Submitted:
11 April 2024
Posted:
12 April 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
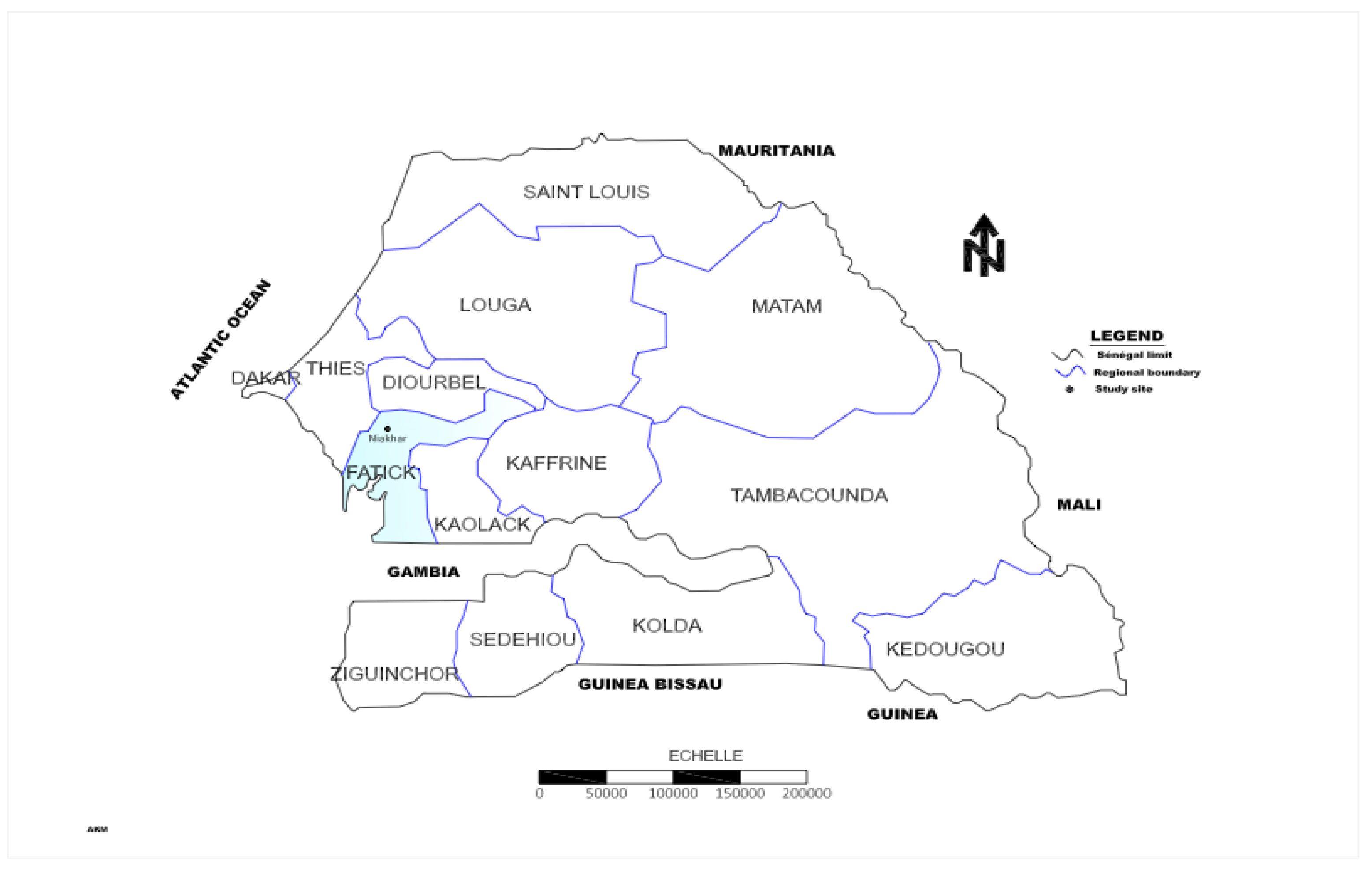
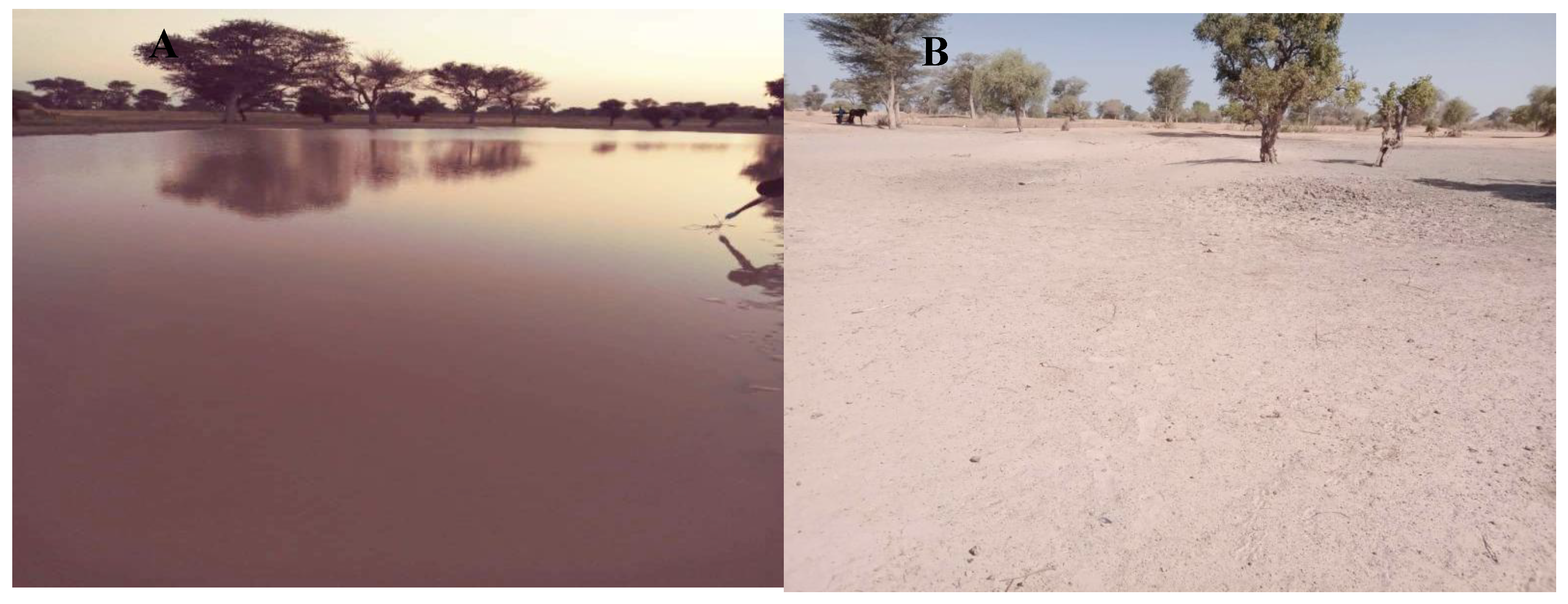
Study Area and Ponds
2.2. Bulinus Snail Collection and Testing for Schistosoma haematobium Infection
- Dry season
- Rainy season
- Cercarial shedding test
- Molecular analysis
2.3. DNA Extraction
2.4. Real-Time PCR (RT-PCR)
2.5. Rapid Diagnostic PCR (RD-PCR) to Detect S. haematobium
- Evaluation of the Bulinus survival to drought under semi experimental conditions
2.6. Statistical Analysis
3. Results
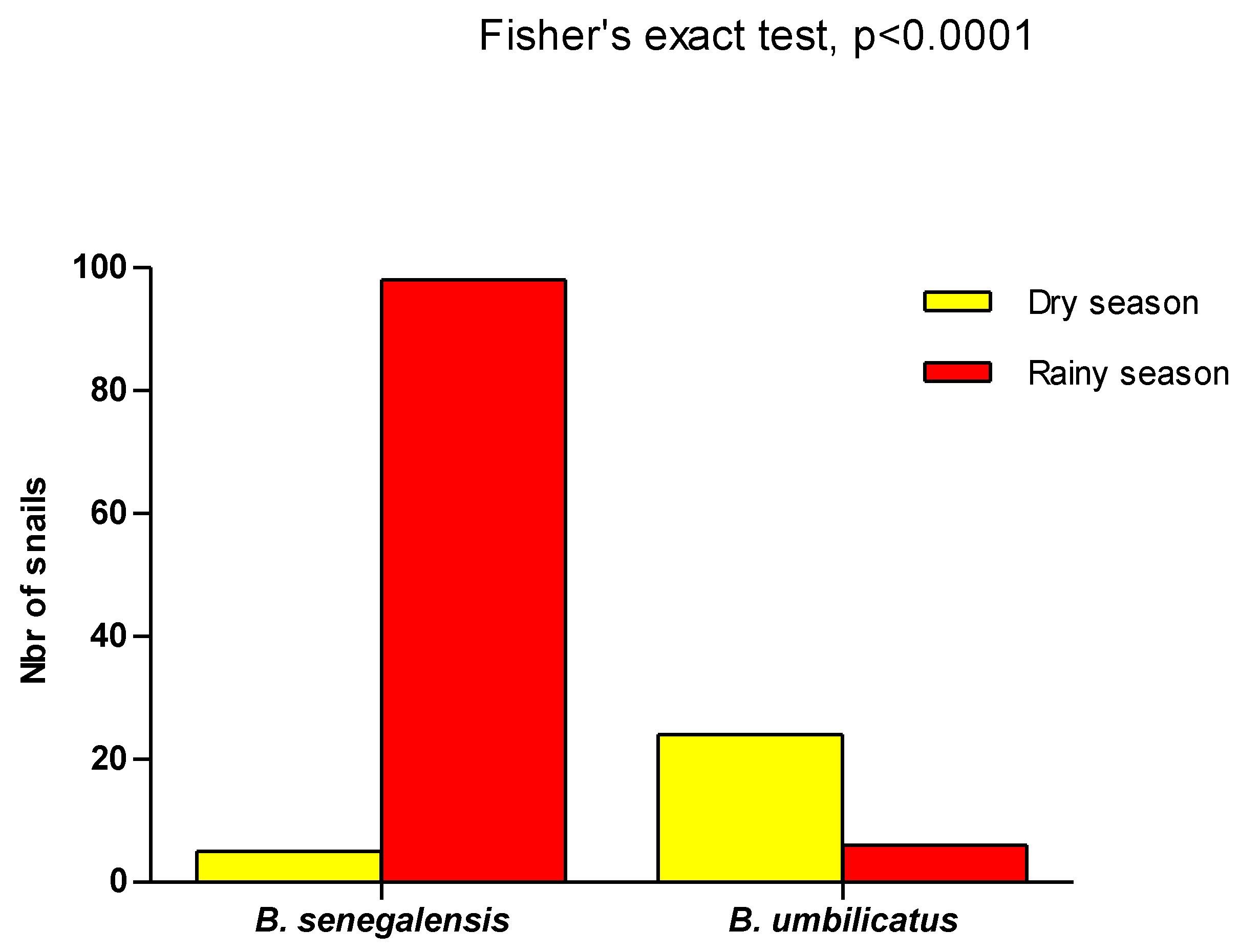
3.1. Snail Species Collected and Infestation Rates
- Dry season
- Rainy season
3.2. Snail Infestation Rates
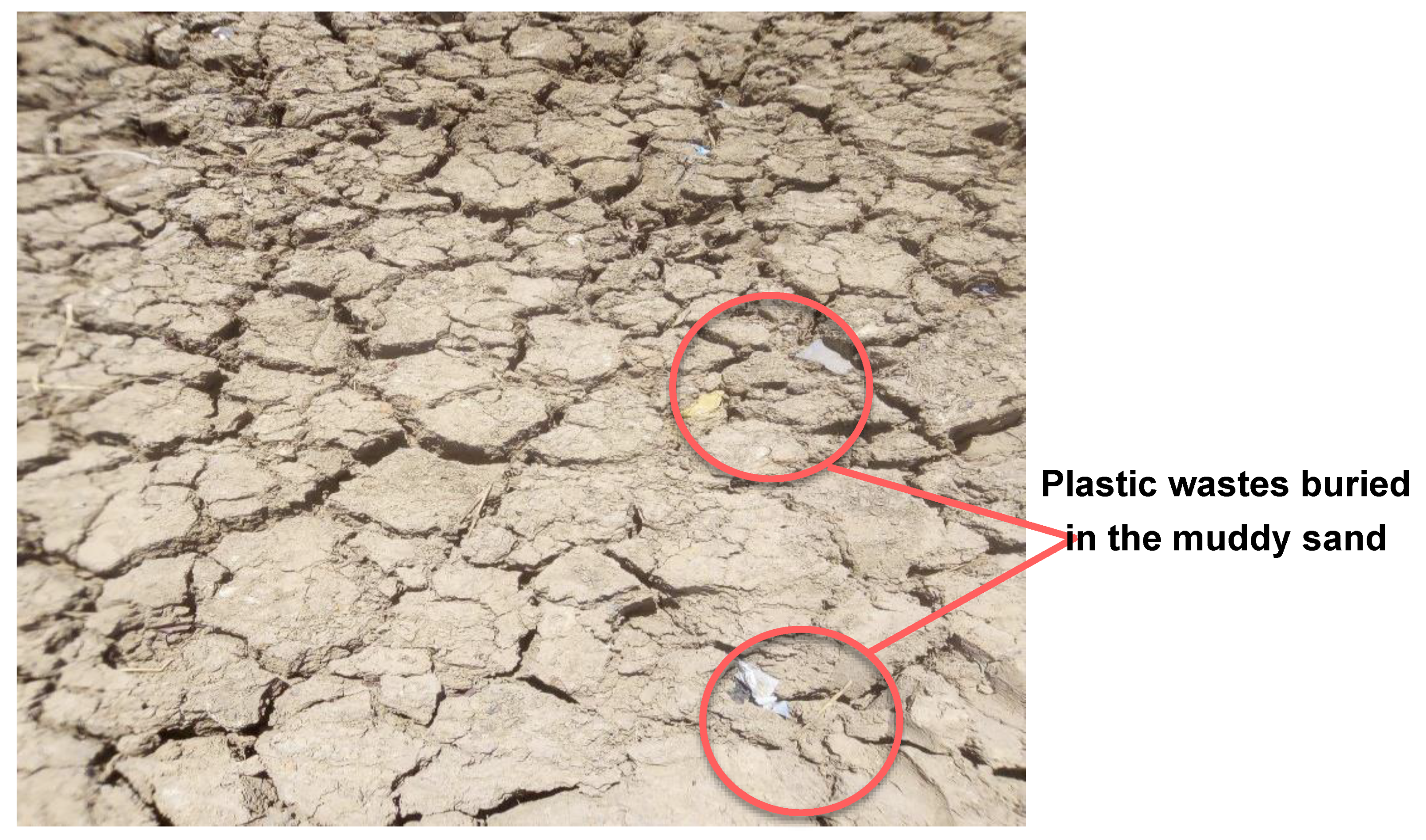
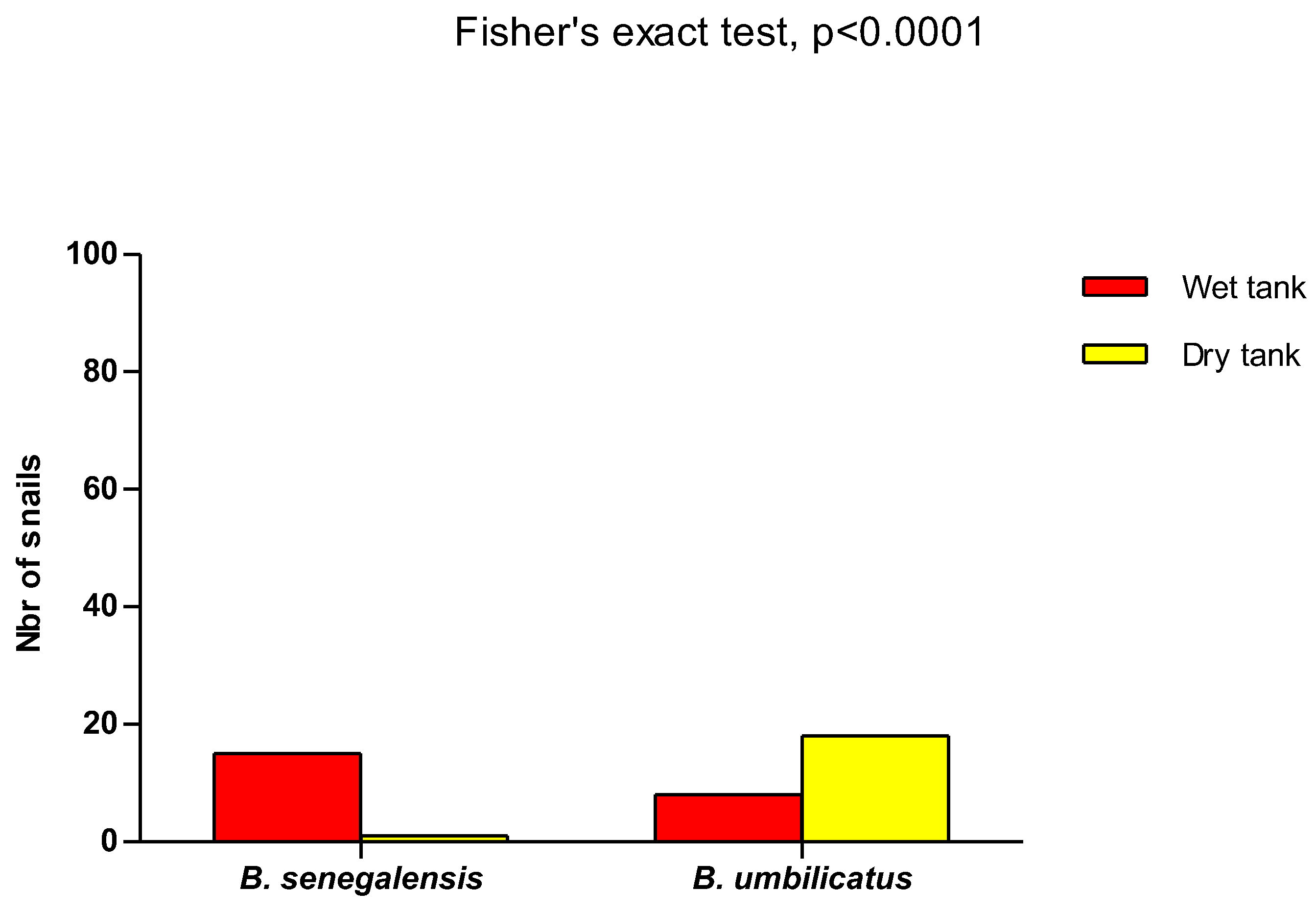
3.3. Evaluation of the Bulinus Survival to Drought under Semi Experimental Conditions
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pennance, T.; Archer, J.; Lugli, E.B.; Rostron, P.; Llanwarne, F.; Ali, S.M.; Amour, A.K.; Suleiman, K.R.; Li, S.; Rollinson, D.; et al. Development of a Molecular Snail Xenomonitoring Assay to Detect Schistosoma Haematobium and Schistosoma Bovis Infections in Their Bulinus Snail Hosts. Molecules 2020, 25, 4011. [Google Scholar] [CrossRef]
- World Health Organization. Available online: https://www.who.int/fr/news-room/fact-sheets/detail/schistosomiasis (accessed on 28 March 2024).
- Montresor, A.; Mwinzi, P.; Mupfasoni, D.; Garba, A. Reduction in DALYs Lost Due to Soil-Transmitted Helminthiases and Schistosomiasis from 2000 to 2019 Is Parallel to the Increase in Coverage of the Global Control Programmes. PLoS Negl Trop Dis 2022, 16, e0010575. [Google Scholar] [CrossRef]
- Rohr, J.R.; Sack, A.; Bakhoum, S.; Barrett, C.B.; Lopez-Carr, D.; Chamberlin, A.J.; Civitello, D.J.; Diatta, C.; Doruska, M.J.; De Leo, G.A.; et al. A Planetary Health Innovation for Disease, Food and Water Challenges in Africa. Nature 2023, 619, 782–787. [Google Scholar] [CrossRef]
- Senghor, B.; Mathieu-Begné, E.; Rey, O.; Doucouré, S.; Sow, D.; Diop, B.; Sène, M.; Boissier, J.; Sokhna, C. Urogenital Schistosomiasis in Three Different Water Access in the Senegal River Basin: Prevalence and Monitoring Praziquantel Efficacy and Re-Infection Levels. BMC Infect Dis 2022, 22, 968. [Google Scholar] [CrossRef]
- Perez-Saez, J.; Mande, T.; Ceperley, N.; Bertuzzo, E.; Mari, L.; Gatto, M.; Rinaldo, A. Hydrology and Density Feedbacks Control the Ecology of Intermediate Hosts of Schistosomiasis across Habitats in Seasonal Climates. Proc Natl Acad Sci U S A 2016, 113, 6427–6432. [Google Scholar] [CrossRef]
- Senghor, B.; Diaw, O.T.; Doucoure, S.; Seye, M.; Talla, I.; Diallo, A.; Bâ, C.T.; Sokhna, C. Study of the Snail Intermediate Hosts of Urogenital Schistosomiasis in Niakhar, Region of Fatick, West Central Senegal. Parasit Vectors 2015, 8, 410. [Google Scholar] [CrossRef]
- Cridland, C.C. Results of Exposure of Batches from Highly Susceptible and Less-Susceptible Strains of Biomphalaria Alexandrina Alexandrina from Egypt to Strains of Schistosoma Mansoni from Cairo and Alexandria. Bull World Health Organ 1968, 39, 955–961. [Google Scholar]
- Lancastre, F.; Vianey-Liaud, M.; Coutris, G.; Bolognini-Treney, J.; Mougeot, G.; Ouaghlissi, J.P. [Resistance to desiccation of Biomphalaria glabrata adults infested by various miracidia of Schistosoma mansoni]. Mem Inst Oswaldo Cruz 1989, 84, 205–212. [Google Scholar] [CrossRef]
- Starkloff, N.C.; Angelo, T.; Mahalila, M.P.; Charles, J.; Kinung’hi, S.; Civitello, D.J. Spatio-Temporal Variability in Transmission Risk of Human Schistosomes and Animal Trematodes in a Seasonally Desiccating East African Landscape. Proc Biol Sci 2024, 291, 20231766. [Google Scholar] [CrossRef]
- Hang, D.-R.; Feng, Y.; Zhang, J.-F.; Wang, Y.-H.; Zhang, B.; Juma, S.; Sleiman, M.M.; Yang, K. Studies on the Ecology of Bulinus Globosus Snails: Evidence against Burrowing into the Soil during the Dry Season. Front. Environ. Sci. 2022, 10. [Google Scholar] [CrossRef]
- Oyeyi, T.I.; Ndifon, G.T. A Note on the Post-Aestivation Biology of Bulinus Rohlfsi (Clessin), an Intermediate Host of Schistosoma Haematobium (Bilharz) in Northern Nigeria. Ann Trop Med Parasitol 1990, 84, 535–536. [Google Scholar] [CrossRef]
- Chu, K.Y.; Bihan, H.; Massoud, J. The Ability of Bulinus Truncatus, Biomphalaria Alexandrina and Lymnaea Gedrosiana to Survive out of Water in the Laboratory. Ann Trop Med Parasitol 1967, 61, 1–5. [Google Scholar] [CrossRef]
- Chu, K.Y.; Arfaa, F.; Massoud, J. The Survival of Bulinus Truncatus Buried in Mud under Experimental Outdoor Conditions. Ann Trop Med Parasitol 1967, 61, 6–10. [Google Scholar] [CrossRef]
- Hang, D.-R.; Feng, Y.; Zhang, J.-F.; Wang, Y.-H.; Zhang, B.; Juma, S.; Sleiman, M.M.; Yang, K. Studies on the Ecology of Bulinus Globosus Snails: Evidence against Burrowing into the Soil during the Dry Season. Front. Environ. Sci. 2022, 10. [Google Scholar] [CrossRef]
- Diaw, O.T.; Seye, M.; Sarr, Y. [Resistance to drought of mollusks of the genus Bulinus, vectors of human and animal trematode infections in Senegal. II. Study under natural conditions in the North-Sudan area. Ecology and resistance to drought of Bulinus umbilicatus and B. senegalensis]. Rev Elev Med Vet Pays Trop 1989, 42, 177–187. [Google Scholar] [CrossRef]
- Gaye, P.M.; Doucoure, S.; Senghor, B.; Faye, B.; Goumballa, N.; Sembène, M.; L’Ollivier, C.; Parola, P.; Ranque, S.; Sow, D.; et al. Bulinus Senegalensis and Bulinus Umbilicatus Snail Infestations by the Schistosoma Haematobium Group in Niakhar, Senegal. Pathogens 2021, 10, 860. [Google Scholar] [CrossRef]
- Brown, D.; Kristensen, T. A Field Guide to African Freshwater Snails. 1. West African species. Danish Bilharziasis Laboratory: Denmark.
- Frandsen, F.; Christensen, N.O. An Introductory Guide to the Identification of Cercariae from African Freshwater Snails with Special Reference to Cercariae of Trematode Species of Medical and Veterinary Importance. Acta Trop 1984, 41, 181–202. [Google Scholar]
- Appleton, C.; Miranda, N. Locating Bilharzia Transmission Sites in South Africa: Guidelines for Public Health Personnel. Southern African Journal of Infectious Diseases 2015, 30, 95–102. [Google Scholar] [CrossRef]
- Cnops, L.; Soentjens, P.; Clerinx, J.; Van Esbroeck, M. A Schistosoma Haematobium-Specific Real-Time PCR for Diagnosis of Urogenital Schistosomiasis in Serum Samples of International Travelers and Migrants. PLoS Negl Trop Dis 2013, 7, e2413. [Google Scholar] [CrossRef]
- Ibironke, O.; Koukounari, A.; Asaolu, S.; Moustaki, I.; Shiff, C. Validation of a New Test for Schistosoma Haematobium Based on Detection of Dra1 DNA Fragments in Urine: Evaluation through Latent Class Analysis. PLoS Negl Trop Dis 2012, 6, e1464. [Google Scholar] [CrossRef]
- Hamburger, J.; He-Na, null; Abbasi, I. ; Ramzy, R.M.; Jourdane, J.; Ruppel, A. Polymerase Chain Reaction Assay Based on a Highly Repeated Sequence of Schistosoma Haematobium: A Potential Tool for Monitoring Schistosome-Infested Water. Am J Trop Med Hyg 2001, 65, 907–911. [Google Scholar] [CrossRef]
- Webster, B.L.; Rollinson, D.; Stothard, J.R.; Huyse, T. Rapid Diagnostic Multiplex PCR (RD-PCR) to Discriminate Schistosoma Haematobium and S. Bovis. J Helminthol 2010, 84, 107–114. [Google Scholar] [CrossRef]
- Webbe, G.; Msangi, A.S. Observations on Three Species of Bulinus on the East Coast of Africa. Ann Trop Med Parasitol 1958, 52, 302–314. [Google Scholar] [CrossRef]
- Senghor, B.; Diaw, O.T.; Doucoure, S.; Seye, M.; Diallo, A.; Talla, I.; Bâ, C.T.; Sokhna, C. Impact of Annual Praziquantel Treatment on Urogenital Schistosomiasis in a Seasonal Transmission Focus in Central Senegal. PLoS Negl Trop Dis 2016, 10, e0004557. [Google Scholar] [CrossRef]
- Gaye, P.M.; Doucoure, S.; Senghor, B.; Faye, B.; Goumballa, N.; Sembène, M.; L’Ollivier, C.; Parola, P.; Ranque, S.; Sow, D.; et al. Bulinus Senegalensis and Bulinus Umbilicatus Snail Infestations by the Schistosoma Haematobium Group in Niakhar, Senegal. Pathogens 2021, 10, 860. [Google Scholar] [CrossRef]
- Vera, C.; Bremond, P.; Labbo, R.; Mouchet, F.; SELLIN, E.; Boulanger, D.; Pointier, J.P.; Delay, B.; Sellin, B. Seasonal Fluctuation in Populations Densities of Bulinus Senegalensis and B. Truncatus (Planorbidae) in Temporary Pools in a Focus of Schistosoma Heamatobium in Niger: Implications for Control. Journal of Molluscan Studies 1995, 61, 79–88. [Google Scholar] [CrossRef]
- Smithers, S.R. On the Ecology of Schistosome Vectors in the Gambia, with Evidence of Their Rôle in Transmission. Trans R Soc Trop Med Hyg 1956, 50, 354–365. [Google Scholar] [CrossRef]
- Brown, D.S. Freshwater Snails Of Africa And Their Medical Importance; CRC Press: London, 2014; ISBN 978-0-429-09494-1. [Google Scholar]
- Knigge, T.; Di Lellis, M.A.; Monsinjon, T.; Köhler, H.-R. Relevance of Body Size and Shell Colouration for Thermal Absorption and Heat Loss in White Garden Snails, Theba Pisana (Helicidae), from Northern France. J Therm Biol 2017, 69, 54–63. [Google Scholar] [CrossRef]
- Diaw, O.T.; Vassiliades, G.; Seye, M.; Sarr, Y. Prolifération de mollusques et incidence sur les trématodoses dans la région du delta et du lac de Guiers après la construction du barrage de Diama sur le fleuve Sénégal. Revue d’élevage et de médecine vétérinaire des pays tropicaux 1990, 43, 499–502. [Google Scholar] [CrossRef]
- Oliver, L.; Barbosa, F.S. Observations on Vectors of Schistosomiasis Mansoni Kept out of Water in the Laboratory. J Parasitol 1956, 42, 277–286. [Google Scholar] [CrossRef]
- Webbe, G. The Transmission of Schistosoma Haematobium in an Area of Lake Province, Tanganyika. Bull World Health Organ 1962, 27, 59–85. [Google Scholar]
- Ohlweiler, F.P.; Kawano, T. Biomphalaria Tenagophila (Orbigny, 1835) (Mollusca): Adaptation to Desiccation and Susceptibility to Infection with Schistosoma Mansoni Sambon, 1907. Rev Inst Med Trop Sao Paulo 2002, 44, 191–201. [Google Scholar] [CrossRef]
- Rubaba, O.; Chimbari, M.; Mukaratirwa, S. The Role of Snail Aestivation in Transmission of Schistosomiasis in Changing Climatic Conditions. African Journal of Aquatic Science 2016, 41, 143–150. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).



