Submitted:
09 April 2024
Posted:
11 April 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
Preparation of External Standards Spiked with Internal Standard
Collection of Cell Culture Media and Lysates
Media for Analysis and Lysates Preparation
LC-MS Analysis
Processing of Spectra
Quantification of Metabolites
Protein Quantification
Statistical Analysis
3. Results
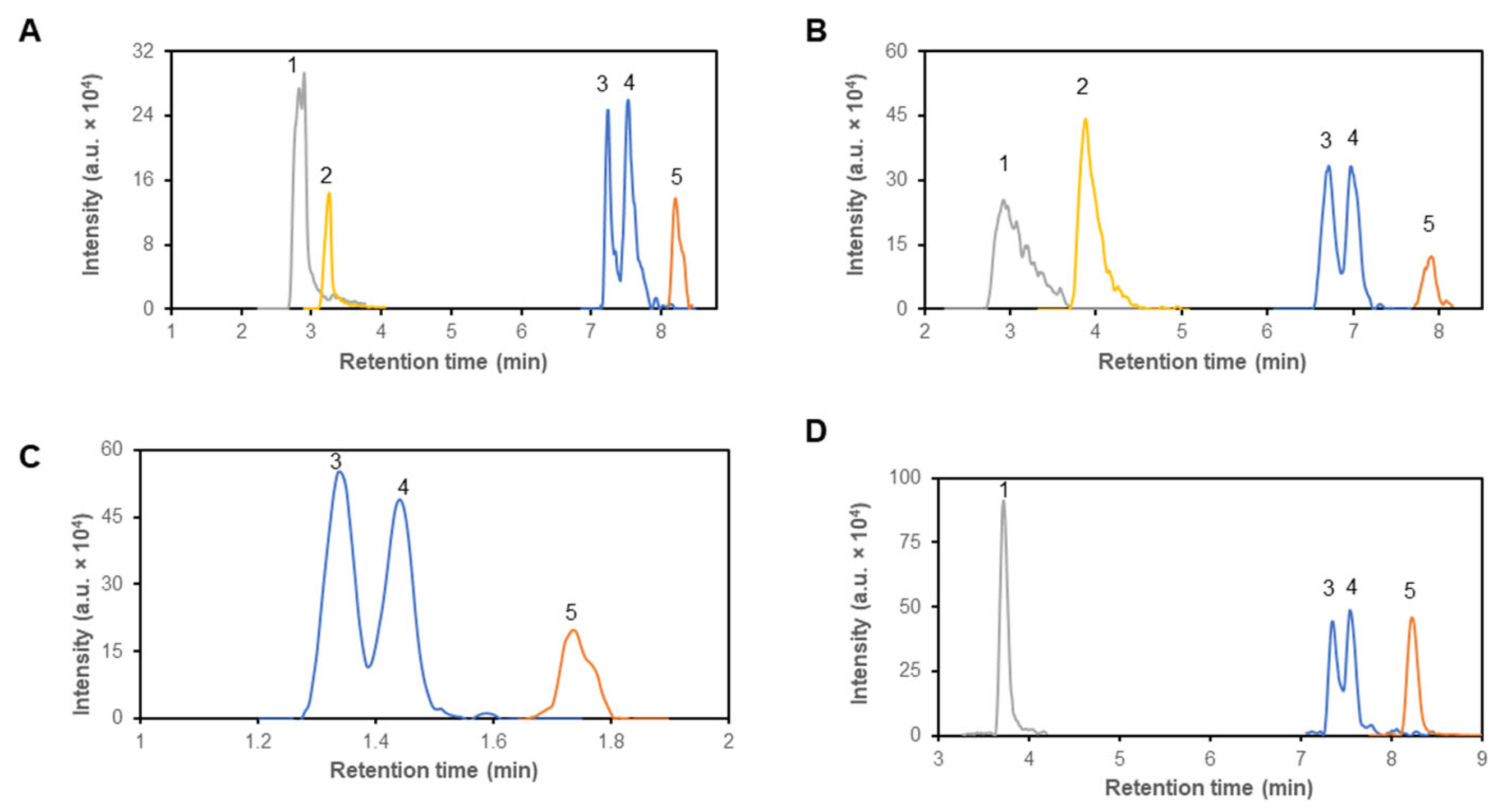
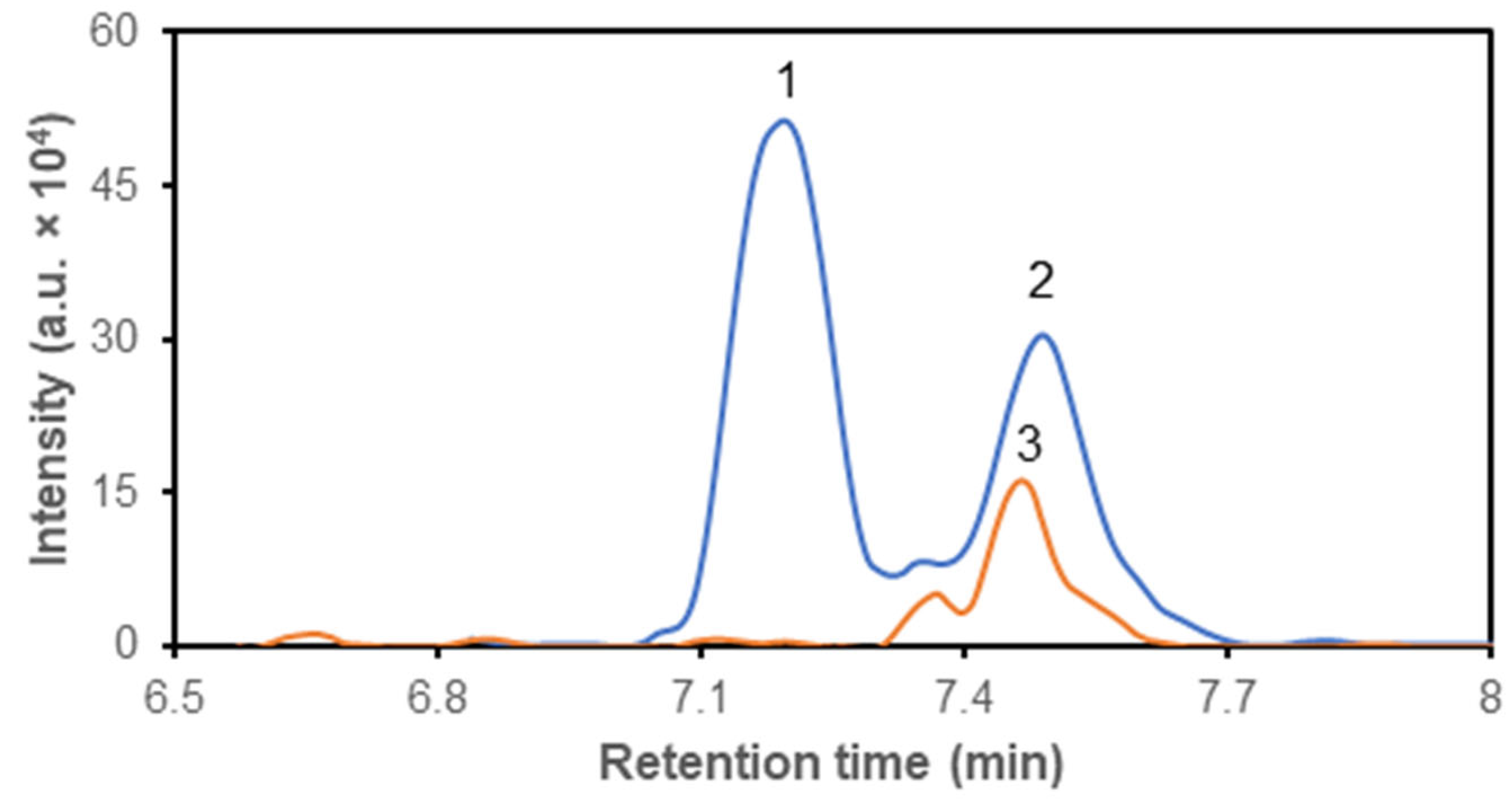
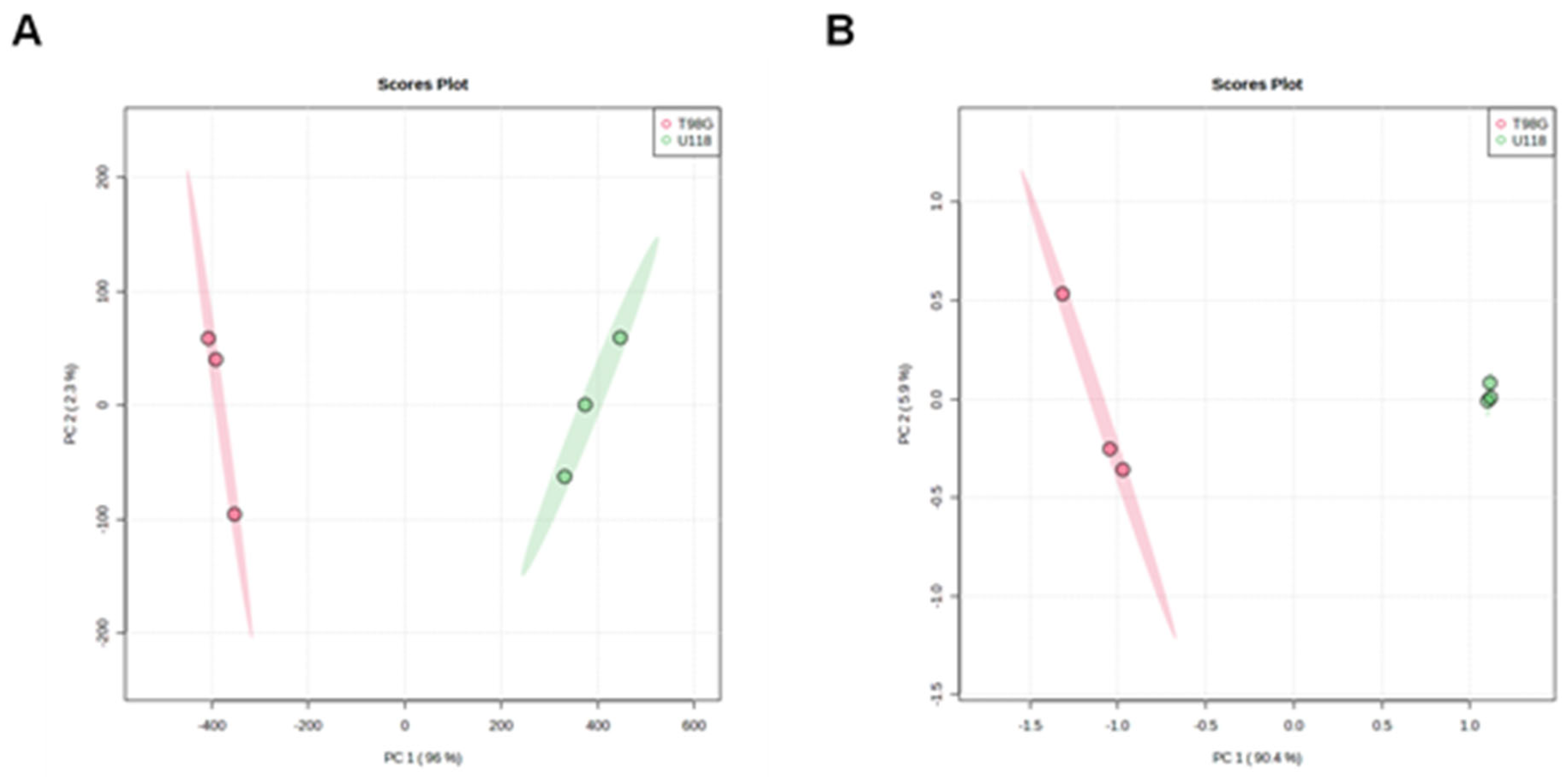
3.1. Design and Testing of LC-MS
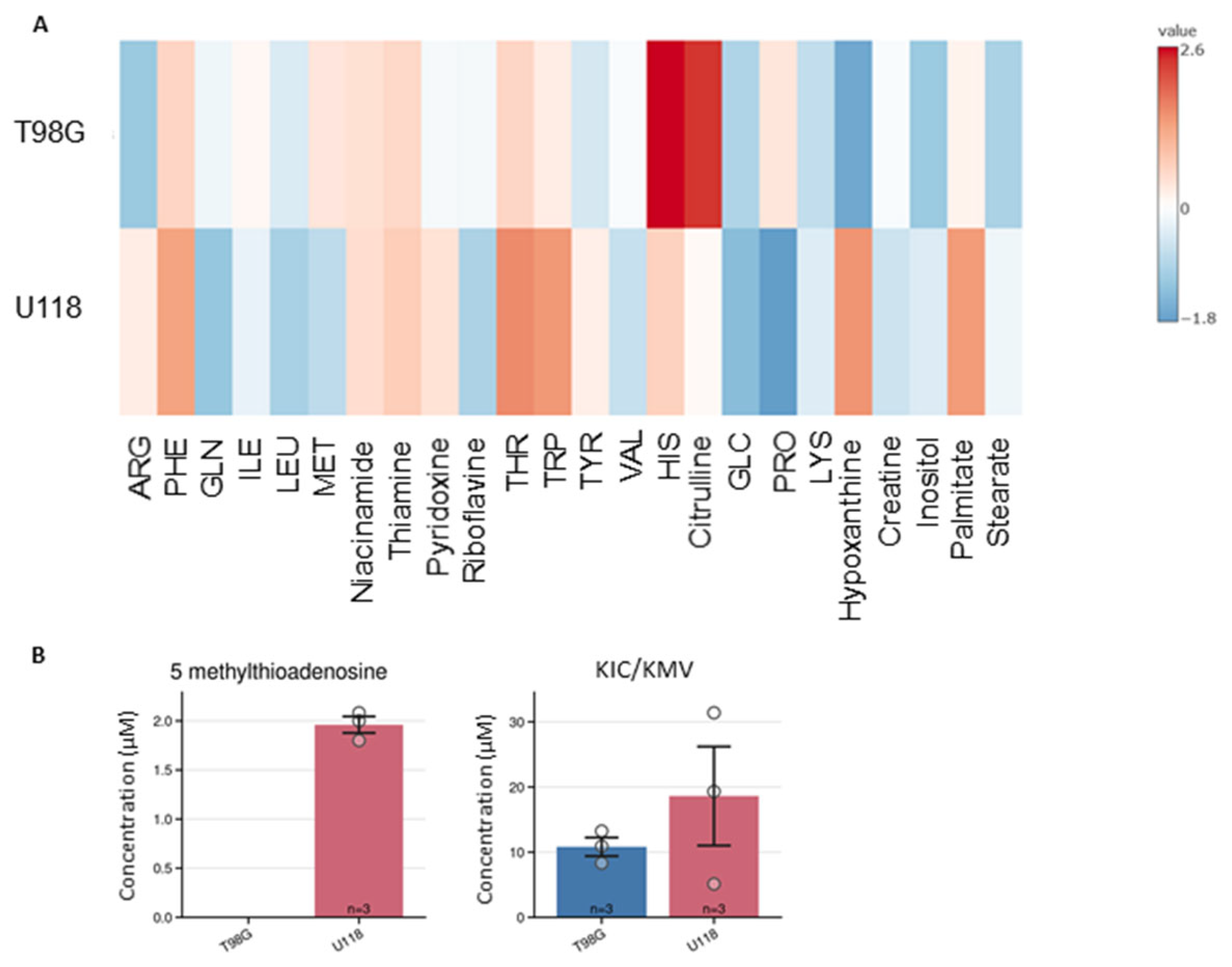
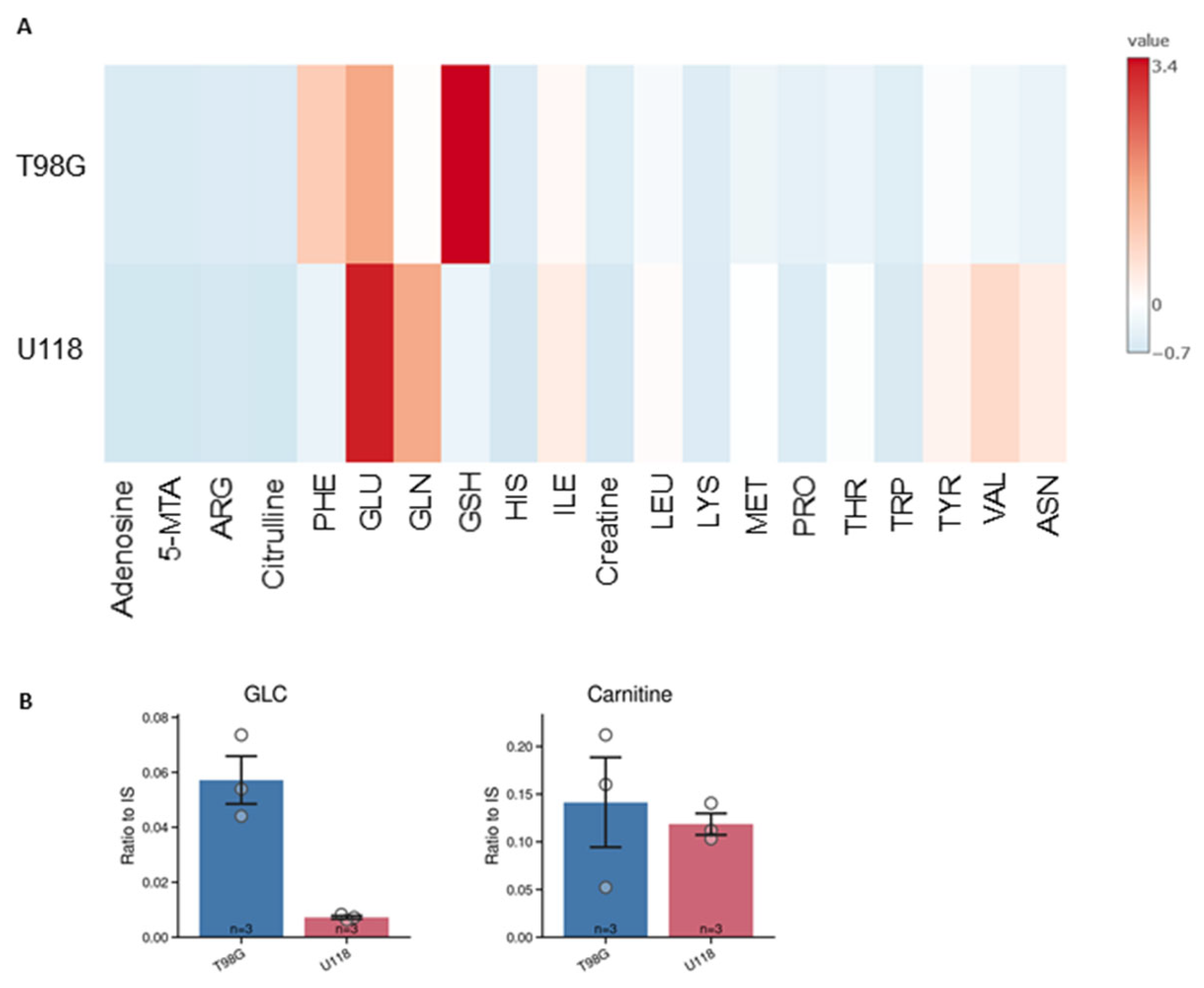
Identification and Quantification of Metabolites in Media
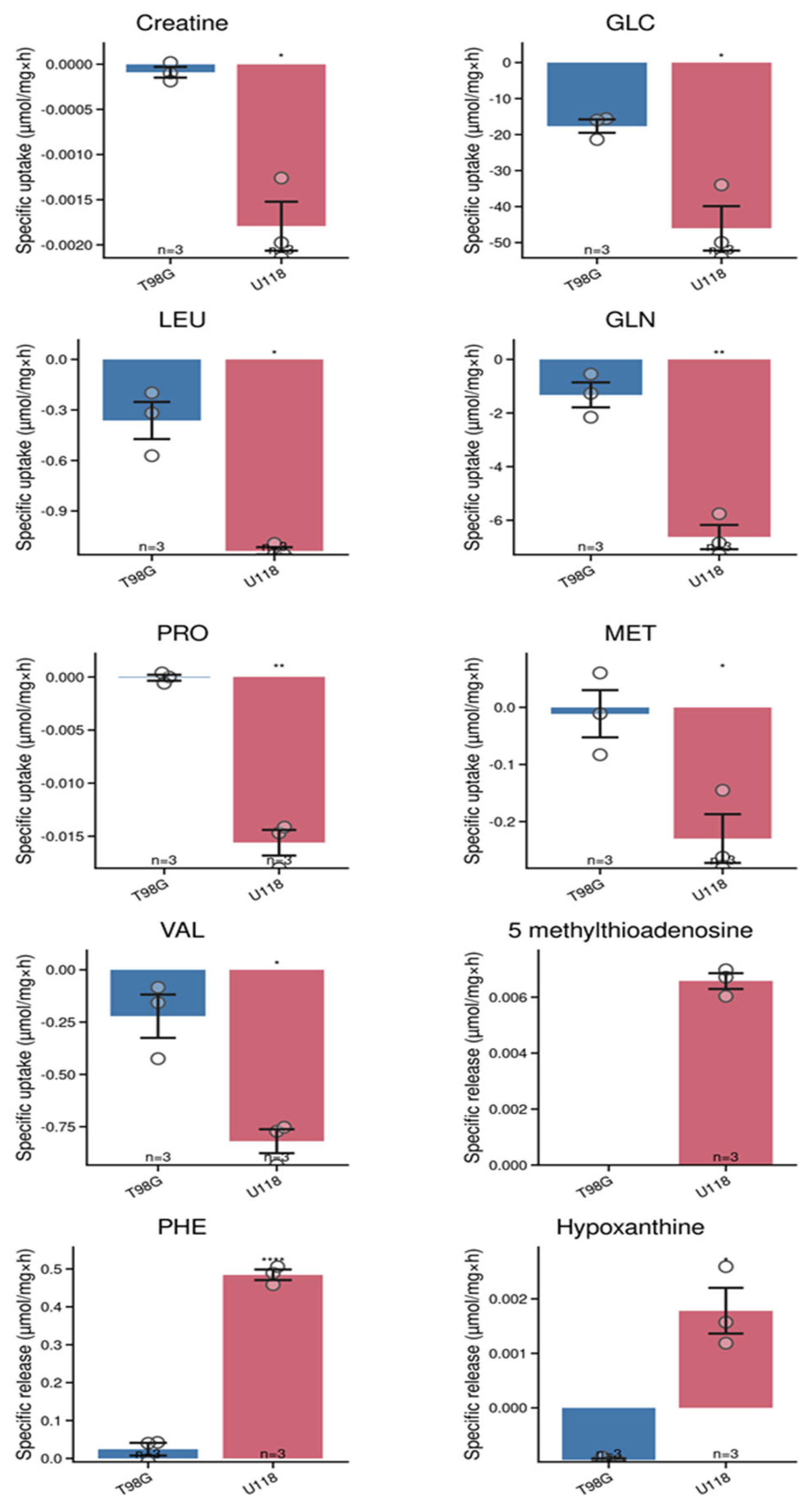
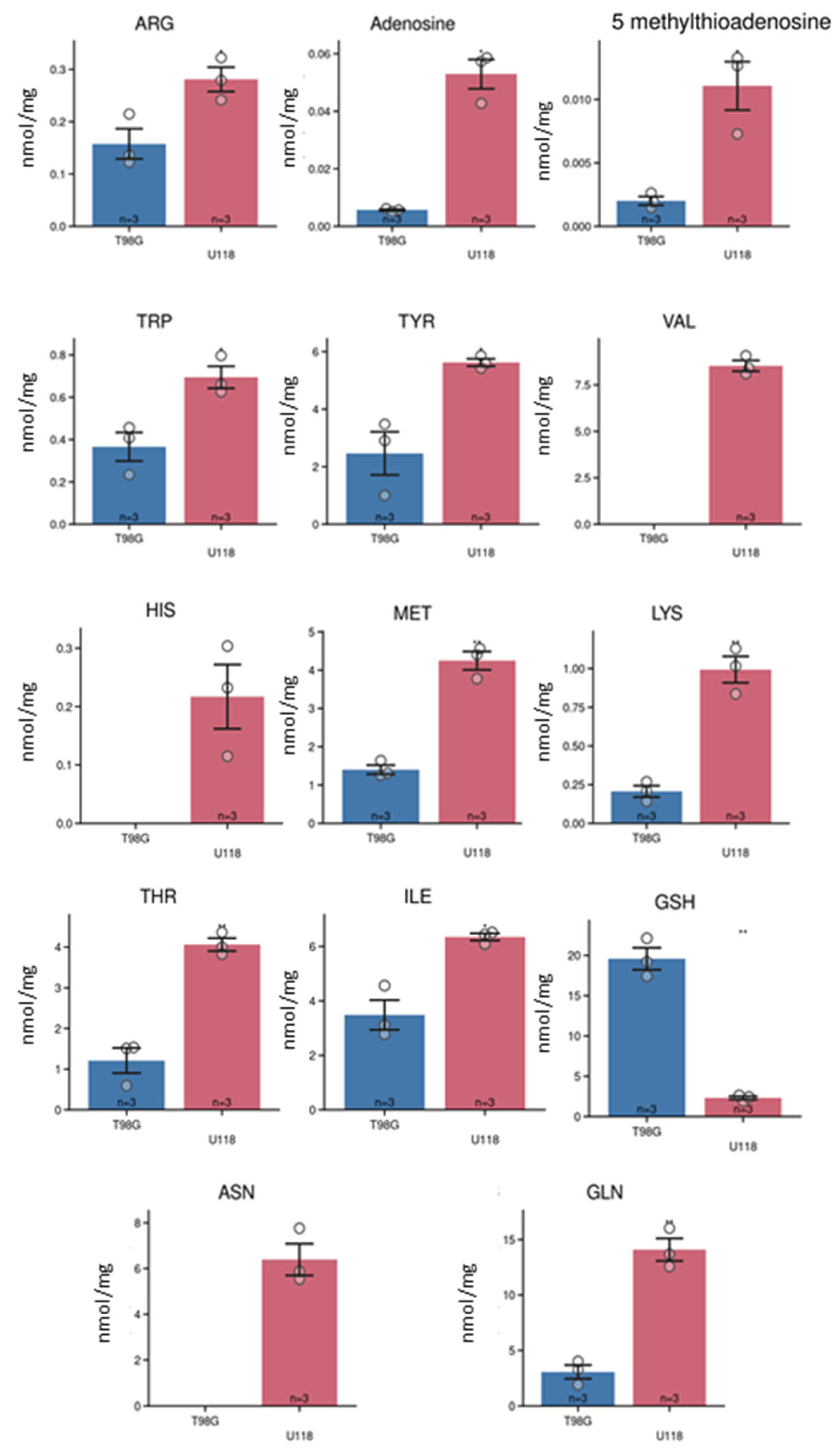
Identification and Quantification of Metabolites in Lysates
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hanif F, Muzaffar K, Perveen kahkashan, et al (2017) Glioblastoma Multiforme: A Review of its Epidemiology and Pathogenesis through Clinical Presentation and Treatment. APJCP 18:. [CrossRef]
- Lapointe S, Perry A, Butowski NA (2018) Primary brain tumours in adults. The Lancet 392:432–446. [CrossRef]
- Bernhard C, Reita D, Martin S, et al (2023) Glioblastoma Metabolism: Insights and Therapeutic Strategies. IJMS 24:9137. [CrossRef]
- Zarzuela L, Durán RV, Tomé M (2023) Metabolism and signaling crosstalk in glioblastoma progression and therapy resistance. Molecular Oncology 1878–0261.13571. [CrossRef]
- Pavlova NN, Zhu J, Thompson CB (2022) The hallmarks of cancer metabolism: Still emerging. Cell Metabolism 34:355–377. [CrossRef]
- Kim J, DeBerardinis RJ (2019) Mechanisms and Implications of Metabolic Heterogeneity in Cancer. Cell Metabolism 30:434–446. [CrossRef]
- Tong Y, Gao W-Q, Liu Y (2020) Metabolic heterogeneity in cancer: An overview and therapeutic implications. Biochimica et Biophysica Acta (BBA) - Reviews on Cancer 1874:188421. [CrossRef]
- Demicco M, Liu X-Z, Leithner K, Fendt S-M (2024) Metabolic heterogeneity in cancer. Nat Metab 6:18–38. [CrossRef]
- Xu E, Ji B, Jin K, Chen Y (2023) Branched-chain amino acids catabolism and cancer progression: focus on therapeutic interventions. Front Oncol 13:1220638. [CrossRef]
- Gondáš E, Kráľová Trančíková A, Baranovičová E, et al (2022) Expression of 3-Methylcrotonyl-CoA Carboxylase in Brain Tumors and Capability to Catabolize Leucine by Human Neural Cancer Cells. Cancers 14:585. [CrossRef]
- Lieu EL, Nguyen T, Rhyne S, Kim J (2020) Amino acids in cancer. Exp Mol Med 52:15–30. [CrossRef]
- Chen J, Cui L, Lu S, Xu S (2024) Amino acid metabolism in tumor biology and therapy. Cell Death Dis 15:42. [CrossRef]
- Chen S, Jiang J, Shen A, et al (2022) Rewired Metabolism of Amino Acids and Its Roles in Glioma Pathology. Metabolites 12:918. [CrossRef]
- Wei Z, Liu X, Cheng C, et al (2021) Metabolism of Amino Acids in Cancer. Front Cell Dev Biol 8:603837. [CrossRef]
- Cheng C, Geng F, Cheng X, Guo D (2018) Lipid metabolism reprogramming and its potential targets in cancer. Cancer Communications 38:1–14. [CrossRef]
- Kou Y, Geng F, Guo D (2022) Lipid Metabolism in Glioblastoma: From De Novo Synthesis to Storage. Biomedicines 10:1943. [CrossRef]
- Vettore L, Westbrook RL, Tennant DA (2020) New aspects of amino acid metabolism in cancer. Br J Cancer 122:150–156. [CrossRef]
- Badr CE, Silver DJ, Siebzehnrubl FA, Deleyrolle LP (2020) Metabolic heterogeneity and adaptability in brain tumors. Cell Mol Life Sci 77:5101–5119. [CrossRef]
- Griguer CE, Oliva CR, Gillespie GY (2005) Glucose Metabolism Heterogeneity in Human and Mouse Malignant Glioma Cell Lines. J Neurooncol 74:123–133. [CrossRef]
- Hensley CT, Faubert B, Yuan Q, et al (2016) Metabolic Heterogeneity in Human Lung Tumors. Cell 164:681–694. [CrossRef]
- Coskun O (2016) Separation Tecniques: CHROMATOGRAPHY. North Clin Istanbul. [CrossRef]
- Holčapek, M, Byrdwell, WmC (2017) Handbook of Advanced Chromatography /mass Spectrometry Techniques.
- Moldoveanu S, David V (2022) Other HPLC separations performed on hydrophobic stationary phases. In: Essentials in Modern HPLC Separations.
- Zhang T-Y, Li S, Zhu Q-F, et al (2019) Derivatization for liquid chromatography-electrospray ionization-mass spectrometry analysis of small-molecular weight compounds. TrAC Trends in Analytical Chemistry 119:115608. [CrossRef]
- Spagou K, Tsoukali H, Raikos N, et al (2010) Hydrophilic interaction chromatography coupled to MS for metabonomic/metabolomic studies. J of Separation Science 33:716–727. [CrossRef]
- Tang D, Zou L, Yin X, Ong CN (2016) HILIC-MS for metabolomics: An attractive and complementary approach to RPLC-MS. Mass Spectrometry Reviews 35:574–600. [CrossRef]
- Gondáš E, Kráľová Trančíková A, Dibdiaková K, et al (2023) Immunodetection of Pyruvate Carboxylase Expression in Human Astrocytomas, Glioblastomas, Oligodendrogliomas, and Meningiomas. Neurochem Res 48:1728–1736. [CrossRef]
- Gondáš E, Kráľová Trančíková A, Majerčíková Z, et al (2021) Expression of pyruvate carboxylase in cultured human astrocytoma, glioblastoma and neuroblastoma cells. gpb 40:127–135. [CrossRef]
- Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. 2: J Biol Chem 193.
- Commisso C, Davidson SM, Soydaner-Azeloglu RG, et al (2013) Macropinocytosis of protein is an amino acid supply route in Ras-transformed cells. Nature 497:633–637. [CrossRef]
- Xu W, Yang H, Liu Y, et al (2011) Oncometabolite 2-Hydroxyglutarate Is a Competitive Inhibitor of α-Ketoglutarate-Dependent Dioxygenases. Cancer Cell 19:17–30. [CrossRef]
- Wanders RJA, Mooyer P (1995) d-2-Hydroxyglutaric acidaemia: identification of a new enzyme,d-2-hydroxyglutarate dehydrogenase, localized in mitochondria. J Inherit Metab Dis 18:194–196. [CrossRef]
- Du X, Hu H (2021) The Roles of 2-Hydroxyglutarate. Front Cell Dev Biol 9:651317. [CrossRef]
- Carbonneau M, M. Gagné L, Lalonde M-E, et al (2016) The oncometabolite 2-hydroxyglutarate activates the mTOR signalling pathway. Nat Commun 7:12700. [CrossRef]
- Gondáš E, Baranovičová E, Bystrický P, et al (2024) Both Enantiomers of 2-Hydroxyglutarate Modulate the Metabolism of Cultured Human Neuroblastoma Cells.
- Yang M, Soga T, Pollard PJ (2013) Oncometabolites: linking altered metabolism with cancer. J Clin Invest 123:3652–3658. [CrossRef]
| Common name | IUPAC name | m/z | tR (min) |
|---|---|---|---|
| Acetoacetate | 3-Oxobutanoic acid | 101.0258 | 5.5 |
| Acetylcarnitine | 3-Acetyloxy-4-trimethylammonio-butanoate | 204.1230 | 13.3 |
| Acetyl-CoA | O1-{(3R)-4-[(3-{[2-(Acetylsulfanyl)ethyl]amino}-3-oxopropyl)amino]-3-hydroxy-2,2-dimethyl-4-oxobutyl} O3-{[(2R,3S,4R,5R)-5-(6-amino-9H-purin-9-yl)-4-hydroxy-3-(phosphonooxy)oxolan-2-yl]methyl} dihydrogen diphosphate | 404.0488 | 12.3 |
| Acetylcholine | 2-Acetoxy-N,N,N-trimethylethanaminium | 146.174 | 8.0 |
| N-Acetylcysteine | 2-Acetamido-3-sulfanylpropanoic aci | 164.0914 | 6.3 |
| Adenosine | 2-(6-Amino-9H-purin-9-yl)-5-(hydroxymethyl)oxolane-3,4-diol | 268.1022 | 3.7 |
| ADP | [(2R,3S,4R,5R)-5-(6-Aminopurin-9-yl)-3,4-dihydroxyoxolan-2-yl]methyl phosphono hydrogen phosphate | 426.0137 | 11.2 |
| AICA Riboside-5-phosphate | 1-(5-Amino-4-carbamoyl-1H-imidazol-1-yl)-1,4-anhydro-D-ribitol 5-(dihydrogen phosphate) | 339.0685 | 10.8 |
| ALA-GLY | 2-(2-Aminopropanoylamino)acetic acid | 218.1135 | 5.5 |
| Alanine | 2-Aminopropanoic acid | 90.055 | 7.3 |
| β-Alanine | 3-Aminopropanoic acid | 161.0909 | 15.6 |
| Alanyl-Glutamine | 5-Amino-2-[[(2S)-2-aminopropanoyl]amino]-5-oxopentanoic acid | 218.1508 | 5.7 |
| γ-Aminobutyric acid | 4-Aminobutanoic acid | 104.0706 | 4.6 |
| Arachidonic acid | Icosa-5,8,11,14-tetraenoic acid | 303.2324 | 1.7 |
| Arachidonic acid methyl ester | Methyl-icosa-5,8,11,14-tetraenoic acid | 319.2225 | 1.7 |
| Arginine | 2-Amino-5-(diaminomethylideneamino)pentanoic acid | 175.119 | 15.9 |
| Ascorbic acid | 3,4-Dihydroxy-5-((S)- 1,2-dihydroxyethyl)furan-2(5H)-one | 175.0238 | 12.7 |
| Asparagine | 2,4-Diamino-4-oxobutanoic acid | 133.0596 | 10.4 |
| Aspartic acid | 2-Aminobutanedioic acid | 134.0476 | 10.9 |
| ATP | [[(2R,3S,4R,5R)-5-(6-Aminopurin-9-yl)-3,4-dihydroxyoxolan-2-yl]methoxy-hydroxyphosphoryl] phosphono hydrogen phosphate | (-)505.9805 | 14.0 |
| 507.9996 | 14.0 | ||
| Betaine | 2-(Trimethylazaniumyl)acetate | 118.0863 | 1.6 |
| Biopterin | 2-Amino-6-(1,2-dihydroxypropyl)-1H-pteridin-4-one | (-)236.0804 | 7.2 |
| 238.1131 | 7.2 | ||
| Biotin | 2-Oxohexahydro-1H-thieno[3,4-d]imidazol-4-yl]pentanoic acid | 245.0954 | 8.8 |
| Butyrylcarnitine | 3-Butanoyloxy-4-(trimethylazaniumyl)butanoate | 232.1543 | 10.8 |
| Caffeine | 1,3,7-Trimethyl-3,7-dihydro-1H-purine-2,6-dion | 195.0937 | 1.8 |
| cAMP | (4aR,6R,7R,7aS)-6-(6-Aminopurin-9-yl)-2-hydroxy-2-oxo-4a,6,7,7a-tetrahydro-4H-furo[3,2-d][1,3,2]dioxaphosphinin-7-ol | (-)328.0414 | 9.7 |
| 330.0603 | 9.7 | ||
| Carnitine | 3-Hydroxy-4-(trimethylazaniumyl)butanoate | 227.1125 | 12.1 |
| 162.1125 | 15.6 | ||
| Carnosine | 2-(3-Aminopropanamido)-3-(3H-imidazol-4-yl)propanoic acid | 227.1125 | 12.1 |
| Cervonic acid | Docosa-4,7,10,13,16,19-hexaenoic acid | 327.2330 | 2.2 |
| Citric acid | 2-Hydroxypropane-1,2,3-tricarboxylic acid | 191.0197 | 21.6 |
| Citrulline | 2-Amino-5-(carbamoylamino)pentanoic acid[ | 176.1029 | 10.3 |
| Coenzyme A | [[(2R,3S,4R,5R)-5-(6-Aminopurin-9-yl)-4-hydroxy-3-phosphonooxyoxolan-2-yl]methoxy-hydroxyphosphoryl] [(3R)-3-hydroxy-2,2-dimethyl-4-oxo-4-[[3-oxo-3-(2-sulfanylethylamino)propyl]amino]butyl] hydrogen phosphate | 357.0813 | 5.3 |
| Creatine | 2-[Carbamimidoyl(methyl)amino]acetic acid | 132.0768 | 2.4 |
| Creatinine | 2-Amino-1-methyl-5H-imidazol-4-one | 114.0662 | 8.5 |
| Cystathionine | S-((R)-2-Amino-2-carboxyethyl)-L-homocysteine | 223.0742 | 13.1 |
| Cytosine | 4-Aminopyrimidin-2(1H)-one | 112.0497 | 6.4 |
| Decanoylcarnitine | 3-Decanoyloxy-4-(trimethylazaniumyl)butanoate | 316.2482 | 7.6 |
| 7,8-Dihydrobiopterin | 2-Amino-6-(1,2-dihydroxypropyl)-7,8-dihydro-1H-pteridin-4-one | 240.1149 | 7.5 |
| Dimethylarginine | (2S)-5-(Diaminomethylideneamino)-2-(dimethylamino)pentanoic acid | 203.1491 | 10.3 |
| Dimethyllysine | 2-Amino-6-(dimethylamino)hexanoic acid | 175.1428 | 17.7 |
| Dodecanoylcarnitine | 3-Decanoyloxy-4-(trimethylazaniumyl)butanoate | 344.2795 | 7.4 |
| Dopamine | 4-(2-Aminoethyl)benzene-1,2-diol | 154.0863 | 2.3 |
| EDTA | 2-[2-[Bis(carboxymethyl)amino]ethyl-(carboxymethyl)amino]acetic acid | (-)291.0769 | 12.1 |
| 293.0982 | 12.1 | ||
| EGTA | 2-[2-[2-[2-[Bis(carboxymethyl)amino]ethoxy]ethoxy]ethyl-(carboxymethyl)amino]acetic acid | 381 | 10.0 |
| Folic acid | 2-[[4-[(2-Amino-4-oxo-1H-pteridin-6-yl)methylamino]benzoyl]amino]pentanedioic acid | 440.1324 | 8.7 |
| Fructose-6-phosphate | 6-O-Phosphono-α-D-fructofuranose | 259.0217 | 11.7 |
| Fumaric acid | (E)-But-2-enedioic acid | 115.0037 | 17.6 |
| Gabapentin | 2-[1-(Aminomethyl)cyclohexyl]acetic acid | 172.1332 | 1.7 |
| Glucose | (3R,4S,5S,6R)-6-(Hydroxymethyl)oxane-2,3,4,5-tetrol | 179.0561 | 7.8 |
| Glucose-6-phosphate | Glucopyranose 6-phosphate | 259.0224 | 19.4 |
| L-Glutamic acid | (2S)-2-Aminopentanedioic acid | 148.0604 | 10.2 |
| L-Glutamine | (2S)-2,5-Diamino-5-oxopentanoic acid | 147.0764 | 9.8 |
| Glutaric acid | Pentanedioic acid | 131.0346 | 12.1 |
| Glutathione | (2S)-2-Amino-5-[[(2R)-1-(carboxymethylamino)-1-oxo-3-sulfanylpropan-2-yl]amino]-5-oxopentanoic acid | 308.0906 | 11.4 |
| Glycerol 1-phosphate | 2,3-Dihydroxyprop yl dihydrogen phosphate | 171.008 | 10.9 |
| Guanine | 2-Amino-1,9-dihydro-6H-purin-6-one | 152.0563 | 7.1 |
| GMP | [(2R,3S,4R,5R)-5-(2-Amino-6-oxo-1H-purin-9-yl)-3,4-dihydroxyoxolan-2-yl]methyl dihydrogen phosphate | 362.0501 | 11.2 |
| Hexanoylcarnitine | 3-Hexanoyloxy-4-(trimethylazaniumyl)butanoate | 260.1856 | 8.9 |
| Histamine | 2-(1H-Imidazol-4-yl)ethanamine | 112.0869 | 11.4 |
| Histidine | 2-Amino-3-(1H-imidazol-5-yl)propanoic acid | 156.0768 | 14.1 |
| Homocarnosine | 2-(4-Aminobutanoylamino)-3-(1H-imidazol-5-yl)propanoic acid | 241.1278 | 12.2 |
| Homocysteine | 2-Amino-4-sulfanylbutanoic acid | 136.0427 | 9.8 |
| α-Hydroxyglutaric acid | 2-Hydroxypentanedioic acid | 147.0293 | 12.0 |
| 3-Hydroxybutyric acid | 3- Hydroxypentanedioic acid | 103.0401 | 2.4 |
| D-2-Hydroxyglutaric acid | (2R)-2-Hydroxypentanedioic acid | 147.0314 | 11.4 |
| L-2-Hydroxyglutaric acid | (2S)-2-Hydroxypentanedioic acid | 147.0314 | 11.4 |
| 3-Hydroxyisobutyric acid | 3-Hydroxy-2-methylpropanoic acid | 103.0401 | 2.4 |
| Hydroxyproline | 4-Hydroxypyrrolidine-2-carboxylic acid | 132.0998 | 10.0 |
| Hypoxanthine | 1,9-Dihydro-6H-purin-6-one | 135.0375 | 5.7 |
| Inosine | 3,4-Dihydroxy-5-(hydroxymethyl)oxolan-2-yl]-6,9-dihydro-3H-purin-6-one | 269.0858 | 6.9 |
| Inosine 5′-monophosphate | [3,4-bis(trimethylsilyloxy)-5-(6-trimethylsilyloxypurin-9-yl)oxolan-2-yl]methyl bis(trimethylsilyl) phosphate | (-)347.041 | 10.7 |
| 349.1287 | 10.7 | ||
| Inositol | Cyclohexane-1,2,3,4,5,6-hexol | 179.0521 | 8.9 |
| Isocitrate | 1-Hydroxypropane-1,2,3-tricarboxylic acid | 191.0197 | 21.6 |
| Isoleucine | 2-Amino-3-methylpentanoic acid | 132.1019 | 7.3 |
| Isovaleryl-CoA | S-{(9R)-1-[(2R,3S,4R,5R)-5-(6-Amino-9H-purin-9-yl)-4-hydroxy-3-(phosphonooxy)tetrahydro-2-furanyl]-3,5,9-trihydroxy-8,8-dimethyl-3,5-dioxido-10,14-dioxo-2,4,6-trioxa-11,15-diaza-3λ5,5λ5- diphosphaheptadecan-17-yl} 2-methylpropanethioate | 850.1486 | 11.1 |
| Itaconic acid | Methylidenebutanedioic acid | 129.0219 | 10.2 |
| α-Ketoisocaproic acid | 4-Methyl-2-oxopentanoic acid | (-)129.0582 | 1.9 |
| α-Ketoisovaleric acid | 3-Methyl-2-oxobutanoic acid | (-)115.0428 | 2.3 |
| α-Keto-β-methylvaleric acid | 2-formylpentanoic acid | (-)129.0582 | 1.9 |
| Kynurenine | 2-Amino-4-(2-aminophenyl)-4-oxo-butanoic acid | 209.0921 | 11.2 |
| Lactic acid | 2-Hydroxypropanoic acid | 89.0244 | 2.0 |
| Leucine | 2-Amino-4-methylpentanoic acid | 132.1019 | 7.5 |
| γ-Linolenic acid | Octadeca-6,9,12-trienoic acid | 277.2173 | 2.2 |
| Linoleic acid | Octadeca-9,12-dienoate | 279.233 | 2.3 |
| Lysine | 2,6-Diaminohexanoic acid | 147.1128 | 15.8 |
| Malic acid | 2-Hydroxybutanedioic acid | 133.0142 | 17.5 |
| Methionine | 2-Amino-4-(methylthio)butanoic acid | 150.0583 | 8.8 |
| 3-Methyladenine | 3-Methyl-7H-purin-6-imine | 157.0774 | 2.4 |
| Methylcrotonyl-CoA | 3′-O-Phosphonoadenosine 5′-[(3R)-3-hydroxy-2-methyl-4-{[3-({2-[(3-methylbut-2-enoyl)sulfanyl]ethyl}amino)-3-oxopropyl]amino}-4-oxobutyl dihydrogen diphosphate] | 850.1654 | 11.3 |
| (-)848.1336 | 11.3 | ||
| 5-Methylcytosine | 4-Amino-5-methylpyrimidin-2(1H)-one | 126.0617 | 4.2 |
| 6-O-Methylguanine | 6-Methoxy-9H-purin-2-amine | 166.0703 | 3.6 |
| 7-Methylguanine | 2-Amino-7-methyl-1,7-dihydro-6H-purin-6-one | 166.071 | 5.5 |
| 5-Methylthioadenosine | 5′-S-Methyl-5′-thioadenosine | 298.0958 | 1.9 |
| Trimethyllysine | (5-Amino-5-carboxypentyl)-trimethylazanium | 189.1598 | 8.5 |
| NAD+ | 5-(6-Aminopurin-9-yl)-3,4-dihydroxyoxolan-2-yl]methoxy-hydroxyphosphoryl] [(2R,3S,4R,5R)-5-(3-carbamoylpyridin-1-ium-1-yl)-3,4-dihydroxyoxolan-2-yl]methyl hydrogen phosphate | 665.1215 | 16.8 |
| NADH | 5-(6-Aminopurin-9-yl)-3,4-dihydroxyoxolan-2-yl]methoxy-oxidophosphoryl] [(2R,3S,4R,5R)-5-(3-carbamoylpyridin-1-ium-1-yl)-3,4-dihydroxyoxolan-2-yl]methyl phosphate | 666.1337 | 16.4 |
| Neopterin | 2-Amino-6-(1,2,3-trihydroxypropyl)pteridin-4(1H)-one | 252.0678 | 8.9 |
| Neopterin | 2-Amino-6-(1,2,3-trihydroxypropyl)pteridin-4(1H)-one | 254.1203 | 8.9 |
| Niacinamide | Pyridine-3-carboxamide | 123.057 | 1.8 |
| N-Methyl-D-aspartic acid | 2-(Methylamino)butanedioic acid | (-)146.0484 | 7.1 |
| 148.0605 | 7.1 | ||
| N-Methyllysine | [(5S)-5-Carboxy-5-(methylamino)pentyl]azanium | 161.1273 | 16.2 |
| N-Methyl phenylalanine | 2-(Methylamino)-3-phenylpropanoic acid | 180.1015 | 7.2 |
| Octanoylcarnitine | 3-Octanoyloxy-4-(trimethylazaniumyl)butanoate | 288.2169 | 8.0 |
| Ornithine | 2,5-Diaminopentanoic acid | 133.0972 | 15.5 |
| 2-Oxoglutarate | 2-Oxopentanedioic acid | 145.0142 | 16.0 |
| Palmitic acid | Hexadecanoic acid | 255.2330 | 2.3 |
| Palmitoylcarnitine | 3-hexadecanoyloxy-4-(trimethylazaniumyl)butanoate | 400.3421 | 7.0 |
| Pantothenic acid | 2,4-Dihydroxy-3,3-dimethylbutanamido]propanoic acid | (-)218.0983 | 7.4 |
| 220.1334 | 7.4 | ||
| Phenylalanine | 2-Amino-3-phenylpropanoic acid | 166.9863 | 7.2 |
| Phenylbutyric acid | 4-Phenylbutanoic acid | 163.076 | 2.2 |
| Phosphocreatine | N-Methyl-N-(phosphonocarbamimidoyl)glycine | 210.0258 | 12.2 |
| Phosphoenolpyruvate | 2-(Phosphonooxy)prop-2-enoic acid | 169.1011 | 1.4 |
| Proline | Pyrrolidine-2-carboxylic acid | 116.0706 | 8.5 |
| Pyridoxine | 4,5-Bis(hydroxymethyl)-2-methylpyridin-3-ol | 170.0941 | 2.7 |
| Pyroglutamic acid | 5-Oxopyrrolidine-2-carboxylic acid | 130.0449 | 10.2 |
| Retinol | 3,7-Dimethyl-9-(2,6,6-trimethylcyclohex-1-en-1-yl)nona-2,4,6,8-tetraen-1-ol | 285.2224 | 3.9 |
| Riboflavin | 7,8-Dimethyl-10-[(2S,3S,4R)-2,3,4,5-tetrahydroxypentyl]benzo[g]pteridine-2,4-dione | 377.1488 | 4.0 |
| Ribose | 5-(Hydroxymethyl)oxolane-2,3,4-triol | 149.047 | 9.9 |
| Serotonin | 3-(2-Aminoethyl)-1H-indol-5-ol | 177.1002 | 3.2 |
| Stearic acid | Octadecanoic acid | 283.2643 | 2.3 |
| Succinyl-CoA | 4-{[1,3-Dihydroxy-1,3-dioxo-3-(3′-O-phosphonoadenosin-5′-O-yl)-1λ5,3λ5-diphosphoxan-1-yl]oxy}-3,3-dimethylbutanamido]propanamido}ethyl)sulfanyl]-4-oxobutanoic acid | 117.0198 | 11.8 |
| Taurine | 2-Aminoethanesulfonic acid | 126 | 10.0 |
| Tetradecanoylcarnitine | 3-Tetradecanoyloxy-4-(trimethylazaniumyl)butanoate | 372.3108 | 7.2 |
| Tetrahydrobiopterin | 2-Amino-6-[(1R,2S)-1,2-dihydroxypropyl]-5,6,7,8-tetrahydropteridin-4(1H)-one | 242.1244 | 7.9 |
| Thiamine | 2-[3-[(4-Amino-2-methylpyrimidin-5-yl)methyl]-4-methyl-1,3-thiazol-3-ium-5-yl]ethanol | 265.1181 | 12.8 |
| Threonine | 2-Amino-3-hydroxybutanoic acid | 120.0655 | 7.7 |
| Tocopherol | 2,5,7,8-Tetramethyl-2-[(4R,8R)-4,8,12-trimethyltridecyl]-3,4-dihydro-2H-1-benzopyran-6-ol | 429.3738 | 2.2 |
| Tryptophane | 2-Amino-3-(1H-indol-3-yl)propanoic acid | 205.0972 | 7.8 |
| Tyrosine | 2-Amino-3-(4-hydroxyphenyl)propanoic acid | 182.0812 | 20.1 |
| Valine | 2-Amino-3-methylbutanoic acid | 118.0863 | 7.7 |
| Vitamin D | 9,10-Secocholesta-5,7,10(19)-trien-3-ol | 383.3319 | 3.0 |
| Vitamin K | 3,7,11,15-Tetramethylhexadec-2-enyl]naphthalene-1,4-dione | 449.3425 | 4.3 |
| Xanthine | 3,7-Dihydro-1H-purine-2,6-dione | 151.0285 | 5.8 |
| Column | tR(ILE) (min) |
tR(LEU) (min) |
Peak resolution |
|---|---|---|---|
| Sequant® ZIC®-cHILIC | 7.3 | 7.5 | 1.28 |
| Sequant® ZIC® pHILIC | 6.7 | 7.0 | 1.08 |
| Raptor Polar X | 1.3 | 1.4 | 1.25 |
| YMC-Triart-Diol-HILIC | 7.4 | 7.5 | 0.55 |
| Analyte | Concentration range (nM) | Linearity | Precision (%) |
| Adenosine | 3.7 – 375 | 0.9920 | 5.4 |
| Arginine | 27.7 – 574 | 0.9664 | 19.2 |
| Asparagine | 7.5 – 751 | 0.9615 | 13.8 |
| Glutamate | 34 – 680 | 0.9734 | 13.5 |
| Glutamine | 68.4 – 685 | 0.9824 | 8.6 |
| Glutathione - reduced | 3.3 – 325 | 0.9820 | 15.9 |
| Histidine | 64.4 – 644 | 0.9923 | 30.5 |
| Hypoxanthine | 7.3 – 735 | 0.9857 | 8.4 |
| Isoleucine | 76.2 – 762 | 0.9690 | 8.7 |
| Leucine | 76.2 – 762 | 0.9637 | 8.2 |
| Methionine | 6.7 – 670 | 0.9718 | 29.7 |
| 5-Methylcytosine | 40 – 800 | 0.9922 | 7.6 |
| 5-Methylthioadenosine | 3.4 – 335 | 0.9978 | 10.2 |
| Phenylalanine | 6.1 – 605 | 0.9768 | 8.2 |
| Threonine | 8.4 – 840 | 0.9735 | 15.0 |
| Tryptophan | 4.9 – 490 | 0.9905 | 13.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).





