Submitted:
12 April 2024
Posted:
15 April 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Diagnostic Criteria
2.3. Clinical and Neuropsychological Data In the PDCD Patients Involved in the Intervention Study
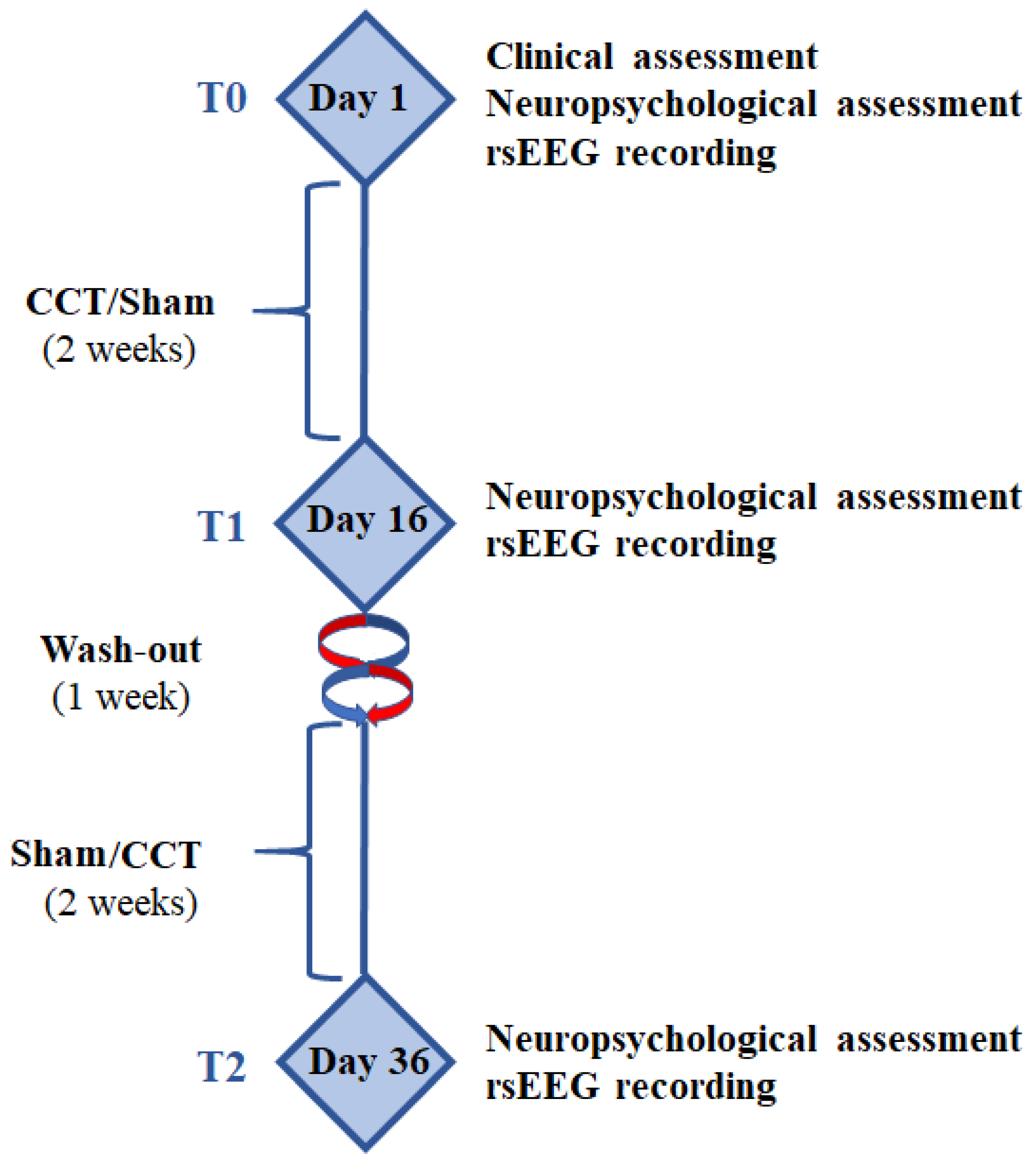
2.4. Experimental Paradigm
2.5. Computerized Cognitive Training (CCT) and Sham Programs
2.6. rsEEG Recordings
2.7. Preliminary rsEEG Data Analysis
2.8. Spectral Analysis of the rsEEG Epochs
2.9. Estimation of the rsEEG Source Solutions
2.10. Statistical Analysis of rsEEG eLORETA Source Activities
3. Results
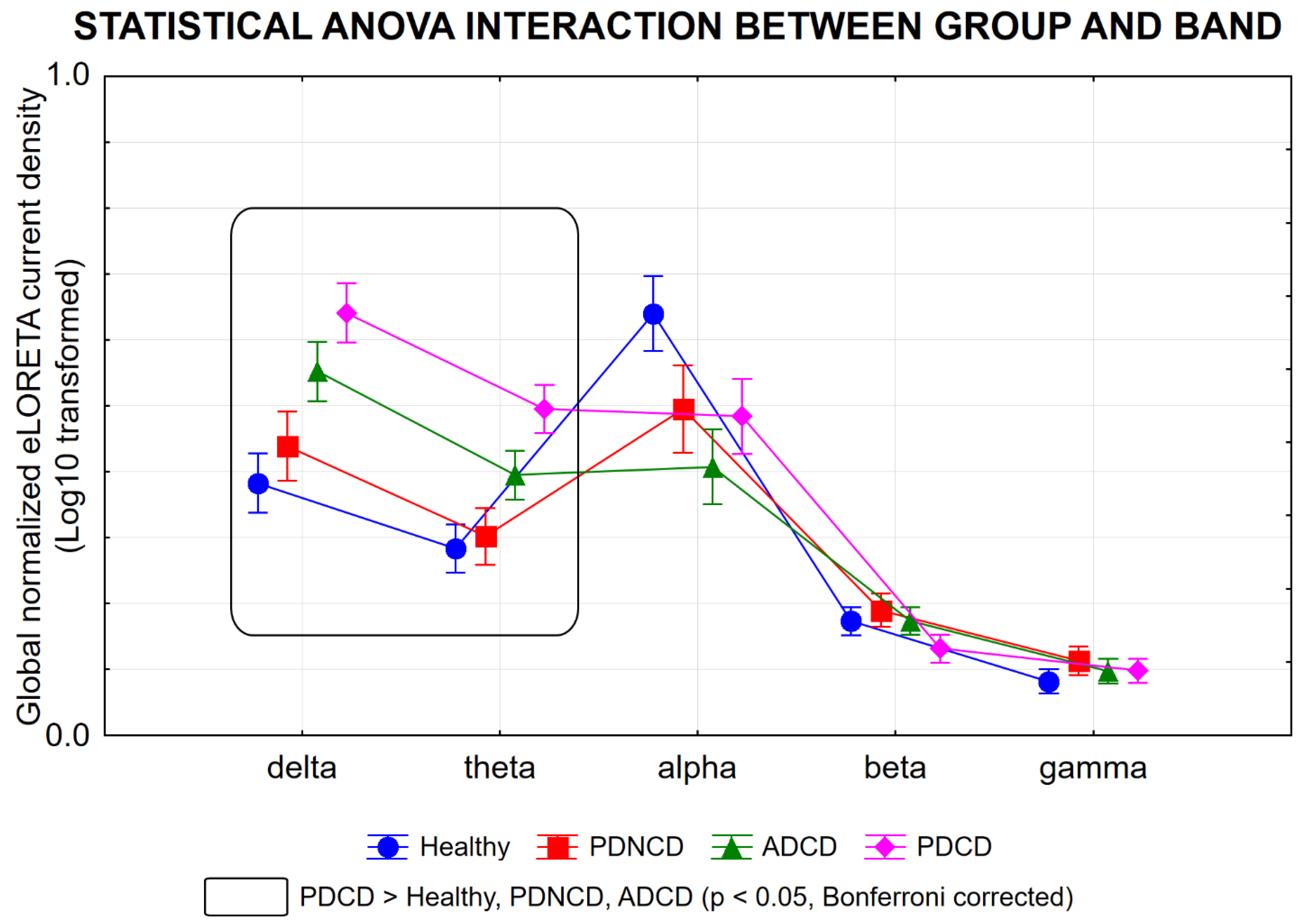
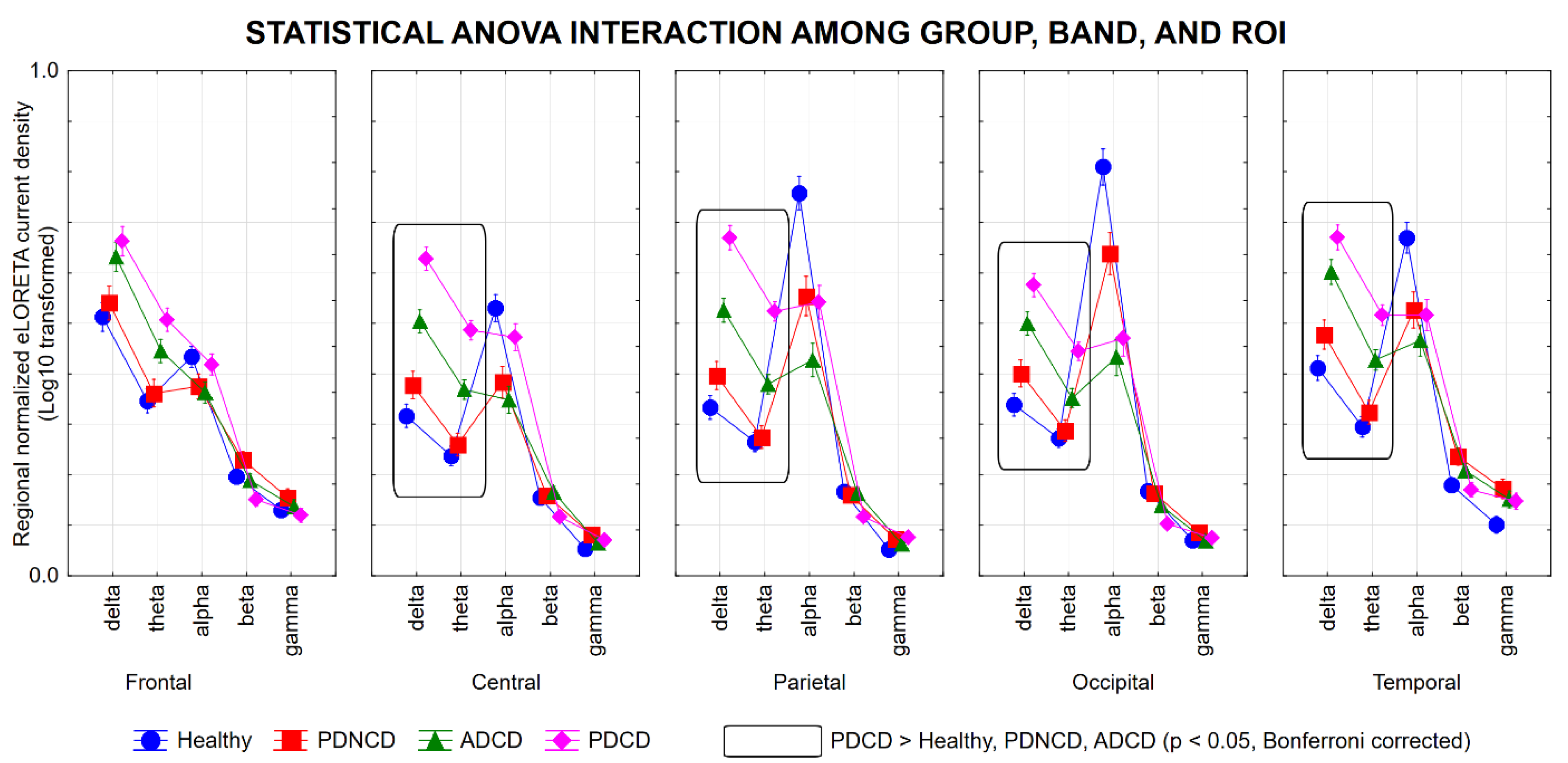
3.1. rsEEG Source Activities in the Healthy, PDNCD, ADCD, and PDCD Participants
3.2. Effects of the CCT Program on Task Performances in PDCD Patients
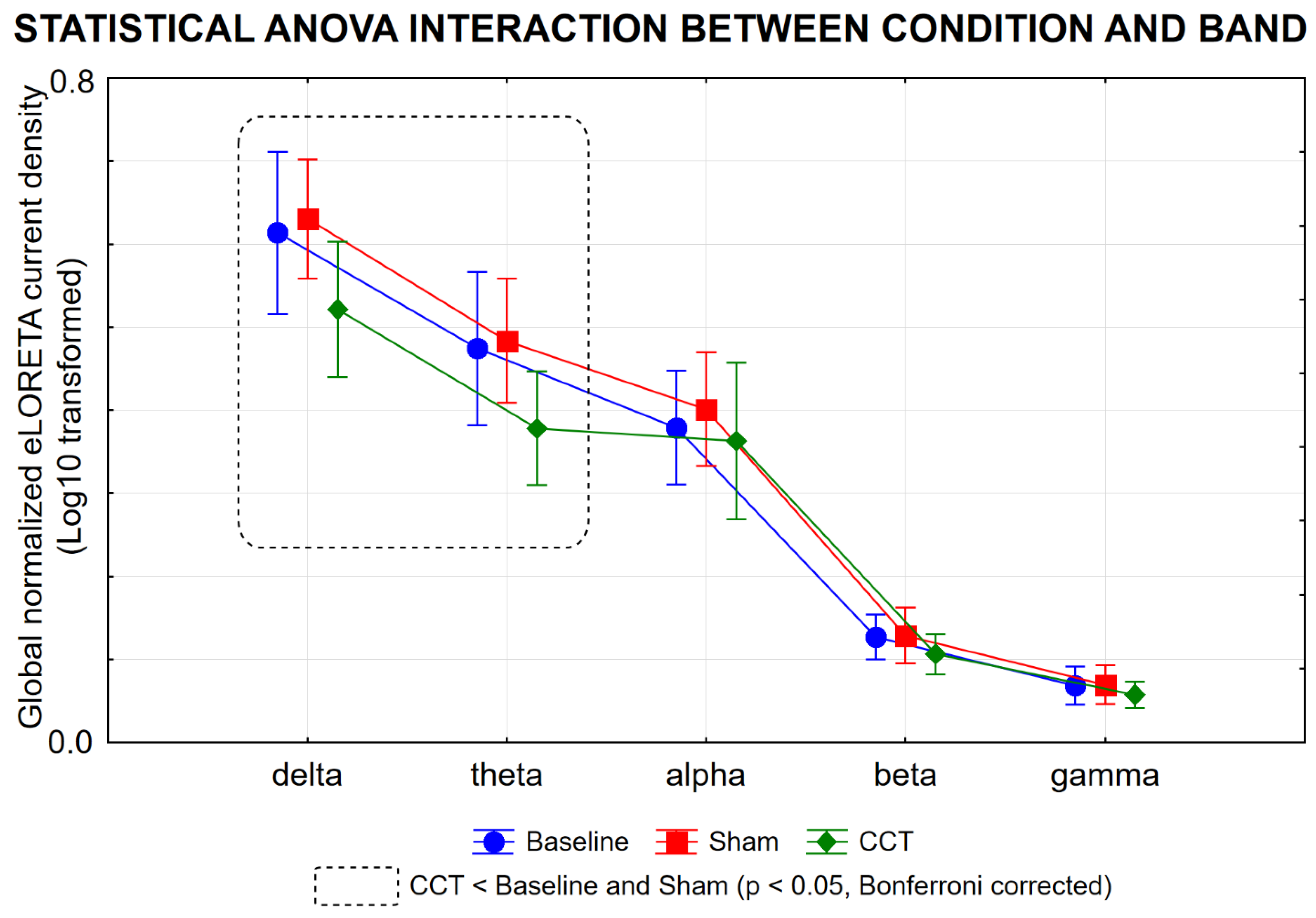
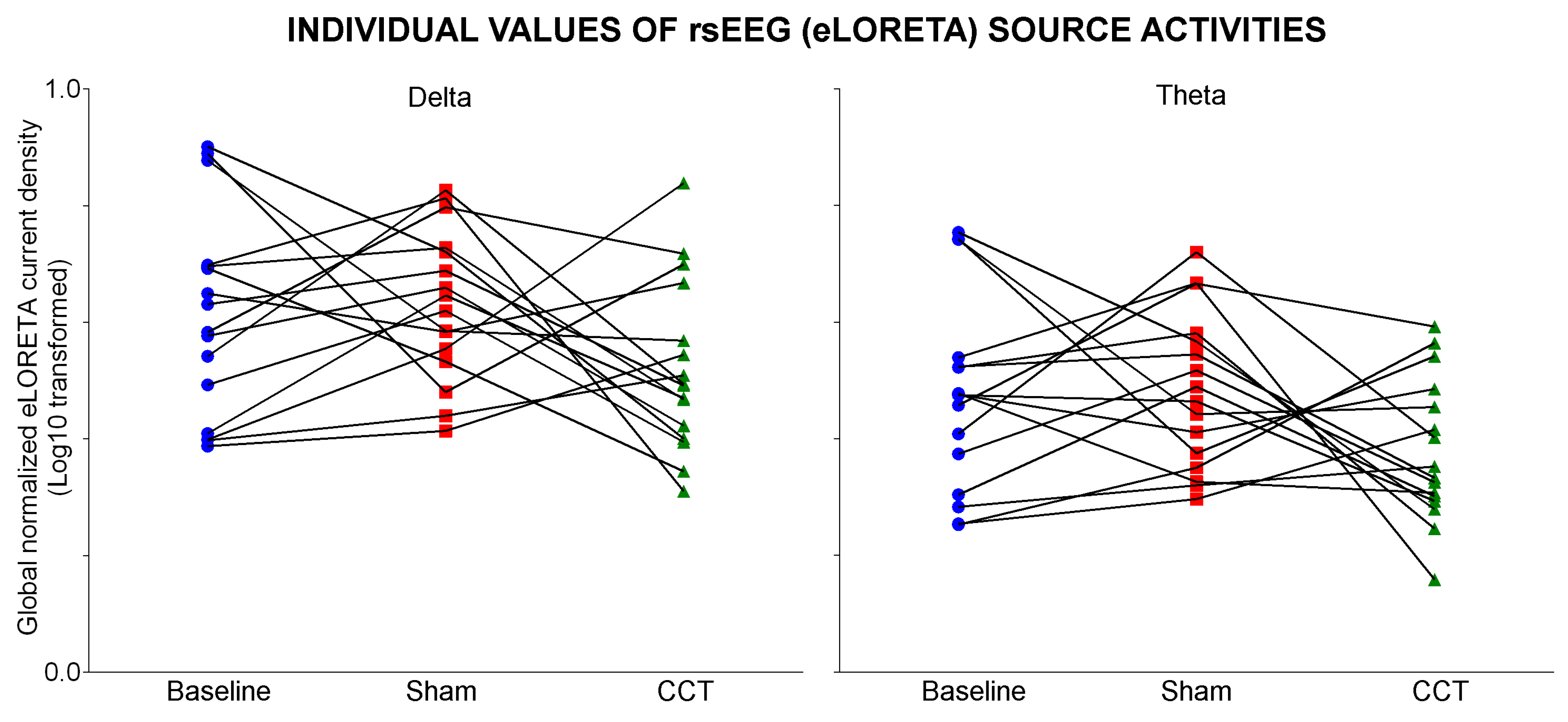
3.3. Effects of the CCT Program on rsEEG Source Activities in PDCD Patients
3.4. Control Analysis
4. Discussion
4.1. The CCT Program Might Mitigate Thalamocortical Dysrhythmia in PDCD Patients
4.2. Cognitive Training and Modulation of Subcortical Ascending Arousing Systems
4.3. Methodological Remarks
5. Conclusions
Authors’ contribution
Funding and Acknowledgments
Informed Consent Statement
Conflicts of interest
References
- World Health Organization Risk Reduction of Cognitive Decline and Dementia: WHO Guidelines; World Health Organization: Geneva, 2019; ISBN 978-92-4-155054-3.
- Aarsland, D.; Batzu, L.; Halliday, G.M.; Geurtsen, G.J.; Ballard, C.; Ray Chaudhuri, K.; Weintraub, D. Parkinson Disease-Associated Cognitive Impairment. Nat Rev Dis Primers 2021, 7, 47. [Google Scholar] [CrossRef] [PubMed]
- Prince, M.; Wimo, A.; Guerchet, M.; Ali, G.-C.; Wu, Y.-T.; Prina, M. World Alzheimer Report 2015.The Global Impact of Dementia: An Analysis of Prevalence, Incidence, Cost and Trends.; Alzheimer’s Disease International, 2015.
- Levy, G.; Tang, M.-X.; Cote, L.J.; Louis, E.D.; Alfaro, B.; Mejia, H.; Stern, Y.; Marder, K. Motor Impairment in PD: Relationship to Incident Dementia and Age. Neurology 2000, 55, 539–544. [Google Scholar] [CrossRef] [PubMed]
- Aarsland, D. Performance on the Dementia Rating Scale in Parkinson’s Disease with Dementia and Dementia with Lewy Bodies: Comparison with Progressive Supranuclear Palsy and Alzheimer’s Disease. Journal of Neurology, Neurosurgery & Psychiatry 2003, 74, 1215–1220. [Google Scholar] [CrossRef]
- Buter, T.C.; van den Hout, A.; Matthews, F.E.; Larsen, J.P.; Brayne, C.; Aarsland, D. Dementia and Survival in Parkinson Disease: A 12-Year Population Study. Neurology 2008, 70, 1017–1022. [Google Scholar] [CrossRef] [PubMed]
- Fang, C.; Lv, L.; Mao, S.; Dong, H.; Liu, B. Cognition Deficits in Parkinson’s Disease: Mechanisms and Treatment. Parkinsons Dis 2020, 2020, 2076942. [Google Scholar] [CrossRef] [PubMed]
- Aarsland, D.; Kurz, M.W. The Epidemiology of Dementia Associated with Parkinson’s Disease. Brain Pathology 2010, 20, 633–639. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, K.F.; Larsen, J.P.; Tysnes, O.-B.; Alves, G. Natural Course of Mild Cognitive Impairment in Parkinson Disease: A 5-Year Population-Based Study. Neurology 2017, 88, 767–774. [Google Scholar] [CrossRef] [PubMed]
- Lawson, R.A.; Yarnall, A.J.; Duncan, G.W.; Breen, D.P.; Khoo, T.K.; Williams-Gray, C.H.; Barker, R.A.; Burn, D.J. Stability of Mild Cognitive Impairment in Newly Diagnosed Parkinson’s Disease. J Neurol Neurosurg Psychiatry 2017, 88, 648–652. [Google Scholar] [CrossRef]
- Zimmermann, R.; Gschwandtner, U.; Benz, N.; Hatz, F.; Schindler, C.; Taub, E.; Fuhr, P. Cognitive Training in Parkinson Disease: Cognition-Specific vs Nonspecific Computer Training. Neurology 2014, 82, 1219–1226. [Google Scholar] [CrossRef]
- Hershey, L.A. Comment: Performance Improvement with Computer Training in Parkinson Disease. Neurology 2014, 82, 1224–1224. [Google Scholar] [CrossRef]
- Edwards, J.D.; Hauser, R.A.; O’Connor, M.L.; Valdés, E.G.; Zesiewicz, T.A.; Uc, E.Y. Randomized Trial of Cognitive Speed of Processing Training in Parkinson Disease. Neurology 2013, 81, 1284–1290. [Google Scholar] [CrossRef]
- Naismith, S.L.; Mowszowski, L.; Diamond, K.; Lewis, S.J.G. Improving Memory in Parkinson’s Disease: A Healthy Brain Ageing Cognitive Training Program. Mov Disord 2013, 28, 1097–1103. [Google Scholar] [CrossRef] [PubMed]
- Dunlosky, J.; Kubat-Silman, A.K.; Hertzog, C. Training Monitoring Skills Improves Older Adults’ Self-Paced Associative Learning. Psychol Aging 2003, 18, 340–345. [Google Scholar] [CrossRef] [PubMed]
- Alloni, A.; Quaglini, S.; Panzarasa, S.; Sinforiani, E.; Bernini, S. Evaluation of an Ontology-Based System for Computerized Cognitive Rehabilitation. International Journal of Medical Informatics 2018, 115, 64–72. [Google Scholar] [CrossRef]
- Vallejo, V.; Wyss, P.; Rampa, L.; Mitache, A.V.; Müri, R.M.; Mosimann, U.P.; Nef, T. Evaluation of a Novel Serious Game Based Assessment Tool for Patients with Alzheimer’s Disease. PLoS ONE 2017, 12, e0175999. [Google Scholar] [CrossRef]
- Ben-Sadoun, G.; Manera, V.; Alvarez, J.; Sacco, G.; Robert, P. Recommendations for the Design of Serious Games in Neurodegenerative Diseases. Front. Aging Neurosci. 2018, 10, 13. [Google Scholar] [CrossRef]
- Bernini, S.; Panzarasa, S.; Barbieri, M.; Sinforiani, E.; Quaglini, S.; Tassorelli, C.; Bottiroli, S. A Double-Blind Randomized Controlled Trial of the Efficacy of Cognitive Training Delivered Using Two Different Methods in Mild Cognitive Impairment in Parkinson’s Disease: Preliminary Report of Benefits Associated with the Use of a Computerized Tool. Aging Clin Exp Res 2021, 33, 1567–1575. [Google Scholar] [CrossRef]
- Kalbe, E.; Folkerts, A.-K.; Ophey, A.; Eggers, C.; Elben, S.; Dimenshteyn, K.; Sulzer, P.; Schulte, C.; Schmidt, N.; Schlenstedt, C.; et al. Enhancement of Executive Functions but Not Memory by Multidomain Group Cognitive Training in Patients with Parkinson’s Disease and Mild Cognitive Impairment: A Multicenter Randomized Controlled Trial. Parkinsons Dis 2020, 2020, 4068706. [Google Scholar] [CrossRef]
- Gavelin, H.M.; Domellöf, M.E.; Leung, I.; Neely, A.S.; Launder, N.H.; Nategh, L.; Finke, C.; Lampit, A. Computerized Cognitive Training in Parkinson’s Disease: A Systematic Review and Meta-Analysis. Ageing Res Rev 2022, 80, 101671. [Google Scholar] [CrossRef]
- Suzuki, K.; Miyamoto, T.; Miyamoto, M.; Okuma, Y.; Hattori, N.; Kamei, S.; Yoshii, F.; Utsumi, H.; Iwasaki, Y.; Iijima, M.; et al. Excessive Daytime Sleepiness and Sleep Episodes in Japanese Patients with Parkinson’s Disease. J Neurol Sci 2008, 271, 47–52. [Google Scholar] [CrossRef]
- Höglund, A.; Broman, J.-E.; Pålhagen, S.; Fredrikson, S.; Hagell, P. Is Excessive Daytime Sleepiness a Separate Manifestation in Parkinson’s Disease? Acta Neurol Scand 2015, 132, 97–104. [Google Scholar] [CrossRef] [PubMed]
- Höglund, A.; Hagell, P.; Broman, J.; Pålhagen, S.; Sorjonen, K.; Fredrikson, S.; Svenningsson, P. Associations Between Fluctuations in Daytime Sleepiness and Motor and Non-Motor Symptoms in Parkinson’s Disease. Movement Disord Clin Pract 2021, 8, 44–50. [Google Scholar] [CrossRef] [PubMed]
- Jost, S.T.; Konitsioti, A.; Loehrer, P.A.; Ashkan, K.; Rizos, A.; Sauerbier, A.; Dos Santos Ghilardi, M.G.; Rosenkranz, F.; Strobel, L.; Gronostay, A.; et al. Non-Motor Effects of Deep Brain Stimulation in Parkinson’s Disease Motor Subtypes. Parkinsonism & Related Disorders 2023, 109, 105318. [Google Scholar] [CrossRef]
- Iijima, M.; Osawa, M.; Yasuda, S.; Kitagawa, K. Association between Excessive Daytime Sleepiness and the Cholinergic Ascending Reticular System in Parkinson’s Disease. Neurodegener Dis 2021, 21, 48–54. [Google Scholar] [CrossRef] [PubMed]
- Shirahige, L.; Berenguer-Rocha, M.; Mendonça, S.; Rocha, S.; Rodrigues, M.C.; Monte-Silva, K. Quantitative Electroencephalography Characteristics for Parkinson’s Disease: A Systematic Review. J Parkinsons Dis 2020, 10, 455–470. [Google Scholar] [CrossRef] [PubMed]
- Pfurtscheller, G.; Lopes da Silva, F.H. Event-Related EEG/MEG Synchronization and Desynchronization: Basic Principles. Clin Neurophysiol 1999, 110, 1842–1857. [Google Scholar] [CrossRef] [PubMed]
- Babiloni, C.; Barry, R.J.; Başar, E.; Blinowska, K.J.; Cichocki, A.; Drinkenburg, W.H.I.M.; Klimesch, W.; Knight, R.T.; Lopes Da Silva, F.; Nunez, P.; et al. International Federation of Clinical Neurophysiology (IFCN) – EEG Research Workgroup: Recommendations on Frequency and Topographic Analysis of Resting State EEG Rhythms. Part 1: Applications in Clinical Research Studies. Clinical Neurophysiology 2020, 131, 285–307. [Google Scholar] [CrossRef] [PubMed]
- Serizawa, K.; Kamei, S.; Morita, A.; Hara, M.; Mizutani, T.; Yoshihashi, H.; Yamaguchi, M.; Takeshita, J.; Hirayanagi, K. Comparison of Quantitative EEGs between Parkinson Disease and Age-Adjusted Normal Controls. J Clin Neurophysiol 2008, 25, 361–366. [Google Scholar] [CrossRef]
- Bonanni, L.; Thomas, A.; Tiraboschi, P.; Perfetti, B.; Varanese, S.; Onofrj, M. EEG Comparisons in Early Alzheimer’s Disease, Dementia with Lewy Bodies and Parkinson’s Disease with Dementia Patients with a 2-Year Follow-Up. Brain 2008, 131, 690–705. [Google Scholar] [CrossRef]
- Kamei, S.; Morita, A.; Serizawa, K.; Mizutani, T.; Hirayanagi, K. Quantitative EEG Analysis of Executive Dysfunction in Parkinson Disease. J Clin Neurophysiol 2010, 27, 193–197. [Google Scholar] [CrossRef]
- Pugnetti, L.; Baglio, F.; Farina, E.; Alberoni, M.; Calabrese, E.; Gambini, A.; Di Bella, E.; Garegnani, M.; Deleonardis, L.; Nemni, R. EEG Evidence of Posterior Cortical Disconnection in PD and Related Dementias. Int J Neurosci 2010, 120, 88–98. [Google Scholar] [CrossRef] [PubMed]
- Babiloni, C.; Del Percio, C.; Lizio, R.; Noce, G.; Cordone, S.; Lopez, S.; Soricelli, A.; Ferri, R.; Pascarelli, M.T.; Nobili, F.; et al. Abnormalities of Cortical Neural Synchronization Mechanisms in Subjects with Mild Cognitive Impairment Due to Alzheimer’s and Parkinson’s Diseases: An EEG Study. JAD 2017, 59, 339–358. [Google Scholar] [CrossRef] [PubMed]
- Babiloni, C.; Del Percio, C.; Lizio, R.; Noce, G.; Cordone, S.; Lopez, S.; Soricelli, A.; Ferri, R.; Pascarelli, M.T.; Nobili, F.; et al. Abnormalities of Cortical Neural Synchronization Mechanisms in Patients with Dementia Due to Alzheimer’s and Lewy Body Diseases: An EEG Study. Neurobiology of Aging 2017, 55, 143–158. [Google Scholar] [CrossRef] [PubMed]
- Babiloni, C.; Blinowska, K.; Bonanni, L.; Cichocki, A.; De Haan, W.; Del Percio, C.; Dubois, B.; Escudero, J.; Fernández, A.; Frisoni, G.; et al. What Electrophysiology Tells Us about Alzheimer’s Disease: A Window into the Synchronization and Connectivity of Brain Neurons. Neurobiology of Aging 2020, 85, 58–73. [Google Scholar] [CrossRef] [PubMed]
- Babiloni, C.; Del Percio, C.; Lizio, R.; Noce, G.; Lopez, S.; Soricelli, A.; Ferri, R.; Pascarelli, M.T.; Catania, V.; Nobili, F.; et al. Levodopa May Affect Cortical Excitability in Parkinson’s Disease Patients with Cognitive Deficits as Revealed by Reduced Activity of Cortical Sources of Resting State Electroencephalographic Rhythms. Neurobiology of Aging 2019, 73, 9–20. [Google Scholar] [CrossRef] [PubMed]
- Styliadis, C.; Kartsidis, P.; Paraskevopoulos, E.; Ioannides, A.A.; Bamidis, P.D. Neuroplastic Effects of Combined Computerized Physical and Cognitive Training in Elderly Individuals at Risk for Dementia: An eLORETA Controlled Study on Resting States. Neural Plast 2015, 2015, 172192. [Google Scholar] [CrossRef] [PubMed]
- Trenado, C.; Trauberg, P.; Elben, S.; Dimenshteyn, K.; Folkerts, A.-K.; Witt, K.; Weiss, D.; Liepelt-Scarfone, I.; Kalbe, E.; Wojtecki, L. Resting State EEG as Biomarker of Cognitive Training and Physical Activity’s Joint Effect in Parkinson’s Patients with Mild Cognitive Impairment. Neurol Res Pract 2023, 5, 46. [Google Scholar] [CrossRef] [PubMed]
- Gelb, D.J.; Oliver, E.; Gilman, S. Diagnostic Criteria for Parkinson Disease. Arch Neurol 1999, 56, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Hoehn, M.M.; Yahr, M.D. Parkinsonism: Onset, Progression, and Mortality. Neurology 1967, 17, 427–427. [Google Scholar] [CrossRef]
- Fahn; Elton The Unified Parkinson’s Disease Rating Scale (UPDRS): Status and Recommendations. Movement Disorders 2003, 18, 738–750. [CrossRef]
- Geser, F.; Wenning, G.K.; Poewe, W.; McKeith, I. How to Diagnose Dementia with Lewy Bodies: State of the Art. Mov Disord. 2005, 20, S11–S20. [Google Scholar] [CrossRef] [PubMed]
- McKeith, I.G.; Galasko, D.; Kosaka, K.; Perry, E.K.; Dickson, D.W.; Hansen, L.A.; Salmon, D.P.; Lowe, J.; Mirra, S.S.; Byrne, E.J.; et al. Consensus Guidelines for the Clinical and Pathologic Diagnosis of Dementia with Lewy Bodies (DLB): Report of the Consortium on DLB International Workshop. Neurology 1996, 47, 1113–1124. [Google Scholar] [CrossRef]
- McKeith, I.G.; Boeve, B.F.; Dickson, D.W.; Halliday, G.; Taylor, J.-P.; Weintraub, D.; Aarsland, D.; Galvin, J.; Attems, J.; Ballard, C.G.; et al. Diagnosis and Management of Dementia with Lewy Bodies: Fourth Consensus Report of the DLB Consortium. Neurology 2017, 89, 88–100. [Google Scholar] [CrossRef]
- Albert, M.S. Changes in Cognition. Neurobiology of Aging 2011, 32, S58–S63. [Google Scholar] [CrossRef]
- Folstein, M.F.; Folstein, S.E.; McHugh, P.R. “Mini-Mental State”. A Practical Method for Grading the Cognitive State of Patients for the Clinician. J Psychiatr Res 1975, 12, 189–198. [Google Scholar] [CrossRef] [PubMed]
- Nasreddine, Z.S.; Phillips, N.A.; Bédirian, V.; Charbonneau, S.; Whitehead, V.; Collin, I.; Cummings, J.L.; Chertkow, H. The Montreal Cognitive Assessment, MoCA: A Brief Screening Tool for Mild Cognitive Impairment. J Am Geriatr Soc 2005, 53, 695–699. [Google Scholar] [CrossRef]
- Novelli, G.; Papagno, C.; Capitani, E.; Laiacona, M. Tre Test Clinici Di Ricerca e Produzione Lessicale. Taratura Su Sogetti Normali. Archivio di psicologia, neurologia e psichiatria, 1986.
- Osterrieth, P.A. Le Test de Copie d’une Figure Complexe; Contribution à l’étude de La Perception et de La Mémoire. [Test of Copying a Complex Figure; Contribution to the Study of Perception and Memory.]. Archives de Psychologie 1944, 30, 206–356. [Google Scholar]
- Benton, A.L. Visuospatial Judgment: A Clinical Test. Arch Neurol 1978, 35, 364. [Google Scholar] [CrossRef]
- Benton, A.; Hamsher, K.; Varney, N.; Spreen, O. Contribution to Neuropsychological Assessment, A Clinical Manual.; Oxford University Press, New York, 1983;
- Stroop, J.R. Studies of Interference in Serial Verbal Reactions. Journal of Experimental Psychology 1935, 18, 643–662. [Google Scholar] [CrossRef]
- Reitan, R.M. VALIDITY OF THE TRAIL MAKING TEST AS AN INDICATOR OF ORGANIC BRAIN DAMAGE. PMS 1958, 8, 271. [Google Scholar] [CrossRef]
- Inouye, S.K. Clarifying Confusion: The Confusion Assessment Method: A New Method for Detection of Delirium. Ann Intern Med 1990, 113, 941. [Google Scholar] [CrossRef]
- Dubois, B.; Slachevsky, A.; Litvan, I.; Pillon, B. The FAB: A Frontal Assessment Battery at Bedside. Neurology 2000, 55, 1621–1626. [Google Scholar] [CrossRef] [PubMed]
- Caffarra, P.; Gardini, S.; Zonato, F.; Concari, L.; Dieci, F.; Copelli, S.; Freedman, M.; Stracciari, A.; Venneri, A. Italian Norms for the Freedman Version of the Clock Drawing Test. Journal of Clinical and Experimental Neuropsychology 2011, 33, 982–988. [Google Scholar] [CrossRef] [PubMed]
- Wechsler, D. WMS-R: Wechsler Memory Scale-Revised: Manual.; Psychological Corporation, 1987;
- Oktem, O. A Verbal Test of Memory Processes: A Preliminary Study. Archives of Neuropsychiatry 1992, 29, 196–206. [Google Scholar]
- Delorme, A.; Makeig, S. EEGLAB: An Open Source Toolbox for Analysis of Single-Trial EEG Dynamics Including Independent Component Analysis. J Neurosci Methods 2004, 134, 9–21. [Google Scholar] [CrossRef]
- Crespo-Garcia, M.; Atienza, M.; Cantero, J.L. Muscle Artifact Removal from Human Sleep EEG by Using Independent Component Analysis. Ann Biomed Eng 2008, 36, 467–475. [Google Scholar] [CrossRef]
- Jung, T.P.; Makeig, S.; Humphries, C.; Lee, T.W.; McKeown, M.J.; Iragui, V.; Sejnowski, T.J. Removing Electroencephalographic Artifacts by Blind Source Separation. Psychophysiology 2000, 37, 163–178. [Google Scholar] [CrossRef] [PubMed]
- Klimesch, W.; Doppelmayr, M.; Schimke, H.; Pachinger, T. Alpha Frequency, Reaction Time, and the Speed of Processing Information. J Clin Neurophysiol 1996, 13, 511–518. [Google Scholar] [CrossRef] [PubMed]
- Klimesch, W.; Doppelmayr, M.; Russegger, H.; Pachinger, T.; Schwaiger, J. Induced Alpha Band Power Changes in the Human EEG and Attention. Neurosci Lett 1998, 244, 73–76. [Google Scholar] [CrossRef]
- Klimesch, W. EEG Alpha and Theta Oscillations Reflect Cognitive and Memory Performance: A Review and Analysis. Brain Research Reviews 1999, 29, 169–195. [Google Scholar] [CrossRef]
- Pascual-Marqui, R.D.; Esslen, M.; Kochi, K.; Lehmann, D. Functional Imaging with Low-Resolution Brain Electromagnetic Tomography (LORETA): A Review. Methods Find Exp Clin Pharmacol 2002, 24 Suppl C, 91–95. [Google Scholar]
- Pascual-Marqui, R.D. Discrete, 3D Distributed, Linear Imaging Methods of Electric Neuronal Activity. Part 1: Exact, Zero Error Localization 2007.
- Leung, I.H.K.; Walton, C.C.; Hallock, H.; Lewis, S.J.G.; Valenzuela, M.; Lampit, A. Cognitive Training in Parkinson Disease: A Systematic Review and Meta-Analysis. Neurology 2015, 85, 1843–1851. [Google Scholar] [CrossRef] [PubMed]
- Aarsland, D.; Creese, B.; Politis, M.; Chaudhuri, K.R.; Ffytche, D.H.; Weintraub, D.; Ballard, C. Cognitive Decline in Parkinson Disease. Nat Rev Neurol 2017, 13, 217–231. [Google Scholar] [CrossRef] [PubMed]
- Van Balkom, T.D.; Van Den Heuvel, O.A.; Berendse, H.W.; Van Der Werf, Y.D.; Vriend, C. The Effects of Cognitive Training on Brain Network Activity and Connectivity in Aging and Neurodegenerative Diseases: A Systematic Review. Neuropsychol Rev 2020, 30, 267–286. [Google Scholar] [CrossRef] [PubMed]
- Kai, T.; Asai, Y.; Sakuma, K.; Koeda, T.; Nakashima, K. Quantitative Electroencephalogram Analysis in Dementia with Lewy Bodies and Alzheimer’s Disease. J Neurol Sci 2005, 237, 89–95. [Google Scholar] [CrossRef]
- Andersson, M.; Hansson, O.; Minthon, L.; Rosén, I.; Londos, E. Electroencephalogram Variability in Dementia with Lewy Bodies, Alzheimer’s Disease and Controls. Dement Geriatr Cogn Disord 2008, 26, 284–290. [Google Scholar] [CrossRef]
- Caviness, J.N.; Hentz, J.G.; Belden, C.M.; Shill, H.A.; Driver-Dunckley, E.D.; Sabbagh, M.N.; Powell, J.J.; Adler, C.H. Longitudinal EEG Changes Correlate with Cognitive Measure Deterioration in Parkinson’s Disease. J Parkinsons Dis 2015, 5, 117–124. [Google Scholar] [CrossRef]
- Schumacher, J.; Thomas, A.J.; Peraza, L.R.; Firbank, M.; Cromarty, R.; Hamilton, C.A.; Donaghy, P.C.; O’Brien, J.T.; Taylor, J.-P. EEG Alpha Reactivity and Cholinergic System Integrity in Lewy Body Dementia and Alzheimer’s Disease. Alzheimers Res Ther 2020, 12, 46. [Google Scholar] [CrossRef] [PubMed]
- Caviness, J.N.; Lue, L.-F.; Hentz, J.G.; Schmitz, C.T.; Adler, C.H.; Shill, H.A.; Sabbagh, M.N.; Beach, T.G.; Walker, D.G. Cortical Phosphorylated α-Synuclein Levels Correlate with Brain Wave Spectra in Parkinson’s Disease. Mov Disord 2016, 31, 1012–1019. [Google Scholar] [CrossRef]
- Caviness, J.N.; Beach, T.G.; Hentz, J.G.; Shill, H.A.; Driver-Dunckley, E.D.; Adler, C.H. Association Between Pathology and Electroencephalographic Activity in Parkinson’s Disease. Clin EEG Neurosci 2018, 49, 321–327. [Google Scholar] [CrossRef]
- Jeanmonod, D.; Magnin, M.; Morel, A. Low-Threshold Calcium Spike Bursts in the Human Thalamus. Common Physiopathology for Sensory, Motor and Limbic Positive Symptoms. Brain, 1996, 119 ( Pt 2), 363–375. [CrossRef]
- Llinás, R.R.; Steriade, M. Bursting of Thalamic Neurons and States of Vigilance. Journal of Neurophysiology 2006, 95, 3297–3308. [Google Scholar] [CrossRef] [PubMed]
- Sarnthein, J.; Jeanmonod, D. High Thalamocortical Theta Coherence in Patients with Parkinson’s Disease. J Neurosci 2007, 27, 124–131. [Google Scholar] [CrossRef] [PubMed]
- Bareš, M.; Kaňovský, P.; Klajblová, H.; Rektor, I. Intracortical Inhibition and Facilitation Are Impaired in Patients with Early Parkinson’s Disease: A Paired TMS Study. Euro J of Neurology 2003, 10, 385–389. [Google Scholar] [CrossRef] [PubMed]
- MacKinnon, C.D.; Gilley, E.A.; Weis-McNulty, A.; Simuni, T. Pathways Mediating Abnormal Intracortical Inhibition in Parkinson’s Disease. Ann Neurol 2005, 58, 516–524. [Google Scholar] [CrossRef] [PubMed]
- Kamble, N.; Bhattacharya, A.; Hegde, S.; Vidya, N.; Gothwal, M.; Yadav, R.; Pal, P.K. Cortical Excitability Changes as a Marker of Cognitive Impairment in Parkinson’s Disease. Behav Brain Res 2022, 422, 113733. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Llinas, R.R.; Lisman, J.E. Inhibition of NMDARs in the Nucleus Reticularis of the Thalamus Produces Delta Frequency Bursting. Front Neural Circuits 2009, 3, 20. [Google Scholar] [CrossRef] [PubMed]
- Vriend, C.; Van Balkom, T.D.; Berendse, H.W.; Van Der Werf, Y.D.; Van Den Heuvel, O.A. Cognitive Training in Parkinson’s Disease Induces Local, Not Global, Changes in White Matter Microstructure. Neurotherapeutics 2021, 18, 2518–2528. [Google Scholar] [CrossRef]
- Haghshomar, M.; Dolatshahi, M.; Ghazi Sherbaf, F.; Sanjari Moghaddam, H.; Shirin Shandiz, M.; Aarabi, M.H. Disruption of Inferior Longitudinal Fasciculus Microstructure in Parkinson’s Disease: A Systematic Review of Diffusion Tensor Imaging Studies. Front. Neurol. 2018, 9, 598. [Google Scholar] [CrossRef] [PubMed]
- Gorges, M.; Müller, H.-P.; Liepelt-Scarfone, I.; Storch, A.; Dodel, R.; LANDSCAPE Consortium; Hilker-Roggendorf, R. ; Berg, D.; Kunz, M.S.; Kalbe, E.; et al. Structural Brain Signature of Cognitive Decline in Parkinson’s Disease: DTI-Based Evidence from the LANDSCAPE Study. Ther Adv Neurol Disord 2019, 12, 1756286419843447. [Google Scholar] [CrossRef]
- Van Balkom, T.D.; Van Den Heuvel, O.A.; Berendse, H.W.; Van Der Werf, Y.D.; Vriend, C. Eight-Week Multi-Domain Cognitive Training Does Not Impact Large-Scale Resting-State Brain Networks in Parkinson’s Disease. NeuroImage: Clinical 2022, 33, 102952. [Google Scholar] [CrossRef]
- Owen, A.M. Cognitive Dysfunction in Parkinson’s Disease: The Role of Frontostriatal Circuitry. Neuroscientist 2004, 10, 525–537. [Google Scholar] [CrossRef] [PubMed]
- Peters, S.K.; Dunlop, K.; Downar, J. Cortico-Striatal-Thalamic Loop Circuits of the Salience Network: A Central Pathway in Psychiatric Disease and Treatment. Front Syst Neurosci 2016, 10, 104. [Google Scholar] [CrossRef] [PubMed]
- Saalmann, Y.B. Intralaminar and Medial Thalamic Influence on Cortical Synchrony, Information Transmission and Cognition. Front. Syst. Neurosci. 2014, 8. [Google Scholar] [CrossRef]
- Wang, L.; Zhou, C.; Cheng, W.; Rolls, E.T.; Huang, P.; Ma, N.; Liu, Y.; Zhang, Y.; Guan, X.; Guo, T.; et al. Dopamine Depletion and Subcortical Dysfunction Disrupt Cortical Synchronization and Metastability Affecting Cognitive Function in Parkinson’s Disease. Hum Brain Mapp 2022, 43, 1598–1610. [Google Scholar] [CrossRef] [PubMed]
- Shine, J.M.; Bissett, P.G.; Bell, P.T.; Koyejo, O.; Balsters, J.H.; Gorgolewski, K.J.; Moodie, C.A.; Poldrack, R.A. The Dynamics of Functional Brain Networks: Integrated Network States during Cognitive Task Performance. Neuron 2016, 92, 544–554. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Criaud, M.; Cho, S.S.; Díez-Cirarda, M.; Mihaescu, A.; Coakeley, S.; Ghadery, C.; Valli, M.; Jacobs, M.F.; Houle, S.; et al. Abnormal Intrinsic Brain Functional Network Dynamics in Parkinson’s Disease. Brain 2017, 140, 2955–2967. [Google Scholar] [CrossRef] [PubMed]
- Fiorenzato, E.; Strafella, A.P.; Kim, J.; Schifano, R.; Weis, L.; Antonini, A.; Biundo, R. Dynamic Functional Connectivity Changes Associated with Dementia in Parkinson’s Disease. Brain 2019, 142, 2860–2872. [Google Scholar] [CrossRef] [PubMed]
- Hirano, S.; Shinotoh, H.; Eidelberg, D. Functional Brain Imaging of Cognitive Dysfunction in Parkinson’s Disease. J Neurol Neurosurg Psychiatry 2012, 83, 963–969. [Google Scholar] [CrossRef]
- Bohnen, N.I.; Albin, R.L.; Müller, M.L.T.M.; Petrou, M.; Kotagal, V.; Koeppe, R.A.; Scott, P.J.H.; Frey, K.A. Frequency of Cholinergic and Caudate Nucleus Dopaminergic Deficits Across the Predemented Cognitive Spectrum of Parkinson Disease and Evidence of Interaction Effects. JAMA Neurol 2015, 72, 194. [Google Scholar] [CrossRef]
- Hirano, S. Clinical Implications for Dopaminergic and Functional Neuroimage Research in Cognitive Symptoms of Parkinson’s Disease. Mol Med 2021, 27, 40. [Google Scholar] [CrossRef]
- Calabresi, P.; Picconi, B.; Parnetti, L.; Di Filippo, M. A Convergent Model for Cognitive Dysfunctions in Parkinson’s Disease: The Critical Dopamine–Acetylcholine Synaptic Balance. The Lancet Neurology 2006, 5, 974–983. [Google Scholar] [CrossRef] [PubMed]
- Bédard, M.A.; el Massioui, F.; Malapani, C.; Dubois, B.; Pillon, B.; Renault, B.; Agid, Y. Attentional Deficits in Parkinson’s Disease: Partial Reversibility with Naphtoxazine (SDZ NVI-085), a Selective Noradrenergic Alpha 1 Agonist. Clin Neuropharmacol 1998, 21, 108–117. [Google Scholar] [PubMed]
- Riekkinen, M.; Kejonen, K.; Jäkälä, P.; Soininen, H.; Riekkinen, P. Reduction of Noradrenaline Impairs Attention and Dopamine Depletion Slows Responses in Parkinson’s Disease. Eur J Neurosci 1998, 10, 1429–1435. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Ganzetti, M.; Wenderoth, N.; Mantini, D. Detecting Large-Scale Brain Networks Using EEG: Impact of Electrode Density, Head Modeling and Source Localization. Front. Neuroinform. 2018, 12, 4. [Google Scholar] [CrossRef] [PubMed]
- Marino, M.; Liu, Q.; Brem, S.; Wenderoth, N.; Mantini, D. Automated Detection and Labeling of High-Density EEG Electrodes from Structural MR Images. J Neural Eng 2016, 13, 056003. [Google Scholar] [CrossRef]
- Michel, C.M.; Brunet, D. EEG Source Imaging: A Practical Review of the Analysis Steps. Front Neurol 2019, 10, 325. [Google Scholar] [CrossRef]
| Mean values (± SE) of demographic and global cognitive status (MMSE) data | |||||
|---|---|---|---|---|---|
| Healthy | PDNCD | ADCD | PDCD | Statistical analysis | |
| N | 40 | 29 | 40 | 40 | - |
| Age (years) | 70.0 (± 1.1 SE) | 70.4 (± 1.3 SE) | 71.7 (± 1.0 SE) | 71.6 (± 0.9 SE) | ANOVA: n.s. |
| Sex (M/F) | 20/20 | 15/14 | 21/19 | 20/20 | Freeman-Halton test: n.s. |
| Education (years) | 10.6(± 0.7 SE) | 9.1 (± 0.3 SE) | 9.0(± 0.6 SE) | 9.5 (± 0.6 SE) | ANOVA: n.s. |
| MMSE score | 28.1 ±(0.2 SE) | 28.0 ±(0.3 SE) | 21.0 (±0.7 SE) | 21.2 (± 0.7 SE) | Kruskal- Wallis test: p < 0.0001 (Healthy, PDNCD > ADCD, PDCD) |
| Clinical data at Baseline in PDCD patients | |||
|---|---|---|---|
| Mean (± SE) | Pathologic cut-off | % Patients with pathologic score | |
| Hoehn and Yahr stage (H & Y) | 2.8 (± 0.1 SE) | ≥ 4 (range 0-5; 1-3 moderate; 4-5 severe) | 100% |
| Unified Parkinson Disease Rating Scale (UPDRS-III Motor Part) | 47.7 (± 4.7 SE) | ≥ 59 (range 0-80; 1-32 mild; 33-58 moderate; 59-80 severe) | 100% |
| Activities of daily living (ADL) | 1.6 (± 0.3 SE) | > 0 (range 0-6; 0 completely autonomous; 6 completely dependent); | 87.5% |
| Instrumental activities of daily living (IADL) | 4.1 (± 0.3 SE) | > 5 (range 0-8; 0 completely autonomous; 8 completely dependent) | 100% |
| Clinical dementia rating scale(CDR) | 0.9 (± 0.1 SE) | > 0 (0.5- 1 mild cognitive impairment; 2-3 moderate to severe impairment) | 93.8% |
| Geriatric depression scale(GDS) | 17.3 (± 1.0 SE) | ≥ 17 (range 0-30; 0-10 depression absent; 11-16 moderate; 17-30 severe) | 93.8% |
| Mini-Mental State Examination (MMSE) | 23.7 (± 0.3 SE) | < 24 (range 0-30) | 56.3% |
| Montreal Cognitive Assessment (MOCA) | 19.4 (± 1.1 SE) | < 26 (range 0-30) | 93.8% |
| Digit span | 4.8 (± 0.2 SE) | < 3.75 (range 0-8) | 6.3% |
| Frontal assessment battery (FAB) | 14.3 (± 0.36 SE) | < 13.5 (range 0-18) | 37.5% |
| Clock drawing | 7.7 (± 0.8 SE) | < 6.55 (range 0-33) | 37.5% |
| Verbal fluency for letters | 29.0 (± 2.0 SE) | < 17 | 0% |
| Verbal fluency for categories | 36.1 (± 2.3 SE) | < 25 | 18.8% |
| Rey wordsImmediate recall | 32.4 (± 1.7 SE) | < 28.53 (range 0-75) | 31.3% |
| Rey wordsdelayed recall | 7.2 (± 0.7 SE) | < 4.69 (range 0-15) | 12.5% |
| Prose memory | 9.1 (± 1.1 SE) | < 14.5 (range 0-28) | 93.8% |
| Rey figures Copy | 21.7 (±3.0 SE) | < 28 (range 0-36) | 50% |
| Rey figuresdelayed recall | 11.5 (± 1.4 SE) | < 6.2 (range 0-36) | 25.0% |
| Mean values (± SE) of the performance of serious video games of CCT program in PDCD patients | ||||
|---|---|---|---|---|
| Task domain | Accuracy (%)/Reaction time (ms) | Baseline | CCT | Wilcoxon test |
| Visual non-spatial reaction time task | Accuracy (%) | 89.0 (± 4.7 SE) | 98.0 (± 1.2 SE) | p = 0.05 |
| Reaction time (ms) | 1.3 (± 0.1 SE) | 1.1 (± 0.1 SE) | n.s. | |
| Visuospatial attention task | Accuracy (%) | 58.0 (± 5.1 SE) | 82.7 (± 3.8 SE) | p = 0.003 |
| Reaction time (ms) | 44.9 (± 1.6 SE) | 43.6 (± 2.3 SE) | n.s. | |
| Visual non-spatial attention task, (iv) short-term visuospatial task | Accuracy (%) | 66.0 (± 5.5 SE) | 86.3 (± 4.8 SE) | p = 0.002 |
| Reaction time (ms) | 43.2 (± 1.6 SE) | 40.5 (± 2.4 SE) | n.s. | |
| Short-term visuospatial task | Accuracy (%) | 75.0 (± 3.0 SE) | 84.0 (± 2.9 SE) | p = 0.01 |
| Reaction time (ms) | 66.3 (± 3.5 SE) | 55.0 (± 4.0 SE) | n.s. | |
| short-term visual non-spatial task | Accuracy (%) | 52.0 (± 4.9 SE) | 66.0 (± 6.1 SE) | p = 0.05 |
| Reaction time (ms) | 50.1 (± 4.9 SE) | 41.6 (± 3.8 SE) | n.s. | |
| A modified Posner’s task | Accuracy (%) | 87.6 (± 3.2 SE) | 97.9 (± 1.3 SE) | p = 0.02 |
| Reaction time (ms) | 1.2 (± 0.1 SE) | 1.0 (± 0.0 SE) | n.s. | |
| A task testing the ability to refrain from impulsive motor responses | Accuracy (%) | 74.5 (± 8.2 SE) | 85.6 (± 7.1 SE) | p = 0.01 |
| Reaction time (ms) | 197.8 (± 13.9 SE) | 152.3 (± 13.9 SE) | p = 0.003 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





