Introduction
Since entering into force in 1975—as the first international treaty to ban an entire class of weapons—the Biological and Toxin Weapons Convention (BWC) has struggled to account for the absence of a treaty protocol, including a verification regime. Without it, the treaty lacks sufficient institutional capacity to support or enforce implementation, and BWC states parties lack a formal ability to assess compliance with treaty obligations. To compensate, states parties established Confidence-Building Measures (CBMs) as a tool to promote information sharing and increase transparency regarding national biological activities, facilities, programs, and capabilities. Ultimately, CBMs aim to ‘enhance confidence in the implementation of [treaty] provisions’ and ‘prevent or reduce the occurrence of ambiguities, doubts and suspicions’ (Second Review Conference 1986).
States parties first agreed to institute CBMs at the Second Review Conference in 1986, and the Ad Hoc Group on Confidence-Building Measures
1—mandated to ‘finalize the modalities for the exchange of information and data [including] appropriate forms’— developed the initial CBM forms in 1987 (BWC Implementation Support Unit 2022). States parties agreed by consensus at the Third Review Conference (1991) to formally implement CBMs, thereby establishing one of the few formal tools under the BWC to facilitate transparency and information sharing about treaty compliance. CBMs comprise a series of forms, to be submitted annually by states parties, that request information on specific activities and capacities relevant to their BWC obligations, including laboratories, biodefense programs, unusual outbreaks, scientific publications, national legislation and regulation, historical offensive biological programs, and vaccine production facilities (BWC Implementation Support Unit 2022).
BWC CBM forms and processes remain largely unchanged from the original iteration, despite radical advancements in biological capabilities and threats over the past several decades. After establishing the original four CBMs in 1987—research centers and laboratories (Form 1), abnormal outbreaks (Form 2), active promotion of contacts (Form 3), and scientific publications (no form; Ad Hoc Meeting of the Scientific and Technical Experts 1987)—states parties reviewed their effectiveness at the Third Review Conference in 1991. This review resulted in the most substantial updates, establishing the modern version of CBM forms: research centers and laboratories (Form A, Part 1), biodefense programs (Form A, Part 2), abnormal outbreaks (Form B), scientific publications (Form C), active promotion of contacts (Form D), national legislation and regulation (Form E), historical offensive biological programs (Form F), and vaccine production facilities (Form G). States parties also added a cover page (Form Zero), which provides options for ‘Nothing to declare’ or ‘Nothing new to declare’ for each form (Third Review Conference 1992). At the Sixth Review Conference, states parties tasked the BWC Implementation Support Unit (ISU) to coordinate the CBM process—including distributing CBM submissions to states parties, reporting on participation, and facilitating support to increase participation—and to develop electronic versions of the CBM forms and an internet platform for submitting and publishing annual CBMs (e-CBM platform; Sixth Review Conference 2006). States parties eliminated Form D (active promotion of contacts) at the Seventh Review Conference (2011), which was the last substantive change to the CBMs. While the Intersessional Programmes (ISPs) ahead of the Eighth (2012-15) and Ninth Review Conferences (2018-21
2) included debate regarding how to strengthen CBMs, no formal changes were adopted. At the 2023 Meeting of States Parties, the ISU announced updates to the e-CBM platform, including to provide additional functionality (e.g. automated translation, improved search capability) for states parties (BWC Implementation Support Unit 2023a).
Despite their existence as a tool dedicated to increasing confidence in compliance, CBMs have not made a meaningful impact in that regard. Low participation, concerns regarding the completeness and accuracy of submissions, a dearth of CBM data analysis, and restricted access to many states parties’ submissions have limited their value from the beginning (Chevrier 1998; Hunger and Dingli 2011). In contrast to declarations and reporting established for other international arms control, disarmament, and nonproliferation treaties, BWC CBMs are not a legally binding obligation. In fact, CBMs were established ‘on the basis of mutual co-operation’, and they were originally described as ‘politically binding measures’ (Second Review Conference 1986). Debate at recent BWC meetings illustrates the disagreement among BWC states parties regarding whether they are obligated or encouraged to participate in ‘politically binding measures’, which likely contributes to historically low annual participation. Notably, CBM submissions did not surpass one-half of states parties in any year until 2021 (BWC Implementation Support Unit 2024a). There is no formal capacity under the BWC to compile, summarize, or synthesize CBM data, leaving that responsibility to the states parties themselves, which face many important competing priorities for limited available resources. It is unclear whether, how, or how much states parties utilize CBM data. The inability or unwillingness to review or analyze CBM data limits CBMs’ ability to actually build confidence in compliance, and the absence of a clear and compelling use case for CBM data could provide further disincentive for states parties to invest the time and resources required to participate. While some CBM submissions are publicly available, most are not. States parties have access to all CBM submissions, but they must elect to make their submissions available to external stakeholders. In fact, of the 104 CBM submissions in 2023,
3 only 31 are publicly available—representing fewer than one-third of submissions and slightly more than one-sixth of all states parties (BWC Implementation Support Unit 2024b)—which limits civil society organizations’ ability to monitor or gain insight regarding states parties’ biological facilities, activities, programs, and capacities, as well as their ability to supplement states parties’ analytic capacity (Hunger and Dingli 2011).
Despite these limitations, CBMs remain a high priority and a frequent topic of debate at BWC meetings, with numerous states parties regularly calling for increased annual participation and improved CBM forms, processes, and use of the data. Crucially, Confidence-Building and Transparency is an agenda item for the 2023-26 Working Group on the Strengthening of the Convention (Working Group), the mandate of which includes developing ‘specific and effective’ proposals for consideration at the Tenth Review Conference in 2027 (Ninth Review Conference 2022). This study builds on our previous BWC assurance research—which documented the landscape of perspectives on issues related to BWC verification and identified priority areas for strengthening the BWC—with a narrower focus on CBMs. Our findings and recommendations—generated from experiences and expertise provided directly by BWC delegations and other international stakeholders—support the Working Group through identifying specific targets for proposals to increase CBM participation and improve the value of CBM data. This research is part of an ongoing series of BWC assurance studies, which aims to characterize challenges around BWC verification and related topics and to strengthen certainty in BWC compliance (Shearer et al. 2022).
Methods
This study utilizes the same mixed-methods analytic methodology used in our previous BWC assurance research (Shearer et al. 2022), with minor updates. The analysis comprises three phases: qualitative coding of interview content, quantitative analysis to identify priority topics, and targeted thematic analysis of coding data. This approach allowed us to systematically and rigorously document the landscape of perceptions associated with BWC CBMs and related concepts, using a previously demonstrated methodology.
From May 2023 to January 2024, we conducted a series of 37 semi-structured, key informant
4 interviews with 53 individuals, including members of BWC delegations and independent experts. The latter category included individuals affiliated with academic institutions and other civil society organizations, the BWC ISU and other nonproliferation fora, and current and former BWC delegation members who participated in their individual capacity. We identified prospective interviewees based on their relevant expertise and institutional affiliations—including participation in BWC and other nonproliferation meetings—utilizing purposive sampling, with a view to including diverse geographic, political, and demographic perspectives. We invited more than 160 individuals and offices across 87 countries, as well as multiple UN offices.
We developed the interview guide (Supplement- Interview Guide) based on a scoping literature review, including historical and current CBM forms and associated publicly available submission data and analysis, as well as our personal experience related to BWC proceedings, statements, and debate. Interview topics centered around the interviewees’ experience with CBMs, CBMs’ purpose and value, CBM forms and processes, and barriers and potential solutions, as well as other information-sharing mechanisms for the BWC and other disarmament fora. The interview guide included specific topics and questions; however, the semi-structured format allowed interviewees to direct the conversation based on their individual experiences and priorities. We conducted the interviews via videoconference, in person, or through written responses, and all interviews were held on a not-for-attribution basis to promote candor and transparency. We recorded audio for the virtual and in-person interviews, with participants’ consent and supplemented the audio with written interview notes. We utilized Otter.ai (Version 3.45.1) to generate automated transcripts and reviewed and corrected all transcripts, as needed, to increase accuracy prior to coding.
The initial thematic coding framework—developed using NVivo qualitative coding software (Release 14.23.2)—was based on topics identified from interview notes, audio recordings, and transcripts. The coding team piloted the coding framework on a subset of interview transcripts and reviewed the results to add, edit, and reorganize codes into a final framework. The final coding framework included 73 codes, organized hierarchically into five broad categories to facilitate coding: subjects, including CBM components, processes, and proposals; values, describing CBMs’ use and purpose; roles, identifying the actors involved in various activities; sentiment, representing interviewees’ perception of various subjects; and feasibility, reflecting factors affecting the possibility or probability of specific actions or changes. Four team members performed qualitative coding on transcripts and written responses, where available, and on interview notes for the remaining interviews. As new themes emerged during the coding process, new codes were added to the framework, and the coders reviewed completed interviews using the new codes. Interviews were classified by interviewee type (i.e. ‘delegation’ or ‘independent expert’); their experience with CBMs and related policy; and for states parties, the BWC regional group (UN Office for Disarmament Affairs n.d.), World Bank income group (World Bank Group n.d.),
5 history of CBM participation, and CBM public availability (BWC Implementation Support Unit 2024a). At least one team member reviewed all coding for quality control, and the coders resolved coding discrepancies and concerns by consensus.
Using NVivo and Microsoft Excel, we quantified the frequency of code usage and co-coding—i.e. when multiple codes were assigned to the same content—to determine the cumulative total instances and number of interviews. We also generated group-specific metrics—weighted inversely by the relative proportion of interviews in each group—to identify themes discussed more often by interviewees from one classification than another, which could signal differences in how various groups prioritize certain topics. For the final thematic analysis, we prioritized individual codes and co-coded pairs utilized in at least 10 interviews and those with a weighted interview difference of 4 or greater between delegations and independent experts. Other group-specific weighted metrics were noted but not used to identify priority codes. We also identified priority thematic codes a priori—based on CBM debate during past BWC meetings and associated literature, specific statements that stood out during the interviews, and our own expertise and observations—which enabled us to include important or interesting content that was not necessarily addressed across numerous interviews. The thematic findings summarized below are data-driven, documenting the interviewees’ comments, and we do not make any judgements regarding the validity or value of any particular position.
The Johns Hopkins University Bloomberg School of Public Health Institutional Review Board determined that this study did not constitute human subjects research (IRB00024211).
Results
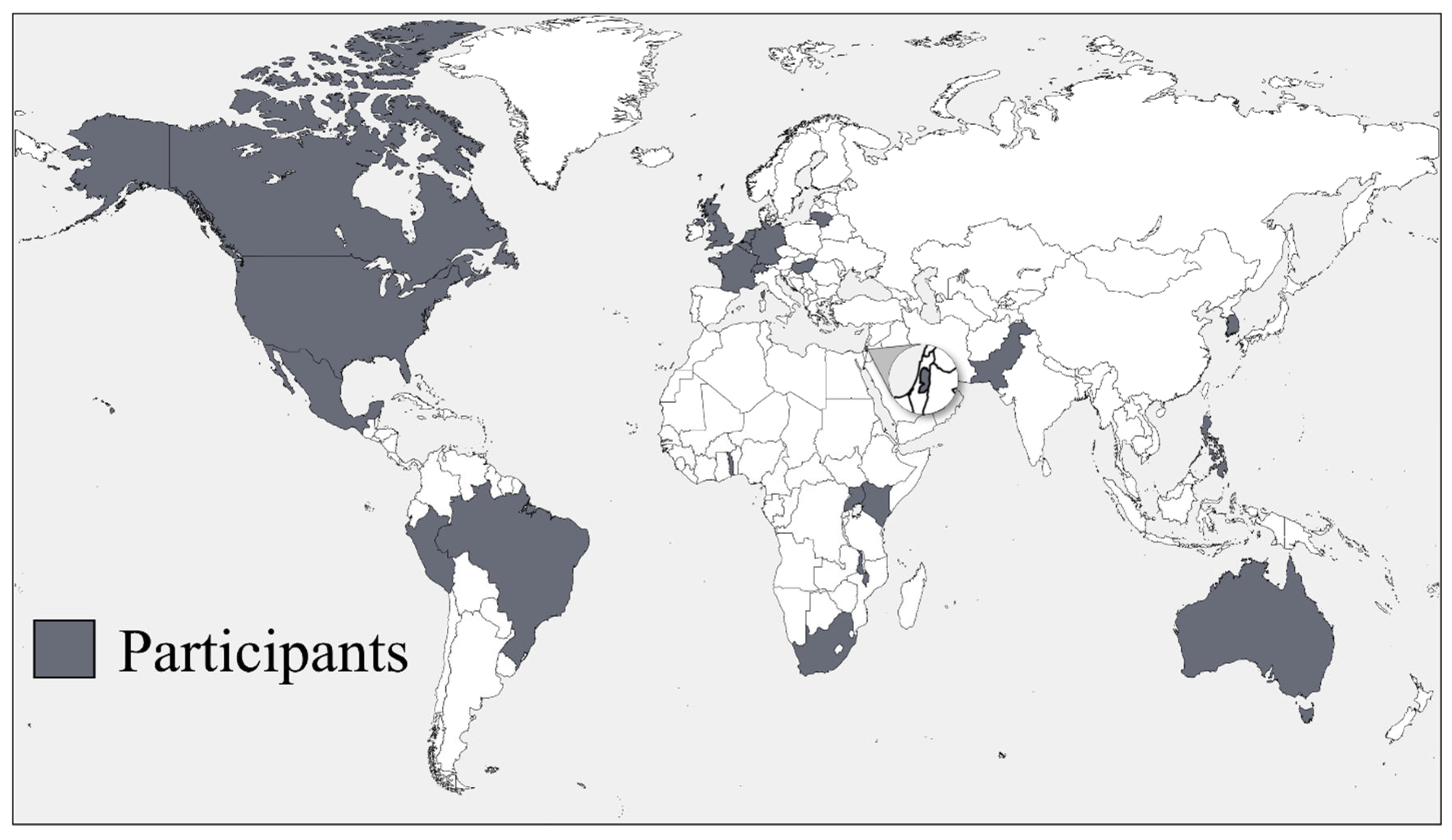
We conducted 37 interviews with a total of 53 international experts—23 interviews with 38 individuals who work on or with BWC national delegations and 14 interviews with 15 independent experts. The interviewees represented 24 countries across 6 continents, as well as all 3 traditional BWC regional groups (
Table 1;
Figure 1). Interviewees’ characteristics are shown in
Table 2. Notably, participating delegations included the current Working Group Chair, as well as the Working Group facilitators for both the Confidence-Building and Transparency and the Verification and Compliance topic areas, which are particularly relevant to CBMs. Three (3) delegation interviewees declined to be listed as participants, and 3 others requested to be listed by their organizational affiliation. One (1) independent expert and 11 states parties declined to participate.
In total, the coding generated 4,419 total coding references (Supplement- Quantitative data) and 8,077 co-coded references (Supplement- Coding matrix). Of the 73 individual codes utilized, 61 codes were addressed in at least 10 interviews as well as 98 co-coded pairs. The use of descriptors below (e.g. ‘several’, ‘numerous’) represent the relative frequency that certain perspectives or topics were addressed by interviewees. These terms pertain only to the interviews themselves and cannot be extrapolated to BWC states parties or other stakeholders, more broadly.
Purpose and Value
Many interviewees discussed CBMs’ current role in the context of their origins, including the evolution of that purpose and CBMs’ value since their inception. CBMs’ purpose was originally described as ‘to prevent or reduce the occurrence of ambiguities, doubts and suspicions, and…to improve international co-operation in the field of peaceful bacteriological (biological) activities’ (Second Review Conference 1986), and interviewees indicated that CBMs remain a key transparency mechanism for the BWC. They emphasized that transparency and information-sharing on biological activities, facilities, programs, and capabilities can mitigate ambiguity and uncertainty around their purpose, contributing to increased confidence in BWC compliance and, ultimately, reducing concern regarding states parties’ biological activities as well as the risk that concern could escalate to conflict. Multiple interviewees also identified information on national legislation and regulatory systems as valuable for providing a window into how states parties approach biosecurity and biodefense, which can yield further insight into BWC compliance by allowing states parties to demonstrate commitment to their BWC obligations. Some interviewees noted, however, that there is a degree of disconnect between CBMs’ original intent and their current role. CBMs arose in the 1980s and are largely representative of Cold War-era capabilities and threats. The world has changed radically since then, including monumental advances in biology and global geopolitical shifts (Koblentz and Chevrier 2011). In discussing these changes, interviewees considered how effectively the existing CBM forms reflect the modern threat landscape and biological capabilities. They generally supported that CBMs contribute to their intended purpose, but there remain major barriers to fully achieving their principal aim.
Beyond the transparency, information sharing, and insight into compliance that benefit other states parties, interviewees highlighted a broad scope of direct benefits to participating states parties. Multiple interviewees emphasized that this may be an overlooked value of CBMs. Numerous delegations indicated that the process of gathering the requested information helps them better understand their own national biosecurity and biodefense systems and their biological research portfolio, including identifying points of contact at relevant organizations or facilities and strengthening those relationships. Additionally, the annual process provides an opportunity to identify changes in capacities and national implementation, including new national legislation or regulatory policies and activities spread across numerous government agencies. Participating in CBMs also aids states parties in identifying gaps in their national implementation, particularly relative to how other states parties approach these challenges and obligations. Interviewees from low- and lower-middle-income countries and those seeking to expand their biological research and industry sectors prioritized CBMs’ role in identifying opportunities for international cooperation and assistance (e.g. under Article X). One interviewee specifically underscored international collaboration as a core tenet of the BWC and essential to achieving its intended objectives and purpose. Similarly, states parties looking to provide aid can use CBM submissions to identify other states parties that might need such support, including those with similar programs or interests, seeking specific capacities or capabilities, or wishing to strengthen national implementation.
Participation
Numerous interviewees lamented poor CBM participation as one of the principal barriers to achieving their intended purpose. The more data are available, the more CBMs can provide insight into global treaty implementation and compliance. Participation is steadily increasing, particularly in recent years, surpassing 100 submissions for the first time ever in 2023 (BWC Implementation Support Unit 2024a); however, nearly half of states parties still do not participate regularly. Interviewees described numerous barriers to increasing participation, particularly with respect to the nature of states parties’ commitment to submit CBMs and the effort required to participate.
Considerable disagreement persists among states parties and stakeholders regarding whether CBM participation is mandatory or voluntary. When CBMs were initially introduced, they were described as ‘politically binding measures’ (Second Review Conference 1986), but states parties disagree whether this means that they are obligated or encouraged to participate, which impacts decisions to submit CBMs. Notably, there was substantial support among interviewees on both sides of this issue, including among delegations. Those who viewed CBMs as an obligation interpreted politically binding commitments as commitments, nonetheless, whereas others viewed them as wholly voluntary, in the absence of a legally binding mandate. CBMs do not appear in the treaty text; however, multiple interviewees argued that their inclusion in the final declarations of the Second and Third Review Conferences, agreed to by consensus, gives them status equal to other Review Conference decisions, such as collecting annual financial contributions and scheduling ISP meetings or future Review Conferences. Some suggested that making CBMs legally binding could stimulate participation, but multiple interviewees maintained that any steps to establish legally binding obligations need to be part of a comprehensive approach that includes a verification regime.
The other major barrier to participation is the effort required to participate, or perhaps more accurately, CBMs’ relative value compared to the required effort. The principal resource barriers include the knowledge and experience necessary to understand national biosecurity and biodefense networks, the time and effort required to collate CBM data, and familiarity with CBM forms and processes. Part of this challenge is identifying the right programs and points of contact—or even to know which programs or capacities qualify as relevant. With biological programs and capacities spread across numerous facilities, agencies, and ministries, as well as private sector or nongovernmental organizations, interviewees described difficulties in understanding exactly what information CBMs are requesting and knowing where to find it. This can be particularly challenging to navigate for states parties completing their first CBM, or their first in a while. Numerous interviewees emphasized that the first CBM is considerably more resource-intensive than subsequent submissions, once sufficient experience and processes are in place. Additionally, multiple delegations indicated that they use the previous year’s submission as a starting point for the current year, making updates where necessary, which requires less effort than starting with blank forms. Interviewees also suggested that states parties’ generally poor perception of CBMs’ value—stemming from low participation, concerns about CBM completeness and accuracy, and a dearth of CBM content analysis—also contributes to low participation. If states parties do not feel that CBMs generate sufficient value, it is more difficult to justify the effort required to participate. Poor participation may, therefore, reinforce poor participation in the future, a cycle that compounds barriers to improving CBMs’ value.
With numerous international arms control treaties and other fora requiring regular declarations, assessments, or other reports, reporting fatigue is another concern affecting CBM participation. Several interviewees noted that these responsibilities often fall on the same few individuals at the national, agency, and facility levels, particularly for states parties with small disarmament delegations. Interviewees listed several examples of responsibilities that contribute to reporting fatigue, including UN Security Council Resolution 1540 (UNSCR 1540), Joint External Evaluations (JEEs), and the International Health Regulations States Parties Self-Assessment Annual Reports (SPARs), all of which cover various aspects of biosecurity and biodefense programs, capacities, and policies. Interviewees suggested that overlap between these various reporting and assessment obligations is a double-edged sword; on one hand, states parties may be duplicating efforts and contributing to reporting fatigue, but on the other, there may exist opportunities to streamline data collection and submission across multiple treaties with overlapping scope. This could actually reduce reporting burden, but this kind of coordination likely requires extensive institutional knowledge and detailed awareness of reporting requirements across diverse government agencies and fora.
Interviewees also discussed opportunities to facilitate increased participation, spanning a broad scope of formal and informal activities and mechanisms. Multiple delegations, from across the spectrum of CBM participation, prioritized providing direct support for states parties in completing their CBM submissions, such as sharing guidance and lessons from their own experiences. This kind of support can be provided by a variety of actors—including states parties themselves, the ISU, or civil society organizations—and could take a variety of forms, ranging from formal bilateral agreements (e.g. via offers in the Article X database) to regional or multilateral workshops to side events at BWC meetings. Several interviewees also discussed a stepwise approach for submitting a first CBM, as outlined in Working Paper #62 from the Ninth Review Conference, led by Japan. This concept encourages states parties to start with a partial submission, such as a single form, and build toward a complete submission over time, as resources allow. Knowing that the first submission is the most resource-intensive, this option spreads that effort across multiple years to decrease the initial burden. Notably, there is no requirement to provide a full submission, so states parties can submit any information that they are able to gather. Proponents argued that some data are better than no data, and states parties need not wait until they can submit a full CBM to contribute to transparency and confidence-building efforts. Interviewees also suggested that improving CBMs’ value could incentivize increased participation, but they consistently emphasized the importance of balancing any additional value against the effort required to participate, specifically that any increase in effort risks decreased participation. Thus, any updates to CBMs need to focus on increasing value without adding undue burden on states parties.
Submission Quality
A combination of factors contributes to CBM quality, namely completeness (whether all relevant information is provided), accuracy (whether information is factually correct), and detail (whether there is sufficient context). Numerous interviewees indicated that inaccurate or omitted information in CBM submissions, deliberate or otherwise, is a concern. Several interviewees argued that the absence of a legally binding mandate results in states parties electing to share only what they want to share, which may or may not provide a full picture of relevant biological activities and national implementation. Interviewees also indicated that states parties are unlikely to share information that may be incriminating or anything that is not already available through other means. Obviously, deliberate efforts to omit, obfuscate, or misrepresent prohibited activities was the most concerning form of quality issue, and interviewees broadly expressed concern in that regard, with respect to certain states parties’ submissions. Not all quality issues are intentional, however. Some result from differences in how states parties understand CBM concepts—such as disparities in what qualifies as biodefense or variations in the translation of key terms—or simply from inadvertent mistakes in information collection or reporting. Multiple interviewees acknowledged that limited resources available for the collection and review of CBM data could hinder states parties’ ability to identify the full scope of relevant activities or programs or to perform quality assurance checks prior to submission. And finally, multiple interviewees indicated that the absence of a formal process to review CBM submissions, assess accuracy and completeness, and follow up with the submitting state party to resolve discrepancies or ambiguities makes it difficult to resolve quality concerns or determine the underlying causes. Numerous interviewees emphasized that CBMs are only one source of data and that any analysis would need to corroborated and integrated with others to help form a more complete picture of a state party’s biological activities and BWC compliance.
Beyond the quality of information submitted by states parties, some interviewees also expressed concern about the CBM forms themselves, specifically whether they request the kinds of information necessary to increase confidence in compliance. The CBM forms are largely unchanged since they were established in the 1980s, despite radical changes in biology and geopolitics, and some interviewees contended that modern capabilities, opportunities, risks, and threats may not be adequately addressed in the current iteration of the forms. For example, the diffusion of biotechnology beyond large, government-sponsored programs—including into academic institutions and private sector business and industry—has resulted in a rapid expansion in the number of facilities and researchers capable of leveraging these capabilities, many of which are not accounted for in CBMs. Several interviewees highlighted that priority pathogens and high-consequence research do not necessarily require BSL-4 containment. Rather, much of this work can be done in BSL-2/3/3+ laboratories, which may not be captured in CBMs. Importantly, it could be prohibitively difficult to provide a comprehensive picture of laboratories below BSL-4, particularly in states parties with large biological research sectors or those with limited reporting capacity; however, interviewees emphasized that there may be other ways of capturing research capacity and priorities in CBMs.
Interviewees also described how technological changes impact how well CBMs reflect national activities and capabilities. In particular, they discussed how modern biotechnology enables research activities that once required large-scale production of dangerous pathogens to be conducted at a much smaller scale. Information such as the number of personnel or laboratory space may no longer be as useful in identifying priority facilities or research programs that could be converted to prohibited offensive activities. Similarly, information about vaccine production facilities may be less applicable to national-level priority disease threats, as a result of modern vaccine manufacturing processes and the global nature of pharmaceutical supply chains. Interviewees also acknowledged that revolutionary changes in the flow and availability of information, including via the internet, may limit CBMs’ value. Specifically, information that was previously difficult to locate or access, such as outbreak reports or scientific publications, is now readily available worldwide. Platforms such as ProMed (for outbreak reports), PubMed or Web of Science (for scientific articles), and social media—in addition to countless agency, institution, and publication websites—provide more timely information about emerging capabilities and events than what is available in annual CBM submissions. Several interviewees argued that these limitations render portions of CBMs outdated and redundant.
CBM Data Analysis
One of the biggest barriers to CBMs’ value is that states parties and other stakeholders make relatively limited use of CBM data, which appears to be largely a function of insufficient resources. Within the BWC itself, the ISU is tasked with providing regular updates on CBM activity; however, that analysis is limited to participation statistics. The ISU staff has neither the resources nor the mandate to analyze submission content. Without a centralized analytic capacity, states parties are responsible for their own CBM review, and the those limited ISU reports may be the only analysis to which some states parties have access. Numerous interviewees indicated that a comprehensive and substantive analysis of CBM content would provide additional insight into states parties’ biological activities and capabilities, but that capacity does not currently exist. In the absence of sufficient resources, states parties must tailor their analyses to their priority interests. Interviewees emphasized that even well-resourced governments may not be able to allocate CBMs the attention necessary to make full use of their data, particularly relative to countless other competing demands. States parties may select submissions from specific states parties, such as those that share similar capabilities or priorities, including neighboring countries or other regional partners, or those suspected of conducting concerning or prohibited activities. States parties may also limit their analysis to specific CBM forms or fields, including to gain insight into dual-use research activities or advanced capabilities, identify opportunities for international cooperation and assistance, or review the characterization of historical offensive biological programs.
The language of CBM submissions also constitutes a major barrier to CBM analysis. While states parties are able to submit CBMs in any of the six official UN languages, submissions are not translated into the others. One interviewee recalled that previous estimates for manual translation of CBM submissions projected the cost around US$1 million per year, which is prohibitively expensive, at nearly half of the BWC’s current annual budget (Secretariat of the Ninth Review Conference 2022). In order to make use of CBM submissions in another language, states parties and other stakeholders must perform their own translation, which some delegations reported doing; however, it requires additional resources to even access CBM data, let alone analyze them.
Even for states parties that do perform their own analysis, there is no formal mechanism to validate the data in CBM submissions or to follow up with other states parties to resolve questions or uncertainties. Interviewees emphasized that the absence of a process to evaluate or validate CBM data contributes to concerns about completeness and accuracy. BWC Article V obligates states parties to consult with one another to resolve concerns, but while a small number of delegations reported bilateral efforts, this kind of cooperation, formally or informally, appears to be limited in the context of CBMs. Multiple interviewees expressed interest in a process to validate CBM data; however, they acknowledged that it could be perceived as confrontational, which could exacerbate tensions between states parties or further disincentivize participation. They emphasized that any process to review and follow up on CBM submissions should be collaborative and collegial, and several noted that it should be a much lower standard—and less official—than formal consultative mechanisms under Article V. Framing questions from the perspective of gaining additional context and clarity—as opposed to an audit of CBM submissions—could facilitate cooperation that could contribute to increased confidence.
Both delegations and independent experts lamented the limited volume of publicly available CBM submissions and its negative impact on CBM analysis. States parties have access to all CBM submissions, but civil society organizations only have access to those from states parties that elect to make theirs publicly available. Multiple interviewees emphasized that restricting access to CBM submissions runs counter to their role as a transparency mechanism. Several interviewees also questioned the reasoning behind decisions to restrict access, particularly considering that states parties routinely disclose similar, or even more-sensitive, information in other fora, including UNSCR 1540 reporting, JEEs, and SPARs. In the absence of analytic capacity within the BWC or at the national level, civil society organizations could supplement efforts to make use of CBM data, but only if submissions are made publicly available. Not surprisingly, this was a high priority among independent experts, but numerous delegations expressed support as well. Multiple delegations suggested that states parties could permit themselves to restrict specific portions of their CBM submissions, while making the rest publicly available, in an effort to expand the publicly available data. Several interviewees indicated that some states parties may not even be aware that their CBM submissions are restricted. In some cases, states parties may have originally decided to restrict their submissions and never revisited that issue. Increasing the number of publicly available CBM submissions not only strengthens the commitment to transparency, but it also enables external stakeholders to support states parties’ efforts to make use of these data.
Interviewees indicated that recent technological advances could facilitate expanded CBM analysis. For example, automated translation could improve the accessibility of CBMs submitted in other languages, potentially at a much lower cost than manual translation. Interviewees warned, however, that unofficial translations could pose challenges, particularly when minor changes in language could impact perceptions of treaty compliance. Advanced computing capabilities, such as machine learning or artificial intelligence, could also facilitate analysis of CBM data, including identifying trends or changes from previous submissions. Notably, these tools tend to rely on large volumes of data to train their models, and the small number of CBMs may render this impractical. Interviewees did acknowledge, however, that it may not be feasible to provide third-party services with access to restricted submissions, which could hinder the use of these kinds of tools. Multiple interviewees also discussed limitations in the e-CBM platform’s search tool and expressed a need for improved functionality, to better enable them to locate desired information among CBM submissions. While technological tools could improve states parties’ ability to analyze CBM content, both technical and political barriers exist to leveraging these capabilities.
Discussion
The challenges facing BWC CBMs largely reflect those facing the treaty as a whole. BWC states parties face uncertainty regarding CBMs’ purpose and scope, which makes it difficult to determine—and agree to—appropriate steps to move forward. And the absence of organizational support and resource limitations, both at the national level and for the BWC itself, hinder participation and efforts to make the fullest use of CBM data. Crucially, it is impossible to extricate CBMs from the verification debate, much like essentially every other aspect of the BWC. While these issues serve as barriers to realizing the full value of CBMs, these similarities also offer multiple avenues, both formal and informal, to take concrete steps forward in that regard. On one end of the spectrum, states parties could make formal changes to the current CBM system, including negotiating updates to CBM forms or establishing analytic capacity under the umbrella of the BWC. But on the other, states parties could take a variety of informal steps—including unilaterally, bilaterally, or multilaterally—to emphasize the value in participating in CBMs, assist others in completing CBM submissions, and facilitate analysis of CBM data.
Focusing on improving the cost-benefit ratio of CBMs addresses the burden on states parties to participate or the value of CBM data—or ideally, both. Notably, each of these aims is multidimensional, with a variety of contributing factors and options to move forward. The burden on states parties applies to both the effort required to compile and submit CBMs and the effort to access and analyze CBM data. Similarly, the value of CBM data depends on the quality of CBM forms, annual submissions themselves, and the benefit of associated analysis. And in addition to building confidence in compliance, states parties can also receive direct value just by participating. Ultimately, the act of compiling and submitting CBMs requires effort, and states parties need to know that their efforts are yielding actual value. States parties can take a myriad of approaches to improving the cost-benefit ratio, but regardless of where they elect to start, those efforts can drive a mutually reinforcing cycle that addresses both sides of the equation. Expanded use of CBM data increases their value, which incentivizes increased participation, which subsequently yields more data and further bolsters CBMs’ value, and so on. The sections below describe several areas in which states parties can focus their efforts to develop and implement concrete solutions to improve CBMs’ cost-benefit ratio. These opportunities span a broad scope of activities, ranging from informal unilateral and bilateral efforts to consensus agreement and formal changes to the CBM system, so states parties have a diverse array of options to ensure that CBMs can effectively serve their primary functions, namely increasing transparency and mitigating ambiguities, with the ultimate goal of building confidence in BWC compliance.
Reduce the Burden of Participation
Perhaps the highest priority in reducing the burden on states parties is supporting increased CBM participation. The more states parties that participate, the more CBM data are available, and the more data available, the more CBMs can be leveraged for a variety of purposes. States parties have made steady progress in increasing participation, particularly in recent years, but there is still considerable room for improvement.
One of the biggest practical barriers to participating is the effort required for a state party to complete its first CBM. Without established processes and contacts in place and without a comprehensive understanding of national biological activities, facilities, programs, and capabilities, the first CBM is a much bigger hurdle than subsequent submissions. Additionally, updating the previous year’s submission is considerably more efficient than starting with blank forms. This is supported by the fact that states parties that submit their first CBM are more likely to continue participating. In fact, among 128 states parties that submitted a CBM between their inaugural year in 2020 or earlier, more than two-thirds (88) participated in half or more of the subsequent years.
6 Even ignoring the early adopters that have submitted CBMs consistently since the first five years, that proportion is still 60% (54 out of 90; BWC Implementation Support Unit 2022).
Easing the burden of submitting an initial CBM could have long-term benefits for increasing participation. One option is to provide additional support for states parties that wish to submit their first CBM. States parties themselves, the ISU, and civil society organizations are all potential sources of assistance for interested states parties. Notably, states parties across the spectrum of participation—from first-time participants to those with a long, consistent history of submissions—can share their experiences and lessons to aid others in identifying appropriate sources of information or establishing effective processes for collating the necessary information. The ISU already supports regional workshops to facilitate this kind of assistance—with funding from states parties or regional organizations (BWC Implementation Support Unit 2023b)—and there are two such offers of assistance in the BWC Article X database, from the US and UK delegations (BWC Implementation Support Unit n.d.a; BWC Implementation Support Unit n.d.b). Civil society organizations around the world already contribute to similar reports and evaluations, such as the JEE, and they could be excellent sources of technical expertise, personnel, and other resources to support states parties’ efforts. So while myriad opportunities exist, additional work is needed to connect states parties with the support they need, including avenues to share concerns and seek assistance as well as to spread awareness of available resources. Another option is to encourage a stepwise approach to completing a first CBM, to enable states parties to spread the initial burden of participation over several years, rather than all at once. There is no minimum threshold for submitting a CBM, so there is no reason why states parties could not submit individual (or even partial) CBM forms, with the goal of steadily building toward a full submission. Crucially, none of these opportunities require consensus agreement by states parties, allowing them to be implemented on an informal basis to strengthen CBMs without getting mired in the myriad political and practical barriers prevalent in the BWC.
There are also opportunities to improve the e-CBM platform and streamline the submission process for all states parties. Clarifying instructions and expectations regarding the information submitted in specific CBM forms and fields could ease the burden on states parties to gather and collate that information. Additionally, updating the e-CBM submission function, including to determine the appropriate methods of entering information could mitigate the burden to participate while improving CBM submission quality. For example, dropdown menus or checkboxes could simplify states parties’ efforts to enter information, whereas text boxes would allow them to submit more detailed and contextual information. Notably, the ISU announced updates to the e-CBM platform at the December 2023 Working Group meeting, which took effect in March 2024, including the ability to upload PDF documents directly via the website (BWC Implementation Support Unit 2023b). This could ease the burden on states parties by mitigating the need to manually transfer information to the electronic forms, but additional study will be necessary to fully understand the impact of these updates, including on the e-CBM search and translation functions.
Reduce Burden of Accessing and Analyzing CBMs
While states parties have access to all CBM submissions, numerous barriers exist to making full and effective use of those submissions, and mitigating this burden could facilitate expanded use of CBM data. The principal mechanism for accessing CBM submissions—for both states parties and civil society—is the e-CBM platform, and updated functionality could mitigate many of these barriers. The 2024 e-CBM platform update is expected to address several key barriers, including the absence of a functional document search capability and the need to translate CBM submissions in other languages. By enhancing the search functionality and integrating an automated translation service, the new e-CBM website will make it easier for states parties to access CBM data and locate information of interest, reducing the manual effort previously required to make use of CBM submissions. Additionally, the update will provide ‘[e]nhanced statistics and graphics’ on CBM participation (BWC Implementation Support Unit 2023b).
Multiple interviewees noted the need for such capabilities, but additional analysis will be needed to determine their effectiveness and identify opportunities for future progress. One immediately apparent limitation is the scope of access to these new tools. Specifically, they are only available to states parties or other registered users (i.e. not civil society organizations), and the automated translation can only be used for publicly available CBMs, since it requires the use of third-party software (BWC Implementation Support Unit 2023b). Opportunities certainly remain to implement centralized CBM analysis capacity, which could also benefit from emerging capabilities in advanced computing (e.g. machine learning, artificial intelligence) and automated tools. While the e-CBM platform updates do ease some burden to accessing and analyzing CBM submissions, additional work is needed to support the fullest use of CBM data.
Establish Analytic Capacity for CBM Submissions
Data only have value if they are analyzed, and the absence of analytic capacity for CBMs remains a major barrier to achieving their full potential. Whether at the national level or within the BWC itself, the limited use of CBM data impedes CBMs’ ability to shed light on biological activities, facilities, programs, and capabilities. The dearth of CBM analysis is largely a result of resource limitations and competing priorities, and even higher-income countries with large delegations must be selective with the analysis they conduct. Ultimately, national governments are responsible for their own CBM analysis, but establishing a formal capacity under the BWC could provide some degree of shared insight that would otherwise be out of reach for many states parties. There are countless options, such as summarizing annual submissions, documenting changes from previous reports, mapping designated facilities or specific categories of research, or reporting on longer-term trends. Centralizing this analysis within the BWC could mitigate the effort required at the national level, which would enable states parties to focus their resources more efficiently on national priorities. This capacity could be housed in a variety of locations, such as within the ISU; with states parties themselves, such as a dedicated working group or other annual meeting; or even in civil society. Some analysis is already performed by civil society organizations (Research Group for Biological Arms Control 2023), but this could be expanded with access to more annual submissions. Emerging capabilities in advanced computing and automated tools could support this analytic capacity as well, potentially with reduced financial or personnel resources, but barriers remain to utilizing third-party tools. Each of these options has its advantages and limitations, but considering the current state of CBM analysis, almost anything is better than the extant capacity.
Despite its status as one of the few formal BWC mechanisms to increase transparency and ambiguity, CBMs’ ability to actually mitigate uncertainty and alleviate concern is limited. To the extent that states parties do engage in analysis of CBM submissions, they lack a process to engage with other states parties to clarify the reported information, a key step in reducing ambiguity. Some of this happens on a bilateral basis, but that does not necessarily help other states parties that have similar questions—or other questions of their own. A dedicated forum or mechanism for states parties to follow up on CBM submissions would allow them to obtain additional context that would improve clarity and contribute to increased confidence in compliance. At the more formal end of the spectrum, this kind of consultation could fall under Article V; however, this could be viewed as too official or even potentially confrontational, which could hinder CBM participation. In contrast, a collaborative follow-up mechanism would allow states parties to ask questions and share information more constructively than an audit of submission content.
At their core, CBMs are intended as a transparency tool, and to some degree, limiting access to submitted information runs counter to that principle. In the absence of a formal analytic capacity, civil society organizations could provide supplemental analysis to support states parties; however, that necessitates access to CBM submissions. Currently, fewer than one-third of annual submissions are publicly available (BWC Implementation Support Unit 2024b), severely limiting civil society organizations’ ability to access and analyze these data. States parties that currently restrict their CBM submissions should revisit that decision, and they may determine that some CBM data are already available in other fora or that they no longer need to be restricted. Alternatively, states parties could establish the ability to designate specific CBM forms or fields as publicly available, while still limiting access to information they feel the need to protect. Civil society organizations have resources and expertise to support CBM analysis, as long as they have access to the data.
Increase Direct Value to Participating States Parties
Beyond the insight gained by analyzing other states parties’ CBM submissions, CBMs can also provide direct value to participating states parties, a potentially undervalued benefit. Through the process of gathering, collating, and reviewing the information for their CBM submissions, national governments build a more complete understanding of the breadth of their respective national biological activities and capabilities and strengthen relationships with key stakeholders, both in and outside of government. This process also affords the opportunity to identify changes to regulatory policies, legislation, and capacities as well as gaps in national implementation. Additionally, submitting a CBM provides other states parties with valuable information regarding research facilities and priorities, industrial capacity, and national implementation needs that can help them identify potential targets for collaboration or support. International cooperation and assistance, including under Article X, is a foundational principle of the BWC, and participating in CBMs can be a pathway to expanding those activities.
Statements and presentations on CBMs at BWC meetings frequently focus on increasing participation—or lamenting low participation—and the value of transparency in building confidence in compliance, largely ignoring the value that participation, in and of itself, can provide to states parties. There are notable exceptions, such as Working Paper #62 at the Ninth Review Conference, led by Japan (Japan 2022), as well as the ISU’s CBM briefing and interventions made by several states parties during the December 2023 Working Group sessions on Confidence-Building and Transparency (BWC Implementation Support Unit 2023b); however, these are relatively less common. Increased focus on the value that participating states parties can derive could provide additional incentive to participate—particularly for states parties that have never submitted a CBM—facilitating more states parties to benefit and expanding the available pool of CBM data. States parties should more regularly share their CBM experiences at BWC meetings, including through national statements and interventions, working papers, and side events. Crucially, these should reach beyond just procedural lessons and focus on how the participating states parties, themselves, benefited from those experiences, such as through improved understanding of national biological activities, strengthened national implementation, and expanded international cooperation and assistance. CBM workshops led by states parties and the ISU are also an excellent forum for sharing these experiences, especially with regional partners. These kinds of statements and events could be particularly impactful from states parties that recently submitted their first CBM, as they can highlight specific considerations and factors in their decision to participate for the first time. These experiences may be received more favorably by states parties with similar governments or biological risks, capabilities, and priorities, which is common among regional partners and allies.
Making Concrete Progress
Formal changes to the existing CBM system could be challenging to enact, particularly in light of their close relationship with verification and compliance assessment, but those are not the only options available. Solutions such as amending CBM forms and establishing an analytic capacity under the BWC would likely require consensus agreement. Numerous states parties, however, argue that a comprehensive and holistic approach is needed to strengthen the treaty, which would make it difficult to improve CBMs without a broader effort to address other aspects of the BWC as well. There is a perception among many stakeholders that progress on CBMs would preclude progress on verification—or similarly, that stalled progress on verification precludes progress on CBMs—but strengthening CBMs and making progress toward verification are not mutually exclusive. There are opportunities to take concrete steps on both, together or independently, to strengthen the treaty. Knowing that verification and a treaty protocol will require time to negotiate, strengthening CBMs could serve as an intermediate step in that process. Additionally, progress on CBMs would have a positive effect on many high-priority aspects of the treaty, including national implementation, reviewing and leveraging emerging science and technology, and international cooperation and assistance. Even if states parties were to finalize a treaty protocol, CBMs may still remain as a transparency mechanism, alongside declarations, inspections, or other activities. While formal changes to CBMs could be prohibitively difficult, states parties have informal options to strengthen CBMs without the need for consensus. There are numerous unilateral, bilateral, and regional opportunities on the table, including to illustrate the direct value to participating states parties, increase assistance for states parties or otherwise ease the burden of submitting a first CBM, make CBM submissions publicly available, and further update the e-CBM platform. States parties have other confidence-building and transparency tools at their disposal as well, including peer review or voluntary site visits, annual Article X reports, and BWC side events or working papers, all of which can provide valuable insight into biological activities, facilities, programs, and capabilities. Ideally, the Working Group will take positive and concrete steps forward on both CBMs and verification, but states parties should not let challenges in one area risk wasting opportunities in the other.
Limitations
While we utilized a systematic and robust analytic methodology to generate our findings and recommendations, several gaps remain. One of the key limitations of this study is that the findings are not necessarily representative of all BWC states parties or other stakeholders. The quantitative analysis enabled us to identify priority topics for subsequent thematic analysis, but the methodology was not designed to yield quantitative or generalizable results. The findings described above are intended to convey the diversity of perspectives on CBMs, rather than represent the full scope of interviewees’ perspectives, or more broadly, those of states parties or other BWC stakeholders. Participant lists for past BWC and other nonproliferation meetings helped identify key state party representatives, but it was not always possible to locate accurate contact information or the appropriate point of contact. While we invited individuals and offices from around the world to participate, approximately half of the invitations to BWC delegations went unanswered. We conducted all interviews on a not-for-attribution basis, but government officials may have been unwilling to speak with a US-based civil society organization about politically sensitive BWC topics, including those closely related to verification. It was not feasible to include participation from all BWC states parties, but we implemented purposive sampling in an effort to incorporate a diverse set of participants, including geographically, politically, socially, and economically. Not surprisingly, the majority of participating states parties have an active interest in CBMs, demonstrated by long histories of participation. We made an explicit effort to include perspectives from states parties that have never submitted a CBM as well as those that recently submitted their first CBM—or their first recent CBM—in order to better capture associated experiences and challenges; however, dedicated study is needed to more fully characterize those barriers.
The use of audio transcription enabled us to ground our analysis in the interviewees’ own words, rather than interview notes, but the automated transcription was not perfect. We reviewed the audio and made necessary corrections to the transcript text, to the best of our ability, including to add appropriate punctuation and correct mis-transcribed statements. This improved readability and reliability, but the audio quality did not allow for 100% accuracy. Additionally, interviewees’ comments, including those from BWC delegations, may not necessarily reflect the official position of their government. All interviews—including written—were conducted in English, which may not have been interviewees’ primary language. We did not necessarily address all topics with each participant; however, the flexibility afforded by the semi-structured interview format allowed participants to discuss their own priorities, which may not have been captured in the interview questions. Our inclusion of select codes identified a priori in the thematic analysis enabled us to better reflect minority positions—in contrast to the more frequently discussed topics identified through the quantitative analysis; however, it was not possible to reflect all positions in the study findings.