Submitted:
13 April 2024
Posted:
15 April 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
1.1. Role of CXCL12 and CXCR4
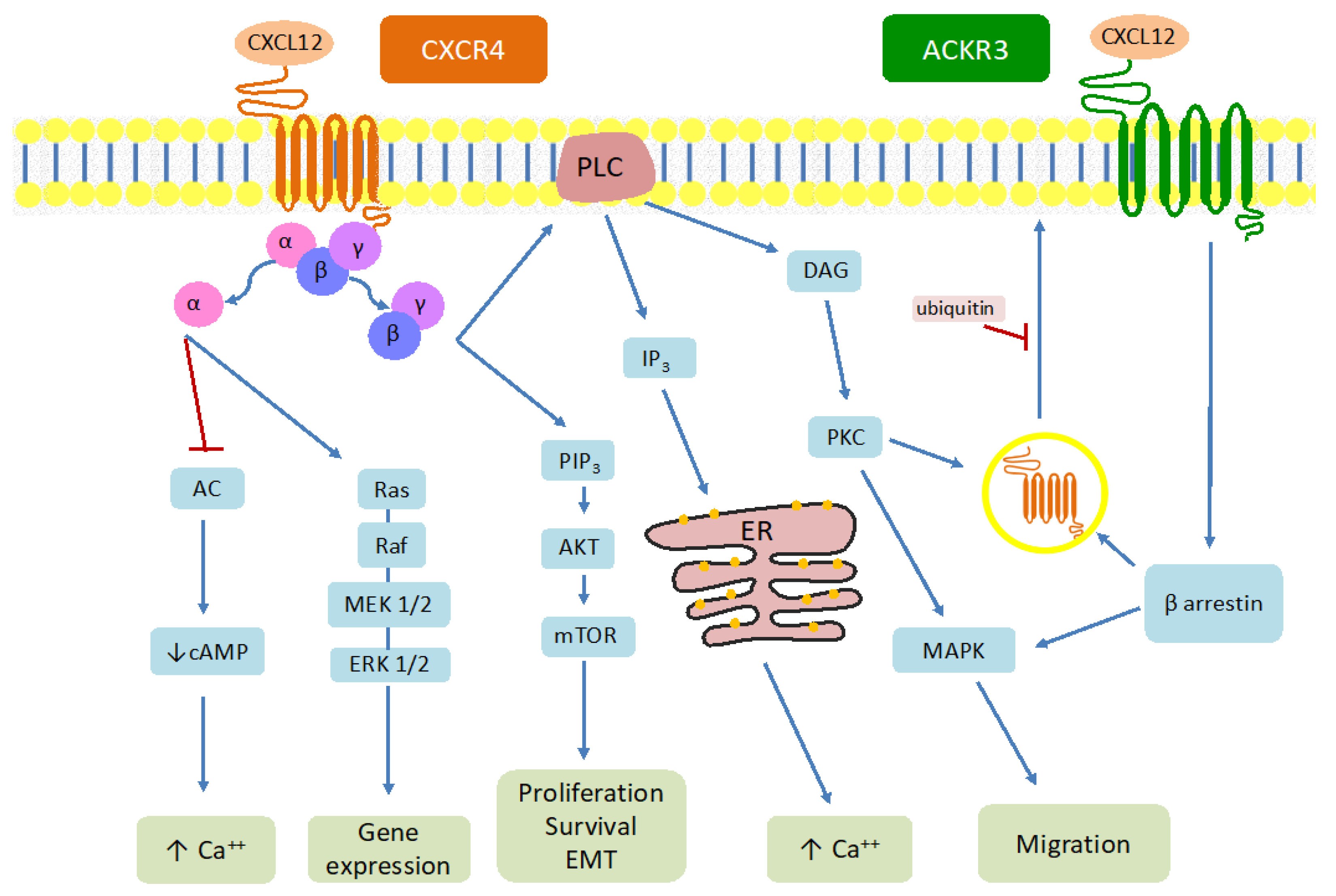
1.2. Structure and Signaling Pathway of the CXCL12-CXCR4-ACKR3 Axis
2. CXCR4 and Cancer
3. CXCR4 and NENs
3.1. Introduction to Neuroendocrine Neoplasms
3.2. Implications of CXCR4 Expression in NENs
3.3. CXCR4 as a Target for Imaging Diagnosis on NENs
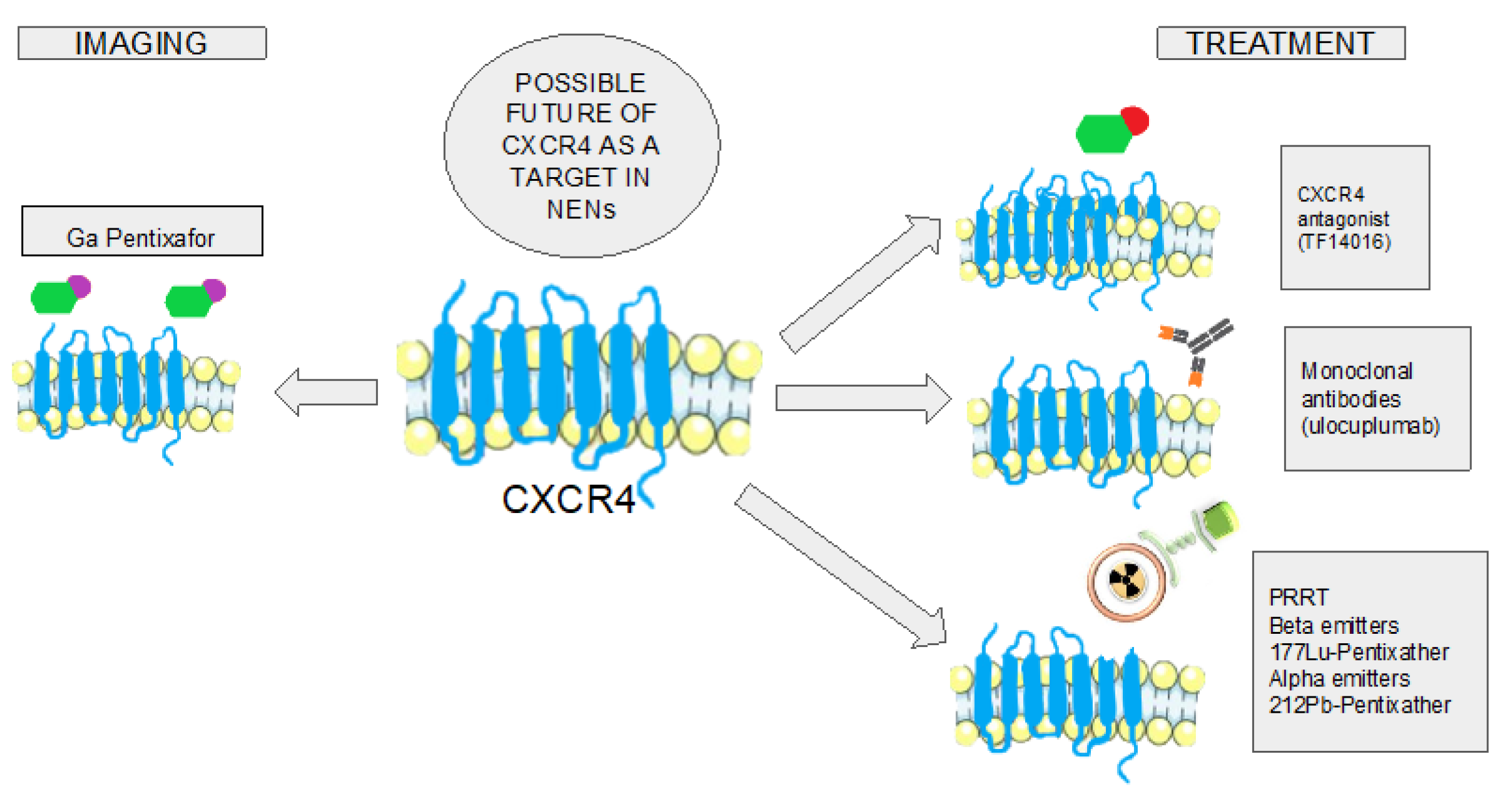
3.4. CXCR4 Targeting as Treatment of NENs
Synthetic Peptides
Monoclonal Antibodies
Peptide Receptor Radionuclide Therapy
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Raman, D.; Baugher, P. J.; Thu, Y. M.; Richmond, A. Role of Chemokines in Tumor Growth. Cancer Lett. 2007, 256, 137–165. [Google Scholar] [CrossRef]
- Le, Y.; Zhou, Y.; Iribarren, P.; Wang, J. Chemokines and Chemokine Receptors: Their Manifold Roles in Homeostasis and Disease. Cell. Mol. Immunol. 2004, 1, 95–104. [Google Scholar]
- Bachelerie, F.; Ben-Baruch, A.; Burkhardt, A. M.; Combadiere, C.; Farber, J. M.; Graham, G. J.; Horuk, R.; Sparre-Ulrich, A. H.; Locati, M.; Luster, A. D.; Mantovani, A.; Matsushima, K.; Murphy, P. M.; Nibbs, R.; Nomiyama, H.; Power, C. A.; Proudfoot, A. E. I.; Rosenkilde, M. M.; Rot, A.; Sozzani, S.; Thelen, M.; Yoshie, O.; Zlotnik, A. International Union of Pharmacology. LXXXIX. Update on the Extended Family of Chemokine Receptors and Introducing a New Nomenclature for Atypical Chemokine Receptors. Pharmacol. Rev. 2014, 66, 1–79. [Google Scholar] [CrossRef]
- Bleul, C. C.; Fuhlbrigge, R. C.; Casasnovas, J. M.; Aiuti, A.; Springer, T. A. A Highly Efficacious Lymphocyte Chemoattractant, Stromal Cell-Derived Factor 1 (SDF-1). J. Exp. Med. 1996, 184, 1101–1109. [Google Scholar] [CrossRef]
- Yu, L.; Cecil, J.; Peng, S.-B.; Schrementi, J.; Kovacevic, S.; Paul, D.; Su, E. W.; Wang, J. Identification and Expression of Novel Isoforms of Human Stromal Cell-Derived Factor 1. Gene 2006, 374, 174–179. [Google Scholar] [CrossRef]
- Righetti, A.; Giulietti, M.; Šabanović, B.; Occhipinti, G.; Principato, G.; Piva, F. CXCL12 and Its Isoforms: Different Roles in Pancreatic Cancer? J. Oncol. 2019, 2019, 9681698. [Google Scholar] [CrossRef] [PubMed]
- Griffith, J. W.; Sokol, C. L.; Luster, A. D. Chemokines and Chemokine Receptors: Positioning Cells for Host Defense and Immunity. Annu. Rev. Immunol. 2014, 32, 659–702. [Google Scholar] [CrossRef] [PubMed]
- Balabanian, K.; Lagane, B.; Infantino, S.; Chow, K. Y. C.; Harriague, J.; Moepps, B.; Arenzana-Seisdedos, F.; Thelen, M.; Bachelerie, F. The Chemokine SDF-1/CXCL12 Binds to and Signals through the Orphan Receptor RDC1 in T Lymphocytes. J. Biol. Chem. 2005, 280, 35760–35766. [Google Scholar] [CrossRef] [PubMed]
- Burns, J. M.; Summers, B. C.; Wang, Y.; Melikian, A.; Berahovich, R.; Miao, Z.; Penfold, M. E. T.; Sunshine, M. J.; Littman, D. R.; Kuo, C. J.; Wei, K.; McMaster, B. E.; Wright, K.; Howard, M. C.; Schall, T. J. A Novel Chemokine Receptor for SDF-1 and I-TAC Involved in Cell Survival, Cell Adhesion, and Tumor Development. J. Exp. Med. 2006, 203, 2201–2213. [Google Scholar] [CrossRef]
- Rajasekaran, D.; Gröning, S.; Schmitz, C.; Zierow, S.; Drucker, N.; Bakou, M.; Kohl, K.; Mertens, A.; Lue, H.; Weber, C.; Xiao, A.; Luker, G.; Kapurniotu, A.; Lolis, E.; Bernhagen, J. Macrophage Migration Inhibitory Factor-CXCR4 Receptor Interactions. J. Biol. Chem. 2016, 291, 15881–15895. [Google Scholar] [CrossRef]
- Tripathi, A.; Saini, V.; Marchese, A.; Volkman, B. F.; Tang, W.-J.; Majetschak, M. Modulation of the CXC Chemokine Receptor 4 Agonist Activity of Ubiquitin through C-Terminal Protein Modification. Biochemistry 2013, 52, 4184–4192. [Google Scholar] [CrossRef]
- Scofield, S. L. C.; Daniels, C. R.; Dalal, S.; Millard, J. A.; Singh, M.; Singh, K. Extracellular Ubiquitin Modulates Cardiac Fibroblast Phenotype and Function via Its Interaction with CXCR4. Life Sci. 2018, 211, 8–16. [Google Scholar] [CrossRef]
- Loetscher, M.; Geiser, T.; O’Reilly, T.; Zwahlen, R.; Baggiolini, M.; Moser, B. Cloning of a Human Seven-Transmembrane Domain Receptor, LESTR, That Is Highly Expressed in Leukocytes. J. Biol. Chem. 1994, 269, 232–237. [Google Scholar] [CrossRef]
- Crump, M. P.; Gong, J. H.; Loetscher, P.; Rajarathnam, K.; Amara, A.; Arenzana-Seisdedos, F.; Virelizier, J. L.; Baggiolini, M.; Sykes, B. D.; Clark-Lewis, I. Solution Structure and Basis for Functional Activity of Stromal Cell-Derived Factor-1; Dissociation of CXCR4 Activation from Binding and Inhibition of HIV-1. EMBO J. 1997, 16, 6996–7007. [Google Scholar] [CrossRef]
- Bernhagen, J.; Krohn, R.; Lue, H.; Gregory, J. L.; Zernecke, A.; Koenen, R. R.; Dewor, M.; Georgiev, I.; Schober, A.; Leng, L.; Kooistra, T.; Fingerle-Rowson, G.; Ghezzi, P.; Kleemann, R.; McColl, S. R.; Bucala, R.; Hickey, M. J.; Weber, C. MIF Is a Noncognate Ligand of CXC Chemokine Receptors in Inflammatory and Atherogenic Cell Recruitment. Nat. Med. 2007, 13, 587–596. [Google Scholar] [CrossRef]
- Saini, V.; Staren, D. M.; Ziarek, J. J.; Nashaat, Z. N.; Campbell, E. M.; Volkman, B. F.; Marchese, A.; Majetschak, M. The CXC Chemokine Receptor 4 Ligands Ubiquitin and Stromal Cell-Derived Factor-1α Function through Distinct Receptor Interactions. J. Biol. Chem. 2011, 286, 33466–33477. [Google Scholar] [CrossRef]
- Rosenbaum, D. M.; Rasmussen, S. G. F.; Kobilka, B. K. The Structure and Function of G-Protein-Coupled Receptors. Nature 2009, 459, 356–363. [Google Scholar] [CrossRef]
- Goldsmith, Z. G.; Dhanasekaran, D. N. G Protein Regulation of MAPK Networks. Oncogene 2007, 26, 3122–3142. [Google Scholar] [CrossRef]
- New, D. C.; Wu, K.; Kwok, A. W. S.; Wong, Y. H. G Protein-Coupled Receptor-Induced Akt Activity in Cellular Proliferation and Apoptosis. FEBS J. 2007, 274, 6025–6036. [Google Scholar] [CrossRef] [PubMed]
- Mellado, M.; Rodríguez-Frade, J. M.; Mañes, S.; Martínez-A, C. Chemokine Signaling and Functional Responses: The Role of Receptor Dimerization and TK Pathway Activation. Annu. Rev. Immunol. 2001, 19. (Volume 19, 2001), 397–421. [Google Scholar] [CrossRef] [PubMed]
- Thelen, M.; Thelen, S. CXCR7, CXCR4 and CXCL12: An Eccentric Trio? J. Neuroimmunol. 2008, 198, 9–13. [Google Scholar] [CrossRef]
- Rajagopal, S.; Kim, J.; Ahn, S.; Craig, S.; Lam, C. M.; Gerard, N. P.; Gerard, C.; Lefkowitz, R. J. β-Arrestin- but Not G Protein-Mediated Signaling by the “Decoy” Receptor CXCR7. Proc. Natl. Acad. Sci. U. S. A. 2010, 107, 628–632. [Google Scholar] [CrossRef]
- Haribabu, B.; Richardson, R. M.; Fisher, I.; Sozzani, S.; Peiper, S. C.; Horuk, R.; Ali, H.; Snyderman, R. Regulation of Human Chemokine Receptors CXCR4: ROLE OF PHOSPHORYLATION IN DESENSITIZATION AND INTERNALIZATION *. J. Biol. Chem. 1997, 272, 28726–28731. [Google Scholar] [CrossRef]
- Signoret, N.; Rosenkilde, M. M.; Klasse, P. J.; Schwartz, T. W.; Malim, M. H.; Hoxie, J. A.; Marsh, M. Differential Regulation of CXCR4 and CCR5 Endocytosis. J. Cell Sci. 1998, 111, 2819–2830. [Google Scholar] [CrossRef]
- Marchese, A.; Chen, C.; Kim, Y.-M.; Benovic, J. L. The Ins and Outs of G Protein-Coupled Receptor Trafficking. Trends Biochem. Sci. 2003, 28, 369–376. [Google Scholar] [CrossRef]
- Signoret, N.; Oldridge, J.; Pelchen-Matthews, A.; Klasse, P. J.; Tran, T.; Brass, L. F.; Rosenkilde, M. M.; Schwartz, T. W.; Holmes, W.; Dallas, W.; Luther, M. A.; Wells, T. N. C.; Hoxie, J. A.; Marsh, M. Phorbol Esters and SDF-1 Induce Rapid Endocytosis and Down Modulation of the Chemokine Receptor CXCR4. J. Cell Biol. 1997, 139, 651–664. [Google Scholar] [CrossRef]
- Marchese, A.; Raiborg, C.; Santini, F.; Keen, J. H.; Stenmark, H.; Benovic, J. L. The E3 Ubiquitin Ligase AIP4 Mediates Ubiquitination and Sorting of the G Protein-Coupled Receptor CXCR4. Dev. Cell 2003, 5, 709–722. [Google Scholar] [CrossRef]
- Burger, J. A.; Burger, M.; Kipps, T. J. Chronic Lymphocytic Leukemia B Cells Express Functional CXCR4 Chemokine Receptors That Mediate Spontaneous Migration beneath Bone Marrow Stromal Cells. Blood 1999, 94, 3658–3667. [Google Scholar] [CrossRef]
- Balkwill, F. Cancer and the Chemokine Network. Nat. Rev. Cancer 2004, 4, 540–550. [Google Scholar] [CrossRef] [PubMed]
- Drury, L. J.; Ziarek, J. J.; Gravel, S.; Veldkamp, C. T.; Takekoshi, T.; Hwang, S. T.; Heveker, N.; Volkman, B. F.; Dwinell, M. B. Monomeric and Dimeric CXCL12 Inhibit Metastasis through Distinct CXCR4 Interactions and Signaling Pathways. Proc. Natl. Acad. Sci. U. S. A. 2011, 108. 17655. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Takeuchi, H.; Lam, S. T.; Turner, R. R.; Wang, H.-J.; Kuo, C.; Foshag, L.; Bilchik, A. J.; Hoon, D. S. B. Chemokine Receptor CXCR4 Expression in Colorectal Cancer Patients Increases the Risk for Recurrence and for Poor Survival. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2005, 23, 2744–2753. [Google Scholar] [CrossRef]
- Scala, S.; Ottaiano, A.; Ascierto, P. A.; Cavalli, M.; Simeone, E.; Giuliano, P.; Napolitano, M.; Franco, R.; Botti, G.; Castello, G. Expression of CXCR4 Predicts Poor Prognosis in Patients with Malignant Melanoma. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2005, 11, 1835–1841. [Google Scholar] [CrossRef]
- Zhao, H.; Guo, L.; Zhao, H.; Zhao, J.; Weng, H.; Zhao, B. CXCR4 Over-Expression and Survival in Cancer: A System Review and Meta-Analysis. Oncotarget 2014, 6, 5022–5040. [Google Scholar] [CrossRef]
- Marquardt, A.; Hartrampf, P.; Kollmannsberger, P.; Solimando, A. G.; Meierjohann, S.; Kübler, H.; Bargou, R.; Schilling, B.; Serfling, S. E.; Buck, A.; Werner, R. A.; Lapa, C.; Krebs, M. Predicting Microenvironment in CXCR4- and FAP-Positive Solid Tumors—A Pan-Cancer Machine Learning Workflow for Theranostic Target Structures. Cancers 2023, 15. (2), 392. [Google Scholar] [CrossRef]
- Janowski, M. Functional Diversity of SDF-1 Splicing Variants. Cell Adhes. Migr. 2009, 3, 243–249. [Google Scholar] [CrossRef]
- Müller, A.; Homey, B.; Soto, H.; Ge, N.; Catron, D.; Buchanan, M. E.; McClanahan, T.; Murphy, E.; Yuan, W.; Wagner, S. N.; Barrera, J. L.; Mohar, A.; Verástegui, E.; Zlotnik, A. Involvement of Chemokine Receptors in Breast Cancer Metastasis. Nature 2001, 410, 50–56. [Google Scholar] [CrossRef]
- Chow, M. T.; Luster, A. D. Chemokines in Cancer. Cancer Immunol. Res. 2014, 2, 1125–1131. [Google Scholar] [CrossRef]
- Darash-Yahana, M.; Pikarsky, E.; Abramovitch, R.; Zeira, E.; Pal, B.; Karplus, R.; Beider, K.; Avniel, S.; Kasem, S.; Galun, E.; Peled, A. Role of High Expression Levels of CXCR4 in Tumor Growth, Vascularization, and Metastasis. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2004, 18, 1240–1242. [Google Scholar] [CrossRef]
- Thiery, J. P. Epithelial-Mesenchymal Transitions in Tumour Progression. Nat. Rev. Cancer 2002, 2, 442–454. [Google Scholar] [CrossRef]
- Bockhorn, J.; Dalton, R.; Nwachukwu, C.; Huang, S.; Prat, A.; Yee, K.; Chang, Y.-F.; Huo, D.; Wen, Y.; Swanson, K. E.; Qiu, T.; Lu, J.; Park, S. Y.; Dolan, M. E.; Perou, C. M.; Olopade, O. I.; Clarke, M. F.; Greene, G. L.; Liu, H. MicroRNA-30c Inhibits Human Breast Tumour Chemotherapy Resistance by Regulating TWF1 and IL-11. Nat. Commun. 2013, 4, 1393. [Google Scholar] [CrossRef] [PubMed]
- Chu, C.-Y. G.; Chung, L. W. K. RANK-Mediated Signaling Network and Cancer Metastasis. Cancer Metastasis Rev. 2014, 33, 497–509. [Google Scholar] [CrossRef]
- Zhu, Y.; Yang, P.; Wang, Q.; Hu, J.; Xue, J.; Li, G.; Zhang, G.; Li, X.; Li, W.; Zhou, C.; Zhao, M.; Wang, D. The Effect of CXCR4 Silencing on Epithelial-Mesenchymal Transition Related Genes in Glioma U87 Cells. Anat. Rec. Hoboken NJ 2007 2013, 296, 1850–1856. [Google Scholar] [CrossRef]
- Cives, M.; Rizzo, F.; Simone, V.; Bisceglia, F.; Stucci, S.; Seeber, A.; Spizzo, G.; Montrone, T.; Resta, L.; Silvestris, F. Reviewing the Osteotropism in Neuroendocrine Tumors: The Role of Epithelial-Mesenchymal Transition. Neuroendocrinology 2016, 103, (3–4). [Google Scholar] [CrossRef]
- Mehrpouri, M. The Contributory Roles of the CXCL12/CXCR4/CXCR7 Axis in Normal and Malignant Hematopoiesis: A Possible Therapeutic Target in Hematologic Malignancies. Eur. J. Pharmacol. 2022, 920, 174831. [Google Scholar] [CrossRef]
- Nagasawa, T.; Hirota, S.; Tachibana, K.; Takakura, N.; Nishikawa, S.; Kitamura, Y.; Yoshida, N.; Kikutani, H.; Kishimoto, T. Defects of B-Cell Lymphopoiesis and Bone-Marrow Myelopoiesis in Mice Lacking the CXC Chemokine PBSF/SDF-1. Nature 1996, 382, 635–638. [Google Scholar] [CrossRef]
- Ma, Q.; Jones, D.; Borghesani, P. R.; Segal, R. A.; Nagasawa, T.; Kishimoto, T.; Bronson, R. T.; Springer, T. A. Impaired B-Lymphopoiesis, Myelopoiesis, and Derailed Cerebellar Neuron Migration in CXCR4- and SDF-1-Deficient Mice. Proc. Natl. Acad. Sci. U. S. A. 1998, 95, 9448–9453. [Google Scholar] [CrossRef]
- Zou, Y. R.; Kottmann, A. H.; Kuroda, M.; Taniuchi, I.; Littman, D. R. Function of the Chemokine Receptor CXCR4 in Haematopoiesis and in Cerebellar Development. Nature 1998, 393, 595–599. [Google Scholar] [CrossRef]
- Wright, D. E.; Bowman, E. P.; Wagers, A. J.; Butcher, E. C.; Weissman, I. L. Hematopoietic Stem Cells Are Uniquely Selective in Their Migratory Response to Chemokines. J. Exp. Med. 2002, 195, 1145–1154. [Google Scholar] [CrossRef]
- Aiuti, A.; Webb, I. J.; Bleul, C.; Springer, T.; Gutierrez-Ramos, J. C. The Chemokine SDF-1 Is a Chemoattractant for Human CD34+ Hematopoietic Progenitor Cells and Provides a New Mechanism to Explain the Mobilization of CD34+ Progenitors to Peripheral Blood. J. Exp. Med. 1997, 185, 111–120. [Google Scholar] [CrossRef] [PubMed]
- Peled, A.; Grabovsky, V.; Habler, L.; Sandbank, J.; Arenzana-Seisdedos, F.; Petit, I.; Ben-Hur, H.; Lapidot, T.; Alon, R. The Chemokine SDF-1 Stimulates Integrin-Mediated Arrest of CD34(+) Cells on Vascular Endothelium under Shear Flow. J. Clin. Invest. 1999, 104, 1199–1211. [Google Scholar] [CrossRef] [PubMed]
- Bilgin, Y. M. Use of Plerixafor for Stem Cell Mobilization in the Setting of Autologous and Allogeneic Stem Cell Transplantations: An Update. J. Blood Med. 2021, 12, 403–412. [Google Scholar] [CrossRef]
- Dasari, A.; Shen, C.; Halperin, D.; Zhao, B.; Zhou, S.; Xu, Y.; Shih, T.; Yao, J. C. Trends in the Incidence, Prevalence, and Survival Outcomes in Patients With Neuroendocrine Tumors in the United States. JAMA Oncol. 2017, 3, 1335–1342. [Google Scholar] [CrossRef]
- Das, S.; Dasari, A. Epidemiology, Incidence, and Prevalence of Neuroendocrine Neoplasms: Are There Global Differences? Curr. Oncol. Rep. 2021, 23. (4), 43. [Google Scholar] [CrossRef]
- Modlin, I. M.; Moss, S. F.; Chung, D. C.; Jensen, R. T.; Snyderwine, E. Priorities for Improving the Management of Gastroenteropancreatic Neuroendocrine Tumors. JNCI J. Natl. Cancer Inst. 2008, 100, 1282–1289. [Google Scholar] [CrossRef]
- Clement, D.; Ramage, J.; Srirajaskanthan, R. Update on Pathophysiology, Treatment, and Complications of Carcinoid Syndrome. J. Oncol. 2020, 2020, 8341426. [Google Scholar] [CrossRef]
- Del Olmo-Garcia, M. I.; Prado-Wohlwend, S.; Andres, A.; Soriano, J. M.; Bello, P.; Merino-Torres, J. F. Somatostatin and Somatostatin Receptors: From Signaling to Clinical Applications in Neuroendocrine Neoplasms. Biomedicines 2021, 9. (12), 1810. [Google Scholar] [CrossRef] [PubMed]
- Gabriel, M.; Decristoforo, C.; Kendler, D.; Dobrozemsky, G.; Heute, D.; Uprimny, C.; Kovacs, P.; Von Guggenberg, E.; Bale, R.; Virgolini, I. J. 68Ga-DOTA-Tyr3-Octreotide PET in Neuroendocrine Tumors: Comparison with Somatostatin Receptor Scintigraphy and CT. J. Nucl. Med. 2007, 48, 508–518. [Google Scholar] [CrossRef]
- Rinke, A.; Müller, H.-H.; Schade-Brittinger, C.; Klose, K.-J.; Barth, P.; Wied, M.; Mayer, C.; Aminossadati, B.; Pape, U.-F.; Bläker, M.; Harder, J.; Arnold, C.; Gress, T.; Arnold, R.; PROMID Study Group. Placebo-Controlled, Double-Blind, Prospective, Randomized Study on the Effect of Octreotide LAR in the Control of Tumor Growth in Patients with Metastatic Neuroendocrine Midgut Tumors: A Report from the PROMID Study Group. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2009, 27, 4656–4663. [Google Scholar] [CrossRef]
- Caplin, M. E.; Pavel, M.; Ćwikła, J. B.; Phan, A. T.; Raderer, M.; Sedláčková, E.; Cadiot, G.; Wolin, E. M.; Capdevila, J.; Wall, L.; Rindi, G.; Langley, A.; Martinez, S.; Blumberg, J.; Ruszniewski, P.; CLARINET Investigators. Lanreotide in Metastatic Enteropancreatic Neuroendocrine Tumors. N. Engl. J. Med. 2014, 371, 224–233. [Google Scholar] [CrossRef]
- Baudin, E.; Horsch, D.; Singh, S.; Caplin, M. E.; Ferone, D.; Wolin, E. M.; Capdevila, J.; Buikhuisen, W. A.; Raderer, M.; Dansin, E.; Grohe, C.; Houchard, A.; Thanh, X.-M. T.; Reidy-Lagunes, D. 1096O Lanreotide Autogel/Depot (LAN) in Patients with Advanced Bronchopulmonary (BP) Neuroendocrine Tumors (NETs): Results from the Phase III SPINET Study. Ann. Oncol. 2021, 32, S906. [Google Scholar] [CrossRef]
- Nagtegaal, I. D.; Odze, R. D.; Klimstra, D.; Paradis, V.; Rugge, M.; Schirmacher, P.; Washington, K. M.; Carneiro, F.; Cree, I. A.; WHO Classification of Tumours Editorial Board. The 2019 WHO Classification of Tumours of the Digestive System. Histopathology 2020, 76, 182–188. [Google Scholar] [CrossRef]
- Travis, W. D.; Brambilla, E.; Nicholson, A. G.; Yatabe, Y.; Austin, J. H. M.; Beasley, M. B.; Chirieac, L. R.; Dacic, S.; Duhig, E.; Flieder, D. B.; Geisinger, K.; Hirsch, F. R.; Ishikawa, Y.; Kerr, K. M.; Noguchi, M.; Pelosi, G.; Powell, C. A.; Tsao, M. S.; Wistuba, I.; WHO Panel. The 2015 World Health Organization Classification of Lung Tumors: Impact of Genetic, Clinical and Radiologic Advances Since the 2004 Classification. J. Thorac. Oncol. Off. Publ. Int. Assoc. Study Lung Cancer 2015, 10, 1243–1260. [Google Scholar] [CrossRef]
- Corleto, V. D.; Falconi, M.; Panzuto, F.; Milione, M.; De Luca, O.; Perri, P.; Cannizzaro, R.; Bordi, C.; Pederzoli, P.; Scarpa, A.; Delle Fave, G. Somatostatin Receptor Subtypes 2 and 5 Are Associated with Better Survival in Well-Differentiated Endocrine Carcinomas. Neuroendocrinology 2009, 89, 223–230. [Google Scholar] [CrossRef]
- Zamora, V.; Cabanne, A.; Salanova, R.; Bestani, C.; Domenichini, E.; Marmissolle, F.; Giacomi, N.; O’Connor, J.; Méndez, G.; Roca, E.; Buenos Aires and La Plata Argentina Argentum Working Group. Immunohistochemical Expression of Somatostatin Receptors in Digestive Endocrine Tumours. Dig. Liver Dis. Off. J. Ital. Soc. Gastroenterol. Ital. Assoc. Study Liver 2010, 42, 220–225. [Google Scholar] [CrossRef]
- Okuwaki, K.; Kida, M.; Mikami, T.; Yamauchi, H.; Imaizumi, H.; Miyazawa, S.; Iwai, T.; Takezawa, M.; Saegusa, M.; Watanabe, M.; Koizumi, W. Clinicopathologic Characteristics of Pancreatic Neuroendocrine Tumors and Relation of Somatostatin Receptor Type 2A to Outcomes. Cancer 2013, 119, 4094–4102. [Google Scholar] [CrossRef]
- Qian, Z. R.; Li, T.; Ter-Minassian, M.; Yang, J.; Chan, J. A.; Brais, L. K.; Masugi, Y.; Thiaglingam, A.; Brooks, N.; Nishihara, R.; Bonnemarie, M.; Masuda, A.; Inamura, K.; Kim, S. A.; Mima, K.; Sukawa, Y.; Dou, R.; Lin, X.; Christiani, D. C.; Schmidlin, F.; Fuchs, C. S.; Mahmood, U.; Ogino, S.; Kulke, M. H. Association Between Somatostatin Receptor Expression and Clinical Outcomes in Neuroendocrine Tumors. Pancreas 2016, 45, 1386–1393. [Google Scholar] [CrossRef]
- Mehta, S.; de Reuver, P. R.; Gill, P.; Andrici, J.; D’Urso, L.; Mittal, A.; Pavlakis, N.; Clarke, S.; Samra, J. S.; Gill, A. J. Somatostatin Receptor SSTR-2a Expression Is a Stronger Predictor for Survival Than Ki-67 in Pancreatic Neuroendocrine Tumors. Medicine (Baltimore) 2015, 94. (40), e1281.. [Google Scholar] [CrossRef]
- Song, K. B.; Kim, S. C.; Kim, J. H.; Seo, D.-W.; Hong, S.-M.; Park, K.-M.; Hwang, D. W.; Lee, J. H.; Lee, Y.-J. Prognostic Value of Somatostatin Receptor Subtypes in Pancreatic Neuroendocrine Tumors. Pancreas 2016, 45, 187–192. [Google Scholar] [CrossRef]
- Circelli, L.; Sciammarella, C.; Guadagno, E.; Tafuto, S.; Del Basso De Caro, M.; Botti, G.; Pezzullo, L.; Aria, M.; Ramundo, V.; Tatangelo, F.; Losito, N. S.; Ieranò, C.; D’Alterio, C.; Izzo, F.; Ciliberto, G.; Colao, A.; Faggiano, A.; Scala, S. CXCR4/CXCL12/CXCR7 Axis Is Functional in Neuroendocrine Tumors and Signals on mTOR. Oncotarget 2016, 7, 18865–18875. [Google Scholar] [CrossRef] [PubMed]
- Yao, J. C.; Lombard-Bohas, C.; Baudin, E.; Kvols, L. K.; Rougier, P.; Ruszniewski, P.; Hoosen, S.; St Peter, J.; Haas, T.; Lebwohl, D.; Van Cutsem, E.; Kulke, M. H.; Hobday, T. J.; O’Dorisio, T. M.; Shah, M. H.; Cadiot, G.; Luppi, G.; Posey, J. A.; Wiedenmann, B. Daily Oral Everolimus Activity in Patients with Metastatic Pancreatic Neuroendocrine Tumors after Failure of Cytotoxic Chemotherapy: A Phase II Trial. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2010, 28, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Pavel, M. E.; Hainsworth, J. D.; Baudin, E.; Peeters, M.; Hörsch, D.; Winkler, R. E.; Klimovsky, J.; Lebwohl, D.; Jehl, V.; Wolin, E. M.; Öberg, K.; Cutsem, E. V.; Yao, J. C. Everolimus plus Octreotide Long-Acting Repeatable for the Treatment of Advanced Neuroendocrine Tumours Associated with Carcinoid Syndrome (RADIANT-2): A Randomised, Placebo-Controlled, Phase 3 Study. The Lancet 2011, 378, 2005–2012. [Google Scholar] [CrossRef] [PubMed]
- Korbecki, J.; Simińska, D.; Gąssowska-Dobrowolska, M.; Listos, J.; Gutowska, I.; Chlubek, D.; Baranowska-Bosiacka, I. Chronic and Cycling Hypoxia: Drivers of Cancer Chronic Inflammation through HIF-1 and NF-κB Activation: A Review of the Molecular Mechanisms. Int. J. Mol. Sci. 2021, 22. (19), 10701.. [Google Scholar] [CrossRef] [PubMed]
- Albadari, N.; Deng, S.; Li, W. The Transcriptional Factors HIF-1 and HIF-2 and Their Novel Inhibitors in Cancer Therapy. Expert Opin. Drug Discov. 2019, 14, 667–682. [Google Scholar] [CrossRef] [PubMed]
- Wicks, E. E.; Semenza, G. L. Hypoxia-Inducible Factors: Cancer Progression and Clinical Translation. J. Clin. Invest. 2022, 132. (11), e159839. [Google Scholar] [CrossRef] [PubMed]
- Jin, Z.; Xiang, R.; Dai, J.; Wang, Y.; Xu, Z. HIF-1α Mediates CXCR4 Transcription to Activate the AKT/mTOR Signaling Pathway and Augment the Viability and Migration of Activated B Cell-like Diffuse Large B-Cell Lymphoma Cells. Mol. Carcinog. 2023, 62, 676–684. [Google Scholar] [CrossRef] [PubMed]
- Guo, M.; Cai, C.; Zhao, G.; Qiu, X.; Zhao, H.; Ma, Q.; Tian, L.; Li, X.; Hu, Y.; Liao, B.; Ma, B.; Fan, Q. Hypoxia Promotes Migration and Induces CXCR4 Expression via HIF-1α Activation in Human Osteosarcoma. PloS One 2014, 9. (3), e90518. [Google Scholar] [CrossRef] [PubMed]
- Arvidsson, Y.; Bergström, A.; Arvidsson, L.; Kristiansson, E.; Ahlman, H.; Nilsson, O. Hypoxia Stimulates CXCR4 Signalling in Ileal Carcinoids. Endocr. Relat. Cancer 2010, 17, 303–316. [Google Scholar] [CrossRef] [PubMed]
- Deschamps, L.; Bacha, D.; Rebours, V.; Mebarki, M.; Bretagnol, F.; Panis, Y.; Bedossa, P.; Ruszniewski, P.; Couvelard, A. The Expression of the Hypoxia Markers CA9 and CXCR4 Is Correlated with Survival in Patients with Neuroendocrine Tumours of the Ileum. Neuroendocrinology 2012, 95, 214–222. [Google Scholar] [CrossRef] [PubMed]
- Kaemmerer, D.; Träger, T.; Hoffmeister, M.; Sipos, B.; Hommann, M.; Sänger, J.; Schulz, S.; Lupp, A. Inverse Expression of Somatostatin and CXCR4 Chemokine Receptors in Gastroenteropancreatic Neuroendocrine Neoplasms of Different Malignancy. Oncotarget 2015, 6, 27566–27579. [Google Scholar] [CrossRef]
- Mai, R.; Kaemmerer, D.; Träger, T.; Neubauer, E.; Sänger, J.; Baum, R. P.; Schulz, S.; Lupp, A. Different Somatostatin and CXCR4 Chemokine Receptor Expression in Gastroenteropancreatic Neuroendocrine Neoplasms Depending on Their Origin. Sci. Rep. 2019, 9. (1), 4339. [Google Scholar] [CrossRef]
- Discipline of Morphopathology, Department of Microscopic Morphology, Victor Babeş University of Medicine and Pharmacy, Timişoara, Romania; Anapatmol Research Center, Victor Babeş University of Medicine and Pharmacy, Timişoara, Romania; Popa, O. ; Tăban, S. M.; Discipline of Morphopathology, Department of Microscopic Morphology, Victor Babeş University of Medicine and Pharmacy, Timişoara, Romania; Anapatmol Research Center, Victor Babeş University of Medicine and Pharmacy, Timişoara, Romania; Dema, A. L. C.; Discipline of Morphopathology, Department of Microscopic Morphology, Victor Babeş University of Medicine and Pharmacy, Timişoara, Romania; Anapatmol Research Center, Victor Babeş University of Medicine and Pharmacy, Timişoara, Romania; Plopeanu, A. D.; Department of Pathology, Pius Brînzeu Emergency County Hospital, Timişoara, Romania; Barna, R. A.; Anapatmol Research Center, Victor Babeş University of Medicine and Pharmacy, Timişoara, Romania; Discipline of Gastroenterology and Hepatology, Department of Internal Medicine II, Victor Babeş University of Medicine and Pharmacy, Timişoara, Romania; Cornianu, M.; Discipline of Morphopathology, Department of Microscopic Morphology, Victor Babeş University of Medicine and Pharmacy, Timişoara, Romania; Anapatmol Research Center, Victor Babeş University of Medicine and Pharmacy, Timişoara, Romania; Dema, S.; Service of Radiotherapy, Emergency City Hospital, Timişoara, Romania. Immunohistochemical Expression of Chemokine Receptor in Neuroendocrine Neoplasms (CXCR4) of the Gastrointestinal Tract: A Retrospective Study of 71 Cases. Rom. J. Morphol. Embryol. 2021, 62, 151–157. [Google Scholar] [CrossRef]
- Kaemmerer, D.; Reimann, C.; Specht, E.; Wirtz, R. M.; Sayeg, M.; Baum, R. P.; Schulz, S.; Lupp, A. Differential Expression and Prognostic Value of the Chemokine Receptor CXCR4 in Bronchopulmonary Neuroendocrine Neoplasms. Oncotarget 2015, 6, 3346–3358. [Google Scholar] [CrossRef] [PubMed]
- Lambert, A. W.; Pattabiraman, D. R.; Weinberg, R. A. Emerging Biological Principles of Metastasis. Cell 2017, 168, 670–691. [Google Scholar] [CrossRef] [PubMed]
- Galván, J. A.; Astudillo, A.; Vallina, A.; Crespo, G.; Folgueras, M. V.; González, M. V. Prognostic and Diagnostic Value of Epithelial to Mesenchymal Transition Markers in Pulmonary Neuroendocrine Tumors. BMC Cancer 2014, 14. (1), 855. [Google Scholar] [CrossRef] [PubMed]
- Galván, J. A.; Astudillo, A.; Vallina, A.; Fonseca, P. J.; Gómez-Izquierdo, L.; García-Carbonero, R.; González, M. V. Epithelial-Mesenchymal Transition Markers in the Differential Diagnosis of Gastroenteropancreatic Neuroendocrine Tumors. Am. J. Clin. Pathol. 2013, 140, 61–72. [Google Scholar] [CrossRef] [PubMed]
- Fendrich, V.; Maschuw, K.; Waldmann, J.; Buchholz, M.; Rehm, J.; Gress, T. M.; Bartsch, D. K.; König, A. Epithelial-Mesenchymal Transition Is a Critical Step in Tumorgenesis of Pancreatic Neuroendocrine Tumors. Cancers 2012, 4, 281–294. [Google Scholar] [CrossRef]
- Cives, M.; Quaresmini, D.; Rizzo, F. M.; Felici, C.; D’Oronzo, S.; Simone, V.; Silvestris, F. Osteotropism of Neuroendocrine Tumors: Role of the CXCL12/CXCR4 Pathway in Promoting EMT in Vitro. Oncotarget 2017, 8, 22534–22549. [Google Scholar] [CrossRef]
- Cives, M.; Pellè, E.; Rinzivillo, M.; Prosperi, D.; Tucci, M.; Silvestris, F.; Panzuto, F. Bone Metastases in Neuroendocrine Tumors: Molecular Pathogenesis and Implications in Clinical Practice. Neuroendocrinology 2021, 111, 207–216. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Li, N.; Li, J.; Lu, M.; Leal, J. P.; Tan, H.; Su, H.; Fan, Y.; Zhang, Y.; Zhao, W.; Zhu, H.; Pomper, M. G.; Zhou, Y.; Yang, Z. The Correlation Between [68Ga]DOTATATE PET/CT and Cell Proliferation in Patients With GEP-NENs. Mol. Imaging Biol. 2019, 21, 984–990. [Google Scholar] [CrossRef]
- Ezziddin, S.; Adler, L.; Sabet, A.; Pöppel, T. D.; Grabellus, F.; Yüce, A.; Fischer, H.-P.; Simon, B.; Höller, T.; Biersack, H.-J.; Nagarajah, J. Prognostic Stratification of Metastatic Gastroenteropancreatic Neuroendocrine Neoplasms by 18F-FDG PET: Feasibility of a Metabolic Grading System. J. Nucl. Med. Off. Publ. Soc. Nucl. Med. 2014, 55, 1260–1266. [Google Scholar] [CrossRef]
- Abgral, R.; Leboulleux, S.; Déandreis, D.; Aupérin, A.; Lumbroso, J.; Dromain, C.; Duvillard, P.; Elias, D.; De Baere, T.; Guigay, J.; Ducreux, M.; Schlumberger, M.; Baudin, E. Performance of 18Fluorodeoxyglucose-Positron Emission Tomography and Somatostatin Receptor Scintigraphy for High Ki67 (≥10%) Well-Differentiated Endocrine Carcinoma Staging. J. Clin. Endocrinol. Metab. 2011, 96, 665–671. [Google Scholar] [CrossRef] [PubMed]
- Pauwels, E. K. J.; Sturm, E. J. C.; Bombardieri, E.; Cleton, F. J.; Stokkel, M. P. M. Positron-Emission Tomography with [18F]Fluorodeoxyglucose. J. Cancer Res. Clin. Oncol. 2000, 126, 549–559. [Google Scholar] [CrossRef] [PubMed]
- Uy, G. L.; Rettig, M. P.; Cashen, A. F. Plerixafor, a CXCR4 Antagonist for the Mobilization of Hematopoietic Stem Cells. Expert Opin. Biol. Ther. 2008, 8, 1797–1804. [Google Scholar] [CrossRef] [PubMed]
- Aghanejad, A.; Jalilian, A. R.; Fazaeli, Y.; Alirezapoor, B.; Pouladi, M.; Beiki, D.; Maus, S.; Khalaj, A. Synthesis and Evaluation of [67Ga]-AMD3100: A Novel Imaging Agent for Targeting the Chemokine Receptor CXCR4. Sci. Pharm. 2014, 82, 29–42. [Google Scholar] [CrossRef] [PubMed]
- Gourni, E.; Demmer, O.; Schottelius, M.; D’Alessandria, C.; Schulz, S.; Dijkgraaf, I.; Schumacher, U.; Schwaiger, M.; Kessler, H.; Wester, H.-J. PET of CXCR4 Expression by a 68Ga-Labeled Highly Specific Targeted Contrast Agent. J. Nucl. Med. 2011, 52, 1803–1810. [Google Scholar] [CrossRef] [PubMed]
- Demmer, O.; Gourni, E.; Schumacher, U.; Kessler, H.; Wester, H.-J. PET Imaging of CXCR4 Receptors in Cancer by a New Optimized Ligand. ChemMedChem 2011, 6, 1789–1791. [Google Scholar] [CrossRef] [PubMed]
- Martin, R.; Jüttler, S.; Müller, M.; Wester, H.-J. Cationic Eluate Pretreatment for Automated Synthesis of [68Ga]CPCR4.2. Nucl. Med. Biol. 2014, 41, 84–89. [Google Scholar] [CrossRef] [PubMed]
- Diagnostische Leistung und Sicherheit von [68Ga]Ga-PentixaFor zur Erkennung und Lokalisation von Chemokin Rezeptor 4 (CXCR4) positiven Tumoren und Metastasen in einem PAN Cancer Ansatz: eine prospektive, multizentrische, internationale, klinische Phase III Studie, die FORPAN Studie | Cochrane Library. [CrossRef]
- Werner, R. A.; Weich, A.; Higuchi, T.; Schmid, J. S.; Schirbel, A.; Lassmann, M.; Wild, V.; Rudelius, M.; Kudlich, T.; Herrmann, K.; Scheurlen, M.; Buck, A. K.; Kropf, S.; Wester, H.-J.; Lapa, C. Imaging of Chemokine Receptor 4 Expression in Neuroendocrine Tumors - a Triple Tracer Comparative Approach. Theranostics 2017, 7, 1489–1498. [Google Scholar] [CrossRef] [PubMed]
- Werner, R. A.; Weich, A.; Schirbel, A.; Samnick, S.; Buck, A. K.; Higuchi, T.; Wester, H.-J.; Lapa, C. Intraindividual Tumor Heterogeneity in NET – Further Insight by C-X-C Motif Chemokine Receptor 4-Directed Imaging. Eur. J. Nucl. Med. Mol. Imaging 2017, 44, 553–554. [Google Scholar] [CrossRef]
- Weich, A.; Werner, R. A.; Buck, A. K.; Hartrampf, P. E.; Serfling, S. E.; Scheurlen, M.; Wester, H.-J.; Meining, A.; Kircher, S.; Higuchi, T.; Pomper, M. G.; Rowe, S. P.; Lapa, C.; Kircher, M. CXCR4-Directed PET/CT in Patients with Newly Diagnosed Neuroendocrine Carcinomas. Diagnostics 2021, 11. (4), 605.. [Google Scholar] [CrossRef]
- Watts, A.; Singh, B.; Singh, H.; Bal, A.; Kaur, H.; Dhanota, N.; Arora, S. K.; Mittal, B. R.; Behera, D. [68Ga]Ga-Pentixafor PET/CT Imaging for in Vivo CXCR4 Receptor Mapping in Different Lung Cancer Histologic Sub-Types: Correlation with Quantitative Receptors’ Density by Immunochemistry Techniques. Eur. J. Nucl. Med. Mol. Imaging 2023, 50, 1216–1227. [Google Scholar] [CrossRef]
- Lewis, R.; Habringer, S.; Kircher, M.; Hefter, M.; Peuker, C. A.; Werner, R.; Ademaj-Kospiri, V.; Gäble, A.; Weber, W.; Wester, H.-J.; Buck, A.; Herhaus, P.; Lapa, C.; Keller, U. Investigation of Spleen CXCR4 Expression by [68Ga]Pentixafor PET in a Cohort of 145 Solid Cancer Patients. EJNMMI Res. 2021, 11. (1), 77.. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, S.; Behnam Azad, B.; Nimmagadda, S. The Intricate Role of CXCR4 in Cancer. In Advances in Cancer Research; Elsevier, 2014; Vol. 124, pp 31–82. [CrossRef]
- Wang, Z.; Ma, Q. Beta-Catenin Is a Promising Key Factor in the SDF-1/CXCR4 Axis on Metastasis of Pancreatic Cancer. Med. Hypotheses 2007, 69, 816–820. [Google Scholar] [CrossRef] [PubMed]
- Kim, J. T.; Li, J.; Jang, E. R.; Gulhati, P.; Rychahou, P. G.; Napier, D. L.; Wang, C.; Weiss, H. L.; Lee, E. Y.; Anthony, L.; Townsend, C. M.; Liu, C.; Evers, B. M. Deregulation of Wnt/β-Catenin Signaling through Genetic or Epigenetic Alterations in Human Neuroendocrine Tumors. Carcinogenesis 2013, 34, 953–961. [Google Scholar] [CrossRef]
- Weich, A.; Rogoll, D.; Gawlas, S.; Mayer, L.; Weich, W.; Pongracz, J.; Kudlich, T.; Meining, A.; Scheurlen, M. Wnt/β-Catenin Signaling Regulates CXCR4 Expression and [68Ga] Pentixafor Internalization in Neuroendocrine Tumor Cells. Diagnostics 2021, 11. [Google Scholar] [CrossRef] [PubMed]
- Martin, M.; Mayer, I. A.; Walenkamp, A. M. E.; Lapa, C.; Andreeff, M.; Bobirca, A. At the Bedside: Profiling and Treating Patients with CXCR4-Expressing Cancers. J. Leukoc. Biol. 2021, 109, 953–967. [Google Scholar] [CrossRef] [PubMed]
- Otani, Y.; Kijima, T.; Kohmo, S.; Oishi, S.; Minami, T.; Nagatomo, I.; Takahashi, R.; Hirata, H.; Suzuki, M.; Inoue, K.; Takeda, Y.; Kida, H.; Tachibana, I.; Fujii, N.; Kumanogoh, A. Suppression of Metastases of Small Cell Lung Cancer Cells in Mice by a Peptidic CXCR4 Inhibitor TF14016. FEBS Lett. 2012, 586, 3639–3644. [Google Scholar] [CrossRef] [PubMed]
- Salgia, R.; Stille, J. R.; Weaver, R. W.; McCleod, M.; Hamid, O.; Polzer, J.; Roberson, S.; Flynt, A.; Spigel, D. R. A Randomized Phase II Study of LY2510924 and Carboplatin/Etoposide versus Carboplatin/Etoposide in Extensive-Disease Small Cell Lung Cancer. Lung Cancer Amst. Neth. 2017, 105, 7–13. [Google Scholar] [CrossRef]
- Robinson, T.; Escara-Wilke, J.; Dai, J.; Zimmermann, J.; Keller, E. T. A CXCR4 Inhibitor (Balixafortide) Enhances Docetaxel-Mediated Antitumor Activity in a Murine Model of Prostate Cancer Bone Metastasis. The Prostate 2023, 83, 1247–1254. [Google Scholar] [CrossRef]
- Bockorny, B.; Macarulla, T.; Semenisty, V.; Borazanci, E.; Feliu, J.; Ponz-Sarvise, M.; Abad, D. G.; Oberstein, P.; Alistar, A.; Muñoz, A.; Geva, R.; Guillén-Ponce, C.; Fernandez, M. S.; Peled, A.; Chaney, M.; Gliko-Kabir, I.; Shemesh-Darvish, L.; Ickowicz, D.; Sorani, E.; Kadosh, S.; Vainstein-Haras, A.; Hidalgo, M. Motixafortide and Pembrolizumab Combined to Nanoliposomal Irinotecan, Fluorouracil, and Folinic Acid in Metastatic Pancreatic Cancer: The COMBAT/KEYNOTE-202 Trial. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2021, 27, 5020–5027. [Google Scholar] [CrossRef]
- Andtbacka, R. H. I.; Wang, Y.; Pierce, R. H.; Campbell, J. S.; Yushak, M.; Milhem, M.; Ross, M.; Niland, K.; Arbeit, R. D.; Parasuraman, S.; Bickley, K.; Yeung, C. C.; Aicher, L. D.; Smythe, K. S.; Gan, L. Mavorixafor, an Orally Bioavailable CXCR4 Antagonist, Increases Immune Cell Infiltration and Inflammatory Status of Tumor Microenvironment in Patients with Melanoma. Cancer Res. Commun. 2022, 2, 904–913. [Google Scholar] [CrossRef] [PubMed]
- Karpova, D.; Bräuninger, S.; Wiercinska, E.; Krämer, A.; Stock, B.; Graff, J.; Martin, H.; Wach, A.; Escot, C.; Douglas, G.; Romagnoli, B.; Chevalier, E.; Dembowski, K.; Hooftman, L.; Bonig, H. Mobilization of Hematopoietic Stem Cells with the Novel CXCR4 Antagonist POL6326 (Balixafortide) in Healthy Volunteers-Results of a Dose Escalation Trial. J. Transl. Med. 2017, 15. (1), 2. [Google Scholar] [CrossRef] [PubMed]
- Crees, Z. D.; Rettig, M. P.; Jayasinghe, R. G.; Stockerl-Goldstein, K.; Larson, S. M.; Arpad, I.; Milone, G. A.; Martino, M.; Stiff, P.; Sborov, D.; Pereira, D.; Micallef, I.; Moreno-Jiménez, G.; Mikala, G.; Coronel, M. L. P.; Holtick, U.; Hiemenz, J.; Qazilbash, M. H.; Hardy, N.; Latif, T.; García-Cadenas, I.; Vainstein-Haras, A.; Sorani, E.; Gliko-Kabir, I.; Goldstein, I.; Ickowicz, D.; Shemesh-Darvish, L.; Kadosh, S.; Gao, F.; Schroeder, M. A.; Vij, R.; DiPersio, J. F. Motixafortide and G-CSF to Mobilize Hematopoietic Stem Cells for Autologous Transplantation in Multiple Myeloma: A Randomized Phase 3 Trial. Nat. Med. 2023, 29, 869–879. [Google Scholar] [CrossRef] [PubMed]
- Badolato, R.; Donadieu, J. Results of a Phase 3 Trial of an Oral CXCR4 Antagonist, Mavorixafor, for Treatment of Patients With WHIM Syndrome. Clin. Immunol. 2023, 250, 109349. [Google Scholar] [CrossRef]
- Bobkov, V.; Arimont, M.; Zarca, A.; De Groof, T. W. M.; Van Der Woning, B.; De Haard, H.; Smit, M. J. Antibodies Targeting Chemokine Receptors CXCR4 and ACKR3. Mol. Pharmacol. 2019, 96, 753–764. [Google Scholar] [CrossRef] [PubMed]
- Pellé, E.; Cives, M.; Quaresmini, D.; Lovero, D.; Felici, C.; Cafforio, P.; Palmirotta, R.; Silvestris, F. CXCR4 Inhibition by Ulocuplumab Prevents EMT of pNET Cells in Vitro. Ann. Oncol. 2017, 28, v154–v155. [Google Scholar] [CrossRef]
- Si, Y.; Guan, J.; Xu, Y.; Chen, K.; Kim, S.; Zhou, L.; Jaskula-Sztul, R.; Liu, X. M. Dual-Targeted Extracellular Vesicles to Facilitate Combined Therapies for Neuroendocrine Cancer Treatment. Pharmaceutics 2020, 12. (11), 1079. [Google Scholar] [CrossRef] [PubMed]
- Sabet, A.; Mader, N.; Bittenbring, J. T.; Khreish, F.; Grünwald, F.; Biersack, H. J.; Ezziddin, S. Prophylactic Peripheral Blood Stem Cell Collection in Patients with Extensive Bone-Marrow Infiltration of Neuroendocrine Tumours Prior to Peptide Receptor Radionuclide Therapy with 177Lu-DOTATATE. Pharmaceuticals 2021, 14. (10), 1022.. [Google Scholar] [CrossRef] [PubMed]
- Strosberg, J.; El-Haddad, G.; Wolin, E.; Hendifar, A.; Yao, J.; Chasen, B.; Mittra, E.; Kunz, P. L.; Kulke, M. H.; Jacene, H.; Bushnell, D.; O’Dorisio, T. M.; Baum, R. P.; Kulkarni, H. R.; Caplin, M.; Lebtahi, R.; Hobday, T.; Delpassand, E.; Van Cutsem, E.; Benson, A.; Srirajaskanthan, R.; Pavel, M.; Mora, J.; Berlin, J.; Grande, E.; Reed, N.; Seregni, E.; Öberg, K.; Lopera Sierra, M.; Santoro, P.; Thevenet, T.; Erion, J. L.; Ruszniewski, P.; Kwekkeboom, D.; Krenning, E.; NETTER-1 Trial Investigators. Phase 3 Trial of 177Lu-Dotatate for Midgut Neuroendocrine Tumors. N. Engl. J. Med. 2017, 376, 125–135. [Google Scholar] [CrossRef]
- Strosberg, J. R.; Caplin, M. E.; Kunz, P. L.; Ruszniewski, P. B.; Bodei, L.; Hendifar, A.; Mittra, E.; Wolin, E. M.; Yao, J. C.; Pavel, M. E.; Grande, E.; Van Cutsem, E.; Seregni, E.; Duarte, H.; Gericke, G.; Bartalotta, A.; Mariani, M. F.; Demange, A.; Mutevelic, S.; Krenning, E. P.; NETTER-1 investigators. 177Lu-Dotatate plus Long-Acting Octreotide versus High-dose Long-Acting Octreotide in Patients with Midgut Neuroendocrine Tumours (NETTER-1): Final Overall Survival and Long-Term Safety Results from an Open-Label, Randomised, Controlled, Phase 3 Trial. Lancet Oncol. 2021, 22, 1752–1763. [Google Scholar] [CrossRef]
- Poschenrieder, A.; Schottelius, M.; Schwaiger, M.; Kessler, H.; Wester, H.-J. The Influence of Different Metal-Chelate Conjugates of Pentixafor on the CXCR4 Affinity. EJNMMI Res. 2016, 6. (1), 36.. [Google Scholar] [CrossRef] [PubMed]
- Schottelius, M.; Osl, T.; Poschenrieder, A.; Hoffmann, F.; Beykan, S.; Hänscheid, H.; Schirbel, A.; Buck, A. K.; Kropf, S.; Schwaiger, M.; Keller, U.; Lassmann, M.; Wester, H.-J. [177Lu]Pentixather: Comprehensive Preclinical Characterization of a First CXCR4-Directed Endoradiotherapeutic Agent. Theranostics 2017, 7, 2350–2362. [Google Scholar] [CrossRef] [PubMed]
- Herrmann, K.; Schottelius, M.; Lapa, C.; Osl, T.; Poschenrieder, A.; Hänscheid, H.; Lückerath, K.; Schreder, M.; Bluemel, C.; Knott, M.; Keller, U.; Schirbel, A.; Samnick, S.; Lassmann, M.; Kropf, S.; Buck, A. K.; Einsele, H.; Wester, H.-J.; Knop, S. First-in-Human Experience of CXCR4-Directed Endoradiotherapy with 177Lu- and 90Y-Labeled Pentixather in Advanced-Stage Multiple Myeloma with Extensive Intra- and Extramedullary Disease. J. Nucl. Med. 2016, 57, 248–251. [Google Scholar] [CrossRef] [PubMed]
- Lapa, C.; Kircher, S.; Schirbel, A.; Rosenwald, A.; Kropf, S.; Pelzer, T.; Walles, T.; Buck, A. K.; Weber, W. A.; Wester, H.-J.; Herrmann, K.; Lückerath, K. Targeting CXCR4 with [68Ga]Pentixafor: A Suitable Theranostic Approach in Pleural Mesothelioma? Oncotarget 2017, 8, 96732–96737. [Google Scholar] [CrossRef] [PubMed]
- Lapa, C.; Hänscheid, H.; Kircher, M.; Schirbel, A.; Wunderlich, G.; Werner, R. A.; Samnick, S.; Kotzerke, J.; Einsele, H.; Buck, A. K.; Wester, H.-J.; Grigoleit, G. U. Feasibility of CXCR4-Directed Radioligand Therapy in Advanced Diffuse Large B-Cell Lymphoma. J. Nucl. Med. Off. Publ. Soc. Nucl. Med. 2019, 60, 60–64. [Google Scholar] [CrossRef] [PubMed]
- Habringer, S.; Lapa, C.; Herhaus, P.; Schottelius, M.; Istvanffy, R.; Steiger, K.; Slotta-Huspenina, J.; Schirbel, A.; Hänscheid, H.; Kircher, S.; Buck, A. K.; Götze, K.; Vick, B.; Jeremias, I.; Schwaiger, M.; Peschel, C.; Oostendorp, R.; Wester, H.-J.; Grigoleit, G.-U.; Keller, U. Dual Targeting of Acute Leukemia and Supporting Niche by CXCR4-Directed Theranostics. Theranostics 2018, 8, 369–383. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, S. M.; Wesseling, P.; de Keizer, B.; Tolboom, N.; Ververs, F. F. T.; Krijger, G. C.; Westerman, B. A.; Snijders, T. J.; Robe, P. A.; van der Kolk, A. G. CXCR4 Expression in Glioblastoma Tissue and the Potential for PET Imaging and Treatment with [68Ga]Ga-Pentixafor /[177Lu]Lu-Pentixather. Eur. J. Nucl. Med. Mol. Imaging 2022, 49, 481–491. [Google Scholar] [CrossRef] [PubMed]
- Fath, M. A.; Liu, D.; Ewald, J. T.; Robles-Planells, C.; Tomanek-Chalkley, A. M.; Graves, S. A.; Howe, J. R.; O’Dorisio, T. M.; Rastogi, P.; Bellizzi, A. M.; O’Dorisio, M. S.; Menda, Y.; Spitz, D. R. Chemokine Receptor CXCR4 Radioligand Targeted Therapy Using 177Lutetium-Pentixather for Pulmonary Neuroendocrine Cancers. Radiat. Res. 2023, 201, 35–47. [Google Scholar] [CrossRef]
- Fath, M. A.; Liu, D.; Robles-Planells, C.; Ewald, J. T.; Christensen, K. A.; Johnson, S. S.; Graves, S. A.; Spitz, D. R.; Menda, Y.; Sue O’Dorisio, M. Abstract 5034: Targeting CXCR4 and Thioredoxin Reductase in High Grade Neuroendocrine Tumors and Neuroendocrine Carcinomas. Cancer Res. 2023, 83. (7_Supplement),5034. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




