1. Introduction
The liver disorders and hepatitis ranked 11
th and 16
th most common cause of deaths, respectively, cumulatively causing 2 million deaths annually [
1]. The prevalence of liver cirrhosis, hepatocellular carcinoma, viral hepatitis, and non-alcoholic fatty liver disease (NAFLD) are affecting communities residing in developing nations. The misuse and abusive consumption of and over the counter (OTC) drugs is one key problem that induces hepatotoxicity [
2]. The etiology, pathogenesis and frequency of hepatic damage encompass numerous factors such as alcohol, obesity, heavy metals, ingestion of toxicants, sedentary lifestyle, poor dietary habits and malnutrition [
3]. The South Asian region specifically Pakistan is battling with malnutrition that has enormous impact on liver damage and poor health. According to report, ~25% of adult population of South Punjab is suffering from liver diseases [
4].
The nature has rewarded mankind with astounding natural herbal sources which have potential to protect liver failure [
5]. Medicinal herbs and plants are cherished benedictions from nature proved their worth in hepatoprotection. Among these herbs
Glycyrrhiza glabra, Silybum marianum, Phyllanthus amarus, Solanum nigrum, Ginkgo biloba, Alstonia scholaris, and
Mangifera indica have been reported with significant hepatoprotective activity against various hepatotoxic substances [
6].
Zingiber officinale (ginger) and
Azadirachta indica (neem) are valuable and efficacious herbs, having effective role in liver protection and improving liver function [
7,
8]. The efficacy of the plants always dependent on their phytochemicals and chemical composition and reports have proved that Z.
officinale and
A. indica are rich source of phytochemicals and can be used to treat disorders associated with free radicals. Phytochemistry of ginger revealed significant bioactive compounds such as Gingerol, shogaols, 6-paradol, 6-gingerdione, 10-gingerdione, 4-gingerdiol and 6-gingerdiol. Whereas, important phytochemical components in neem are azadirachtin, nimbandiol, Quercetin, azadirachtin-A, isoazadirolide, nimbaflavone, and 6-desacetylnimbinene [
9,
10].
The liver is most significant part of body gifted by nature to human and performs several functions that include bile formation, cholesterol and blood plasma proteins synthesis, glucose conversion, glycogen storage, hemoglobin production and detoxification of health hazards [
11]. It is involved in the metabolism of nutrients and drugs that occurs in two phases i.e., first phase includes Cytochrome P450 (CYP) enzymes found in microsomes and second phase is carried out through Glucuronosyltransferase (UGT), N-acetyltransferase (NAT) and Glutathione S-transferase (GST) present in cytosol [
12,
13]. In this research, efforts were made to elaborate hepatoprotective role of neem and ginger against paracetamol induced liver damage. Moreover, antioxidant potential of ginger and neem is limelight of current research study. Biochemical analysis especially liver function test (LFT) is conducted to prove effectiveness of neem and ginger in hepatic protection and function improvement.
2. Materials and Methods
The A. indica leaves were collected from Department of Agriculture, Bahauddin Zakariya University Multan, Z. officinale rhizome was obtained from the local market and unnecessary material was isolated from the sample. A. indica leaves were washed, rhizome was cleaned, followed by shade sun-drying for 48 hours (12 hours/day). After drying samples were ground into fine powder in grinder (Model BJ-9176) and placed into polyethylene zip bags for further use. The antioxidant activity of both plants has been determined in Human Nutrition Lab, and efficacy has been conducted in Department of Human Nutrition, Bahauddin Zakariya University, Multan.
Determination of Anti-Oxidants
Antioxidant-rich extracts of dried neem and ginger powder were formulated by mixing the sample with solvent,
i.e., distilled water, ethanol, acetone and hexane. Samples were mixed and homogenized for 5-6 hours by using an orbital shaker (Model, BSOT-604), and rest overnight. The filtered (through Whatman No. 4 filter paper) extracts were concentrated on a rotary evaporator (Model, RE202) and then were ready to execute the anti-oxidant protocols, showed in
Figure 1. The Total Phenolic Contents (TPC) of
Z. officinale and
A. indica were determined by the modified Folin–Ciocalteau assay. The outcomes were expressed as mgGAE /g (mg gallic acid-equivalent/g of extract). The absorbance (Abs) was determined by spectrophotometry (Model, 823-0210 P-2-R) at a wavelength (λ) of 760nm and against a control (without sample).
DPPH (2, 2-Diphenyl-1-picrylhydrazyl)-Free Radical Scavenging Activity Assay
To determine the anti-oxidant capacity of
Z. officinale and
A. indica, a DPPH-free radical scavenging assay was used. The following
Equation I was used to determine the percentage of radical scavenging activity. Accordingly, the absorbance was determined by spectrophotometry (Model 823-0210 P-2-R) at a wavelength (λ) of 517 nm against a control (without sample).
Ferric Ion Reducing Antioxidant Power (FRAP) Assay
The metal chelating ability of the extracted fractions were measured using a FRAP assay and ferric reducing power (µMFe/g) was calculated using the following
Equation II and expressed as µMFe/g sample. Accordingly, the absorbance was determined by spectrophotometry (Model 823-0210 P-2-R) at a wavelength of 593 nm against a control (without sample).
Efficacy Studies and Housing of Rats
The 6–8-weeks old Sprague Dawley rats were purchased from Pharmacy Department, Bahauddin Zakariya University Multan, Pakistan after physical inspection. The 20 animals (Weighed 120-140g) were equally divided into 4 groups and were fed on control diet and water for one-week acclimatization period demonstrated in
Figure 2. The temperature was maintained at (25±2
oC), relative humidity (55±3%) in well-ventilated room with 12 hours’ light-dark cycle.
Induction of Liver Damage and Experimental Diets
Liver damage was induced orally through water dissolved paracetamol 400mg/kg body weight for consecutive seven days by following the protocol of [
14] and showed in
Figure 3. The four groups were fed on their respective diets i.e., D
1 (Negative Control fed on control diet), D
2 (Paracetamol induced hepato-toxic fed on control diet), D
3: (Paracetamol induced hepato-toxic fed on 2.5 % ginger) and D
4: (Paracetamol induced hepato-toxic fed on 2.0% neem). The ingredients for diet include corn oil (10.0%), corn starch (66.50%), wheat bran (5.0%), cellulose (5.0%), casein protein (10.0%), vitamins (2.5%), and minerals (1.0%). The daily monitoring of water and feed intake were done and body weights were monitored weekly during experimental study.
Sacrificing of Animals
The rats were anesthetized and sacrificed after 6 weeks’ study using ketamine as an anesthesia showed in
Figure 4. The blood was collected by heart puncture in ethylenediaminetetraacetic acid (EDTA) and gel vials and organs (liver, heart, kidneys, heart, lungs, and spleen) were removed, cleaned and weighed (GX-600, Japan). Blood was collected for hematological analysis and biochemical examination.
Liver Function Test (LFT)
Liver Function Test (LFT) including liver enzymes like Alanine transaminase (ALT), Aspartate transaminase
(AST), and Alkaline phosphatase (ALP) and bilirubin were measured as these enzymes indicate the normal functionality of liver [
15]. The liver enzymes were determined in an in an ELISA reader (Bio-Tek, Winooski, VT, USA) following the protocol of [
16].
Renal Function Test (RFT) and Serum Electrolytes
The normal levels of serum creatinine, blood urea nitrogen (BUN) depict the proper renal functionality and measured using commercially available kits. The serum electrolytes were measured following the procedure of [
17] involving spectrophotometry using specific colorimetric kits.
Blood Lipid Profile, Blood Glucose and Serum Protein
Serological analysis comprising blood glucose, lipid profile and protein profile. Blood lipid profile covers triglycerides (TG), total cholesterol (TC), high density lipoproteins (HDL), low-density lipoproteins (LDL) and very low-density lipoproteins (VLDL). These parameters were analyzed by enzymatic hydrolysis and oxidation of the sample and sample was checked at 456 nm [
18,
19,
20]. Serum protein analysis contains serum albumin and globulin level and analyzed by using automatic chemistry analyzer (BS-240 VET, Mindray, China).
Hematological Analysis
The hematological analysis includes red blood cells (RBC’s), hemoglobin (Hb), hematocrit, white blood cells (WBC’s) and different white blood cells were determined [
21,
22]. Briefly, blood samples were collected in tubes indicated in
Figure 5(a), containing ethylenediaminetetraacetic acid (EDTA) as an anticoagulant, centrifuged
Figure 5(b) and examination was conduct by using automated hematology analyzer (Sysmex KX-21N, Japan).
Statistical Analysis
Data obtained was analyzed using statistical package i.e., Cohort V-6.1 (Co-Stat-2003). Analysis of Variance (ANOVA) technique was applied to determine the level of significance. Means were separated using Duncan’s Multiple Range test (DMRt).
3. Results
Phytochemistry referred as chemical composition predominantly secondary metabolites of plants that are also known as phytochemicals or bioactive compounds. The rich phytochemistry provides effective health benefits and protects humans against various pathogens. The phytochemicals are potent antioxidant present in natural products, capable of counteracting the damaging effects of free radicals and oxidation. The pathogenesis of diseases such as liver cirrhosis, diabetes mellitus, cancer and chronic or acute kidney failure is mainly linked with damage caused by free radicals and oxygen reactive species. Antioxidants can reduce such damage by making them stable via donating or accepting electron.
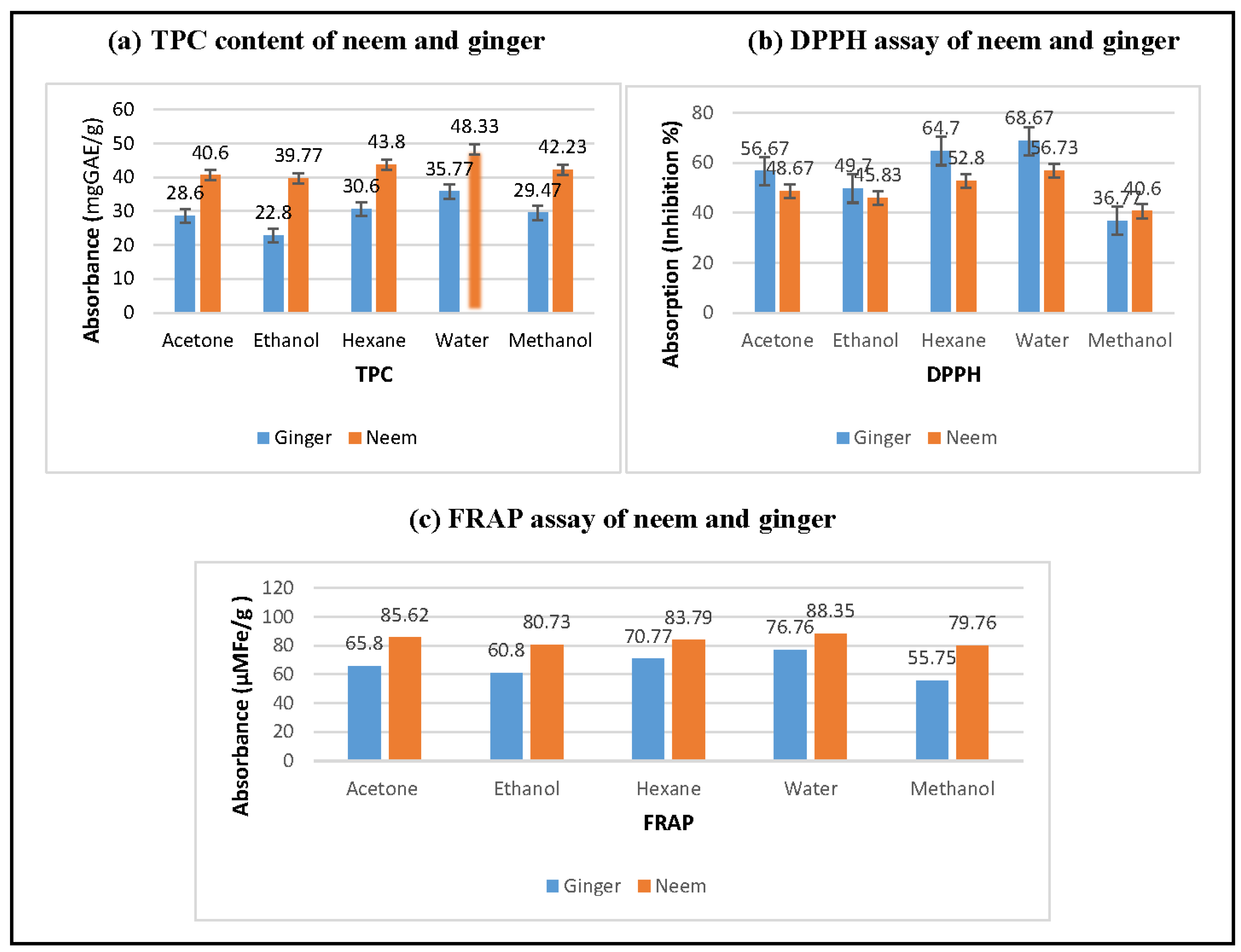
The first phase of research, the extracts were prepared that were analyzed for the antioxidant parameters i.e., total polyphenol (TPC) (a), 2, 2-diphenyl-1-picrylhydrazyl (DPPH) (b), ferric reducing antioxidant power assay (FRAP) (c). The results concerning total polyphenol (TPC), 2, 2-diphenyl-1-picrylhydrazyl (DPPH) and ferric reducing antioxidant power assay (FRAP) indicated the significant differences as a function of solvents and plants i.e., neem and ginger. The results indicated that the neem contain higher total polyphenols as compared to ginger, and extraction with water resulted in higher recovery (48.33 mgGAE/g). The DPPH test measures the scavenging capacity and present research intervention showed that ginger and neem contain several bioactive compounds that are responsible for strong antioxidant potential. The solvent fractions of ginger recorded maximum inhibition (68.67 %) in water extract and lowest (36.77 %) in methanolic extract. The DPPH inhibition in the range of (40.60 %) to (56.73 %) was recorded in bioactive rich fractions of neem. Overall, ginger has more scavenging capacity than neem but the methanolic extract of neem showed slightly higher value. FRAP is another antioxidant assay that is used to measure the metal chelating capacity of foods, beverages and plants. The FRAP assay explicated higher anti-oxidant potential of neem than ginger especially in water and acetone with the recorded values of (88.35 and 85.63 µM Fe/g), respectively.
Figure 6.
Antioxidant potential of Neem and Ginger.
Figure 6.
Antioxidant potential of Neem and Ginger.
Efficacy Study
This phase of research study includes hepatoprotective effect of neem and ginger against paracetamol (acetaminophen) induced liver damage in Sprague Dawley rats. The rats were fed with paracetamol 400mg/kg body weight for consecutive seven days to damage liver and treated with neem and ginger for six weeks.
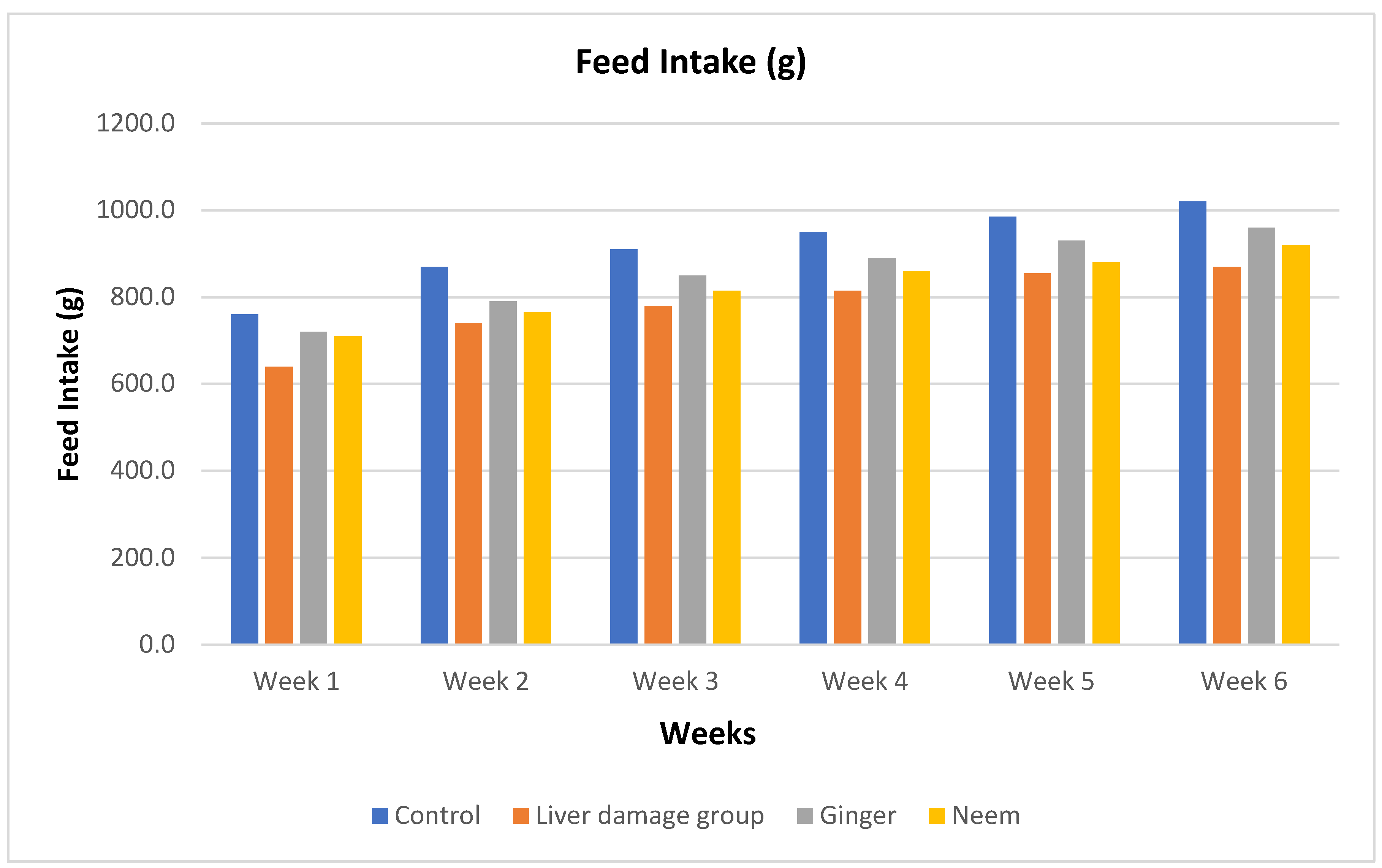
Feed Intake
Feed intake data collected on daily basis during the entire study period and described weekly in
Figure 7. The data demonstrated that in 1
st week maximum feed intake was recorded in control. However, liver damage group (positive control) consumed lower amounts of feed and same trend was recorded for whole study duration. Feed intake constantly reduced in liver injured rats however, treated animals showed increased intake prominently in ginger group. During 2
nd to 4
th week, liver damages showed recovery as indicated from increased feed intake. In the last two weeks, maximum feed intake was noticed in ginger group that reflects the promising effects of ginger against paracetamol induced liver injury.
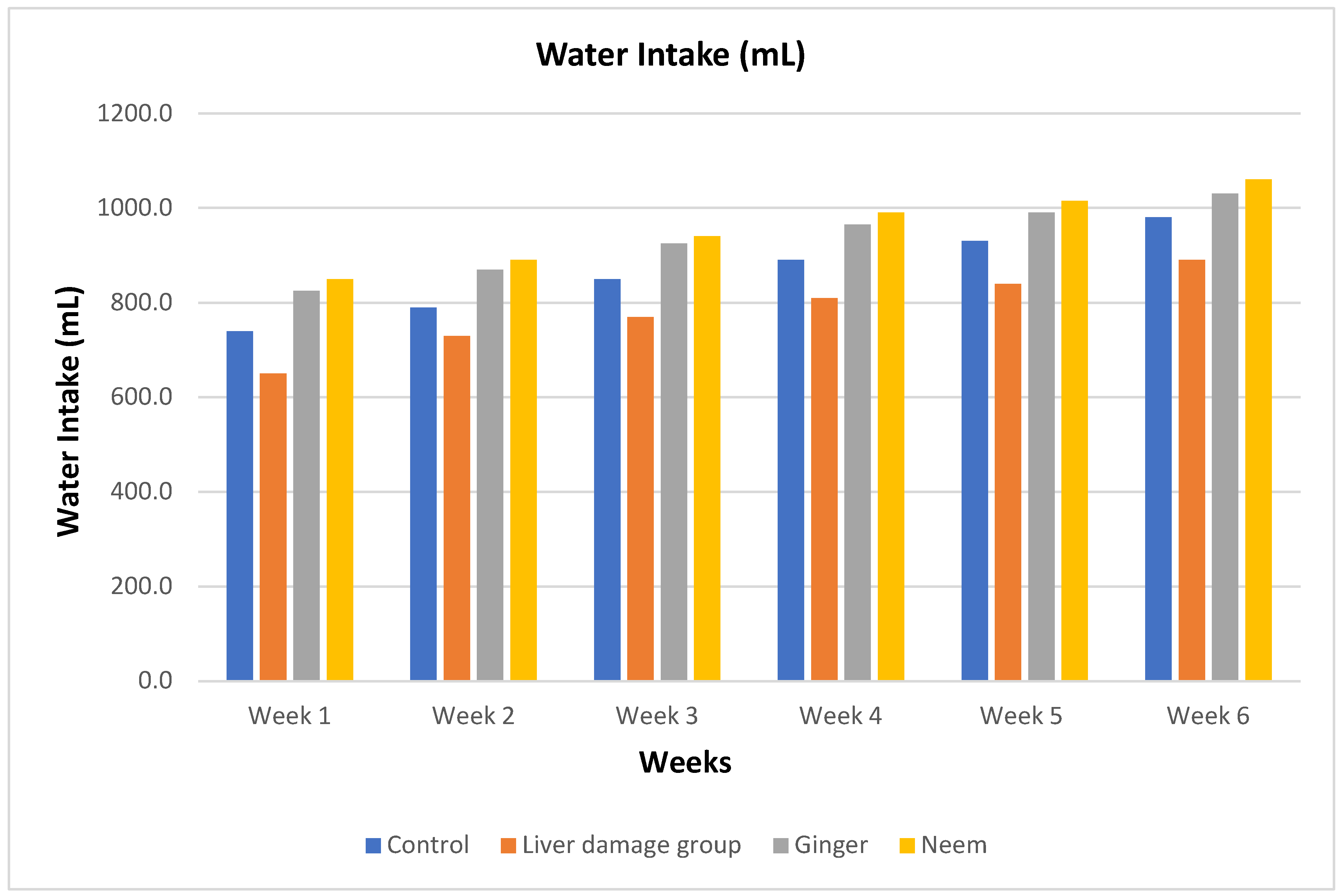
Water Intake
The water intake presented in
Figure 8. revealed non-significant changes in 1
st week of study in ginger and neem groups. However, water intake reduced in liver damage group as compare to others throughout the study. In 2
nd, 3
rd and 4
th week of study, water consumption increased especially in normal control and treated groups. Water consumption continuously elevated in 5
th and 6
th weeks of study. The water intake data revealed excess water consumption in groups of rats fed on 2.5% ginger powder and 2% neem powder followed by placebo, whilst minimum water intake was recorded in the paracetamol induced liver injured rats.
Body and Organ Weight (g)
The paracetamol induced liver injured rats fed with ginger and neem indicated significant changes in body weight and body to organ weight ratio. The results regarding body weight and organ weight (
Table 1) in 42 days study revealed the lowest body weight of 130.78±7.43 gm in liver injury group as compared to placebo (173.03±9.03 gm). However, weight improved in treated groups as it increased to 148.33±4.77 and 144.52±6.70 gm in ginger and neem groups, respectively. Liver weight ratio significantly increased due to liver injury but after treated with ginger and neem it declines and maximum reduction was noticed in ginger (3.09±0.32 gm/100 gm). Results regarding right and left kidney expounded major variations especially in neem group with recorded values of 0.46±0.05 and 0.45±0.05 gm/100 gm, respectively. Spleen and lungs demonstrated non-significant changes however, in ginger group organ to body weight ratio of lung increased with the value of (0.86±0.11 gm/100 gm).
Glucose and Serum Lipid Profile
Serum glucose and lipid profile revealed significant variations as glucose, triglycerides, total cholesterol and LDL increased in liver damage group but significantly decreased in ginger and neem group in
Table 2. Serum glucose increased to (95.30±5.05 mg/dL) in liver damage rats while reduced to (78.97±8.03 mg/dL) in ginger and (89.37±9.27 mg/dL) in neem group. Triglycerides (TG) and total cholesterol (TC) elevated to (91.18±6.61 mg/dL) and (105.43±7.69 mg/dL), respectively. The TG reduced in ginger and neem i.e., (76.68±11.35, 76.97±5.50 mg/dL), whilst total cholesterol decreased to (92.34±15.71 and 96.06±5.97 mg/dL), respectively. The HDL slightly fall in liver damage group, whereas improved in neem group and LDL amplified in paracetamol induced liver damage rats with the value of (52.06±2.56 mg/dL) decreased to (42.09±2.75 mg/dL) in ginger and (44.12±5.02 mg/dL) in neem groups.
Serum Protein Profile
Serum protein analysis covering total protein, albumin and globulin demonstrated significant variations in results and values are displayed in
Table 3. Data concerning total protein revealed decline in liver damage group from normal control, while increased in ginger and neem groups and the recorded values are 6.39±0.40, 7.06±0.33, 6.58±0.29 and 6.38±0.37 g/dL respectively. Serum albumin decreased to (2.85±0.34 g/dl) from (3.50±0.33 g/dl) but improved in ginger and neem group with the values of (3.18±0.27 g/dL) and (2.95±0.18 g/dL). Meanwhile, Serum globulin decreased to (3.11±0.33 g/dl) from (3.23±0.21 g/dl) but improved in ginger and neem group with the recorded values of (3.15±0.18 g/dL) and (3.04±0.48 g/dL).
Renal Function Test
The paracetamol induced renal damage as well and results are also supporting the claims. The results regarding renal function test (RFT) revealed significant variations in values i.e., serum creatinine, urea and serum electrolytes increased within liver damage group. The maximum increase in serum creatinine (1.26±0.03 mg/dL) and urea (44.09±1.90 mg/dL) was recorded in liver damage rats. The improved values for creatinine in ginger and neem groups are (1.03±0.05 and 0.98±0.06 mg/dL). The values of Urea also improved in rats fed with ginger and neem and the values are (36.07±1.87 and 38.04±2.02 mg/dL), respectively (
Table 4). Serum electrolytes declined in paracetamol induced liver damage group, however, slight improvements were observed in treatment groups with the values of 132.75±15.03 mg/dL for sodium and 38.12±3.53 mg/dL for potassium.
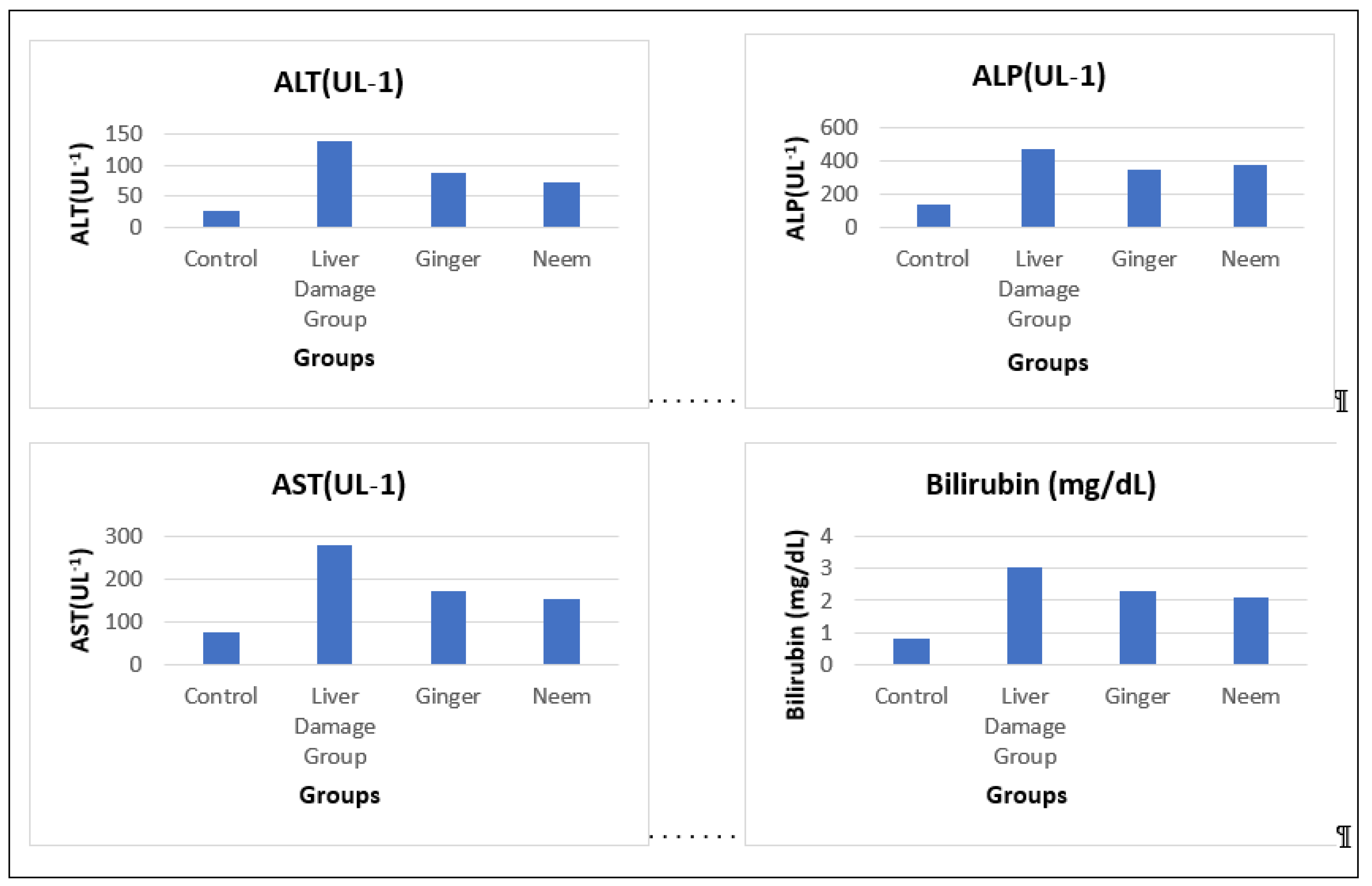
Liver Function Tests
Liver function test (LFT) comprises bilirubin and liver enzymes aspartate aminotransferase (AST), alkaline phosphatase (ALP) and alanine aminotransferase (ALT) are major indicator of liver damage and hepatotoxicity. Elevated liver enzymes and bilirubin level unveiled liver damage but after treated with ginger and neem improved liver function was noticed. The augmented value of bilirubin was (3.06±0.19 mg/dL) but reduced to (2.32±0.1 mg/dL) in ginger and (2.09±0.12 mg/dL) in neem group. In case of AST and ALT values dropped to (172.77±7.65 U/L), (152.90±8.92 U/L), (86.70±6.33 U/L) and (72.62±5.70 U/L) in ginger and neem respectively. Alkaline phosphatase (ALP) value amplified to (468.93±36.55 U/L) but decreased to (347.51±25.17 U/L) in ginger and (375.08±31.35 U/L) in neem group and results are presented in
Table 5 and
Figure 9.
Hematological Profile
Hematological profile of liver damage rats predicted significant changes, especially red blood cells (RBC’s), Hemoglobin (Hb) and Hematocrit (HCT). Red blood cells reduced to (4.06±0.18 million/mm
3) in liver injured rats but improved in ginger and neem with the values of 4.53±0.31 and 4.31±0.30 million/mm
3 respectively, shown in
Table 6. Hemoglobin value declined in liver injured rats, while increased in treatment groups and the recorded value are 10.27±0.80, 11.73±0.17, and 11.35±1.24 g/dL. Results regarding MCV and MCH displayed minor variations with non-significant impact as values lessened in liver damage rats but improved with treatment of ginger and neem.
White Blood Cells
White blood cells (WBCs) and their supportive cells i.e., neutrophils, lymphocytes, monocytes, eosinophils and basophils revealed significant changes in results. White blood cells increased to (5.02±0.29 10
3/μL) from (3.97±0.6010
3/μL) indicating liver damage and inflammation but the values reduced to (4.73±0.23 10
3/μL)) in ginger group, however increased in neem group having a value of (5.96±0.68 10
3/μL). Neutrophils decreased within liver damage group (28.26±2.17 %) but increased in ginger group with the value of (30.60±2.44 %). Monocytes elevated in liver injured rats but declined in treated groups and the recorded results are 4.66±0.46, 2.66±0.25, and 2.89±0.10 % respectively, shown in
Table 7.
4. Discussion
The acetaminophen (paracetamol) is non-opioid analgesic drug used to treat fever and pain but its excessive use can damage liver. The current study plan was designed to identify potent side effects of acetaminophen on liver, and hepatoprotective properties of
Z. officinale and
A. indica. The six weeks study revealed significant augmentation in liver parameters in positive control group, however, values dropped in treatment groups. The hepatoprotective role of ginger and neem was due to their antioxidant potential in different solvents. The results of antioxidant activity are advocated by the findings of [
23] worked on anti-cancer property of neem and highlighted antioxidant potential of neem. The antioxidant activity of ginger was proven by [
24] highlighted TPC and flavonoid content of ginger. The feed and water intake reduced in liver damage groups, though, the consumption improved in experimental groups fed on ginger and neem. The hepatic damage reduced the feed intake and loss of appetite could further result in malnutrition thus exuberating the consequences of liver injury. The addition of ginger and neem in the experimental diets partially recovered the loss of appetite. The plant leaves powder might increase water intake because consumption of plant powder can cause dryness of mouth due to presence of high concentration of minerals and bioactive compounds. Moreover, plant leaves contain high protein contents, which increased body temperature through activation of β-adrenergic receptor signaling, results in increase water intake [
25]. Low feed and higher water intake cause weight reduction in liver injured rats but improved in treated groups. However, liver weight and size increased due to liver damage, but reduced after treated with ginger and neem. Other body organs displayed minor changes in weight reduction and the result of improved body organs weight was advocated by the findings of [
26] studied CCl
4 induced hepatotoxicity and treated with olive oil and black cumin.
Serum glucose and lipid profile revealed significant variations as serum glucose, triglycerides, total cholesterol and LDL increased in liver damage group but significantly decreased in treated groups. The decline bile production due to liver damage outcomes in elevated serum cholesterol, because bile is involved in the digestion, absorption and regulation of lipids [
27]. Accretion of excessive fat in liver results in insulin resistance, decreases insulin sensitivity, and increase blood glucose level [
28,
29]. The result of reduced serum glucose and lipid profile was supported the findings of [
30] studied polychlorinated biphenyls (PCBs) induced acute hepatotoxicity in rats and treated with aqueous extract of ginger. At the end of study, they stated significant reduction in serum lipid and glucose profile. The results of serum total protein decline in liver damage group while increased in ginger and neem groups. Serum globulin also declined in liver damage group, but improved in ginger group. Liver is vital organ in protein and amino acids metabolism thus, poor liver function and damage cause impaired protein metabolism and proteinuria [
31]. Hepato-renal syndrome (HRS) or progressive kidney failure mainly occur due to hepatic damage cause loss of total protein, albumin and globulin but serum protein profile improved in treated groups. [
32] researched on hepatoprotective effects of neem in hepatotoxic rats caused by CCl
4. They treated rats with seed oil of neem with the dose of 0.25 mL/kg, 0.5 mL/kg and 1.0 mL/kg for two weeks and stated improved hepato and renal functions in rats.
Results regarding renal function test (RFT) indicated significant variations in values as, serum creatinine, urea and serum electrolytes decreased within liver damage group but improved in treatment groups. Poor liver function interferes with creatine production, as hepatic malfunction cause low production and storage of creatine hence results in declined serum creatinine. Injured liver is unable to metabolize amino acids and urea due to low production of urease enzyme, substrates of urea cycle and low hepatic mass [
33]. Furthermore, liver damage outcomes in electrolytes imbalance. Improved renal function was proved by [
34] investigated on neem leaves ethanolic extract in hepatotoxic rats and affirmed improved creatinine and urea. The hepatic biomarkers significantly increased in liver damage group, due to liver injury. Liver is the main organ involved in metabolism and detoxification of all substances either food or drug. Paracetamol augments oxidative stress through the production of free radical species and lipid peroxidation, moreover pathogenicity of disease includes expression of numerous inflammatory cytokines and pathways such as, IL-6, COX-2, NF-κB, and TNF-α [
35]. Inflammation and stress enhance the expression of nuclear factor kappa B (NF-κB), a key transcription factor which regulates cytokines. The gene mainly responsible for inflammation is COX-2, regulate NF-κB and Kupffer cells are accountable for the expression of COX-2 gene [
36]. The protective mechanism against these inflammatory elements is transcription factor Nrf
2 as this factor is intricate in the regulation of cytoprotective enzymes and promoted cellular protection against these deleterious species [
37]. The aqueous extract of ginger and neem leaves accredited efficacious against liver necrosis caused by paracetamol in rats [
38]. Later, [
39] and [
40] investigated nutraceutical potential of ginger phenolic extracts and ginger nanoparticles respectively, reported hepatoprotection and significant rehabilitation of liver functions. The effects are due to improved antioxidant enzyme activity, enhanced detoxification, and reduced lipid peroxidation.
Hematological profile of liver damage rats predicted significant changes especially red blood cells (RBCs), Hemoglobin (Hb) and Hematocrit (HCT). Red blood cells reduced in positive control group but improved in treated groups. Hepatic dysfunction leads towards bone marrow alterations such as low erythropoietin production and decrease iron storage in hepatocytes. Moreover, poor renal function or kidney damage due to HRS also contributes in low erythropoietin production thus cause decline blood count and hemoglobin (Hb) [
41]. White blood cells (WBC’s) and their supportive cells i.e., neutrophils, lymphocytes, monocytes, eosinophils and basophils increased in liver damage group, but decreased in treatment groups. Augmented white blood cells and supportive cells especially monocytes and lymphocytes indicating liver inflammation. Leukocytes are first line border defense mechanism of body and protect against infections and invaders. After treated with ginger and neem hematological profile improved and leukocytes decline showing rehabilitation and recovery or animals. Our result of improved hematology was supported by the results of [
42].
5. Conclusion
The study concluded that Z. officinale and A. indica have significant antioxidant activity and hepatoprotective potential. Moreover, the plants having substantial therapeutic attributes because of rich phytochemistry. Thus, consumption of neem and ginger in hepatic damage can prove efficacious by lowering hepatic biomarkers and can restore liver function.
Author Contributions
Conceptualization, M.T.S.; methodology, S.S., R.A.; formal analysis, S.S. C.C; software, M.T.S.; validation, M.T.S. M.I; original draft preparation, S.S., A.M.N.; writing and editing, A.M.N. R.N.; reviewing, M.T.S., A.M.N.; visualization, M.I. All authors have read and agreed to this original manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research did not receive any funding.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
| OTC |
over the counter |
| NAFLD |
non-alcoholic fatty liver disease |
| CYP |
cytochrome P450 |
| UGT |
glucuronosyltransferase |
| NAT |
N-acetyltransferase |
| GST |
glutathione S-transferase |
| LFT |
liver function test |
| TPC |
total phenolic contents |
| DPPH |
2, 2-Diphenyl-1-picrylhydrazyl |
| EDTA |
ethylene diamine tetra acetic acid |
| ALT |
alanine transaminase |
| AST |
aspartate transaminase |
| ALP |
alkaline phosphatase |
| RFT |
renal function test |
| TG |
triglycerides |
| TC |
total cholesterol |
| HDL |
high density lipoproteins |
| LDL |
low-density lipoproteins |
| VLDL |
very low-density lipoproteins |
| RBC’s |
red blood cells |
| Hb |
hemoglobin |
| HCT |
hematocrit |
| WBC’s |
white blood cells |
| ANOVA |
analysis of variance |
| DMRt |
duncan’s multiple range test |
| FRAP |
ferric reducing antioxidant power |
| PCBs |
polychlorinated biphenyls |
| HRS |
hepato-renal syndrome |
| NF-Κb |
nuclear factor kappa B |
References
- Asrani, S.K.; Devarbhavi, H.; Eaton, J.; Kamath, P.S. Burden of liver diseases in the world. J. Hepatol., 2019, 70, 151–171. [Google Scholar] [CrossRef] [PubMed]
- Bourhia, M.; Ullah, R.S.; Alqahtani, A.; Ibenmoussa, S. Evidence of drug-induced hepatotoxicity in the Maghrebian population. Drug Chem. Toxicol., 2022, 45, 985–989. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Wang, N.; Tan, H.Y.; Li, S.; Zhang, C.; Feng, Y. Function of Akkermansia muciniphila in obesity: interactions with lipid metabolism, immune response and gut systems. Front. microbiol., 2020, 11, 219. [Google Scholar] [CrossRef] [PubMed]
- Butt, A.S. Epidemiology of viral hepatitis and liver diseases in Pakistan. EJOHG, 2015, 5, 43. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Niu, Y.; Xing, P.; Wang, C. Bioactive polysaccharides from natural resources including Chinese medicinal herbs on tissue repair. Chin. Med., 2018, 13, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Zhang, G.; Song, M.; Wang, J.; Shen, C.; Chen, Z.; Liu, C. Activation of farnesoid X receptor by schaftoside ameliorates acetaminophen-induced hepatotoxicity by modulating oxidative stress and inflammation. ARS, 2020, 33, 87–116. [Google Scholar] [CrossRef] [PubMed]
- Islas, J.F.; Acosta, E.; Zuca, G.; Delgado-Gallegos, J.L.; Moreno-Treviño, M.G.; Escalante, B.; Moreno-Cuevas, J.E. An overview of Neem (Azadirachta indica) and its potential impact on health. J. Funct. Foods, 2020, 74, 104171. [Google Scholar] [CrossRef]
- Shahrajabian, M.H.; Sun, W.; Cheng, Q. Clinical aspects and health benefits of ginger (Zingiber officinale) in both traditional Chinese medicine and modern industry. ACTA AGR SCAND B-S, 2019, 69, 546–556. [Google Scholar] [CrossRef]
- Adebayo, S.A.; Amoo, S.O.; Mokgehle, S. N.; Aremu, A.O. Ethnomedicinal uses, biological activities, phytochemistry and conservation of African ginger (Siphonochilus aethiopicus): A commercially important and endangered medicinal plant. J. Ethnopharmacol, 2021, 266, 113459. [Google Scholar] [CrossRef] [PubMed]
- Patil, S.M.; Shirahatti, P.S.; Ramu, R. Azadirachta indica A. Juss (neem) against diabetes mellitus: a critical review on its phytochemistry, pharmacology, and toxicology. JPP 2021. [Google Scholar] [CrossRef] [PubMed]
- Wan, L.; Xia, T.; Du, Y.; Liu, J.; Xie, Y.; Zhang, Y.; Shi, C. Exosomes from activated hepatic stellate cells contain GLUT1 and PKM2: a role for exosomes in metabolic switch of liver nonparenchymal cells. FASEB J., 2019, 33, 8530–8542. [Google Scholar] [CrossRef] [PubMed]
- Gong, L.; Zhou, H.; Wang, C.; He, L.; Guo, C.; Peng, C.; Li, Y. Hepatoprotective effect of forsythiaside a against acetaminophen-induced liver injury in zebrafish: Coupling network pharmacology with biochemical pharmacology. J. Ethnopharmacol., 2021, 271, 113890. [Google Scholar] [CrossRef] [PubMed]
- Gao, Q.; Gu, Y.; Jiang, Y.; Fan, L.; Wei, Z.; Jin, H.; Liu, Y. Long non-coding RNA Gm2199 rescues liver injury and promotes hepatocyte proliferation through the upregulation of ERK1/2. Cell Death Dis, 2018, 9, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.T.; Quispe, C.; Islam, M.A.; Ali, E.S.; Saha, S.; Asha, U.H.; Sharifi-Rad, J. Effects of nerol on paracetamol-induced liver damage in Wistar albino rats. Biomed Pharmacother, 2021, 140, 111732. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.; Zhang, J.; Liang, S.; Du, Z.; Liu, J.; Sun, Z.; Duan, J. Metabolomic characteristics of hepatotoxicity in rats induced by silica nanoparticles. Ecotoxicol Environ Saf, 2021, 208, 111496. [Google Scholar] [CrossRef] [PubMed]
- Kucukler, S.; Darendelioğlu, E.; Caglayan, C.; Ayna, A.; Yıldırım, S.; Kandemir, F.M. Zingerone attenuates vancomycin-induced hepatotoxicity in rats through regulation of oxidative stress, inflammation and apoptosis. Life Sci., 2020, 259, 118382. [Google Scholar] [CrossRef] [PubMed]
- Cortes, A. L.; Gonsalez, S.R.; Rioja, L.S.; Oliveira, S.S.; Santos, A.L.; Prieto, M.C.; Lara, L.S. Protective outcomes of low-dose doxycycline on renal function of Wistar rats subjected to acute ischemia/reperfusion injury. Biochim Biophys Acta, 2018, 1864, 102–114. [Google Scholar] [CrossRef] [PubMed]
- Adegbola, P.I.; Fadahunsi, O.S.; Ajilore, B.S.; Akintola, A.O.; Olorunnisola, O.S. Combined ginger and garlic extract improves serum lipid profile, oxidative stress markers and reduced IL-6 in diet induced obese rats. Obes. Med., 2021, 23, 100336. [Google Scholar] [CrossRef]
- Silveira, L.; Borges, R.D.C.F.; Navarro, R.S.; Giana, H.E.; Zângaro, R.A.; Pacheco, M.T.T.; Fernandes, A.B. Quantifying glucose and lipid components in human serum by Raman spectroscopy and multivariate statistics. LIMS, 2017, 32, 787–795. [Google Scholar] [CrossRef] [PubMed]
- Ullah, H.; Jahan, S.; Ain, Q.U.; Shaheen, G.; Ahsan, N. Effect of bisphenol S exposure on male reproductive system of rats: A histological and biochemical study. Chemosphere, 2016, 152, 383–391. [Google Scholar] [CrossRef] [PubMed]
- Etim, E.A.; Adebayo, Y.A.; Ifeanyi, O.E. Effect of Luffa cylindrica leaf extract on hematological parameters of Swiss Albino mice. Med Aromat Plants, 2018, 7, 2167–0412. [Google Scholar]
- Ujah, G.A. Effect of aqueous leaf extract of Mangifera indica on differential white blood cell count. IJIRAS, 2017, 3, 123–125. [Google Scholar]
- Poompavai, S.; Gowri Sree, V.; RamSugitha, B. Enhanced Extraction and Improved Anticancer Activity of Neem (Azadirachta indica) Leaves by Electroporation Technique. IETE J. Res. 2021, 1–11. [Google Scholar] [CrossRef]
- Ali, A.M.A.; El-Nour, M.E.M.; Yagi, S.M. Total phenolic and flavonoid contents and antioxidant activity of ginger (Zingiber officinale Rosc.) rhizome, callus and callus treated with some elicitors. JGEB, 2018, 16, 677–682. [Google Scholar] [CrossRef] [PubMed]
- M Aborehab, N.; A Boshra, S. Hepatoprotective effect of ginger and grape seed, alone and in combination orally, in paracetamol induced acute liver toxicity in rats. IJEB 2019. [Google Scholar]
- Al-Seeni, M.N.; El Rabey, H.A.; Zamzami, M.A.; Alnefayee, A.M. The hepatoprotective activity of olive oil and Nigella sativa oil against CCl4 induced hepatotoxicity in male rats. BMC Complement Altern Med, 2016, 16, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Adewale, O.O.; Samuel, E.S.; Manubolu, M.; Pathakoti, K. Curcumin protects sodium nitrite-induced hepatotoxicity in Wistar rats. Toxicol. Rep, 2019, 6, 1006–1011. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Wang, P.; Yu, R.; Lu, J.; Jiang, M.; Zhou, K. Long-term exposure of Psoralen and Isopsoralen induced hepatotoxicity and serum metabolites profiles changes in female rats. Metabolites, 2019, 9, 263. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Sun, L.; Wang, L.; Hassan, H.M.; Wang, X.; Hylemon, P.B.; Jiang, Z. Activation of Sirt1/FXR signaling pathway attenuates triptolide-induced hepatotoxicity in rats. Front. pharmacol., 2017, 8, 260. [Google Scholar] [CrossRef] [PubMed]
- Ahd, K.; Dhibi, S.; Akermi, S.; Bouzenna, H.; Samout, N.; Elfeki, A.; Hfaiedh, N. Protective effect of ginger (Zingiber officinale) against PCB-induced acute hepatotoxicity in male rats. RSC Adv., 2019, 9, 29120–29130. [Google Scholar] [CrossRef] [PubMed]
- Mahaldar, K.; Hossain, A.; Islam, F.; Islam, S.; Islam, M.A.; Shahriar, M.; Rahman, M.M. Antioxidant and hepatoprotective activity of Piper retrofractum against Paracetamol-induced hepatotoxicity in Sprague-Dawley rat. Nat. Prod. Res., 2020, 34, 3219–3225. [Google Scholar] [PubMed]
- Okojie, S.; Idu, M.; Ovuakporie-Uvo, O. Protective effects of neem (Azadirachta indica A. Juss) seed oil on carbon tetrachloride-induced hepatotoxicity in Wistar rats. JOMPED, 2017, 1, 1–5. [Google Scholar]
- Bektur, N.E.; Sahin, E.; Baycu, C.; Unver, G. Protective effects of silymarin against acetaminophen-induced hepatotoxicity and nephrotoxicity in mice. Toxicol. Ind. Health., 2016, 32, 589–600. [Google Scholar] [CrossRef] [PubMed]
- Seriana, I.; Akmal, M.; Darusman, D.; Wahyuni, S.; Khairan, K.; Sugito, S. Neem leaf (Azadirachta indica A. Juss) ethanolic extract on the liver and kidney function of rats. Sci. World J. 2021. [Google Scholar] [CrossRef] [PubMed]
- Mani, V.; Arivalagan, S.; Siddique, A.I.; Namasivayam, N. Antioxidant and anti-inflammatory role of zingerone in ethanol-induced hepatotoxicity. Mol. Cell. Biochem., 2016, 421, 169–181. [Google Scholar] [CrossRef] [PubMed]
- Mishra, A.; Paul, S.; Swarnakar, S. Downregulation of matrix metalloproteinase-9 by melatonin during prevention of alcohol-induced liver injury in mice. Biochimie, 2011, 93, 854–866. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Wang, X.; Vikash, V.; Ye, Q.; Wu, D.; Liu, Y.; Dong, W. ROS and ROS-mediated cellular signaling. Oxid Med Cell Longev T 2016. [Google Scholar] [CrossRef] [PubMed]
- Maqbool, M.; Rasool, S.; Dar, M.A.; Bashir, R.; Khan, M. Hepatotoxicity and Hepatoprotective agents: A Mini review. PharmaTutor, 2019, 7, 34–40. [Google Scholar]
- Bakr, A.F.; Abdelgayed, S.S.; El-Tawil, O.S.; Bakeer, A.M. Assessment of ginger extract and ginger nanoparticles protective activity against acetaminophen-induced hepatotoxicity and nephrotoxicity in rats. Pak. Vet. J., 2019, 39, 479–486. [Google Scholar] [CrossRef]
- Vipin, A.V.; Rao, R.; Kurrey, N.K.; KA, A.A.; Venkateswaran, G. Protective effects of phenolics rich extract of ginger against Aflatoxin B1-induced oxidative stress and hepatotoxicity. Biomed Pharmacothe, 2017, 91, 415–424. [Google Scholar]
- Yarjou, S.; Sadeghpour, O.; Nazem, E.; Emami, A.H. Liver function and anemia pathogenesis in Iranian traditional medicine. IRCMJ, 2015, 17. [Google Scholar] [CrossRef] [PubMed]
- Morvaridzadeh, M.; Sadeghi, E.; Agah, S.; Fazelian, S.; Rahimlou, M.; Kern, F.G.; Heshmati, J. Effect of ginger (Zingiber officinale) supplementation on oxidative stress parameters: A systematic review and meta-analysis. J. Food Biochem., 2021, 45, e13612. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).