Submitted:
14 April 2024
Posted:
15 April 2024
You are already at the latest version
Abstract
Keywords:
Introduction
Methods
Ethics Approval Statement
Study Population and Design
Initial Reaction
Candidate Patients for SDD
The SDD Protocol
SDD Premedication and Concomitant Drugs
SDD Location
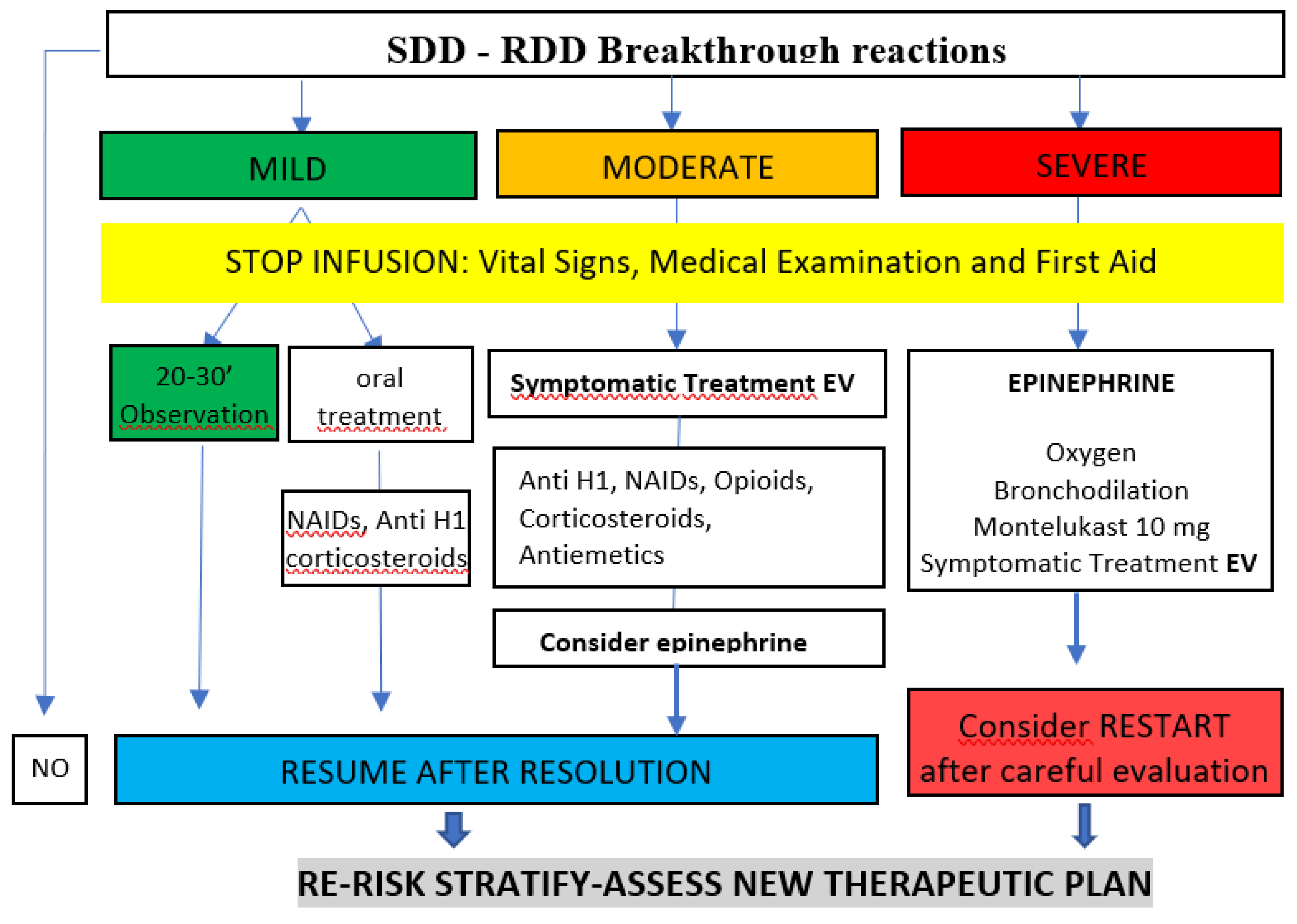
SDD Breakthrough Reactions
Diagnostic Protocol: Skin Test, Serological Biomarkers and Drug Provocation Test (DPT)
Results
Patient Characteristics
SDD Outcomes
Discussion
Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Doroshow JH, Kummar S. Translational research in oncology-10 years of progress and future prospects. Nat Rev Clin Oncol. 2014, 11, 649–662. [Google Scholar] [CrossRef] [PubMed]
- Desmond-Hellmann S, Sawyers CL, Cox DR et al. Toward Precision Medicine: Building a Knowledge Network for Biomedical Research and a New Taxonomy of Disease. Washington, DC: The National Academies Press; 2011.
- Rosell R, Monzó M, Alberola V, Taron M, Barnadas A, Sánchez JM, et al. Determinants of response and resistance to cytotoxics. Semin Oncol. 2002, 29, 110–118. [Google Scholar] [CrossRef] [PubMed]
- Matzka M, Köck-Hódi S, Jahn P, Mayer H. Relationship among symptom clusters, quality of life, and treatment-specific optimism in patients with cancer. Support Care Cancer. 2018, 26, 2685–2693. [Google Scholar] [CrossRef] [PubMed]
- Mayorga C, Ariza A, Muñoz-Cano R, Sabato V, Doña I, Torres MJ. Biomarkers of immediate drug hypersensitivity. Allergy. 2024, 79, 601–612. [Google Scholar] [CrossRef]
- Borrás J, Farzanegan R, Torres MC, Germán A et al. Same-Day Desensitization in Patients Who Experience Their First Reaction to a Platin Agent at the Oncology Day Unit: A Pilot Study to Safely Include This Technique Within the Multidisciplinary Pathways for the Diagnosis & Management of Hypersensitivity to Platin Agents. Front Allergy. 2022, 3, 1–7. [Google Scholar]
- Cernadas JR, Brockow K, Romano A, Aberer W, Torres MJ, Bircher A, et al. General considerations on rapid desensitization for drug hypersensitivity - a consensus statement. Allergy. 2010, 65, 1357–1366. [Google Scholar] [CrossRef]
- Jensen-Jarolim E, Bax HJ, Bianchini R, Capron M, Corrigan C, Castells M, et al. AllergoOncology - the impact of allergy in oncology: EAACI position paper. Allergy. 2017, 72, 866–887. [Google Scholar] [CrossRef]
- Gastaminza G, de la Borbolla JM, Goikoetxea MJ, Escudero R, Antón J, Espinós J, et al. A new rapid desensitization protocol for chemotherapy agents. J Investig Allergol Clin Immunol. 2011, 21, 108–112. [Google Scholar]
- Borras J, El-Qutob D. Experience With Rapid Desensitization to Chemotherapy in a Type B Hospital. J Investig Allergol Clin Immunol. 2016, 26, 271–273. [Google Scholar] [CrossRef]
- Madrigal-Burgaleta R, Bernal-Rubio L, Berges-Gimeno MP, Carpio-Escalona LV, Gehlhaar P, Alvarez-Cuesta E. A large single-hospital experience using drug provocation testing and rapid drug desensitization in hypersensitivity to antineoplastic and biological agents. J Allergy Clin Immunol Pract. 2019, 7, 618–632. [Google Scholar] [CrossRef]
- Broyles AD, Banerji A, Castells M. Practical guidance for the evaluation and management of drug hypersensitivity: general concepts. J Allergy Clin Immunol Pract. 2020, 8, S3–S15. [Google Scholar] [CrossRef]
- Lee JH, Moon M, Kim YC, Chung SJ, Oh J, Kang D-Y, et al. A one-bag rapid desensitization protocol for paclitaxel hypersensitivity: a noninferior alternative to a multi-bag rapid desensitization protocol. J Allergy Clin Immunol Pract. 2020, 8, 696–703. [Google Scholar] [CrossRef] [PubMed]
- Vidal C, Méndez-Brea P, López-Freire S, Bernárdez B, Lamas MJ, Armisén M, et al. A modified protocol for rapid desensitization to chemotherapy agents. A modified protocol for rapid desensitization to chemotherapy agents. J Allery Clin Immunol Pract. 2016, 4, 1003–1005. [Google Scholar]
- Sala-Cunill A, Molina-Molina G, Verdesoto J, Labrador-Horrillo M, Luengo O, Galvan-Blasco P, et al. One-dilution rapid desensitization protocol to chemotherapeutic and biological agents: a five-year experience. J Allergy Clin Immunol Pract. 2021, 9, 4045–4054. [Google Scholar] [CrossRef]
- Vázquez-Revuelta P, Lleonart-Bellfill R, Molina-Mata K, Múñoz-Sánchez C, Rey-Salido M, Madrigal-Burgaleta R, et al. A Pilot Experience Using a 1-Bag Intravenous Rapid Desensitization Protocol for Chemotherapy and Biologics in a Cohort of Patients With Access to a Delabeling Pathway. J Investig Allergol Clin Immunol. 2023, 33, 289–291. [Google Scholar] [CrossRef] [PubMed]
- Yang BC, Castells MC. The Who, What, Where, When, Why, and How of Drug Desensitization. Immunol Allergy Clin North Am. 2022, 42, 403–420. [Google Scholar] [CrossRef]
- Berges-Gimeno MP, Carpio-Escalona LV, Longo-Muñoz F, Bernal-Rubio L, López-Gónzalez P, Gehlhaar P, et al. Does Rapid Drug Desensitization to Chemotherapy Affect Survival Outcomes? J Investig Allergol Clin Immunol. 2020, 30, 254–263. [Google Scholar] [CrossRef]
- Castells M, Matulonis U, Horton T. Infusion reactions to systemic chemotherapy. UpToDate. O: Version 23 (Accessed, 20 January 2023.
- Mohamed OE, Baretto RL, Walker I, Melchior C, Heslegrave J, Mckenzie R, et al. Empty mast cell syndrome: fallacy or fact? J Clin Pathol. 2020, 73, 250–256. [Google Scholar] [CrossRef]
- Brown SGA. Clinical features and severity grading of anaphylaxis. J Allergy Clin Immunol. 2004, 114, 371–376. [Google Scholar] [CrossRef]
- Roselló S, Blasco I, García Fabregat L, Cervantes A, Jordan K. Management of infusion reactions to systemic anticancer therapy: ESMO Clinical Practice Guidelines. Ann Oncol. 2017, 28, iv100–iv118. [Google Scholar]
- Adnan A, Acharya S, Alenazy LA, de las Vecillas L, Giavina Bianchi P, Picard M, et al. Multistep IgE Mast Cell Desensitization Is a Dose- and Time-Dependent Process Partially Regulated by SHIP-1. J Immunol. 2023, 210, 709–720. [Google Scholar] [CrossRef] [PubMed]
- Vega A, Jiménez-Rodríguez T-W, Barranco R, Bartra J, Diéguez MC, Doña I, et al. Hypersensitivity Reactions to Cancer Chemotherapy: Practical Recommendations of ARADyAL for Diagnosis and Desensitization. J Investig Allergol Clin Immunol. 2021, 31, 364–384. [Google Scholar] [CrossRef] [PubMed]
- Gorgulu Akin B, Erkoc M, Korkmaz ET, Ozdel Ozturk B, Colak S, Ozalp Ates FS, et al. Rapid drug desensitization with platin-based chemotherapy: Analysis of risk factors for breakthrough reactions. World Allergy Organ J. 2021, 15, 1–12. [Google Scholar]
- Broyles AD, Banerji A, Bermettler S, Biggs CM, Blumenthal K, Brennan PJ, et al. Practical Guidance for the Evaluation and Management of Drug Hypersensitivity: Specific Drugs. J Allergy Clin Immunol Pract. 2020, 8, S16–S116. [Google Scholar] [CrossRef] [PubMed]
- García JC, Rodríguez E. Capítulo 12: Técnicas diagnósticas in vivo, En: Dávila I, Jáuregui I, eds. Tratado de Alergología. TOMO I. 2da Edición. Madrid (2015): 151-160.
- Pagani M, Bavbek S, Alvarez-Cuesta E, Berna Dursun A, Bonadonna P, Castells M, et al. Hypersensitivity reactions to chemotherapy: an EAACI Position Paper. Allergy 2022, 77, 388–403. [Google Scholar] [CrossRef] [PubMed]
- Madrigal-Burgaleta R, Vázquez-Revuelta P, Martí-Garrido J, Lleonart R, Ali MBBS FR, Alvarez-Cuesta E, et al. Importance of Diagnostics Prior to Desensitization in New Drug Hypersensitivity: Chemotherapeutics and Biologicals. Curr Treat Options Allergy. 2020, 7, 1–13. [Google Scholar] [CrossRef]
- Jiménez-Rodríguez T-W, Marco de la Calle F-M, Lozano-Cubo I, Montoyo-Anton R-A, Soriano-Gomis V, González-Delgado P, et al. Converter Phenotype: A New Profile That Is Not Exclusive to Taxanes. Front Allergy. 2022, 12, 785259. [Google Scholar]
- Silver J, Garcia-Neuer M, Lynch DM, Pasaoglu G, Sloane DE, Castells M. Endophenotyping oxaliplatin hypersensitivity: personalizing desensitization to the atypical platin. J Allergy Clin Immunol Pract. 2020, 8, 1668–1680. [Google Scholar] [CrossRef] [PubMed]
- Mayorga C, Celik G, Rouzaire P, Whitaker P, Bonadonna P, Rodrígues-Cernadas J, et al. In vitro tests for drug hypersensitivity reactions: an ENDA/EAACI Drug Allergy Interest Group position paper. Allergy. 2016, 71, 1103–1134. [Google Scholar] [CrossRef]
- Jakubovic BD, Sanchez-Sanchez S, Hamadi S, Lynch D, Castells M. Interleukin-6: a novel biomarker for monoclonal antibody and chemotherapy-associated hypersensitivity confirms a cytokine release syndrome phenotype-endotype association. Allergy. 2021, 76, 1571–1573. [Google Scholar] [CrossRef]
- Sabato V, Ebo DG, Van Der Poorten MM, Toscano A, Van Gasse AL, Mertens C et al. Allergenic and mas-related G protein-coupled receptor X2-activating properties of drugs: resolving the two. J Allergy Clin Immunol Pract. 2023, 11, 395–404. [Google Scholar] [CrossRef] [PubMed]
- Álvarez-Cuesta E, Madrigal-Burgaleta R, Broyles AD, Cuesta-Herranz J, Guzman-Melendez MA, Maciag MC, et al. Standards for practical intravenous rapid drug desensitization & delabeling: A WAO committee statement. World Allergy Organ J. 2022, 15, 100640.
- Jutel M, Agache I, Zemelka-Wiacek M, Akdis M, Chivato T, Del Giacco S, et al. Nomenclature of allergic diseases and hypersensitivity reactions: Adapted to modern needs: An EAACI position paper. Allergy. 2023, 78, 2851–2874. [Google Scholar] [CrossRef] [PubMed]
- Madrigal-Burgaleta R, Guzman-Melendez MA, Alvarez-Cuesta E. New Technical Aspects Used in the Management of Desensitization to Antineoplastic Drugs. Curr Treat Options Allergy. 2022, 9, 204–218. [Google Scholar] [CrossRef]
- Jimenez-Rodriguez TW, Garcia-Neuer M, Alenazy LA, Castells M. Anaphylaxis in the 21st century: phenotypes, endotypes, and biomarkers. J Asthma Allergy. 2018, 20, 121–142. [Google Scholar]
- Madrigal-Burgaleta R and Castells, M. Editorial: Diagnosis and management of allergy to chemotherapy and biologics. Front Allergy. 2023, 11, 1205345. [Google Scholar] [CrossRef]
|
5 mg/ml | |||||
|
100 mg (20 ml) | |||||
|
270 ml | |||||
|
0.37 mg/ml | |||||
| Example when the reaction appears at 40 ml of volume infused: | 14.8 mg (40 ml) | |||||
|
85.2mg (230ml) | |||||
| STEP | Rate ml/hour | Time (min) | Administered volumen (ml) | Administered dose (mg) | Cumulative dose infused (mg) | |
| 1 | 0.6 | 15 | 0.15 | 0.06 | 0.06 | |
| 2 | 1.2 | 15 | 0.3 | 0.11 | 0.17 | |
| 3 | 2.4 | 15 | 0.6 | 0.22 | 0.39 | |
| 4 | 4.8 | 15 | 1.2 | 0.44 | 0.83 | |
| 5 | 9.6 | 15 | 2.4 | 0.89 | 1.72 | |
| 6 | 19.2 | 15 | 4.8 | 1.78 | 3.50 | |
| 7 | 38.4 | 15 | 9.6 | 3.56 | 7.06 | |
| 8 | 76.8 | 15 | 19.2 | 7.11 | 14.17 | |
| 9 | 100 | 15 | 25 | 9.26 | 23.43 | |
| 10 | 120 | 83.37 | 166.75 | 61.76 | 85.19 | |
| TOTAL (SDD) | 218.4 | 230 | 85.20 | 85.20 | ||
| Previous to reaction: | 40 ml | 14.8 mg | ||||
| TOTAL (Administered) | 270 ml | 100 mg | ||||
| Drug | SPT | IDT |
|---|---|---|
| Paclitaxel 6 mg/mL |
1/10: 0.1 - 0.6 mg/mL 1/1: 1 - 6 mg/mL |
1/1000: 0.001- 0.006 mg/mL 1/100: 0.01 - 0.06 mg/mL 1/10: 0.6 mg/mL |
| Carboplatin 10 mg/mL |
1/1: 10 mg/mL | 1/100: 0.1 mg 1/10: 1 mg |
| Oxaliplatin 5 mg/mL |
1/1: 5 mg/mL | 1/100: 0.05 mg 1/10: 0.5 mg 1/1: 5 mg |
| Cetuximab 5 mg/mL |
1/1: 5 mg/mL | 1/10: 0.5 mg/mL 1/1: 5 mg/mL |
| Doxorubicin | unrealized | unrealized |
| Total n=35 | ||
|---|---|---|
| Sex | ||
| Male | 18 | (51%) |
| Female | 17 | (49%) |
| Age | ||
| Average | 58 | |
| Range | 37-79 | |
| Diagnosis | ||
| Colorectal | 18 | (51%) |
| Gastric | 8 | (23%) |
| Ovarian | 4 | (11%) |
| Breast | 1 | (3%) |
| Lung | 1 | (3%) |
| Esophagus | 1 | (3%) |
| Pancreas | 1 | (3%) |
| Urologic | 1 | (3%) |
| TNM staging | ||
| T2 | 2 | (6%) |
| T3 | 11 | (31%) |
| T4 | 22 | (63%) |
| Associated mutations | ||
| BRCA | 0 | |
| BRAF | 6 | |
| RAS | 2 | |
| HER2 | 2 | |
| Drug | ||
| Oxaliplatin | 28 | (80%) |
| Carboplatin | 3 | (9%) |
| Paclitaxel | 2 | (6%) |
| Doxorubicin | 1 | (3%) |
| Cetuximab | 1 | (3%) |
| Cycle in which the reaction was presented | ||
| Average | 4 | |
| Range | 1-12 | |
| Periordicity of cycles | ||
| weekly (7) | 1 | (3%) |
| every 15 days | 13 | (37%) |
| every 21 days | 20 | (57%) |
| every 28 days | 1 | (3%) |
| Retreatment | ||
| Yes | 24 | (69%) |
| No | 11 | (31%) |
| Volume infused (in the initial HR) | ||
| Average | 76,57 | ml |
| Range | 7-256 | ml |
| Type of infusion (according to oncological prescription) | ||
| Normal | 13 | (37%) |
| Slow | 22 | (63%) |
| Suspected phenotype | ||
| Type 1 reaction | 31 | (88%) |
| Infusional reaction | 2 | (6%) |
| Cytokine release reaction | 0 | |
| Mixed reaction | 2 | (6%) |
| Final diagnosis | ||
| Type 1 reaction | 27 | (77%) |
| Infusional reaction | 5 | (14%) |
| Cytokine release reaction | 0 | |
| Mixted reaction | 3 | (9%) |
| Oxaliplatin (n=28) | Carboplatin (n =3) | Paclitaxel(n=2) | Doxorubicin(n=1) | Cetuximab(n=1) | |
|---|---|---|---|---|---|
| Symptomatology | |||||
| Shivering | 1 | 0 | 0 | 0 | 0 |
| Hypertension | 4 | 0 | 2 | 0 | 0 |
| Flushing | 5 | 0 | 1 | 0 | 0 |
| Cutaneous | 22 | 3 | 1 | 1 | 1 |
| Digestive | 6 | 1 | 0 | 0 | 1 |
| Respiratory | 4 | 2 | 0 | 1 | 1 |
| Cardiac | 0 | 1 | 0 | 1 | 0 |
| Hemodynamic inestability* | 2 | 0 | 1 | 1 | 1 |
| Severity according to Brown's scale | |||||
| Grade 1 | 21 (75%) | 1 (33%) | 1 (50%) | ||
| Grade 2 | 5 (18%) | 2 (67%) | |||
| Grade 3 | 1 (100%) | ||||
| Severity according to NCI | |||||
| Grade 1 | 2 (7%) | ||||
| Grade 2 | 1 (50%) | 1 (100%) | |||
| Grade 3 | |||||
| Grade 4 | |||||
| Grade 5 | |||||
| Positive skin tests | N=35 | |
|---|---|---|
| Prick | 4 | (11%) |
| ID 1/100 | 10 | (29%) |
| ID 1/10 | 11 | (31%) |
| ID 1/1 | 2 | (6%) |
| Negative | 5 | (14%) |
| not performed | 3 | (9%) |
| Post reaction tryptase (ng/ml) | ||
| Average | 8,5 | |
| Range | 1-39,8 | |
| Basal tryptase (ng/ml) | ||
| Average | 5,9 | |
| Range | 2,3-11,9 | |
| Post reaction IL-6 (pg/ml) | ||
| Average | 93 | |
| Range | 1,2-2370 | |
| IL-6 Basal (pg/ml) | ||
| Average | 14,25 | |
| Range | 0,9-61 | |
| IgE total (IU/ml) | ||
| Average | 418 | |
| Range | 2,8-3891 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





