRP94, AKR1C3 (an enzyme that inactivates the activity of chemotherapeutic agents by metabolizing HO), and P53 (product of a tumor suppressor gene). On the other hand, the 3 factors unique to the LBCL Urayasu classification are CYP3A4 (an enzyme that metabolizes and inactivates the activities of the CHOP regimen), MDR1, and MRP1, with both the latter being HO efflux pumps.
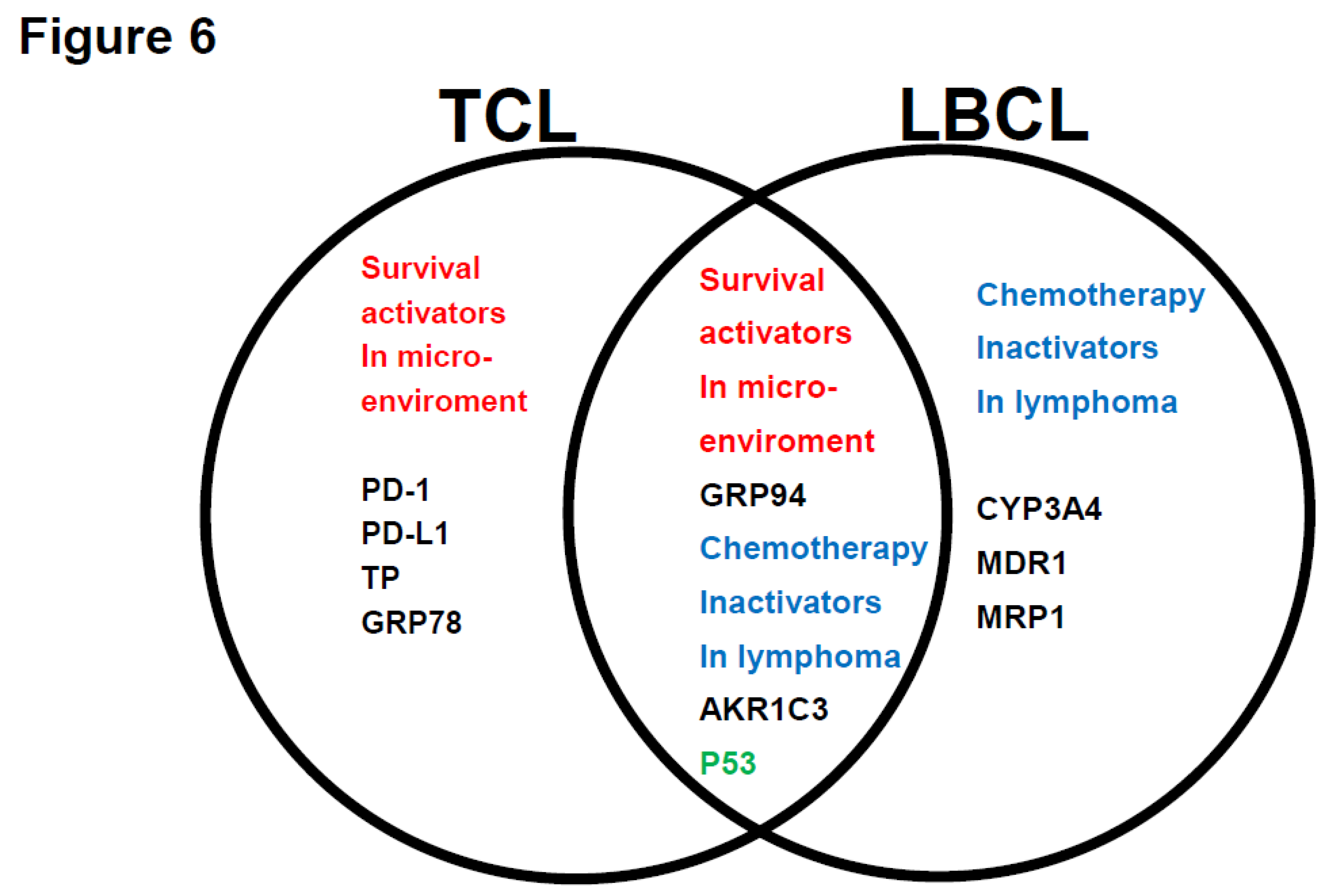
Table 1.
Characteristics of the patients enrolled in this analysis.
Table 1.
Characteristics of the patients enrolled in this analysis.
| |
n = 16 |
| Age Mean (Range) |
69.3(33-79) |
| Age > 60 years (%) |
12 (75%) |
| Male (%) |
9 (56%) |
| Histology |
|
| PTCL (NOS) |
7 (44%) |
| AITL |
6 (38%) |
| ALCL |
3 (19%) |
| Stage |
|
| Stage 1–2 |
6 (38%) |
| Stage 3–4 |
10 (63%) |
| IPI |
|
| 1-2 Low risk |
7 (44%) |
| 3-4 High risk |
9 (56%) |
| PIT |
|
| Group 1–2 Low risk |
4 (25%) |
| Group 3–4 High risk |
12 (75%) |
| CHOP outcomes |
|
| CR |
8 (50%) |
| PD |
8 (50%) |
| PD + relapse within 1 year |
10 (63%) |
| Median OS (range) |
38M (2–114) |
Table 3.
Summary of the tumor immunohistochemical findings in the patients with TCL (n = 16).
Table 3.
Summary of the tumor immunohistochemical findings in the patients with TCL (n = 16).
| Table 2 |
|
|
|
|
|
|
| Category |
Factors (♯Significant difference:) |
n |
Median OS(months) |
p value |
Figure |
Reference |
| Total |
TCL |
16 |
34 |
|
1-A |
|
| Disease |
PTCL (NOS) (♯) |
7 |
72 |
*p < 0.05 |
1-B |
|
| |
AITL (♯) |
6 |
34 |
*p < 0.05 |
1-B |
|
| |
ALCL (♯) |
3 |
6 |
*p < 0.05 |
1-B |
|
| Prognostic factor |
IPI High risk |
7 |
17 |
p > 0.05 |
1-C |
|
| |
PIT High risk |
4 |
21 |
p > 0.05 |
1-D |
|
| Result |
non-CR1 (PD) (♯) |
8 |
10 |
*p < 0.01 |
1-E |
|
| |
PD or within 1Y relapse (♯) |
10 |
14 |
*p < 0.01 |
1-F |
|
| ER stress proteins |
GRP94 (♯) |
10 |
14 |
*p < 0.01 |
2-B |
4-6 |
| |
TGFβ1 |
7 |
25 |
p > 0.05 |
|
9,10 |
| |
GRP78 |
10 |
14 |
p > 0.05 |
|
7,8 |
| |
TNFα1 |
4 |
16 |
p > 0.05 |
|
11 |
| OH metabolic enzyme |
AKR1C3 (♯) |
6 |
10 |
*p < 0.01 |
2-D |
12-16 |
| |
AKR1B1 |
4 |
21 |
p > 0.05 |
|
|
| |
AKR1B10 |
10 |
21 |
p > 0.05 |
|
|
| C metabolic enzyme |
CYP2B6 (♯) |
4 |
14 |
*p < 0.05 |
2-F |
19 |
| CHOP metabolic enzyme |
CYP3A4 |
0 |
|
|
|
17,18 |
| OH efflux pump |
MDR1 |
1 |
24 |
p > 0.05 |
|
20-22 |
| |
MRP1 |
0 |
|
|
|
23,24 |
| MTX efflux pump |
MRP4 |
0 |
|
|
|
25 |
| Immune check point molecules |
PD-1 |
5 |
52 |
p > 0.06 |
|
|
| |
PD-L1 (♯) |
4 |
6 |
*p < 0.01 |
2-C |
|
| |
PD-L2 |
1 |
53 |
p > 0.05 |
|
|
| Others |
TP (♯) |
3 |
6 |
*p < 0.01 |
2-E |
28 |
| |
p53 |
2 |
12 |
p > 0.05 |
|
|
| |
GST |
13 |
25 |
p > 0.05 |
|
27 |
| |
MYC |
3 |
6 |
p > 0.05 |
|
|
| |
ENT-1 |
16 |
34 |
p > 0.05 |
|
|
| |
Fibrosis (Silver stain positive) |
14 |
39 |
p > 0.05 |
|
29 |
| Significant combination |
|
|
|
|
|
|
| |
non-CR1 plus GRP94+ (♯) |
8 |
10 |
**p < 0.01 |
2-A |
|
| |
PD relapse < 1 year plus GRP94 (♯) |
10 |
10 |
**p < 0.01 |
2-B |
|
| |
GRP94+ plus PD-L1+ (♯) |
4 |
4 |
**p < 0.01 |
3-A |
|
| |
GRP94+ plus TP+ (♯) |
3 |
5 |
**p < 0.01 |
3-B |
23,24,26 |
| |
GRP94+ plus AKR1C3+ (♯) |
6 |
10 |
**p < 0.01 |
3-C |
|
| |
GRP94+ plus P53+ (♯) |
2 |
4 |
**p < 0.01 |
3-D |
|
| |
GRP94+ plus PD-L1+ (♯) |
7 |
10 |
**p < 0.01 |
3-E |
|
| |
GRP94+ plus GRP78+ (♯) |
8 |
11 |
**p < 0.01 |
3-F |
|
| |
PD relapse < 1 year plus AKR1C3+ (♯) |
6 |
10 |
**p < 0.01 |
|
|
| |
PD relapse < 1 year plus PD-L1+ (♯) |
4 |
5 |
**p < 0.01 |
|
|
| |
IPI High-risk, GRP94+ (♯) |
5 |
10 |
**p < 0.01 |
|
|
| |
PIT High-risk, PD-L1+ (♯) |
4 |
8 |
**p < 0.01 |
|
|
| |
TCL-Urayasu G1 (♯) |
6 |
88 |
**p < 0.01 |
4-A |
|
| |
TCL-Urayasu G2 |
5 |
25 |
p > 0.05 |
4-B |
|
| |
TCL-Urayasu G3 (♯) |
5 |
10 |
**p < 0.01 |
4-C |
|
| |
TCL-Urayasu G3 PTCL (NOS) |
2 |
10 |
*p < 0.05 |
4-D |
|
| |
TCL-Urayasu G3 AITL |
0 |
|
**p < 0.01 |
4-E |
|
| |
TCL-Urayasu G3 ALCL |
3 |
6 |
p < 0.05 |
4-F |
|
Table 3.
Summary of three representative cases shown in
Figure 5 classified according to the histological prognostic index (TCL Urayasu classification).
Table 3.
Summary of three representative cases shown in
Figure 5 classified according to the histological prognostic index (TCL Urayasu classification).
| Figure 5 |
Case |
Age |
Disease |
IPI |
PIT |
TCL-Urayasu |
Main factor |
|
|
Additional 6 factors |
Treatment |
Outcome |
OS |
| |
|
Sex |
|
|
|
Figure 4A-F |
GRP94 |
PD-L1 |
TP |
ANR1C3 |
P53 |
PD-1 |
GRP78 |
|
|
months |
| A |
1 |
73F |
PTCL |
High |
G1 |
Group 1 |
(-) |
(-) |
(-) |
(-) |
(-) |
(-) |
(-) |
CHOP x 6 |
CR1 |
114 |
| |
|
|
(NOS) |
|
|
Relatively good prognosis |
|
|
|
|
|
|
|
|
Alive |
|
| B |
2 |
65F |
AITL |
HI |
G3 |
Group 2 |
(+) |
(-) |
(-) |
(+) |
(-) |
(-) |
(+) |
CHOP x 3 |
non-CR |
25 |
| |
|
|
|
|
|
Poor prognosis |
|
|
|
|
|
|
|
5 regimens |
Dead |
|
| C |
3 |
4F |
ALCL |
LI |
G3 |
Group 3 |
(+) |
(+) |
(+) |
(-) |
(-) |
(-) |
(+) |
CHOP x 2 |
PD |
2 |
| |
|
|
|
|
|
Very poor prognosis |
|
|
|
|
|
|
|
ESHAP x 1 |
Dead |
|












