1. Introduction
The role of the human vermiform appendix is contentious. Long thought to be a vestigial organ, nascent research now suggests that the appendix is more than just an evolutionary remnant of the caecum. Rather, it is an active immunological organ made up of gut-associated lymphoid tissue and immune cells with a potential luminal reservoir[
1,
2,
3,
4,
5]. Further, the appearance of the appendix early in gestation[
6], the rarity of appendiceal malformation or absence (agenesis)[
7], and the continuous presence of the appendix in humans (and appendix-like structures in other mammals) across millennia[
1], contradicts evolutionary redundancy and suggests ongoing biological relevance.
There is growing evidence to support the concept of the colonic gut microbiota being essential for host health, with disruptions to gut ecosystem homeostasis commonly associated with diseases including colorectal cancer[
8], obesity[
9], and mental disorders[
10]. A study of extracted appendices revealed a rich appendiceal microbiota, with greater diversity than that of the colonic microbiota, including bacteria only previously found in oral cavities[
11]. Thus, it has been postulated that a function of the appendix may be to act as a microbial reservoir and to protect important gut bacteria during gastrointestinal disruption, such as infection or diarrhoeal disease[
4,
12]. Indeed, the positioning of the appendix at the ileo-caecal junction leaves it relatively protected from the faecal stream[
6,
12], further supporting the potential for it to act as a microbial ‘safe house’ protecting commensal gut bacteria.
In addition to this potential structural protective function, the appendix supports the production and maintenance of biofilms[
12]. Biofilms are abundant in the appendix and caecum, then decrease in density along the length of the large intestine, with limited to no presence in the distal colon and rectum[
12]. Biofilms comprise an extracellular matrix of host and microbial components that line portions of the gastrointestinal tract and assist with defence against pathogenic invasion[
12]. These biofilms undergo constant cycles of shedding and regeneration and are believed to release bacteria from their matrix into the intestinal lumen when required[
2,
12,
13]. Thus, it is postulated that the appendix may facilitate reinoculation of the gut microbiota after disruption through the release of biofilms into the colon, which can then adhere to the luminal surfaces and shed bacteria to assist with homeostatic regulation and colonisation resistance[
4,
12].
Most studies examining the influence of the appendix in humans have been observational and have investigated the association between appendicectomy status and disease states, such as ulcerative colitis[
14,
15,
16,
17,
18], Crohn’s disease[
14,
15,
19],
Clostridioides difficile infection[
20,
21,
22,
23], colorectal cancer[
24,
25,
26], diverticular disease[
27], ischaemic heart disease[
28], myocardial infarction[
29], rheumatoid arthritis[
30], Parkinson’s disease[
31], and mood disorders[
32]. Findings in studies of each disorder have been equivocal, with little consensus as to whether prior appendicectomy is likely protective, a risk factor, or of little relevance to disease. Additionally, years since appendicectomy and the presence of acute appendicitis appears to influence some of these associations[
17,
19,
24,
27]. One study has further examined the relationship between appendicectomy, disease outcomes, and gut microbiome composition[
33]. They observed that those with prior appendicectomy were at an increased risk of colorectal cancer, and that they had both an enrichment of colorectal cancer-promoting bacterial species, and depletion of health-associated commensals compared to those without prior appendicectomy[
33]. However, to date, there is a paucity of studies that have attempted to explore the actual mechanistic functions of the appendix as a bacterial reservoir, and whether people without an appendix have different responses to colonic disruption compared to those with an appendix is so far unknown.
Thus, the aim of this study was to examine the possible influence of prior appendicectomy on gut microbiota composition and reestablishment after induced disruption via bowel preparation and colonoscopy (hereafter referred to as the ‘procedure’). We firstly aimed to explore whether people without an appendix (i.e., with prior appendicectomy) had a different gut microbiota composition at baseline compared to those with an appendix. Secondly, we aimed to examine whether the absence of an appendix influenced the trajectory of gut microbiota reestablishment post-procedure.
2. Materials and Methods
2.1. Study Design
The data presented in this manuscript originate from the Micro-Scope study, a pre–post intervention study designed to investigate changes in depressive symptoms and gut microbiota composition following bowel preparation and colonoscopy. The findings are reported in accordance with established guidelines, including the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement for observational studies[
34] and the Strengthening the Organising and Reporting of Microbiome Studies (STORMS) checklist[
35]. Ethical approval for this study was obtained from the Barwon Health (#15-129), Epworth Healthcare (#EH2016-146), and Deakin University (#2016-391) human research ethics committees. Microbial data were not shared in an online public repository due to the absence of consent for data sharing. This study was pre-registered on the Open Science Framework (OSF):
https://osf.io/j69tk.
2.2. Study Participants and Setting
The study included community-dwelling adults referred for colonoscopy between May 2017 and November 2018 at University Hospital Geelong, Australia. Participants were recruited during their initial outpatient consultation with the General Surgery or Gastroenterology services. Any adults referred for colonoscopy during the study period were eligible for inclusion, unless they were heavily reliant on medical care or unable to provide informed consent (e.g., due to language barriers, significant intellectual or cognitive disability). No exclusions were made based on recent antibiotic usage. Eligible participants were referred to a research team member for detailed study discussions and consent. Colonoscopies were conducted at University Hospital Geelong or Epworth Hospital Geelong. Participants diagnosed with cancer post-colonoscopy were withdrawn, and their follow-up data were not included.
2.3. Data Collection
Baseline data were obtained one week before the procedure. Participants completed paper-based questionnaires and collected a fresh faecal sample in a sterile collection jar with a scoop lid at home. The sample was stored in their home freezer (−20℃) for approximately one week until it was transported on ice to a research team member on the day of their colonoscopy. Upon receipt, it was transferred to a −80℃ freezer for storage until DNA extraction. One month after the procedure, participants completed another set of questionnaires and provided a final faecal sample at home as above.
2.4. Intervention
Bowel preparation followed standard procedures prescribed by the colonoscopy service. All participants were advised to initiate a low-fibre (‘white’) diet two days before their colonoscopy, followed by a 12–24-hour period of clear fluids before the procedure. They were instructed to consume a sodium picosulfate-based bowel preparation product in three separate doses. The adequacy of bowel preparation was assessed during the colonoscopy by the endoscopist using a modified overall Boston Bowel Preparation Scale score[
36].
2.5. Outcome Measures
The primary outcome of this study was the composition of the gut bacterial microbiota. Alpha-diversity of the gut bacterial microbiota was assessed using the Shannon index—a composite measure of richness and evenness within a sample—as well as the count of taxa present at the genus level. Beta-diversity was quantified using the Aitchison distance metric, and differential taxonomic abundances at the genus level were evaluated using centred-log ratio transformed count abundance data.
2.6. Covariates
Age (in years) at the time of recruitment and sex (male/female/other) were extracted from medical records. A triage nurse recorded participant height and weight during their initial outpatient consultation, facilitating the computation of body mass index (BMI; kilograms/meter²). Participants self-reported their residential postcode and suburb, allowing for the determination of socioeconomic status via an area-based metric called the Index of Relative Socio-economic Advantage and Disadvantage (IRSAD)[
37]. Each suburb has an IRSAD classification on a scale of 1-10, where a lower score suggests greater disadvantage. Self-reported information included smoking status, lifetime medical conditions (including depression), and current medication usage. Diet quality was assessed using the Simple Dietary Questionnaire[
38], with the total score (out of 100) indicating adherence to the Australian Dietary Guidelines; higher scores denoted greater adherence[
39]. The ROME III Diagnostic Questionnaire for Adult Functional Gastrointestinal Disorders was used to establish whether participants met criteria for irritable bowel syndrome (IBS) at baseline[
40]. Depressive symptoms were assessed using the depression sub-score of the Hospital Anxiety and Depression Scale (HADS)[
41]; the severity of depressive symptoms was measured using the Patient Health Questionnaire-9 (PHQ-9) [
42]; anxiety symptoms were evaluated using the anxiety sub-score of the HADS[
41]; overall quality of life, as well as psychosocial and physical quality of life, were measured using the Assessment of Quality of Life-8 (AQOL-8D) [
43]; and stress levels were quantified using the Perceived Stress Scale (PSS) [
44]. Details on colonoscopy indications and outcomes were extracted from medical records.
2.7. DNA Extraction
Microbial DNA extraction from stool was performed using the commercial QIAamp Fast DNA Stool Mini Kit (QIAGEN, Germany) according to manufacturer instructions, with an additional mechanical lysis step using PowerBead tubes (QIAGEN, Germany). Extracted DNA was stored at −80℃ until couriered on dry ice to the Australian Genomic Research Facility (AGRF) for sequencing.
2.8. Sequencing and Annotation
Sequencing of the 16S rRNA gene sequence was performed using the Illumina MiSeq platform. The V1–V3 hypervariable region of the 16S rRNA gene was amplified by polymerase chain reaction using 27F (AGAGTTTGATCMTGGCTCAG) and 519R (GWATTACCGCGGCKGCTG) primers with a read length of 300 base pairs. Diversity profiling analysis was performed with QIIME 2 2019.7[
45]. The demultiplexed raw reads were primer trimmed and quality filtered as per AGRF protocols using the cutadapt plugin followed by denoising with DADA2 (via q2--dada2)[
46]. Taxonomy was assigned to amplicon sequence variants (ASVs) using the q2--feature--classifier[
47] classify--sklearn naïve Bayes taxonomy classifier. Taxonomy was assigned using the SILVA (v.132) database.
2.9. Pre-Processing
Pre-processing and filtering was performed in line with recommendations from Callahan et al.[
48]. Zero count bacterial features and non-bacterial taxa were removed prior to calculating the alpha-diversity metrics Shannon index and observed genera. Additional filtering removed low prevalence taxa (those present in less than 5% of samples), and data were centred-log ratio transformed to calculate Aitchison distances (i.e., beta-diversity) and for differential abundance testing.
2.10. Statistical Analyses
Statistical analyses were performed within the RStudio[
49] 4.3.1 environment. Packages used for analysis are listed within the
Supplementary Material. Only participants with baseline and follow-up data (i.e., complete cases) were included in analyses.
We aimed to explore whether people without an appendix (i.e., with prior appendicectomy) had a different gut microbiota composition at baseline or follow-up compared to those with an appendix (i.e., between-group differences). We also aimed to examine whether the absence of an appendix influenced gut microbiota reestablishment post-procedure (i.e., within-group changes. Both between-group differences and within-group changes in alpha-diversity were calculated via generalised estimating equations (GEE) assuming a Gaussian distribution with an AR(1) correlation structure to account for within participant autocorrelation. Robust standard error estimates are reported for all GEE models. Between-group differences and within-group changes in beta-diversity were calculated using permutational analysis of variance (adonis2) with 999 permutations, stratified by participant ID when appropriate considering the paired nature of the data. Differential abundance analyses at the genus level were calculated using general linear mixed models using the Maaslin2 package with minimum abundance and prevalence set to zero, and a centred–log ratio transformation. For between-group differences in genera at baseline and follow up, appendicectomy group was included as the fixed effect in the model. For estimating within-group changes in genera pre-post procedure, time point was the fixed effect, and participant identifier the random effect. The Benjamini-Hochberg procedure was applied to control the false discovery rate, with associations yielding an adjusted p-value (i.e., q-value) under 0.05 reported in results.
We also conducted additional post-hoc exploratory analyses to explore whether prior appendicectomy influenced the reestablishment of any bacteria at the genus level. These analyses were calculated using the Maaslin2 package with minimum abundance and prevalence set to zero, a centred–log ratio transformation, participant identifier as the random effect, and appendicectomy status, time point, and an appendicectomy status by time point interaction. As this was an exploratory analysis, we did not control the false discovery rate, with taxa below a p-value of 0.05 reported in results.
Given the small sample size in our study, it is important to interpret the results cautiously. Statistical significance should be considered in conjunction with effect sizes and the context of the study.
3. Results
3.1. Participant Characteristics
A total of 136 participants provided informed consent at the time of their initial outpatient appointment, of which 86 participants were successfully contacted prior to colonoscopy and provided baseline data. Twenty participants were lost to follow-up, five participants were excluded from analyses due to inadequate bowel preparation (rated as ‘poor’ by their endoscopist), two were excluded due to cancer diagnosis post-procedure, and one was excluded due to uncertainty regarding appendicectomy status. Therefore, a total of 58 participants were included in the present analyses and provided both pre- and post-procedure stool samples, with 13 participants (22%) reporting prior appendicectomy (i.e., no appendix). Baseline characteristics are presented in
Table 1 and procedural outcomes are described in
Table S1.
Those without an appendix had a lower average BMI compared to those with an appendix (p=0.007) and had lower average depression symptom scores (p=0.002), lower average depression severity scores (p=0.005), and weak evidence of lower average anxiety scores (p=0.054). Those without an appendix also reported higher average psychosocial (p=0.040), physical (p=0.014), and total (p=0.006) quality of life scores compared to those with an appendix.
There were no statistically significant differences between groups with respect to age, sex, smoking status, meeting the diagnostic criteria for irritable bowel syndrome, or average diet quality.
3.2. Between-Group Differences in Gut Microbiota Composition at Baseline and Follow Up
There was no statistical evidence of a difference in alpha-diversity between groups at baseline or follow up (Table S2). However, there was weak evidence of a difference in beta-diversity between groups at baseline (R2 = 0.02, p=0.084), and this had attenuated at follow up (R2 = 0.02, p=0.154) (Table S3).
At baseline, higher average CLR-transformed abundances of an uncultured Coriobacteriales incertae sedis genus (β = 2.67, 95%CI: 1.45, 3.88, q=0.008) and Ruminococcaceae DTU089 (β = 1.77, 95%CI: 0.81, 2.73, q=0.043) were observed in those without an appendix compared to those with an appendix, after adjusting for multiple comparisons. At follow up, there were no between-group differences in the CLR-transformed abundances of any taxa before or after adjusting for multiple comparisons.
3.3. Within-Group Changes in Gut Microbiota Composition Post-Procedure
There was statistical evidence of reduced richness one-month post-procedure compared to baseline for both groups (Table S4). The effect estimates were slightly greater in magnitude for those without an appendix (β= −155, 95%CI: −220, −89.7) than those with an appendix (β= −128, 95%CI: −166, −89.4). There was no change in either group for the Shannon index (Table S4).
In those with an appendix, there was evidence of an effect of bowel preparation and colonoscopy on beta-diversity one-month post-procedure compared to baseline (R2 = 0.004, p=0.047) (Table S5). There was stronger evidence of an effect on beta-diversity in those without an appendix (R2 = 0.01, p=0.019), however the explained variance for both was very small.
After adjusting for multiple comparisons, differential abundance analyses identified 22 genera in those with an appendix that were differentially abundant one-month post-procedure compared to baseline, including increases in the annotated genera Cutibacterium, Megamonas, Ruminococcaceae UCG-009, Gordonibacter, Megasphaera, Ruminococcaceae DTU-089, Lachnospiraceae UCG-010, and Oxalobacter (Table S6). In those without an appendix, there were 11 genera that were differentially abundant one-month post-procedure compared to baseline, including increases in the annotated genera Ruminococcaceae CAG-352, Lachnospiraceae NK4B4 group, Megamonas, Tyzzerella, Holdemanella, Mogibacterium, and Oxalobacter (Table S7).
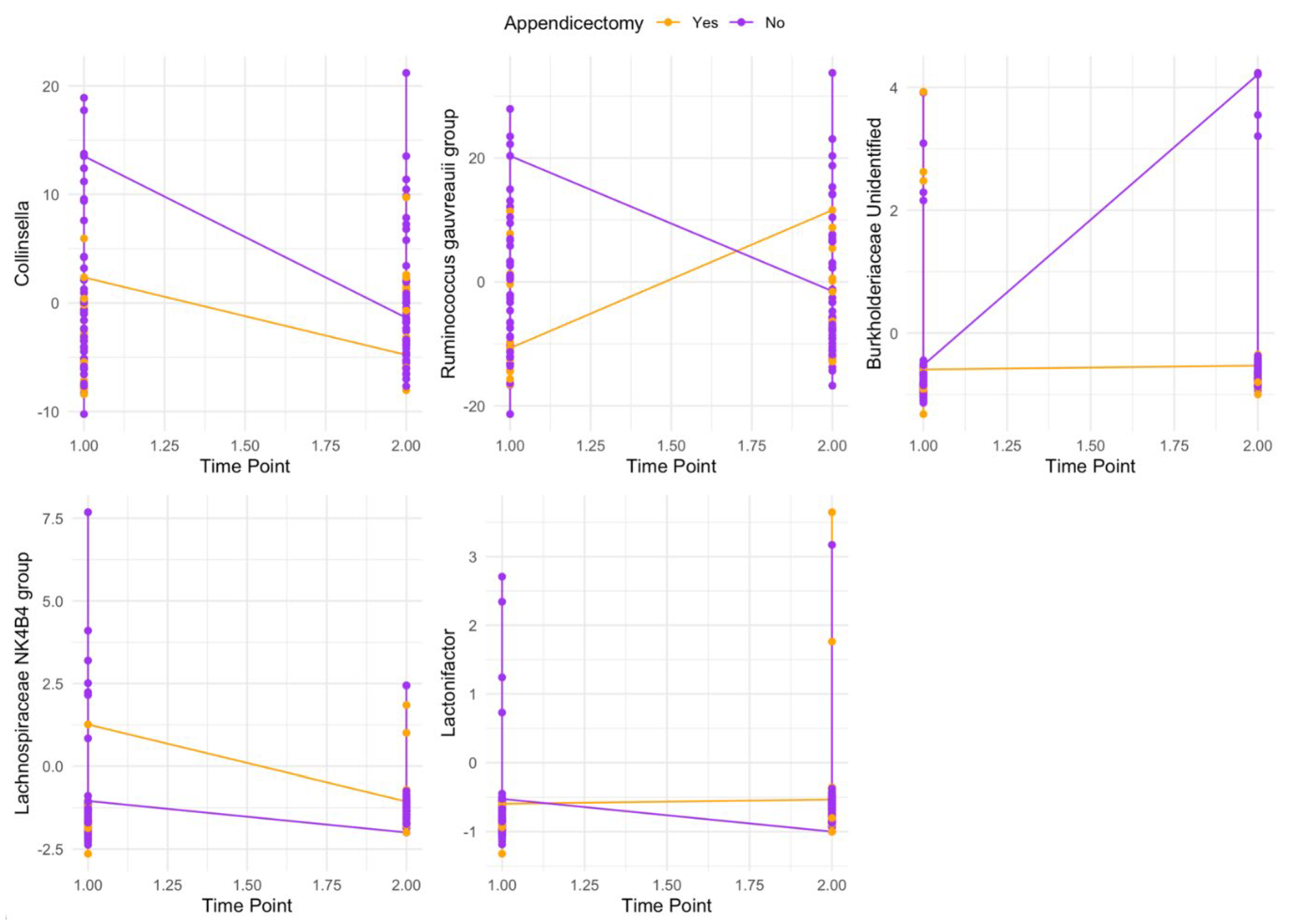
3.4. Post-Hoc Analyses of Appendicectomy as an Effect Modifier of Genus Re-Establishment
Exploratory analyses provided some evidence (not adjusting for multiple comparisons) of two-way interactions between appendicectomy status and the re-establishment of
Collinsella,
Ruminococcus gauvreauii group,
Lachnospiraceae NK4B4 group,
Lactonifactor, and an unidentified
Burkholderiaceae (
Figure 1; Table S8).
4. Discussion
This is the first study to investigate gut microbiota reestablishment in those with and without an appendix after bowel preparation and colonoscopy, aiming to explore the evolutionary survival and potential functionality of the appendix as a microbial safe house. At baseline, we observed some differences in gut microbiota composition between those with and without an appendix, however these differences were no longer apparent one-month post-procedure. We observed within-group reductions in richness (i.e., alpha diversity) and shifts in beta diversity in both groups after one month, with stronger evidence of these changes in those without an appendix. Exploratory analyses identified five bacterial genera whose re-establishment post-procedure appeared to be moderated by appendicectomy status. Although limited by small sample size and the experimental nature of the study, these data provide preliminary evidence of a potentially differential re-establishment of the colon after bowel preparation and colonoscopy in those with and without an appendix that warrants further investigation.
Our findings suggest a potentially greater impact of bowel preparation and colonoscopy on those without an appendix, including richness and beta diversity. This somewhat supports the hypothesis that the appendix may be acting as a microbial safe house for bacteria and may assist with re-establishment of the colon after perturbation. Whether the appendix may potentially contain important and/or common gut commensals, or alternatively a specific bacterial composition that may reflect an individual’s core gut microbiota, warrants further investigation. Both groups experienced changes in the CLR-transformed abundances of specific genera, however due to the difference in group size in those with and without an appendix (i.e., 45 versus 13 people, respectively), the comparison or significance of these is unclear. We did observe weak evidence that the re-establishment of five bacterial genera appeared to be moderated by appendicectomy status. Further studies with larger sample sizes powered to detect between group differences in the change in gut microbiota composition are warranted.
When comparing differences in gut microbiota composition between those with and without an appendix at baseline, there was strong evidence of higher abundances of two genera – an uncultured Coriobacteriales incertae sedis genus and
Ruminococcaceae DTU089 – in participants without an appendix. Bacteria belonging to the order Coriobacteriales are diverse, including many genera that are known to ferment glucose into lactic acid, including Atopobium, Coriobacterium, Olsenella, and Collinsella. Taxa belonging to the family
Ruminococcaceae are also frequently associated with health, as this family includes many butyrate-producing bacteria such as
Ruminococcus,
Faecalibacterium, and
Butyricicoccus. Higher
Ruminococcus,
Faecalibacterium, and
Butyricicoccus have previously been observed in studies comparing gut microbiota composition in those with and without an appendix[
50,
51], however one study observed lower Faecalibacterium in those without an appendix[
52]. Our findings suggest a potentially more beneficial composition of gut microbiota in those without an appendix, with this finding requiring replication.
We did not observe any differences in alpha diversity between those with and without an appendix. Our finding is consistent with two previous studies[
50,
51], although one other study reported lower alpha diversity in those without an appendix[
52], and another reported lower alpha diversity if the appendicectomy was less than two years prior to gut microbiota testing[
50]. We observed weak evidence of a difference in beta diversity between groups, which is consistent with two previous studies[
50,
52], but not a third[
51]. One-month post-procedure, we observed that the differences in gut microbiota between groups at baseline were no longer apparent, suggesting that bowel preparation and colonoscopy may have ‘reset’ the gut microbiota and that potential appendix-related differences in composition may take longer than one month to develop. However, the importance of these findings, or their meaning in the context of human physiology, is unclear.
Unexpectedly, those without an appendix appeared to have better health status at baseline. Participants without an appendix had statistically significantly lower body mass index than those with an appendix. Lower body mass index has been observed previously in those with prior appendicectomy compared to those without appendicectomy in China[
50], however higher body mass index was observed in those that had undergone appendicectomy in the United States[
51]. Additionally, lower depression scores and higher quality of life scores (i.e., better mental health) were observed in those without an appendix. These findings are somewhat discordant with a previous study that observed that childhood appendicectomy increased the risk of adult psychiatric disorders[
32]. There is potential reverse causation, as those that have had an appendicectomy might have higher help-seeking behaviours, which may drive higher rates of surgery and other adaptive health behaviours. However, as both body weight[
9] and mental disorders[
10] have been associated with differences in gut microbiota composition, future studies investigating the associations between appendicectomy and health outcomes are needed to clarify potential additional functions of the appendix in the context of human physiology.
There are substantial challenges to studying the appendix experimentally in humans. It has been postulated that the proposed role of the appendix to re-inoculate the gut may be of greater relevance in developing nations where sanitation is poorer and diarrhoeal diseases are more common[
1,
4,
12]. Modern hygiene practices, better diet quality, access to clean drinking water, and greater access to health care after infection, may all contribute to the apparent functional redundancy of the appendix in Western society and, in turn, make investigation of its potential role in this context difficult. Thus, our results may not be generalisable to other populations and may not be reflective of what would occur in other circumstances, such as following infection or other perturbations. An additional reason that we may not have observed pronounced differences between groups in the reestablishment of the gut microbiota after bowel preparation is the location of the appendix. As previously noted, the appendix is located proximally in the colon at the ileo-caecal junction. Biofilms are produced by the appendix, which undergo shedding and release bacteria into the colon, and these biofilms decrease in density as they move through the colon[
2,
12,
13]. Thus, it is possible that the results obtained from faecal samples in our study have not captured what is happening more proximally in the colon, which may have greater association with appendicectomy status. Future advances in sampling methodologies and studies with longer follow-up periods are required in the field of gut microbiota research to provide greater insight into what might be occurring more proximally to the appendix.
This study is the first to attempt to elucidate the mechanistic role of reinoculation of the colon by the human appendix. However, there are limitations that need to be noted. Our sample size was very small, particularly for the appendicectomy group (n=13), thus was not adequately powered to detect moderate to small differences. The present study used 16S rRNA gene sequencing, which is subject to variability in sequencing depth and typically has only genus-level resolution. As mentioned previously, our results may not be generalisable to other populations or to other forms of perturbation, such as diarrhoea, and considering the proximal location of the appendix whether the differences we observed are related to appendicectomy or some other aspect of health is unclear.
5. Conclusions
In conclusion, there was some evidence to support the appendix as an evolutionarily conserved bacterial safe house as we observed greater changes in gut microbiota composition in those without an appendix compared to those with an appendix. Those without an appendix appeared to have better mental health, lower BMI, and some differences in gut microbiota composition at baseline compared to those with an appendix. Bowel preparation and colonoscopy appeared to attenuate these gut microbiota differences, and the reestablishment of some bacterial genera were moderated by appendicectomy status. However, the importance of these findings, or their meaning in the context of human physiology or evolution, is unclear. Future studies that challenge the widely repeated chestnut that the appendix lacks any function and is evolutionarily redundant are warranted.
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on Preprints.org.
Author Contributions
Amelia McGuinness: Conceptualization (Supporting); Data curation (Lead); Formal analysis (Lead); Investigation (Equal); Methodology (Equal); Project administration (Lead); Writing – original draft (Lead); Writing – review & editing (Lead). Martin O’Hely: Data curation (Supporting); Formal analysis (Supporting); Methodology (Supporting); Writing – review & editing (Equal). Douglas Stupart: Conceptualization (Equal); Funding acquisition (Supporting); Investigation (Equal); Methodology (Equal); Project administration (Supporting); Supervision (Supporting); Writing – review & editing (Equal). David Watters: Conceptualization (Equal); Funding acquisition (Supporting); Investigation (Equal); Methodology (Equal); Project administration (Supporting); Supervision (Supporting); Writing – review & editing (Equal). Samantha Dawson: Formal analysis (Supporting); Methodology (Supporting); Writing – review & editing (Equal). Chris Hair: Conceptualization (Equal); Investigation (Equal); Methodology (Equal); Project administration (Supporting); Supervision (Supporting); Writing – review & editing (Equal). Michael Berk: Conceptualization (Supporting); Supervision (Supporting); Writing – review & editing (Equal). Mohammadreza Mohebbi: Formal analysis (Supporting); Methodology (Supporting); Supervision (Supporting); Writing – review & editing (Equal). Amy Loughman: Formal analysis (Supporting); Methodology (Supporting); Supervision (Supporting); Writing – review & editing (Equal). Glenn Guest: Conceptualization (Equal); Funding acquisition (Supporting); Investigation (Equal); Methodology (Equal); Project administration (Supporting); Supervision (Supporting); Writing – review & editing (Supporting). Felice Jacka: Conceptualization (Lead); Data curation (Supporting); Formal analysis (Supporting); Funding acquisition (Lead); Investigation (Supporting); Methodology (Equal); Project administration (Supporting); Supervision (Lead); Writing – review & editing (Equal).
Funding
This research received no external funding. AJM has received funding from Australian Rotary Health via the Australian Rotary Health/Ten Island Tassie Tag Along Tour Funding Partner PhD Scholarship. MB is supported by a NHMRC Senior Principal Research Fellowship (1156072). MB has received Grant/Research Support from the NIH, Cooperative Research Centre, Simons Autism Foundation, Cancer Council of Victoria, Stanley Medical Research Foundation, Medical Benefits Fund, National Health and Medical Research Council, Medical Research Futures Fund, Beyond Blue, Rotary Health, A2 milk company, Meat and Livestock Board, Woolworths, Avant and the Harry Windsor Foundation, has been a speaker for Abbot, Astra Zeneca, Janssen and Janssen, Lundbeck and Merck and served as a consultant to Allergan, Astra Zeneca, Bioadvantex, Bionomics, Collaborative Medicinal Development, Janssen and Janssen, Lundbeck Merck, Pfizer and Servier – all unrelated to this work. AL is supported by a Deakin Dean’s Postdoctoral Research Fellowship. AL has received grant, research or travel support from Deakin University, The University of Melbourne, RMIT University, National Health and Medical Research Council, Australian Academy of Science, The Jack Brockhoff Foundation, Epilepsy Foundation of Australia, American Epilepsy Society and has received speakers’ honoraria from European Space Agency and Swisse Australia – all unrelated to this work. MM has received Grant/research support from the NHMRC, Deakin University School of Medicine, Deakin Biostatistics Unit, Institute for Mental and Physical Health and Clinical Translation, Stroke Foundation and Medibank Health Research Fund. FNJ has received: competitive Grant/Research support from the Brain and Behaviour Research Institute, the National Health and Medical Research Council (NHMRC), Australian Rotary Health, the Geelong Medical Research Foundation, the Ian Potter Foundation, The University of Melbourne; industry support for research from Meat and Livestock Australia, Woolworths Limited, the A2 Milk Company, Be Fit Foods; philanthropic support from the Fernwood Foundation, Wilson Foundation, the JTM Foundation, the Serp Hills Foundation, the Roberts Family Foundation, the Waterloo Foundation and; travel support and speakers honoraria from Sanofi-Synthelabo, Janssen Cilag, Servier, Pfizer, Network Nutrition, Angelini Farmaceutica, Eli Lilly and Metagenics. She is on the Scientific Advisory Board of the Dauten Family Centre for Bipolar Treatment Innovation and Zoe Limited. FNJ has written two books for commercial publication. She is currently supported by an NHMRC Investigator Grant L1 (#1194982). The Food & Mood Centre has received Grant/Research support from the a2 Milk Company, Be Fit Foods, Meat and Livestock Australia, and Woolworths Limited, and philanthropic support from the Fernwood Foundation, Wilson Foundation, the JTM Foundation, the Serp Hills Foundation, the Roberts Family Foundation, and the Waterloo Foundation.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Institutional Review Board (or Ethics Committee) of Barwon Health (#15-129), Epworth Healthcare (#EH2016-146), and Deakin University (#2016-391).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The datasets presented in this article are not readily available because consent for data sharing was not obtained from participants.
Acknowledgments
We would like to thank Genevieve Moseley, Helene Nauwelaers, Elisa Pruss, Johanna Mousely, Madeline West, and Athanasia Outsikas for their assistance with data collection, and Eileen Moore for their assistance with preparing the ethics application.
Conflicts of Interest
MOH has a financial interest in Prevatex Pty Ltd., a company developing probiotic-based biotherapeutics. AL is a named inventor on a patent relating to Prevotella. FNJ has written two books for commercial publication. The remaining authors declare no conflicts of interest.
References
- Girard-Madoux, M.J.H.; Gomez de Aguero, M.; Ganal-Vonarburg, S.C.; Mooser, C.; Belz, G.T.; Macpherson, A.J.; Vivier, E. The immunological functions of the Appendix: An example of redundancy? Semin Immunol 2018, 36, 31–44. [Google Scholar] [CrossRef] [PubMed]
- Kooij, I.A.; Sahami, S.; Meijer, S.L.; Buskens, C.J.; Te Velde, A.A. The immunology of the vermiform appendix: a review of the literature. Clin Exp Immunol 2016, 186, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Vitetta, L.; Chen, J.; Clarke, S. The vermiform appendix: an immunological organ sustaining a microbiome inoculum. Clin Sci (Lond) 2019, 133, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Laurin, M.; Everett, M.L.; Parker, W. The cecal appendix: one more immune component with a function disturbed by post-industrial culture. Anat Rec (Hoboken) 2011, 294, 567–579. [Google Scholar] [CrossRef] [PubMed]
- Spencer, J.; Finn, T.; Isaacson, P.G. Gut associated lymphoid tissue: a morphological and immunocytochemical study of the human appendix. Gut 1985, 26, 672–679. [Google Scholar] [CrossRef] [PubMed]
- Schumpelick, V.; Dreuw, B.; Ophoff, K.; Prescher, A. Appendix and cecum. Embryology, anatomy, and surgical applications. Surg Clin North Am 2000, 80, 295–318. [Google Scholar] [CrossRef] [PubMed]
- Vieira, E.d.P.L.; Bonato, L.M.; Silva, G.G.P.d.; Gurgel, J.L. Congenital abnormalities and anatomical variations of the vermiform appendix and mesoappendix. Journal of Coloproctology 2019, 39, 279–287. [Google Scholar] [CrossRef]
- Wong, S.H.; Yu, J. Gut microbiota in colorectal cancer: mechanisms of action and clinical applications. Nat Rev Gastroenterol Hepatol 2019, 16, 690–704. [Google Scholar] [CrossRef]
- Pinart, M.; Dotsch, A.; Schlicht, K.; Laudes, M.; Bouwman, J.; Forslund, S.K.; Pischon, T.; Nimptsch, K. Gut Microbiome Composition in Obese and Non-Obese Persons: A Systematic Review and Meta-Analysis. Nutrients 2021, 14. [Google Scholar] [CrossRef] [PubMed]
- McGuinness, A.J.; Davis, J.A.; Dawson, S.L.; Loughman, A.; Collier, F.; O’Hely, M.; Simpson, C.A.; Green, J.; Marx, W.; Hair, C.; et al. A systematic review of gut microbiota composition in observational studies of major depressive disorder, bipolar disorder and schizophrenia. Molecular Psychiatry 2022. [Google Scholar] [CrossRef] [PubMed]
- Guinane, C.M.; Tadrous, A.; Fouhy, F.; Ryan, C.A.; Dempsey, E.M.; Murphy, B.; Andrews, E.; Cotter, P.D.; Stanton, C.; Ross, R.P. Microbial composition of human appendices from patients following appendectomy. mBio 2013, 4. [Google Scholar] [CrossRef] [PubMed]
- Randal Bollinger, R.; Barbas, A.S.; Bush, E.L.; Lin, S.S.; Parker, W. Biofilms in the large bowel suggest an apparent function of the human vermiform appendix. J Theor Biol 2007, 249, 826–831. [Google Scholar] [CrossRef] [PubMed]
- Palestrant, D.; Holzknecht, Z.E.; Collins, B.H.; Parker, W.; Miller, S.E.; Bollinger, R.R. Microbial biofilms in the gut: visualization by electron microscopy and by acridine orange staining. Ultrastruct Pathol 2004, 28, 23–27. [Google Scholar] [CrossRef] [PubMed]
- Chung, W.S.; Chung, S.; Hsu, C.Y.; Lin, C.L. Risk of Inflammatory Bowel Disease Following Appendectomy in Adulthood. Front Med (Lausanne) 2021, 8, 661752. [Google Scholar] [CrossRef] [PubMed]
- Radford-Smith, G.L.; Edwards, J.E.; Purdie, D.M.; Pandeya, N.; Watson, M.; Martin, N.G.; Green, A.; Newman, B.; Florin, T.H. Protective role of appendicectomy on onset and severity of ulcerative colitis and Crohn’s disease. Gut 2002, 51, 808–813. [Google Scholar] [CrossRef] [PubMed]
- Koutroubakis, I.E.; Vlachonikolis, I.G.; Kouroumalis, E.A. Role of appendicitis and appendectomy in the pathogenesis of ulcerative colitis: a critical review. Inflamm Bowel Dis 2002, 8, 277–286. [Google Scholar] [CrossRef] [PubMed]
- Andersson, R.E.; Olaison, G.; Tysk, C.; Ekbom, A. Appendectomy and protection against ulcerative colitis. N Engl J Med 2001, 344, 808–814. [Google Scholar] [CrossRef] [PubMed]
- Cosnes, J.; Carbonnel, F.; Beaugerie, L.; Blain, A.; Reijasse, D.; Gendre, J.P. Effects of appendicectomy on the course of ulcerative colitis. Gut 2002, 51, 803–807. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, G.G.; Pedersen, B.V.; Andersson, R.E.; Sands, B.E.; Korzenik, J.; Frisch, M. The risk of developing Crohn’s disease after an appendectomy: a population-based cohort study in Sweden and Denmark. Gut 2007, 56, 1387–1392. [Google Scholar] [CrossRef] [PubMed]
- Essrani, R.; Saturno, D.; Mehershahi, S.; Essrani, R.K.; Hossain, M.R.; Ravi, S.J.K.; Berger, A.; Mehmood, A. The Impact of Appendectomy in Clostridium difficile Infection and Length of Hospital Stay. Cureus 2020, 12, e10342. [Google Scholar] [CrossRef] [PubMed]
- Im, G.Y.; Modayil, R.J.; Lin, C.T.; Geier, S.J.; Katz, D.S.; Feuerman, M.; Grendell, J.H. The appendix may protect against Clostridium difficile recurrence. Clin Gastroenterol Hepatol 2011, 9, 1072–1077. [Google Scholar] [CrossRef] [PubMed]
- Fujii, L.; Fasolino, J.; Crowell, M.D.; DiBaise, J.K. Appendectomy and Risk of Clostridium difficile Recurrence. Infectious Diseases in Clinical Practice 2013, 21. [Google Scholar] [CrossRef]
- Merchant, R.; Mower, W.R.; Ourian, A.; Abrahamian, F.M.; Moran, G.J.; Krishnadasan, A.; Talan, D.A. Association Between Appendectomy and Clostridium difficile Infection. Journal of Clinical Medicine Research 2012, 4. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.-C.; Chen, W.T.-L.; Muo, C.-H.; Ke, T.-W.; Fang, C.-W.; Sung, F.-C. Association between Appendectomy and Subsequent Colorectal Cancer Development: An Asian Population Study. PLOS ONE 2015, 10, e0118411. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.Y.; Lee, K.Y.; Oh, S.T.; Park, S.H.; Han, K.D.; Lee, J. A link between appendectomy and gastrointestinal cancers: a large-scale population-based cohort study in Korea. Sci Rep 2020, 10, 15670. [Google Scholar] [CrossRef] [PubMed]
- Rothwell, J.A.; Mori, N.; Artaud, F.; Fournier, A.; Conte, M.; Boutron-Ruault, M.C.; Chan, S.S.M.; Gunter, M.J.; Murphy, N.; Severi, G. Colorectal cancer risk following appendectomy: a pooled analysis of three large prospective cohort studies. Cancer Commun (Lond) 2022, 42, 486–489. [Google Scholar] [CrossRef] [PubMed]
- Skoldberg, F.; Olen, O.; Ekbom, A.; Schmidt, P.T. Appendectomy and Risk of Subsequent Diverticular Disease Requiring Hospitalization: A Population-Based Case-Control Study. Dis Colon Rectum 2018, 61, 830–839. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.H.; Tsai, M.C.; Lin, H.C.; Lee, H.C.; Lee, C.Z.; Chung, S.D. Appendectomy increased the risk of ischemic heart disease. J Surg Res 2015, 199, 435–440. [Google Scholar] [CrossRef] [PubMed]
- Janszky, I.; Mukamal, K.J.; Dalman, C.; Hammar, N.; Ahnve, S. Childhood appendectomy, tonsillectomy, and risk for premature acute myocardial infarction--a nationwide population-based cohort study. Eur Heart J 2011, 32, 2290–2296. [Google Scholar] [CrossRef] [PubMed]
- Tzeng, Y.M.; Kao, L.T.; Kao, S.; Lin, H.C.; Tsai, M.C.; Lee, C.Z. An appendectomy increases the risk of rheumatoid arthritis: a five-year follow-up study. PLoS One 2015, 10, e0126816. [Google Scholar] [CrossRef] [PubMed]
- Ishizuka, M.; Shibuya, N.; Takagi, K.; Hachiya, H.; Tago, K.; Suda, K.; Aoki, T.; Kubota, K. Appendectomy Does Not Increase the Risk of Future Emergence of Parkinson’s Disease: A Meta-analysis. The American Surgeon 2021, 87, 1802–1808. [Google Scholar] [CrossRef] [PubMed]
- Ekstrom, L.D.; Ekstrom, H.; Dal, H.; Kosidou, K.; Gustafsson, U.O. Childhood appendectomy and adult mental disorders: A population-based cohort study. Depress Anxiety 2020, 37, 1108–1117. [Google Scholar] [CrossRef] [PubMed]
- Shi, F.; Liu, G.; Lin, Y.; Guo, C.l.; Han, J.; Chu, E.S.H.; Shi, C.; Li, Y.; Zhang, H.; Hu, C.; et al. Altered gut microbiome composition by appendectomy contributes to colorectal cancer. Oncogene 2023, 42, 530–540. [Google Scholar] [CrossRef] [PubMed]
- von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gotzsche, P.C.; Vandenbroucke, J.P.; Initiative, S. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement: guidelines for reporting observational studies. Int J Surg 2014, 12, 1495–1499. [Google Scholar] [CrossRef] [PubMed]
- Mirzayi, C.; Renson, A.; Genomic Standards, C.; Massive, A.; Quality Control, S.; Zohra, F.; Elsafoury, S.; Geistlinger, L.; Kasselman, L.J.; Eckenrode, K.; et al. Reporting guidelines for human microbiome research: the STORMS checklist. Nat Med 2021, 27, 1885–1892. [Google Scholar] [CrossRef] [PubMed]
- Lai, E.J.; Calderwood, A.H.; Doros, G.; Fix, O.K.; Jacobson, B.C. The Boston bowel preparation scale: a valid and reliable instrument for colonoscopy-oriented research. Gastrointest Endosc 2009, 69, 620–625. [Google Scholar] [CrossRef] [PubMed]
- Statistics, A.B.o. 2033.0.55.001 - Census of Population and Housing: Socio-Economic Indexes for Areas (SEIFA), Australia, 2016. 2018.
- Parletta, N.; Zarnowiecki, D.; Cho, J.; Wilson, A.; Bogomolova, S.; Villani, A.; Itsiopoulos, C.; Niyonsenga, T.; Blunden, S.; Meyer, B. A Mediterranean-style dietary intervention supplemented with fish oil improves diet quality and mental health in people with depression: A randomized controlled trial (HELFIMED). Nutritional neuroscience 2019, 22, 474–487. [Google Scholar] [CrossRef] [PubMed]
- Dawson, S.L.; Mohebbi, M.; Craig, J.M.; Dawson, P.; Clarke, G.; Tang, M.L.; Jacka, F.N. Targeting the perinatal diet to modulate the gut microbiota increases dietary variety and prebiotic and probiotic food intakes: results from a randomised controlled trial. Public Health Nutr 2021, 24, 1129–1141. [Google Scholar] [CrossRef] [PubMed]
- Mostafa, R. Rome III: The functional gastrointestinal disorders, third edition, 2006. World J Gastroenterol 2008, 14, 2124–2125. [Google Scholar] [CrossRef]
- Zigmond, A.S.; Snaith, R.P. The hospital anxiety and depression scale. Acta Psychiatr Scand 1983, 67, 361–370. [Google Scholar] [CrossRef]
- Kroenke, K.; Spitzer, R.L.; Williams, J.B. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med 2001, 16, 606–613. [Google Scholar] [CrossRef] [PubMed]
- Richardson, J.; Iezzi, A.; Khan, M.A.; Maxwell, A. Validity and reliability of the Assessment of Quality of Life (AQoL)-8D multi-attribute utility instrument. Patient 2014, 7, 85–96. [Google Scholar] [CrossRef] [PubMed]
- Cohen, S.; Kamarck, T.; Mermelstein, R. A global measure of perceived stress. J Health Soc Behav 1983, 24, 385–396. [Google Scholar] [CrossRef] [PubMed]
- Bolyen, E.; Rideout, J.R.; Dillon, M.R.; Bokulich, N.A.; Abnet, C.C.; Al-Ghalith, G.A.; Alexander, H.; Alm, E.J.; Arumugam, M.; Asnicar, F.; et al. Author Correction: Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat Biotechnol 2019, 37, 1091. [Google Scholar] [CrossRef] [PubMed]
- Callahan, B.J.; McMurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.; Holmes, S.P. DADA2: High-resolution sample inference from Illumina amplicon data. Nat Methods 2016, 13, 581–583. [Google Scholar] [CrossRef] [PubMed]
- Bokulich, N.A.; Dillon, M.R.; Zhang, Y.; Rideout, J.R.; Bolyen, E.; Li, H.; Albert, P.S.; Caporaso, J.G. q2-longitudinal: Longitudinal and Paired-Sample Analyses of Microbiome Data. mSystems 2018, 3. [Google Scholar] [CrossRef] [PubMed]
- Callahan, B.J.; Sankaran, K.; Fukuyama, J.A.; McMurdie, P.J.; Holmes, S.P. Bioconductor Workflow for Microbiome Data Analysis: from raw reads to community analyses. F1000Res 2016, 5, 1492. [Google Scholar] [CrossRef] [PubMed]
- RStudio Team. RStudio: Integrated Development Environment for R. RStudio, PBC, Boston, MA 2022. Available online: http://www.rstudio.com/.
- Cai, S.; Fan, Y.; Zhang, B.; Lin, J.; Yang, X.; Liu, Y.; Liu, J.; Ren, J.; Xu, H. Appendectomy Is Associated With Alteration of Human Gut Bacterial and Fungal Communities. Front Microbiol 2021, 12, 724980. [Google Scholar] [CrossRef] [PubMed]
- Goedert, J.J.; Hua, X.; Yu, G.; Shi, J. Diversity and composition of the adult fecal microbiome associated with history of cesarean birth or appendectomy: Analysis of the American Gut Project. EBioMedicine 2014, 1, 167–172. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Alcoholado, L.; Fernandez-Garcia, J.C.; Gutierrez-Repiso, C.; Bernal-Lopez, M.R.; Ocana-Wilhelmi, L.; Garcia-Fuentes, E.; Moreno-Indias, I.; Tinahones, F.J. Incidental Prophylactic Appendectomy Is Associated with a Profound Microbial Dysbiosis in the Long-Term. Microorganisms 2020, 8. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).