1. Introduction
The closely related QT interval and Heart Rate (HR) are considered, even with some controversial evidence, predictors of death [
1,
2].
The QT interval on the surface electrocardiogram (ECG) represents the time from the onset of ventricular depolarization to the end of repolarization and is mediated by ion channels, molecular structures in the myocardial cells membrane. Both QT shortening and QT prolongation correlate with a poor prognosis, being associated with potentially fatal arrhythmias such as torsade de pointes, ventricular tachyarrhythmias and sudden cardiac death [
3]. QT prolongation has two main aetiologies: congenital (i.e. inherited long-QT syndrome) [
4] and acquired. Different factors have been related to the acquired long-QT, including demographic risk factors, pollution, drugs, cardiovascular risk factors, electrolyte disorders, stroke, altered autonomic tone and structural heart diseases [
5,
6,
7,
8,
9]. The estimation of the QT interval on surface ECG may be difficult, as the beginning of the QRS complex and/or the end of the T wave may not be easy to identify but the digitalization of ECG traces could help clinicians. Moreover, the QT interval changes, according to HR, increasing at low HR and decreasing at high HR. To normalize such variability, many methods, currently available in clinical practice, have been devised to correct the QT interval according to HR (QTc) [
10,
11].
Conversely, HR is a simple, inexpensive and easily measurable biological parameter. Similar to the QT interval, HR is influenced by many conditions such as genetic, cardiovascular risk factors and cardiovascular diseases, infections, inflammatory diseases, drug use, physical activity and sympathetic and vagal imbalance [
12,
13,
14,
15]. Also HR has been described as a relevant predictor of all-cause and cardiovascular death, despite it is often overlooked [
2].
In practice the demand for ECG reporting for QTc is growing exponentially. This study would like to assess whether the simple evaluation of HR, the main correction parameter of QT interval, had a prognostic value comparable with QTc according to Bazett, Fridericia and Framingham methods in a large, non-selected, primary, adult, European population subdivided into age-groups.
2. Material and Methods
The study was performed according to the Ethical Standards of the 1975 Helsinki Declaration revised in 2013 and was approved by the local Ethic Committee of Modena (AVEN), protocol number 2605/2021, date of approval 2021, September, 21. Due to the anonymous and observational nature of the study, informed consent could not be obtained from the enrolled patients.
Study Population: the metropolitan area of Modena, located in south-central Emilia-Romagna, Italy, has a population of 702,635 people (
www.provincia.modena.it, last accessed 2022, October 1st) spread across 47 municipalities. National health general practitioners refer their patients to National Health System core facilities for clinical tests. ECGs are recorded in the Emergency Departments, in Hospitals (surgical and medical units, day-hospital, day-services, pre-operative screening), and in- and out-of-hospital patients clinics.
Patients with a digitized ECG archived in any facility in the metropolitan area of Modena from January 2008 to October 2022 were eligible.
Cardiovascular risk factors in the resident population were: diabetes (the group of metabolic disorders characterized by hyperglycemia resulting from defects in insulin secretion or action or both), systemic arterial hypertension (the increase of systolic blood pressure above 140 mmHg and/or diastolic blood pressure above 90 mmHg), dyslipidemia (a disorder in lipoprotein metabolism resulting in elevation of the blood concentration of cholesterol and/or triglycerides) and tobacco smoke (the active exposure to tobacco products).
The main cardiovascular diseases in the resident population were: heart failure (the syndrome with symptoms and or signs caused by structural and/or functional cardiac abnormalities), coronary artery diseases (the group of diseases characterized by the reduction of blood flow into the heart muscle causing the partial or complete blockage of the coronary flow) and stroke (a neurological deficit due to a cerebrovascular cause).
The main comorbidities in the resident population were: chronic obstructive pulmonary disease (the chronic inflammatory lung disease obstructing airflow), dementia (the chronic deterioration of cognitive function not expected from the consequences of biological ageing), cancer (the large group of diseases starting in any tissue of the body when abnormal cells grow uncontrollably and can invade other organs) and chronic kidney disease (a kidney damage or glomerular filtration rate lower than 60 mL/min/1.73m2 for more than three months).
The prevalence of CV risk factors in the resident population was: diabetes 5.7%; systemic arterial hypertension 23.1%; dyslipidemia 38.8%; tobacco smoke 17.1%; that of the main CV diseases and comorbidities: CV diseases 7.9%; cerebrovascular diseases 1.5%; chronic obstructive pulmonary diseases 7.5%; dementia 3.1%; cancer 5.1%; chronic kidney disease 1.2%.
Age and sex were also anonymously collected.
Electrocardiography: ECGs were recorded at rest in the supine position using a standard 12-lead tracing at 25-mm/s speed, 10mm/mV amplitude, with a sampling rate of at least 500 Hz and were archived into a “MUSEⓇ” electronic archive (GE Marquette Medical System, Milwaukee, Wisconsin, USA). Automated analyses were performed through a digitized multi-channel computer-assisted program (GE 12SL ECG Analysis), a healthcare system that uses validated algorithms. ECG diagnoses were supervised and confirmed by trained cardiologists.
ECGs were discarded when turned out to be incomplete or when they had technical problems such as a bad signal quality (causing failure in the evaluation of ECG parameters), waveform recognition errors and electrode interchanges or in the presence of a pacemaker or implantable cardioverter-defibrillator. ECGs were also discarded in patients suffering from atrial fibrillation or atrial flutter, supraventricular or ventricular tachycardia, Wollf-Parkinson-White syndrome, second- or third-degree A-V block, with complete or incomplete left or right bundle-branch block, with a QRS duration greater than 140 msec, with a QTc duration greater than 650 msec or lower than 280 msec due to artifacts and in the presence of more than three premature beats.
In the case of patients with multiple ECGs archived in the dataset, only the first was used for the present study.
HR was automatically calculated from the stored ECGs and QTc was digitally measured utilizing Bazett, Fridericia and Framingham correction from the mean of the QT interval of the 12 ECG leads.
The quality control on the ECGs consisted of two steps: the first was provided by the GE healthcare analysis system and the second by the validation made by clinicians.
Other inclusion/exclusion criteria: young patients (up to 25 years of age) and very old people (more than 85 years of age) were excluded. Since the whole population was not homogeneous, the enrolled patients were also divided into three age-groups: group 1 was composed of subjects with age from 25 to 45 years of age, group 2 was composed of subjects from 46 to 65 years, and group 3 was composed of subjects with age from 66 to 85.
Follow-up: all-cause mortality and emigrations were retrospectively evaluated through an anonymous numeric personal identification code utilizing electronic medical records of the Health Authority and Services of the Province of Modena.
All emigrated people were excluded from the follow-up.
Statystical analysis: Continuous variables are displayed as mean ± standard deviation, while categorical data are displayed as frequencies.
T-test was used to compare means for clinical and ECG data to check the null hypothesis that the average of two subpopulations were different. The three mean values of the different QTc formulas were compared using one-way ANOVA analysis, followed by pairwise t-test comparisons. Linear regression was used to test the independence of the QTc values from the corresponding R wave to R wave interval (RR interval = 60/HR in milliseconds).
To establish the optimal operating point the Receiver Operating Characteristic (ROC) curves for QTc methods and for HR were constructed.
The survival analysis was performed for the end point of all-cause mortality at follow-up. Rates of mortality were computed for the individuals whose QTc and HR values were, respectively, below the 25th percentile and above the 75th percentile in the whole population and in the three age-groups. Cumulative endpoint event rates were analyzed through the Kaplan-Meier event-free survival method.
In the whole population, according to the three age-groups and stratifying for sex, a forward Cox proportional hazard model was used to estimate the relative risk, denoted Exp B, and to assess the performance of a multivariable model for HR and for the three considered QTc methods.
All the statistical tests with a p-value <0.05 were considered significant and the confidence intervals were taken at level 95%.
3. Results
From January 2008 to October 2022, in the National Health System facilities of Modena, 375.207 ECGs were archived in the GE Marquette Healthcare Analysis Program. From these ECGs, after exclusions, 131.627 subjects were enrolled.
Table 1 shows the clinical and ECG characteristics of the enrolled patients and exclusion criteria. Females had longer QTc values and higher HR with respect to males while males had an older mean age and higher rates of mortality with respect to females. In the entire population and in the three facilities Bazett’s QTc values were longer than those obtained with other correction methods.
Table 2 shows ANOVA analysis between the three mean QTc values and pairwise t-test comparison between QTc and RR interval revealing different R values [
2].
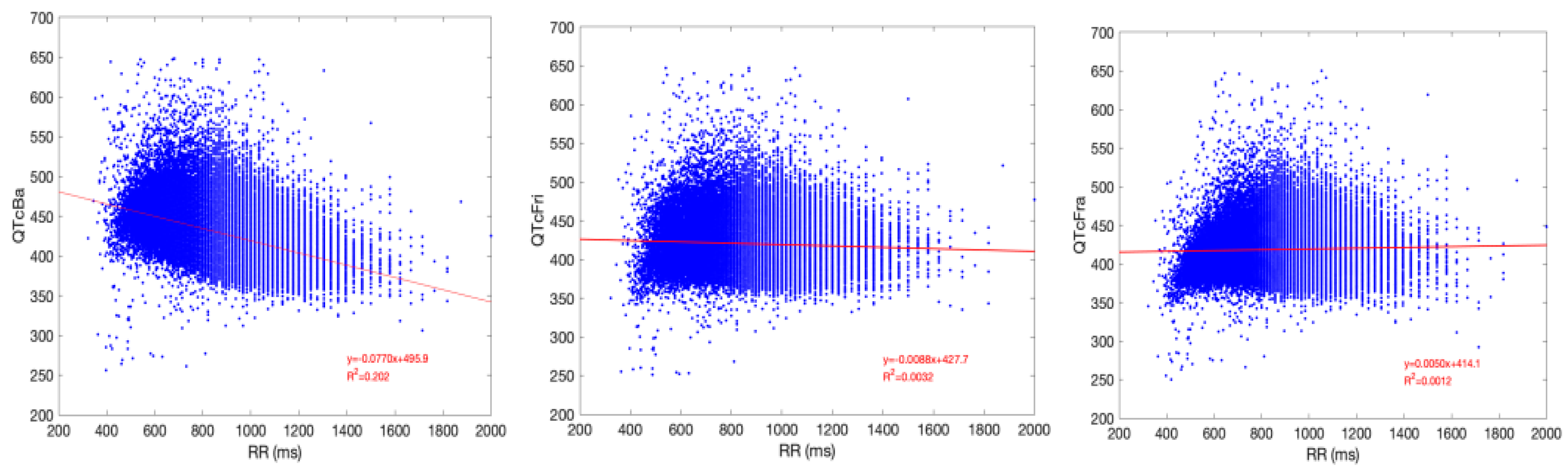
Figure 1 represents linear regression scatterplots between QTc methods and RR interval. Framingham’s correction had greater independence from RR with respect to the other methods.
During the follow-up period (mean follow-up 1641.4 days) 11.727 subjects (8.9%) had died of all-cause mortality (6370 men and 5357 women, see
Table 1).
Table 3 shows rates of mortality for the three QTc methods and for HR according to the 25
th and the 75
th percentile in the whole population and in the three age-groups. In the whole population, the increase of relative risk of mortality (Exp B) for QTc methods and for HR was statistically significant over the 75
th percentile, with Bazett’s correction performing better. Instead, in each of the three age-groups, the relative risk of mortality (Exp B) over the 75
th percentile was significantly greater for HR and for Bazett’s method.
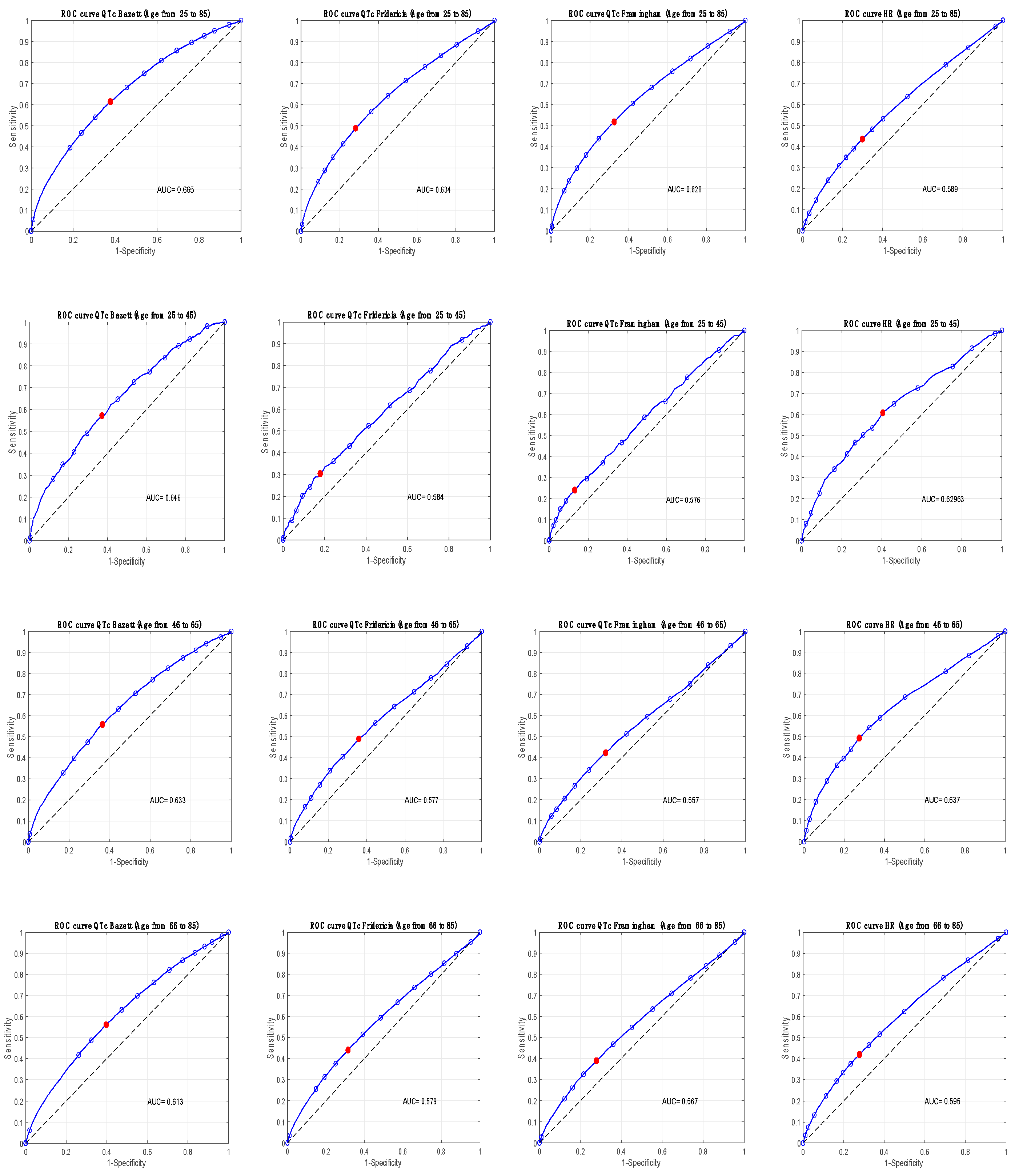
Figure 2 shows ROC curves for the considered ECG parameters in the whole population and in the three age-groups; the optimal operating points were superimposable to the 75
th percentile.
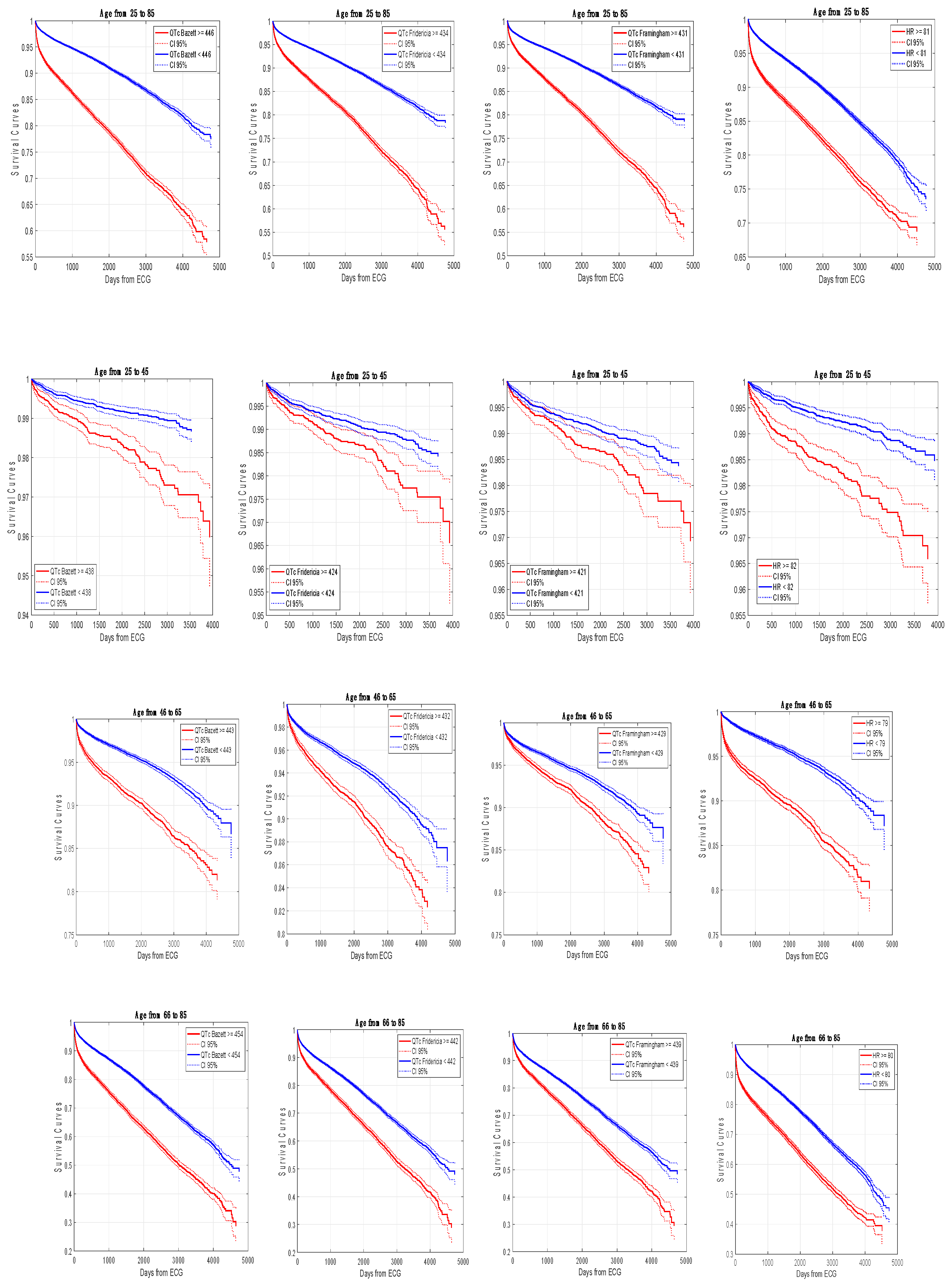
Figure 3 shows Kaplan-Meier event-free survival curves in the whole population and in the three age-groups according to the 75
th percentile/optimal operating points revealing the same significant trends.
Table 4 shows the multivariable forward Cox proportional hazard model, in the whole population and in the three age-groups, stratifying for sex, revealing the greater relative risk of all-cause mortality for Bazett’s correction and especially for HR in the entire population and in the three age-groups.
4. Discussion
In this large non-selected adult population, the three considered QT correction methods (Bazett, Fridericia, Framingham) were statistically associated with all-cause mortality, mainly in the whole population. However, quite unexpectedly, HR had the same or a greater correlation with all-cause mortality than any QTc method especially in younger subjects (groups 1 and 2).
For many years, clinicians corrected the QT interval based on HR and researched the best QTc methods: therefore, it was somehow surprising that the simple evaluation of HR performed equally or, sometimes, even better than QTc.
Previous studies not compared prognostically HR and QTc but the simple evaluation of HR could provide an additional prognostic value and running many ECGs for QTc estimation would reduce its meaning.
Many studies already detected the association between QTc and mortality, sometimes with controversial results: none identified the most predictive correction method but some of them revealed the worse performance of Bazett’s method [
16]. Vandemberk and coll. in 6.609 hospitalized patients observed that five QTc methods were related to mortality, but Bazett’s correction had the worse performance; moreover, in multivariable analysis, age, HR and QTc were independent predictors of mortality and once more Bazett's correction performed worst [
17].
Bazett’s method is the oldest proposed for QT correction, being studied in 1920. It divides the QT interval by the square root of the RR interval and it’s commonly used in clinical practice despite the known over-correction at fast HR and the under-correction at slow HR; so, it should not be used for patients utilizing HR modifying drugs [
18,
19].
In our study Bazett’s correction confirmed a lower independence from HR but a stronger correlation with all-cause mortality with respect to Fridericia and Framingham methods in each age-group. These results could not be easily explained, but the overestimation of QTc through Bazett’s method comprised a greater number of patients over the 75th percentile: the strength of Bazett’s method could be represented by the overestimation of the QTc, especially with HR increase.
Conversely, in a digital ECG re-examination of the Framingham population, Noseworthy and coll. observed that Bazett’s method had a greater correlation with all-cause and cardiovascular mortality than other QTc methods, but the association was attenuated after adjustment for cardiovascular risk factors [
20]. The authors suggested that QTc contributed weakly to mortality, but reported an incremental risk with increasing QTc. Also Yazdanpanah and coll. recently observed that amongst five different QTc methods, Bazett’s formula had the best correlation with cardiovascular mortality in 7071 non-hospitalised Iranian patients [
21].
Our study confirms the results of Noseworthy and Yazdanpanah and the better prognostic value of Bazett’s correction, in a twentyfold larger population that included hospitalized and non-hospitalized patients. Therefore, the first practical message from our study is that clinicians should pay attention to QTc values greater than 440 msec in young subjects and greater than 455 msec in old subjects (over the 75
th percentile in age-groups 1 and 3, see
Table 3) remembering that young females have longer QTc values with respect to males [
22]. In this population-study mortality increased already from lower QTc values when compared to the QTc reference values [
20,
23].
Other relevant findings concern HR. Meanwhile, QTc and HR are obviously related and have different trends with increasing age: QTc has a linear increase while HR has smaller and not-linear changes [
22].
HR is linked to genetics, lifestyle factors such as physical activity, diet, stress, and quality of sleep and is influenced by many confounding factors such as cardiovascular diseases, infections, anemia, thyroid function, and by the use of HR modifying drugs [
12,
13,
14,
15]. The increase of HR enhances oxygen consumption, imbalances the parasympathetic and sympathetic activity, provokes a faster progression of coronary diseases, reduces left ventricular function, increases the risk of ventricular arrhythmias and increases inflammatory markers [
2,
24]. Despite all this evidence, HR evaluation is not recommended in clinical guidelines for risk stratification.
In a recent meta-analysis Zhang and coll. observed that an increase of resting HR greater than 10 beats per minute (bpm) increased the risk of all-cause and cardiovascular mortality respectively by 9% and 8 %, already from normal HR values [
25]. Likewise, Ristow and coll. observed an increased risk of all-cause mortality for a within-person increase of HR greater than 2.8 bpm [
26], while Aune and coll. reported an association between an at rest HR increase greater than 10 bpm with cardiovascular mortality, stroke, all-cause death, and cancer [
27].
In general population studies, a resting HR higher than 90 bpm was described as potentially harmful and the probability of reaching age 85 was 40% lower in subjects with HR greater than 80 bpm compared with subjects with HR lower than 60 bpm [
28,
29].
In conclusion, there is a large body of evidence showing that HR is an independent predictor of mortality. Therefore, the second practical message from our study is that the simple evaluation of HR may be equally or more important than QTc evaluation, and clinicians should consider as potentially harmful a resting HR higher than 81 bpm (75
th percentile of the whole population and of the three age-groups, see
Table 3). It should be considered that a resting HR of 81 bpm is currently retained in the upper range of normality.
Despite this evidence, no human prospective studies demonstrated the efficacy, the risk-benefit ratio, or the cost-effectiveness of HR lowering treatment in a general population even though, in patients with cardiovascular diseases (i.e. heart failure and coronary heart diseases), the beneficial effects of lowering HR on symptoms and prognosis are well known.
Anyway, the precise definition of reference values for HR and for QTc is very difficult due to the molteplicity of confounding factors. Probably, reference values should be further addressed and will be reconsidered when more research data will be available. In our population we observed that a resting HR higher than 81 bpm and QTc values longer than 455 msec should be focused, as they have a higher overall risk of mortality and clinicians should reduce the use of QTc modifying drugs already from borderline values.
Due to its anonymous nature and to the privacy policies applied by our institutions, this work has the following limitations: the precise prevalence of cardiovascular risk factors, cardiovascular diseases and comorbidities, the unknown prevalence of patients utilizing QTc modifying drugs, HR modifying drugs and the unknown exposure to environmental factors. For the same reason, we could not exactly collect the appearance of MACES and the specific causes of death.
5. Conclusions
The results of this study are intriguing and could be useful in clinical practice, as they were obtained in a much larger real-world population with respect to previous studies. First of all, we observed that Bazett’s QT correction better predicts all-cause mortality with respect to other methods despite its lower independence from HR. QTc values greater than 440 msec in young subjects and greater than 455 msec in old subjects should be considered potentially harmful. The evaluation of HR at rest had the same or a greater prognostic value with respect to the QTc. HR is very simple to assess and clinicians should improve its evaluation with an alert for resting HR above 81 bpm. However, both QTc and HR have great variability and depend from many confounding factors, so it’s very difficult to define normal and pathological values but ECG is increasingly becoming a prognostic tool more than a diagnostic test.
Author Contributions
Conceptualization, P.G., C.V., E.T., G.S. and C.G.; Methodology, P.G., C.V., S.R., G.S. and C.G.; Software, C.V., F.S. and C.G.; Validation, C.V. and C.G.; Formal Analysis, P.G., C.V., E.T., G.S., F.S. and C.G.; Investigation, P.G., C.V., S.R. and C.G.; Resources, P.G.,E.T., G.S. and F.S.; Data Curation, C.V. and F.S.; Writing - original draft preparation, P.G., S.R., E.T. and G.S.; Writing - Review & Editing, P.G., C.V., S.R., E.T., G.S, F.S. and C.G.; Visualization, P.G., S.R., and G.S., Supervision, P.G., C.V. and C.G.; Project administration, P.G., C.V., and F.S.; Funding acquisition none.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki, and was approved by the local Ethic Committee of Modena (AVEN), protocol number 2605/2021, date of approval 2021, September, 21.
Informed Consent Statement
Due to the anonymous and observational nature of the study the informed consent could not be obtained from the enrolled patients.
Data Availability Statement
The data underlying this article could be shared on request to the corresponding author with the permission of the Health Authority and Services of Modena.
Declaration of Generative AI and AI-Assisted Technologies in the Writing Process
No generative AI or AI-assisted technologies were used in manuscript elaboration and in the writing process.
Conflicts of Interest
The authors declare non conflicts of interest.
References
- Montanez A: Ruskin JN, Hebert PR, Lamas GA, Hennekens CH. Prolonged QTc interval and risks of total and cardiovascular mortality and sudden death in the general population: a review and qualitative overview of the prospective cohort studies. Arch Intern Med 2004;164:943–8. [CrossRef]
- Arnold JM, Fitchett DH, Howlett JG, Lonn EM, Tardif J-C. Resting heart rate: a modifiable prognostic indicator of cardiovascular risk and outcomes? Can J Cardiol 2008;24 Suppl A:3A-8A. [CrossRef]
- Nielsen JB, Graff C, Rasmussen PV, Pietersen A, Lind B, Olesen MS, et al. Risk prediction of cardiovascular death based on the QTc interval: evaluating age and gender differences in a large primary care population. Eur Heart J 2014;35:1335–44. [CrossRef]
- Priori SG, Napolitano C, Schwartz PJ. Low penetrance in the long-QT syndrome: clinical impact. Circulation 1999;99:529–33. [CrossRef]
- Vink AS, Clur S-AB, Wilde AAM, Blom NA. Effect of age and gender on the QTc-interval in healthy individuals and patients with long-QT syndrome. Trends Cardiovasc Med 2018;28:64–75. [CrossRef]
- Mehta AJ, Kloog I, Zanobetti A, Coull BA, Sparrow D, Vokonas P, et al. Associations between Changes in City and Address Specific Temperature and QT Interval - The VA Normative Aging Study. PLoS ONE 2014;9:e106258. [CrossRef]
- Al-Khatib SM, LaPointe NMA, Kramer JM, Califf RM. What clinicians should know about the QT interval. JAMA 2003;289:2120–7. [CrossRef]
- El-Sherif N, Turitto G. Electrolyte disorders and arrhythmogenesis. Cardiol J 2011;18:233–45. [CrossRef]
- Shah SR, Park K, Alweis R. Long QT Syndrome: A Comprehensive Review of the Literature and Current Evidence. Curr Probl Cardiol 2019;44:92–106. [CrossRef]
- Malik M. Problems of Heart Rate Correction in Assessment of Drug-Induced QT Interval Prolongation. J Cardiovasc Electrophysiol 2001;12:411–20. [CrossRef]
- Strohmer B, Schernthanere C, Paulweber B, Pichler M. Gender-specific comparison of five QT correction formulae in middle-aged participants in an atherosclerosis prevention program. Med Sci Monit Int Med J Exp Clin Res 2007;13:CR165-171.
- Rogowski O, Shapira I, Shirom A, Melamed S, Toker S, Berliner S. Heart rate and microinflammation in men: a relevant atherothrombotic link. Heart 2007;93:940–4. [CrossRef]
- Larsen A-I. The pulse; from adagio to prestissimo; the prognostic importance of heart rate increase and its associations with cardiovascular risk factors. Eur J Prev Cardiol 2020;27:520–5. [CrossRef]
- Hermansen R, Jacobsen BK, Løchen M-L, Morseth B. Leisure time and occupational physical activity, resting heart rate and mortality in the Arctic region of Norway: The Finnmark Study. Eur J Prev Cardiol 2019;26:1636–44. [CrossRef]
- Jensen MT, Suadicani P, Hein HO, Gyntelberg F. Elevated resting heart rate, physical fitness and all-cause mortality: a 16-year follow-up in the Copenhagen Male Study. Heart Br Card Soc 2013;99:882–7. [CrossRef]
- Chiladakis J, Kalogeropoulos A, Arvanitis P, Koutsogiannis N, Zagli F, Alexopoulos D. Heart rate-dependence of QTc intervals assessed by different correction methods in patients with normal or prolonged repolarization. Pacing Clin Electrophysiol PACE 2010;33:553–60. [CrossRef]
- Vandenberk B, Vandael E, Robyns T, Vandenberghe J, Garweg C, Foulon V, et al. Which QT Correction Formulae to Use for QT Monitoring? J Am Heart Assoc 2016;5:e003264. [CrossRef]
- Desai M, Li L, Desta Z, Malik M, Flockhart D. Variability of heart rate correction methods for the QT interval. Br J Clin Pharmacol 2003;55:511–7. [CrossRef]
- Malik M. The imprecision in heart rate correction may lead to artificial observations of drug induced QT interval changes. Pacing Clin Electrophysiol PACE 2002;25:209–16. [CrossRef]
- Noseworthy PA, Peloso GM, Hwang S-J, Larson MG, Levy D, O’Donnell CJ, et al. QT interval and long-term mortality risk in the Framingham Heart Study. Ann Noninvasive Electrocardiol Off J Int Soc Holter Noninvasive Electrocardiol Inc 2012;17:340–8. [CrossRef]
- Yazdanpanah MH, Naghizadeh MM, Sayyadipoor S, Farjam M. The best QT correction formula in a non-hospitalized population: the Fasa PERSIAN cohort study. BMC Cardiovasc Disord 2022;22:52. [CrossRef]
- Giovanardi P, Vernia C, Tincani E, Giberti C, Silipo F, Fabbo A. Combined Effects of Age and Comorbidities on Electrocardiographic Parameters in a Large Non-Selected Population. J Clin Med 2022;11:3737. [CrossRef]
- Rautaharju PM, Surawicz B, Gettes LS. AHA/ACCF/HRS Recommendations for the Standardization and Interpretation of the Electrocardiogram. Circulation 2009;119:e241–50. [CrossRef]
- Palatini P, Benetos A, Grassi G, Julius S, Kjeldsen SE, Mancia G, et al. Identification and management of the hypertensive patient with elevated heart rate: statement of a European Society of Hypertension Consensus Meeting. J Hypertens 2006;24:603–10. [CrossRef]
- Zhang D, Shen X, Qi X. Resting heart rate and all-cause and cardiovascular mortality in the general population: a meta-analysis. CMAJ Can Med Assoc J J Assoc Medicale Can 2016;188:E53–63. [CrossRef]
- Ristow B, Doubell A, Derman W, Heine M. Change in resting heart rate and risk for all-cause mortality. Eur J Prev Cardiol 2022;29:e249–54. [CrossRef]
- Aune D, Sen A, ó’Hartaigh B, Janszky I, Romundstad PR, Tonstad S, et al. Resting heart rate and the risk of cardiovascular disease, total cancer, and all-cause mortality - A systematic review and dose-response meta-analysis of prospective studies. Nutr Metab Cardiovasc Dis NMCD 2017;27:504–17. [CrossRef]
- Conroy RM, Pyörälä K, Fitzgerald AP, Sans S, Menotti A, De Backer G, et al. Estimation of ten-year risk of fatal cardiovascular disease in Europe: the SCORE project. Eur Heart J 2003;24:987–1003. [CrossRef]
- Chen X, Barywani SB, Hansson P-O, Thunström EÖ, Rosengren A, Ergatoudes C, et al. Impact of changes in heart rate with age on all-cause death and cardiovascular events in 50-year-old men from the general population. Open Heart 2019;6:e000856. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).