1. Introduction:
LSCC is the most common type of laryngeal cancer, accounting for approximately 95% of cases [
1]. The incidence of LSCC varies regionally, primarily due to high rates of smoking and alcohol consumption in some geographic regions [
2]. The clinical outcome of LSCC is influenced by various factors, including tumor stage, the patient's overall health, and chosen treatments [
3]. The cumulative 5-year survival rate for LSCC is estimated to be around 60%; however, this prognosis is notably impacted by the tumor stage and the specific treatment modality employed [
4].
Identifying individuals at high risk of relapse is crucial for personalized therapeutic regimens and improved patient survival.
MicroRNA-449a (miR-449a) is a non-coding RNA that regulates gene expression across various tissues and cells, and its dysregulation has been linked to malignancy [
5]. MiR-449a possesses many biological functions, among which its paramount role lies in inhibiting tumor growth and metastasis. By selectively targeting the genes implicated in the malignant proliferation and dissemination of cancer cells, miR-449a effectively suppresses their expression [
6]. This action hampers the division, dissemination, and invasion of malignant cells, ultimately impeding cancer progression [
7]. MiR-449a plays a crucial role in inducing apoptosis in cancer cells by selectively targeting genes that regulate anti-apoptotic pathways, suppressing their expression. At the same time, miR-449a activates pro-apoptotic pathways, promoting the activation of cellular mechanisms that drive cancer cell death. This orchestrated molecular interplay ultimately leads to the demise of cancer cells, contributing to the inhibition of tumor growth and progression [
5]. The unique characteristics of miR-449a make it a promising target for cancer treatment, providing potential avenues for effective therapeutic strategies [
8]. In addition, miR-449a has the remarkable ability to promote the differentiation of cancer cells, causing them to transform into normal cells. This is significant in cancer treatment, as the main objective is to eliminate cancer cells and restore the normal functioning of tissues. The versatile nature of miR-449a makes it a highly promising candidate for developing effective cancer therapies [
9].
This research aims to assess the efficacy of combining chemotherapy and radiation therapy as a treatment approach for LSCC. Additionally, the study seeks to explore the role of miR-449a in LSCC and examine its potential as a biomarker for predicting treatment response, prognosis, and survival outcomes among LSCC patients.
2. Patients and Methods
From April 2016 to November 2022, the Clinical Oncology Department and Head and Neck Surgical Oncology Unit at Zagazig University Hospitals conducted a study involving 81 patients with locally LSCC and 50 healthy individuals as control. All participants voluntarily joined and gave written consent. The study was approved by Zagazig University's Faculty of Medicine Ethics Committee under approval number (2016-April-213) and adhered to the Code of Ethics of the World Medical Association (Declaration of Helsinki).
To be eligible for this study, patients must meet the following criteria:
They have not yet received any treatment.
A biopsy has confirmed the presence of LSCC
They have T3 or T4 cancer with N0, N1, or N2, as defined by the American Joint Committee on Cancer Staging [
10].
Their ECOG scale performance rating is 2 [
11].
Their liver, kidneys, heart, and bone marrow function normally.
They have good nutritional and hearing status.
They have provided written informed consent.
They are under the age of 70.
During the initial assessment, various tests were conducted to determine the extent and severity of the condition. These tests included a physical examination, complete blood cell count, routine serum chemistries, creatinine clearance, chest x-ray, C.T. scan or MRI of the head and neck, and bone scan. In addition, a triple endoscopy was performed to evaluate the local tumor extent and regional metastases. Biopsies were taken from the tumor and surrounding normal tissues during the procedure. These biopsies were collected for further analysis. Additionally, blood samples were collected from all patients as well as 50 healthy control subjects. The serum from these blood samples will be preserved to investigate miR-449a.
The treatment involved taking three doses of docetaxel at 75 mg/m2 on day one and cisplatin at 75 mg/m2 on the same day. Additionally, a continuous infusion of fluorouracil at 500 mg/m2 per day was given from days 1 to 5, and this treatment was repeated every four weeks.
Before administering the next cycle of chemotherapy, the patient's response was evaluated both clinically through endoscopic inspection and radiologically through CT or MRI after the second cycle. Additionally, palpable lymph nodes were evaluated through clinical examination and palpation. After undergoing chemotherapy, patients who showed complete or partial response were given a maximum of three courses of treatment before receiving definitive radiation therapy. If there was any indication of disease progression, patients underwent surgical resection followed by radiation therapy. Those who showed complete or partial response received radiation therapy after the third cycle of chemotherapy. All patients received radiotherapy either immediately after chemotherapy if they achieved a complete or partial response or after surgery if they did not respond to chemotherapy. The radiation treatment included both sides of the neck and the primary site. Patients were given standard megavoltage radiation in a lying position, with one fraction of 2 Gy per day for five days a week. Those who underwent irradiation after chemotherapy received a total dose of 5000cGy and an additional dose of 2000cGy at the tumor site and any palpable nodes. Following surgery, a radiation dose of 5000cGy was administered, followed by a booster dose of 14 Gy at areas where positive margins, extracapsular spread, or three or more involved lymph nodes were detected. After twelve weeks of completing the radiation therapy, the response was re-evaluated. If a patient had persistent laryngeal disease, they would undergo a salvage laryngectomy. However, if their primary tumor were under control, but they still had neck disease, they would only need neck dissection. The scope of the surgical resection was determined based on the initial evaluation of the tumor's size before chemotherapy. Classic wide-field total laryngectomy was performed for all primary tumors, and regional neck dissection was carried out on all surgical patients except for those with T3N0 or midline supraglottic T4N0 tumors, where it was not clear which side of the neck was at risk for occult metastases. Patients who underwent salvage surgery were assessed by biopsy to confirm the presence of residual primary tumors. Follow-up appointments were scheduled every three months for the first year after treatment and every six months thereafter to monitor and evaluate all patients. Complete or partial response was evaluated by tissue biopsy after neoadjuvant chemotherapy and after radiation therapy.
2.1. Quantitative Real Time-Polymerase Chain Reaction (qRT-PCR) for miR-449a
To obtain total RNA, including miRNAs, we utilized the TRIzol reagent (Invitrogen) and followed the manufacturer's instructions from tumor tissue, adjacent normal tissues and from serum. The cDNA was produced using the Transcript First-Strand Synthesis Supermix (TransGen Biotech, AT301) in accordance with the manufacturer's protocol. We used TaKaRa reagents and an Applied Biosystems 7900 Fast Real-Time PCR System to conduct qRT-PCR. U6 was utilized as the internal control. The primers sequences are shown in
Table 1.
Each sample was analyzed three times for accuracy. The expression was presented as the Δ cycle threshold (2-△Ct) value. The relative expression of miR-449a was calculated using the comparative Ct method and miR-449a values were normalized to the internal reference U6.
The RT-PCR system is composed of a total volume of 20 μL and includes the following components: 10 μL of 2x Ultra SYBR one-step qRT-PCR Buffer, 2 μL of RNA template, 1 μL each of upstream and downstream primers, 0.5 μL of Super enzyme mix, and 5.5 μL of nuclease-free water. The RT-PCR reaction is performed with the following conditions: pre-denaturation at 95ºC for 10 minutes, denaturation at 60ºC for 15 seconds, annealing and extension at 60ºC for 1 minute, for a total of 40 cycles.
2.2. Statistical Analysis
The data analysis was done using the IBM SPSS Statistics software, version 27. To compare the means and standard deviations of different groups, the student's t-test was used. In order to evaluate the diagnostic accuracy of miR-449a, receiver operating characteristic (ROC) analysis was performed on both tissue and serum samples. The survival rate of LSCC patients was estimated using the Kaplan-Meier curve, and the significance of the survival differences was evaluated by employing the log-rank test.
3. Results
Table 2 presents the patient's gender, age, and performance level.
Table 3 contains data on the T and N stages of patients diagnosed with LSCC. The T stage (T2, T3, T4) describes the size and extent of the primary tumor, while the N stage (N0, N1, N2) shows the presence and extent of lymph node involvement. The table also provides the total number of patients.
Of 81 patients, 11 had T2-stage tumors, 45 had T3-stage tumors, and 25 had T4-stage tumors. Among them, 36 patients had N1-stage disease, while 24 had N2-stage disease. The remaining 21 patients had N0-stage disease, implying no lymph node involvement. Additionally, the table showcases the number of patients for every T and N-stage combination, for instance, 12 patients with T3N0 (stage III) and 10 with T4N1 (stage IVa).
Table 4 displays the response rates of LSCC patients to neoadjuvant chemotherapy and radiotherapy. Of the 81 patients who received neoadjuvant chemotherapy, 25 individuals (31%) achieved a complete response, indicating the absence of any cancer after treatment. Additionally, 40 patients (49%) showed a partial response, indicating a reduction in tumor size. There were 16 patients (20%) exhibited stable disease, indicating no significant change in tumor size following treatment. These findings are particularly significant as they demonstrate that 25 patients with advanced LSCC successfully preserved their larynxes and retained their voice function.
Table 4 also presents a summary of radiotherapy outcomes in 65 patients. Out of these patients, 48 (74%) had a complete response, 13 (20%) showed a partial response, and 4 (6%) had stable disease after the radiation therapy.
Several surgical salvage options are available for patients with LSCC who did not respond well to the initial treatment. These include total laryngectomy with partial pharyngectomy (4 cases), total laryngectomy alone (5 cases), hemilaryngectomy (7 cases), bilateral, regional neck dissection (3 cases), and unilateral regional neck dissection (4 cases). The details of these procedures are presented in
Table 5.
After chemotherapy and radiation therapy, patients had different surgical salvage options. None underwent a total laryngectomy. Four patients had hemilaryngectomy, with one case involving unilateral neck dissection and three cases involving bilateral neck dissection. Additionally, 13 patients underwent neck dissection, with eight cases involving unilateral neck dissection and five cases involving bilateral neck dissection.
The findings highlight the importance of individualized treatment plans, considering factors such as disease extent, medical history, and treatment response, are crucial for optimizing outcomes in LSCC patients.
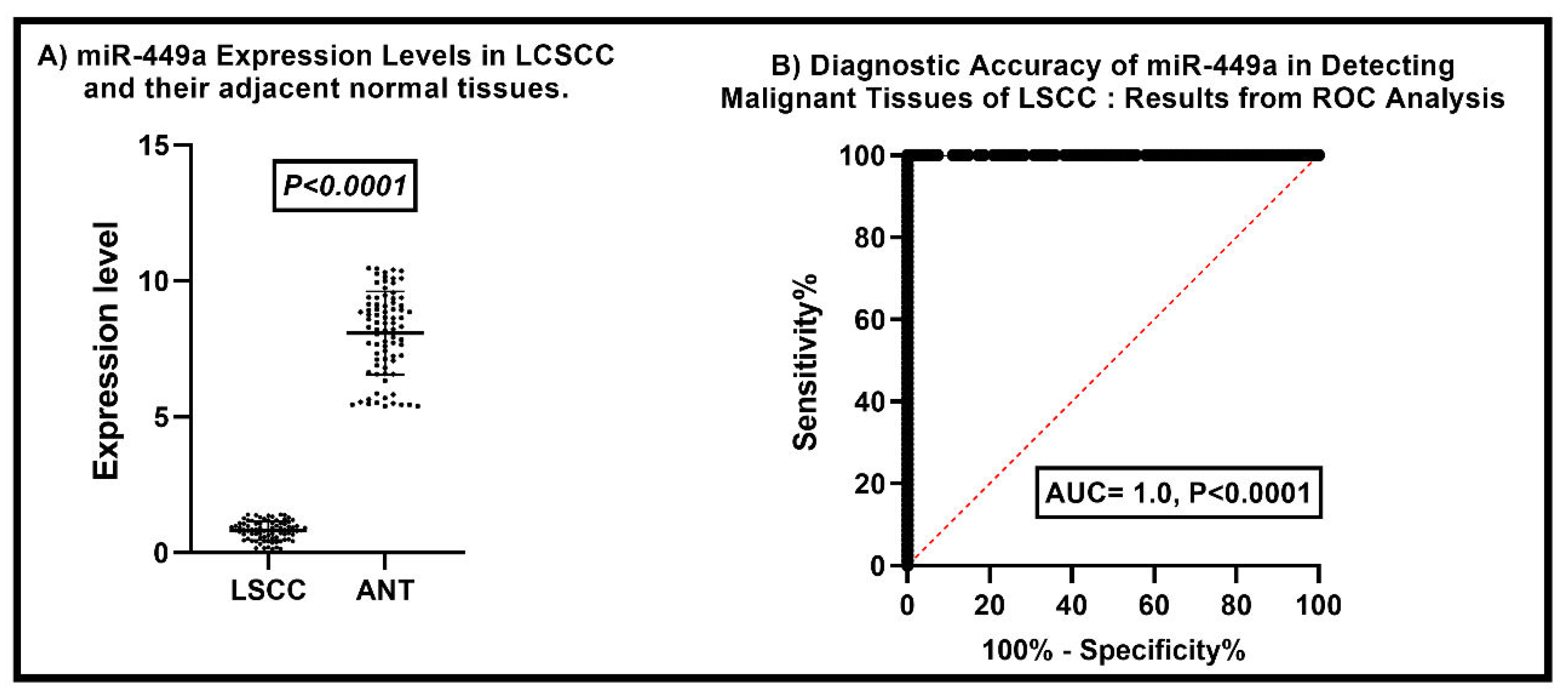
Comparative Analysis of miR-449a Expression Levels in LSCC and Adjacent Normal Tissues
Our findings indicate a significant difference in the levels of miR-449a expression between LSCC and adjacent normal tissue. The average miR-449a expression level in LSCC was 8.078 ± 0.3434, whereas in adjacent normal laryngeal tissues, it was 8.079 ± 1.525 (P < 0.0001). These results demonstrate a statistically significant discrepancy, indicating the downregulation of miR-449a expression in LSCC compared to normal tissue (
Figure 1A).
Correlation between miR-449a Expression and Clinical Features in Patients With Laryngeal Squamous Cell Carcinoma
Table 6 shows the expression levels and statistical significance of miR-449a across various clinical subgroups of LSCC patients. The study found significant differences in the expression of miR-449a among subgroups based on age, gender, smoking status, tumor grade, tumor stage, and lymph node involvement.
The expression of miR-449a was lower in the age group of 35-45 years compared to the age group of 46-69 years. Moreover, the study observed that males had lower miR-449a expression than females, and smokers had lower expression levels than non-smokers.
The study found that low-grade tumors exhibited higher miR-449a expression than high-grade tumors. Additionally, T1-T2 tumors had higher expression levels than T3-T4 tumors. N0-N1 tumors showed higher expression levels than N2-N3 tumors; stage III tumors had higher expression levels than stage IV tumors.
These findings suggest that miR-449a could be a useful prognostic indicator associated with various clinical characteristics in LSCC patients.
Changes in Serum miR-449a Expression Profile in Laryngeal Squamous Cell Carcinoma Patients after Neoadjuvant Chemotherapy and Radiation Therapy
Our study evaluated the expression of miR-449a in the serum of LSCC patients before and after neoadjuvant chemotherapy and radiation therapy. (
Table 7).
Our study found significant changes in miR-449a levels in the serum of LSCC patients after receiving neoadjuvant chemotherapy and radiation therapy. Before treatment, patients who achieved a complete or partial response had lower miR-449a levels. However, after treatment, both groups showed a significant increase in miR-449a levels, with a more significant increase observed in the partial response group. Similarly, before radiation therapy, the mean miR-449a levels were lower in both complete and partial response groups. Nonetheless, following radiation therapy, both groups experienced a significant increase in miR-449a levels, with a more pronounced increase observed in the complete response group. These findings indicate that combined chemotherapy and radiation therapy affect miR-449a expression, highlighting its potential as a therapeutic response biomarker for LSCC patients.
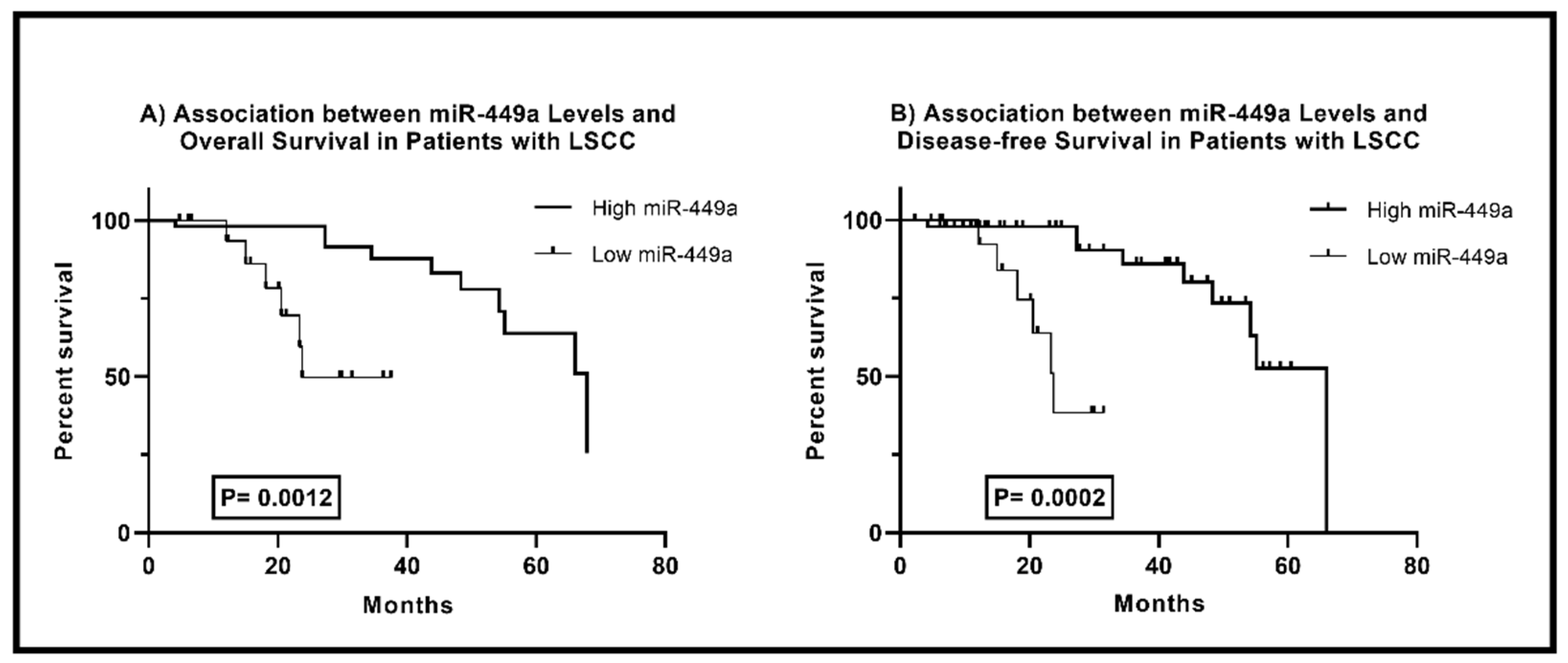
Correlation between miR-449a Levels and Survival in Laryngeal Squamous Cell Carcinoma Patients
The comparison of survival rates in LSCC patients based on miR-449a levels is shown in
Figure 3. According to the Log-rank test, there is a significant difference in overall survival (OA) between low and high miR-449a groups (P = 0.0012). Patients with low miR-449a levels have a median OA time of 23.74 months, while those with high levels have a median survival time of 67.82 months. A significant difference in disease-free survival (DFS) rates was also observed. Patients with high miR-449a levels had 2.781 times longer DFS, with a median time of 66.03 months (95% CI: 0.9899-7.814), compared to those with low levels. These findings indicate that miR-449a expression is a valuable prognostic factor in LSCC patients. A higher expression level of miR-449a is associated with improved overall and disease-free survival.
4. Discussion
Numerous studies have been conducted across various cancer types, including breast, lung, ovarian, and head and neck cancers, which have concluded that miR-449a is crucial in inhibiting cancer growth and invasion. This exceptional molecule can target multiple pathways and oncogenes involved in cancer development and progression. It has been observed that miR-449a can increase the sensitivity of ovarian cancer cells to chemotherapy. Additionally, in the case of head and neck squamous cell carcinoma, low levels of miR-449a have been linked to a poor prognosis, highlighting its potential as a prognostic marker. These findings are promising and suggest that miR-449a could be a valuable target for cancer treatment [9, 12, 13].
The main objective of this research is to evaluate how effective the combination of chemotherapy and radiation therapy is in treating LSCC. Furthermore, the study intends to investigate the function of miR-449a in LSCC and determine its potential as a biomarker for predicting treatment response, prognosis, and survival rates in patients with LSCC.
Out of the 81 patients examined, 25 (31%) attained a complete response to chemotherapy, 40 (49%) demonstrated a partial response, and 16 (20%) had stable disease. After receiving radiation therapy, 48 (74%) of the 65 patients achieved a complete response, 13 (20%) had a partial response, and 4 (6%) maintained stable disease. Notably, 73 patients responded completely, allowing them to preserve their voices and avoid radical, mutilating surgery. These findings emphasize the effectiveness of the combined treatment approach in managing LSCC and its potential to improve patient's quality of life. By understanding how each patient responds to these treatments, doctors can create personalized and optimized treatment plans for LSCC patients, considering the tumor's characteristics, preferences, and possible side effects. Our study's findings align with the research conducted by Mesía et al. regarding the effects of chemotherapy and radiation therapy on LSCC. According to their study, the overall response rate to chemotherapy was 54.4%, with a complete response rate of 28.9% and a partial response rate of 25.5%. These outcomes are consistent with our observations, further confirming chemotherapy's effectiveness in treating LSCC patients [
14]. It is important to note that our study shares similarities with previous research, strengthening our findings' reliability and generalizability. The consistent responses observed in LSCC patients undergoing chemotherapy emphasize the potential of this treatment modality in achieving favorable outcomes. This emphasizes the need for further research to optimize chemotherapy use for LSCC patients. Our research confirms the results of Yokota et al.'s study regarding the impact of radiation therapy on locally advanced LSCC. According to Yokota et al.'s report, patients who received radiation therapy demonstrated a complete response rate of 45% and a partial response rate of 23% [
15]. Our study's findings are consistent with these results and further support the effectiveness of radiation therapy as a viable treatment option for LSCC patients. Following chemotherapy, a notable 25 patients experienced a complete response, contributing to the preservation of their larynx and vocal function. This outcome serves as a testament to the efficacy of chemotherapy in the treatment of LSCC, enabling patients to retain their laryngeal integrity and voice. Following radiation therapy for LSCC, 48 patients achieved a complete response while concurrently maintaining their laryngeal function. This outcome underscores the profound influence of preserving laryngeal function on the overall quality of life experienced by individuals who have undergone radiation therapy.
The current study underscores the importance of maintaining laryngeal function to enhance patients' quality of life after radiation therapy. This supports previous studies showing altered miR-449a expression in various cancers, underscoring our findings' importance [
16]. Extensive research has shown the dysregulation of miR-449a in various cancers, indicating its potential role in cancer development and progression, specifically HNSCC [17-19]. Our study reaffirms the paramount importance of miR-449a in the context of LSCC and cancer biology as a whole. This noteworthy finding accentuates the immense potential for pioneering diagnostic tools and therapeutic approaches to provide substantial benefits to patients grappling with this complex condition. In both HNSCC tissues and cell lines, the expression of miR-449a was reduced. However, reintroducing miR-449a inhibited the growth and invasion of HNSCC cells, indicating its potential as a therapeutic approach against HNSCC aggressiveness [
20]. Our study suggests miR-449a as a diagnostic or therapeutic target in LSCC. Further research is needed to understand its potential.
Our research has revealed the potential of miR-449a as a diagnostic biomarker for LSCC by successfully distinguishing it from normal laryngeal tissue. Additionally, we observed its ability to discriminate between LSCC and healthy controls in serum samples accurately. These findings underscore the promising prospects of miR-449a as a valuable diagnostic tool for LSCC. Our findings support previous research on miR-449a's diagnostic abilities in various cancer types, confirming its substantial role in cancer research [17, 21]. Our research has revealed a compelling correlation between miR-449a expression and various clinical factors in patients diagnosed with LSCC. Notably, we observed a significant association between lower levels of miR-449a and higher tumor grades, T-stages, N-stages, and TNM stages. These findings provide valuable insights into the potential involvement of miR-449a in the development and progression of LSCC, thereby underscoring its promising role as a potential biomarker in this specific context. Extensive research has explored the potential of miRNAs in the diagnosis, prognostication, and treatment of cancer [22-24]. In Head and Neck Squamous Cell Carcinoma (HNSCC) specifically, there is a strong correlation between reduced levels of miR-449a in tumor tissues and the presence of lymph node metastasis and an unfavorable prognosis [25, 26]. Earlier studies have confirmed the crucial role of miR-449a in HNSCC [
17]. It has been observed that miR-449a expression is significantly reduced in gastric cancer tumor tissues compared to non-tumor tissues. This observation was made after thoroughly analyzing tumor tissue and serum samples collected from patients who had undergone surgical interventions [
21]. A study conducted by Meng et al. further substantiates our findings. Their investigation revealed a significant reduction in miR-449a expression levels in lung cancer tissues, strongly correlated with tumor stage, lymph node metastasis, and patient survival. These compelling results underscore the potential utility of miR-449a as a valuable biomarker for evaluating disease progression and predicting patient outcomes within lung cancer [
27]. Studies in the intestinal-type sinonasal adenocarcinoma have consistently demonstrated a notable downregulation of miR-449a expression in malignant tissues, in contrast to non-malignant counterparts. This substantial decrease in miR-449a expression is robustly linked with unfavorable disease-free survival (DFS) and overall survival (OS) outcomes, enriching our understanding of the disease's progression and its impact on patient experiences [
28]. The results of previous studies consistently show that miR-449a is downregulated, indicating a potential link between its reduced expression and increased tumor aggressiveness [17, 21, 26, 27]. These findings demonstrate the potential of miR-449a as a valuable biomarker in LSCC, providing insights into the clinical characteristics of the disease. Additionally, targeting miR-449a for therapeutic intervention shows promise in LSCC treatment. The observed association between miR-449a expression levels and various clinical criteria in patients diagnosed with LSCC reinforces the significance of miR-449a in the context of this disease. Our research has identified miR-449a as a potential indicator for treatment effectiveness in patients with LSCC. We found a significant increase in miR-449a expression levels in patients with a complete or partial response to chemotherapy and radiation therapy. This suggests that monitoring miR-449a expression could help predict treatment outcomes and optimize treatment strategies for LSCC patients. These findings are important for improving patient outcomes in managing LSCC. These findings align with a prior study investigating miR-449a expression in osteosarcoma patients who underwent chemotherapy and radiation therapy [
29]. Zhang et al. conducted a study that revealed lower levels of miR-449a in patients diagnosed with osteosarcoma before surgical tumor resection. However, there was a significant increase in miR-449a levels after surgical intervention. These findings demonstrate that miR-449a expression is dynamic and responsive to surgical intervention in the context of osteosarcoma [
29]. Our study and the results of Zhang et al. [
29] indicate that miR-449a holds potential as a biomarker for assessing treatment response in LSCC patients undergoing chemotherapy and radiation therapy.
Our study has provided strong evidence indicating a significant correlation between the levels of miR-449a and the survival rates of patients diagnosed with LSCC, including both overall and disease-free survival. Notably, patients with higher levels of miR-449a have shown longer overall and disease-free survival durations than those with lower levels. These findings emphasize the potential of miR-449a as a prognostic biomarker in LSCC, offering valuable insights into patient outcomes and survival rates. Our study findings align with prior research emphasizing the prognostic value of miR-449a in various cancer types. A study conducted by Ishikawa et al. examining gastric cancer patients revealed that individuals with low expression levels of miR-449a experienced inferior cancer-specific survival outcomes compared to those with high levels [
21]. These consistent outcomes across studies reinforce the significance of miR-449a as a predictive biomarker for prognosis assessment in cancer patients. Reduced levels of miR-449a have been observed in various types of cancer [
30]. Although the exact mechanisms are yet to be fully understood, miR-449a is believed to be vital in regulating essential cellular processes such as cell cycle progression, apoptosis, and metastasis. Further studies are required to discover the specific molecular pathways and targets that miR-449a affects in cancer biology. This understanding will aid in comprehending the importance of miR-449a and its potential therapeutic applications.
5.Conclusions
The combination of chemotherapy and radiotherapy has proven efficacy in treating locally advanced LSCC, effectively preserving vocal function and enhancing patient outcomes, thereby improving their overall quality of life.
Furthermore, the remarkable potential of miR-449a as a valuable biomarker in LSCC has been consistently demonstrated. The strong association between miR-449a expression levels, clinical outcomes, and treatment response in LSCC patients highlights its diagnostic value, prognostic significance, and potential as an indicator of treatment efficacy.
Author Contributions
Abdulraheem A. Almalki, Amal F Gharib & Wael H Elsawy: Conceptualization, Methodology, Software Amani A. Alrehaili , Afaf Alharthi , Ohud Alsalmi, Rasha L. Etewa, Mutaib M Mashraqi, Shaimaa A. Alharthi: Data curation, Writing- Original draft preparation. Amani A. Alrehaili, Amal F Gharib, Mutaib M Mashraqi & Wael H Elsawy. Amal F Gharib: Supervision.: Amani A. Alrehaili: Software, Validation.: Abdulraheem A. Almalki, Amal F Gharib &Wael H. Elsawy: Writing- Reviewing and Editing, all authors have read the manuscript and approved the submission.
Funding
This research was funded by Taif University, Saudi Arabia, project No. (TU-DSPP-2024-54).
Data Availability Statement
All data will be available upon request.
Acknowledgments
The authors extend their appreciation to Taif University, Saudi Arabia, for supporting this work through project number (TU-DSPP-2024-54)
Conflicts of Interest
There are no conflicts of interests.
Ethics approval and Consent to participate
The study received approval from Zagazig University's Faculty of Medicine Ethics Committee under approval number (2016- April- 213).
Ethics declaration
All methods were conducted according to relevant guidelines and regulations, and all subjects signed informed consent forms.
Patients consent for publication
Not applicable.
References
- de la Cour CD, Munk C, Aalborg GL, Kjaer SK. Base of tongue/tonsillar and laryngeal cancer in Denmark 1994–2018: Temporal trends in incidence according to education and age. Oral Oncology. 2022, 128, 105832. [Google Scholar] [CrossRef] [PubMed]
- Lin C, Cheng W, Liu X, Li H, Song Y. The global, regional, national burden of laryngeal cancer and its attributable risk factors (1990–2019) and predictions to 2035. European Journal of Cancer Care. 2022, 31, e13689. [Google Scholar] [CrossRef]
- Gormley M, Creaney G, Schache A, Ingarfield K, Conway DI. Reviewing the epidemiology of head and neck cancer: definitions, trends and risk factors. British Dental Journal. 2022, 233, 780–6. [Google Scholar] [CrossRef] [PubMed]
- Zhang Q, Wang H, Zhao Q, Zhang Y, Zheng Z, Liu S, et al. Evaluation of risk factors for laryngeal squamous cell carcinoma: a single-center retrospective study. Frontiers in oncology. 2021, 11, 606010. [Google Scholar] [CrossRef]
- Gupta S, Panda PK, Hashimoto RF, Samal SK, Mishra S, Verma SK, et al. Dynamical modeling of miR-34a, miR-449a, and miR-16 reveals numerous DDR signaling pathways regulating senescence, autophagy, and apoptosis in HeLa cells. Scientific Reports. 2022, 12, 4911. [Google Scholar] [CrossRef]
- Zhong P, Guo A, Wang L, Lin X, Feng M. Circular RNA CDK6 suppresses cervical cancer proliferation and metastasis by sponging miR-449a. Bioengineered. 2022, 13, 4885–97. [Google Scholar] [CrossRef] [PubMed]
- Zhang J, Liu W, Ji P, Zhang Y. Silencing of long chain noncoding RNA paternally expressed gene (PEG10) inhibits the progression of neuroblastoma by regulating microRNA-449a (miR-449a)/ribosomal protein S2 (RPS2) axis. Bioengineered. 2022, 13, 6309–22. [Google Scholar] [CrossRef]
- Reinkens T, Stalke A, Huge N, Vajen B, Eilers M, Schäffer V, et al. Ago-RIP sequencing identifies new microrna-449a-5p target genes increasing sorafenib efficacy in hepatocellular carcinoma. Journal of Cancer. 2022, 13, 62. [Google Scholar] [CrossRef]
- Noureddine S, Nie J, Schneider A, Menon V, Fliesen Z, Dhahbi J, et al. microRNA-449a reduces growth hormone-stimulated senescent cell burden through PI3K-mTOR signaling. Proceedings of the National Academy of Sciences. 2023, 120, e2213207120. [Google Scholar] [CrossRef]
- Wang X, Cao K, Guo E, Mao X, An C, Guo L, et al. Integrating DOI in T classification improves the predictive performance of laryngeal cancer staging. Cancer Biology & Therapy. 2023, 24, 2169040. [Google Scholar] [CrossRef]
- 11. Allende-Pérez S, Rodríguez-Mayoral O, Peña-Nieves A, Bruera E. Performance status and survival in cancer patients undergoing palliative care: Retrospective study. BMJ Supportive & Palliative Care. [CrossRef]
- Miśkiewicz J, Mielczarek-Palacz A, Gola JM. MicroRNAs as Potential Biomarkers in Gynecological Cancers. Biomedicines. 2023, 11, 1704. [Google Scholar] [CrossRef]
- Mondal P, Meeran SM. Emerging role of non-coding RNAs in resistance to platinum-based anti-cancer agents in lung cancer. Frontiers in Pharmacology. 2023, 14, 1105484. [Google Scholar] [CrossRef] [PubMed]
- Mesía R, Garcia-Saenz JA, Lozano A, Pastor M, Grau JJ, Martínez-Trufero J, et al. Could the addition of cetuximab to conventional radiation therapy improve organ preservation in those patients with locally advanced larynx cancer who respond to induction chemotherapy? An organ preservation Spanish Head and Neck Cancer Cooperative Group Phase 2 study. International Journal of Radiation Oncology* Biology* Physics. 2017, 97, 473–80. [Google Scholar] [CrossRef]
- Yokota T, Kato K, Hamamoto Y, Tsubosa Y, Ogawa H, Ito Y, et al. Phase II study of chemoselection with docetaxel plus cisplatin and 5-fluorouracil induction chemotherapy and subsequent conversion surgery for locally advanced unresectable oesophageal cancer. British journal of cancer. 2016, 115, 1328–34. [Google Scholar] [CrossRef]
- Veena MS, Raychaudhuri S, Basak SK, Venkatesan N, Kumar P, Biswas R, et al. Dysregulation of hsa-miR-34a and hsa-miR-449a leads to overexpression of PACS-1 and loss of DNA damage response (DDR) in cervical cancer. Journal of Biological Chemistry. 2020, 295, 17169–86. [Google Scholar] [CrossRef] [PubMed]
- Piotrowski I, Zhu X, Saccon TD, Ashiqueali S, Schneider A, de Carvalho Nunes AD, et al. miRNAs as Biomarkers for Diagnosing and Predicting Survival of Head and Neck Squamous Cell Carcinoma Patients. Cancers. 2021, 13, 3980. [Google Scholar] [CrossRef]
- Jiang T, Zhao R, Du Y, Shi Z, Cheng H. MiR-449a-5p regulates the proliferation of esophageal carcinoma cell by targeting B-cell lymphoma 2. Translational Cancer Research. 2020, 9, 7236. [Google Scholar] [CrossRef]
- Wu D, Liu J, Chen J, He H, Ma H, Lv X. miR-449a suppresses tumor growth, migration, and invasion in non-small cell lung cancer by targeting a HMGB1-mediated NF-κB signaling pathway. Oncology research. 2019, 27, 227. [Google Scholar] [CrossRef] [PubMed]
- Thomaidou AC, Batsaki P, Adamaki M, Goulielmaki M, Baxevanis CN, Zoumpourlis V, et al. Promising Biomarkers in Head and Neck Cancer: The Most Clinically Important miRNAs. International Journal of Molecular Sciences. 2022, 23, 8257. [Google Scholar] [CrossRef]
- Ishikawa D, Yoshikawa K, Takasu C, Kashihara H, Nishi M, Tokunaga T, et al. Expression level of MicroRNA-449a predicts the prognosis of patients with gastric cancer. Anticancer Research. 2020, 40, 239–44. [Google Scholar] [CrossRef]
- Chakrabortty A, Patton DJ, Smith BF, Agarwal P. miRNAs: Potential as Biomarkers and Therapeutic Targets for Cancer. Genes 2023, 14, 1375. [Google Scholar] [CrossRef] [PubMed]
- Falco M, Tammaro C, Takeuchi T, Cossu AM, Scafuro G, Zappavigna S, et al. Overview on molecular biomarkers for laryngeal cancer: looking for new answers to an old problem. Cancers. 2022, 14, 1716. [Google Scholar] [CrossRef] [PubMed]
- Sharma PC, Gupta A. MicroRNAs: potential biomarkers for diagnosis and prognosis of different cancers. Translational cancer research. 2020, 9, 5798. [Google Scholar] [CrossRef] [PubMed]
- Supic G, Stefik D, Ivkovic N, Sami A, Zeljic K, Jovic S, et al. Prognostic impact of miR-34b/c DNA methylation, gene expression, and promoter polymorphism in HPV-negative oral squamous cell carcinomas. Scientific reports. 2022, 12, 1296. [Google Scholar] [CrossRef] [PubMed]
- Re M, Tomasetti M, Monaco F, Amati M, Rubini C, Sollini G, et al. MiRNome analysis identifying miR-205 and miR-449a as biomarkers of disease progression in intestinal-type sinonasal adenocarcinoma. Head & Neck. 2022, 44, 18–33. [Google Scholar] [CrossRef]
- Meng H, Huang Q, Zhang X, Huang J, Shen R, Zhang B. MiR-449a regulates the cell migration and invasion of human non-small cell lung carcinoma by targeting ADAM10. OncoTargets and therapy. 2019, 12, 3829. [Google Scholar] [CrossRef] [PubMed]
- Li J, Lu M, Jin J, Lu X, Xu T, Jin S. miR-449a suppresses tamoxifen resistance in human breast cancer cells by targeting ADAM22. Cellular Physiology and Biochemistry. 2018, 50, 136–49. [Google Scholar] [CrossRef] [PubMed]
- Zhang H, Wang Z, Liu L, Zhang F, Song Y, Qian Y. Expression Changes, Prognostic Analysis and Risk Factors of miR-625-3p and miR-449a in Osteosarcoma Patients after Surgery. Oncologie. 2020, 22, 23–33. [Google Scholar] [CrossRef]
- Fu D, Chen Y, Xu D. Circulating miR-449a predicts survival outcome for colorectal cancer following curative resection: An observational study. Medicine. 2021, 100. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).