1. Introduction
Our sense of time is subjective and relative. On some occasions, a day feels like an eternity; on others, it goes by in a flash. An identical situation may seem to pass very quickly or very slowly depending on diverse situational factors (Ahn et al., 2009; Vohs & Schmeichel, 2003).
There is no human clinical condition that can be defined solely as a disorder of timing and time perception per se. However, distortions in time perception are present in many people and may accompany differences in other aspects of sensory processing, as well as in developmental, cognitive, and behavioral profiles (e.g., Allman, 2011; Ballotta et al., 2018; Montalemberta et al., 2016; Myrseth et al., 2010).
There are also changes in time perception according to individual circumstances. For example, emotions change the subjective sense of time (Campbell & Bryant, 2007; Droit-Volet & Meck, 2007; Smith et al., 2011; Tse et al., 2004; Wittmann & van Wessenhove, 2009). Several studies have demonstrated an interaction between time perception and emotion (Dirnberger et al., 2012; Droit-Volet et al., 2004; Lee et al., 2011; Noulhiane et al., 2007; Smith et al., 2011; Tipples, 2008). The sense of passage of time varies depending on whether we are in an emotional or a neutral context (Dirnberger et al., 2012). Emotional sounds are judged as being longer than neutral sounds (Noulhiane et al., 2007) and the exposure time to unpleasant images seems longer than to neutral images (Angrilli et al., 1997).
The effects of emotions on time perception seem to differ according to the valence of stimuli. In time estimation tasks, pleasant and unpleasant images with high activation power were compared to neutral images and, overall, participants overestimated (estimated time > real time) the duration of unpleasant images to a greater degree than that of pleasant images, even when controlling for the activation level of the images (e.g., Angrilli et al., 1997). These effects have been demonstrated in several previous studies with adults and children (Gil & Droit-Volet, 2011a, 2011b), in individuals with negative emotionality (Tipples, 2008, 2011) and in depression (Gil & Droit-Volet, 2009).
Although it is still unclear which are the neurobiological mechanisms that allow to account for the passage of time, several areas of the brain seem to constitute fundamental elements of a time interval timing system in the order of seconds (a “neuronal clock”). Several brain regions are normally activated in imaging studies on time perception that suggest that different processing components are involved, such as paying attention to time, coding an interval and maintaining the representation of duration in working memory, as well as decision processes (Harrington et al., 2004; Rubia & Smith, 2004). Fronto-striated circuits, which are modulated by the dopaminergic system, are crucial for temporal processing in the order of seconds (Coull et al., 2004; Matell & Meck, 2004). Individuals with structural injuries to the frontal lobes (Kagerer et al., 2002) or traumatic brain injuries predominantly affecting the frontal areas (Pouthas & Perbal, 2004) show a poor estimate of time intervals of several seconds. Neuroimaging studies with healthy participants show that temporal processing is predominantly associated with activation in the prefrontal and striated regions on the right side (Coull et al., 2004; Hinton & Meck, 2004). Thus, areas that are activated during duration processing are similar to those that are activated in delay discount tasks. Other regions of the brain, such as the cerebellum, also appear to play a decisive role in processing duration; however, the involvement of these regions is probably restricted to the time range from milliseconds to a few seconds (Ivry & Spencer, 2004). In addition, other studies indicate that several areas of the brain can contribute to the processing of duration in the range of milliseconds (e.g., Mauk & Buonomano, 2004).
The cognitive models that presuppose the performance of an “internal clock” as a type of pacemaker, producing subjective time units, have been very successful in interpreting the results of studies of the perception of time in humans and of the temporally organized behavior of animals. According to the aforementioned models, the subjective duration of time is estimated as a function of the number of temporal units produced by the so-called “internal clock” and accumulated over a given period of time. When individuals are asked to judge a certain interval during a time estimation task, an overestimate corresponds to the accumulation of a greater number of temporal units over that period. The cognitive models of temporal perception propose two mechanisms that can explain the increase in the number of units of time in a supposed accumulator: (a) focusing attention, as opposed to distracting time, leads to an accumulation of more units over a period of time; (b) a higher rhythm of units emitted by the internal pacemaker, namely related to a greater activation (i.e., the accelerated rhythm of the internal clock) leads to a faster accumulation of temporal units over time (Wittmann & Paulus, 2008). In fact, the overestimation of time associated with altered psychological conditions, for example by the increase in body temperature, was explained by the increase in the pacemaker pace (Wearden & Penton-Voak, 1995).
Time estimation has been measured by the theta index (), which is the ratio of the subjective time estimate by the objective time (estimated time/real time), so that: = 1 indicates perfect time estimation of objective time; > 1 indicates an overestimation of time, as it results from an estimation of the passage of time that is greater than real time; and < 1 indicates an underestimation of time or an estimation of the passage of time that is lower than real time (Block et al., 1999, Block et al., 2000; Klein et al., 2003). Therefore, if the speed of this marker is precise, it will track the passage of time in a duration that corresponds to that of chronological time. On the other hand, if the timer is faster, it will take less time than real time to produce a desired duration, but it will estimate a faster passage of time than real time, that is, the individual estimate of a certain time interval will be longer than the real duration of this interval. The inverse condition is for a slower timer, which leads us to estimate that a target interval has a shorter duration than the real one, or to produce intervals with a longer duration than real time (Pande & Pati, 2010).
With the systematic review by Moreira, Pinto, Almeida, and Barbosa (2016), it is possible to verify that most studies use a forced response scheme with a reduced number of response options, often only four response options. In addition, of the existing studies that report on experimentally manipulated emotional conditions, to the best of our knowledge, none has used, as experimental conditions, pleasant, unpleasant and neutral stimuli and their comparison with a condition without interference. Herein lies the relevance and innovation of this study.
In sum, the aim of the present study was to examine the effect of emotional stimuli on time estimation. We formulated the hypotheses that (a) individuals overestimate time more markedly when they are under the influence of unpleasant and pleasant images, compared to neutral images and the time estimation condition without emotional interference; (b) individuals overestimate time more when they are under the influence of unpleasant images compared to pleasant, neutral images and the condition without emotional interference.
2. Materials and Methods
2.1. Participants
One hundred and twenty adult participants were recruited from the community. The sample size was calculated based on statistical power analyzes conducted in G*Power, Version 3.1.9.7 (Faul et al., 2009; Faul et al., 2007). We excluded six participants who scored below the cut-off point suggested for the Portuguese population on the MoCA (Simões et al., 2008), as well as three participants who obtained z > |3| in the value of θ in at least one of the experimental conditions. Thus, the final sample comprised 111 participants (M = 30.3 years, SD = 8.70, of which 73 were women), having an average of 14.7 years of schooling (SD = 4.08). No other participants were eliminated after the application of other exclusion criteria (screened by self-report), namely abuse of alcohol or other psychoactive substances, psychopathologies or neuropathologies, or sensory-motor impairments that may interfere in the performance of the tasks.
The study was approved by the Ethics Committee of the Faculty of Psychology and Educational Sciences of the University of Porto and, after a description of the study and its objectives, written informed consent was obtained from all participants. No compensation was given for participating in the study.
2.2. Materials
The stimuli consisted of 45 color images extracted from the Nencki Affective Picture System (NAPS; Marchewka et al., 2014). The images from this database are pre-evaluated in terms of valence on a 9-point scale, where values closer to 1 indicate negative valence and values closer to 9 indicate positive valence (values around 5 are neutral). The images used were: POSITIVE IMAGES: Faces_104_h, Landscapes_035_v, Objects_074_v, Faces_351_h, Landscapes_064_h, Landscapes_166_h, Faces_116_h, People_169_h, People_154_h, Landscapes_097_v, Landscapes_137_h, Animals_131_h, Faces_140_h, People_185_h, People_043_h; NEGATIVE IMAGES: People_238_h, People_198_h, People_237_h, Faces_371_v, Faces_364_v, People_218_v, Faces_143_v, People_208_h, People_221_h, Faces_365_v, People_220_h, Animals_077_h, People_127_h, Faces_010_h, People_200_h; IMAGENS NEUTRAS: Faces_167_v, Landscapes_170_h, Animals_133_h, Objects_210_h, Animals_081_h, Objects_147_v, Faces_039_h, Faces_216_h, Faces_305_h, Objects_314_h, Objects_057_h, People_146_h, Objects_213_h, Faces_218_h, Objects_112_h.
The set of stimuli included equal numbers of: (1) positive valence images (M = 7.94, SD = 0.20), which made up the pleasant stimuli condition; (2) negative valence images (M = 1.94, SD = 0.25), which constituted the unpleasant stimuli condition; and (3) neutral images (valence: M = 5.07, SD = 0.14), which made up the neutral stimuli condition. This rating was based on the valence ratings provided with the NAPS image database (Marchewka et al., 2014).
All stimuli were presented at the center of a 17’’ computer monitor. Participants sat approximately 80 cm from the monitor. All tasks were programmed using the software E-Prime 2.0 (2011, Psychology Software Tools, Inc., Sharpsburg, PA, USA), through which the stimuli were administered and the responses were collected.
2.3. Design and Procedure
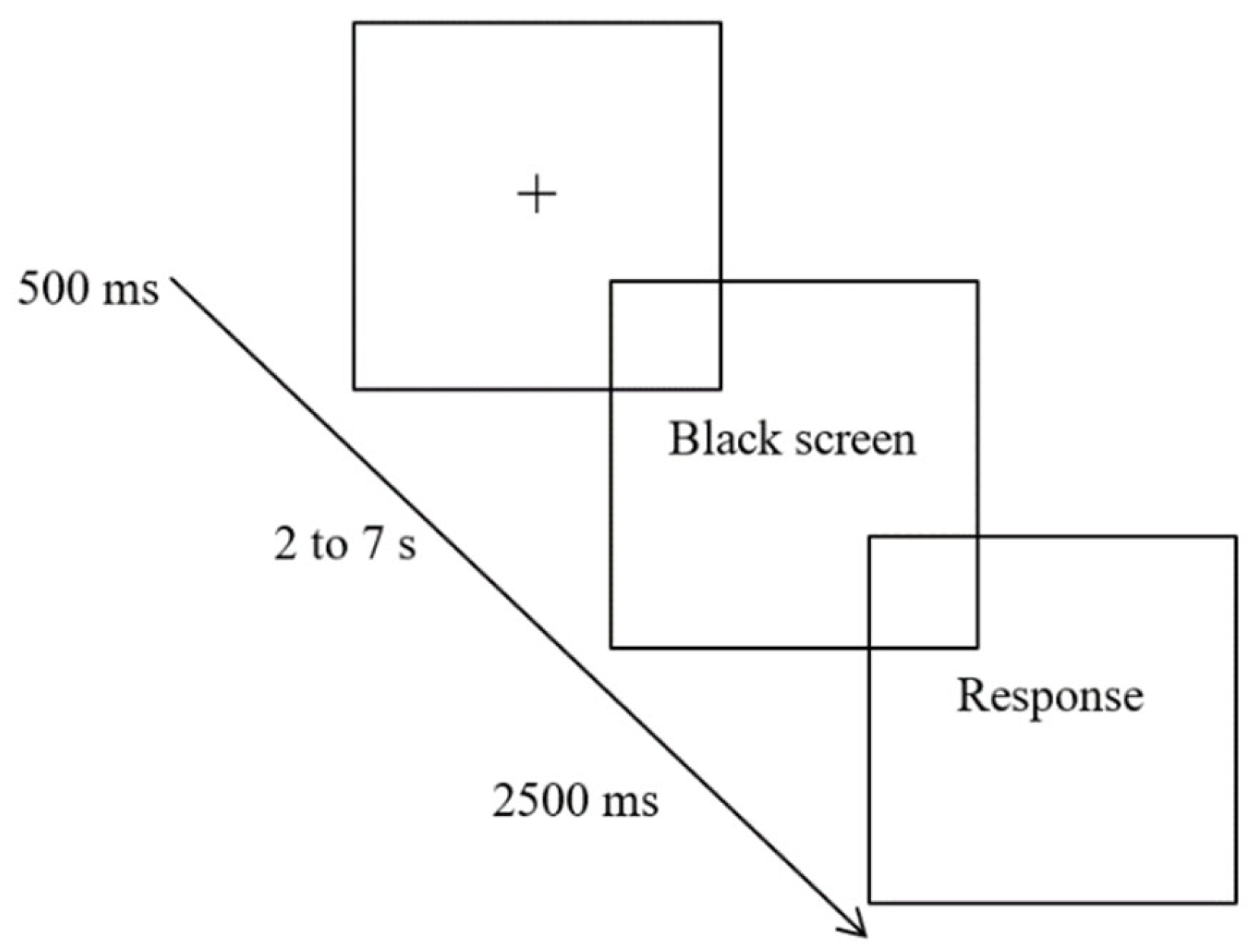
Four experimental conditions were administered. One condition consisted of a time estimation task without emotional interference, and participants estimated the exposure time of a black screen, presented after a 500 ms fixation cross. Fifteen trials were presented for each time interval (2, 3, 4, 5, 6 and 7 s), adding up to a total of 90 trials (15 trials * 6 time intervals). The trials were pseudo-randomized, avoiding two consecutive trials with exposure times of the same duration. Participants responded freely with whole numbers between 1 and 9, having 2.5 s to produce their answer, after which it immediately proceeded to the next trial. Participants were instructed to not count aloud or use any kind of body movement that would help estimate the time (
Figure 1).
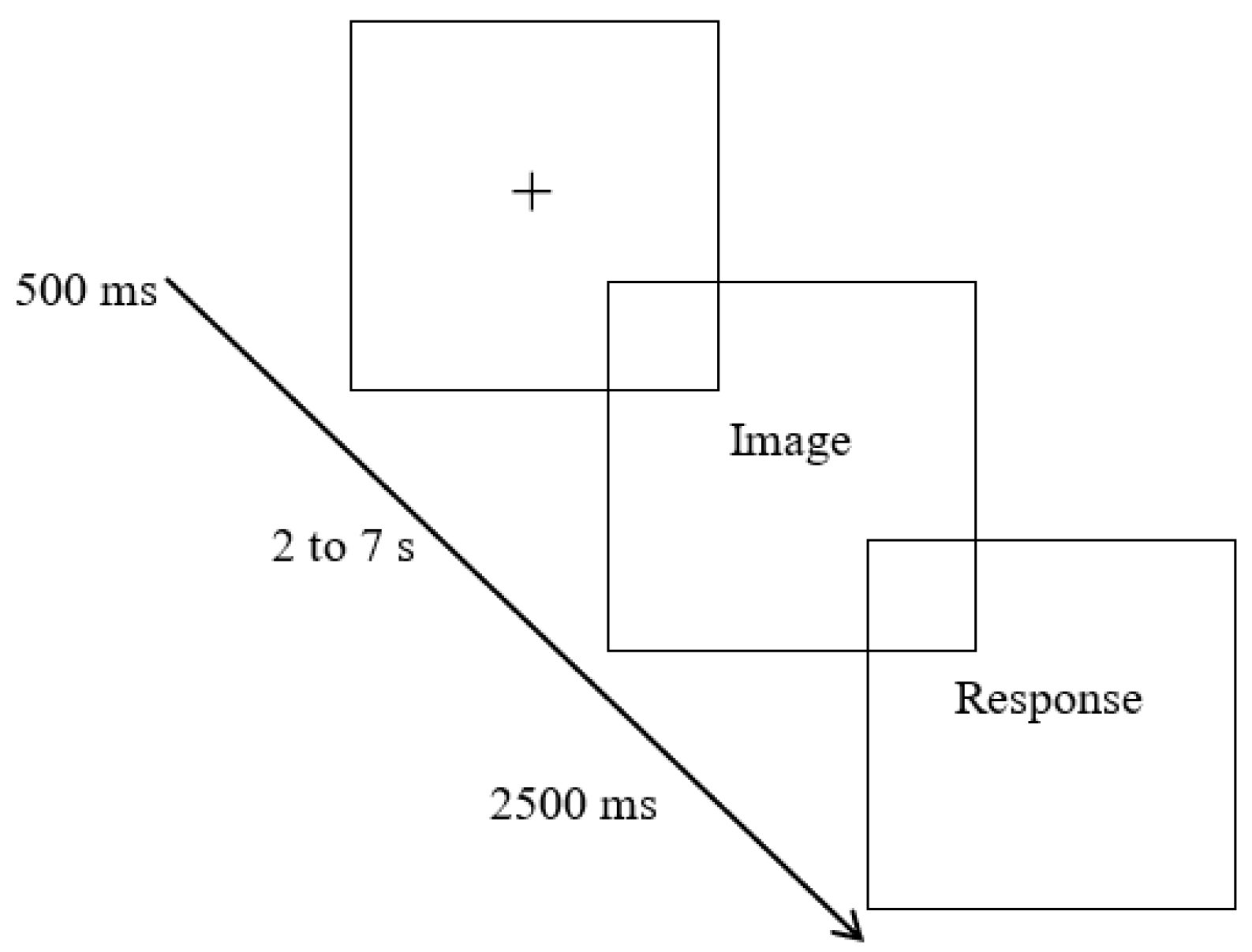
The other conditions were based on a time estimation task with emotional interference (
Figure 2), in which the trials were organized in the same manner as the previous condition but, instead of the black screen, there was a projection of images that induced emotions of positive (pleasant condition), negative (unpleasant condition) or neutral valence (neutral condition), to which participants were instructed to focus their attention. The 15 images of each condition were organized into blocks (pleasant, unpleasant and neutral) and repeated for each time interval, totaling 90 trials per block (15 images * 6 time intervals).
The image blocks of the four conditions were administered in a counterbalanced order across participants to reduce order effects, with no break between blocks. All participants had the opportunity to perform practice trials to become familiarized with the task.
2.4. Data Analysis
In the first phase of data treatment and analysis, central tendency (M) and dispersion (SD) measures were calculated. To test the hypothesis, a Repeated Measures ANOVA was used, with the condition (pleasant, unpleasant, neutral, without interference) as the within-subjects factor and time estimation (measured by θ) as the dependent variable.
For the analysis and treatment of data, the statistical software Statistical Packages for Social Sciences (SPSS, Versão 28, IBM Corp., Armony, NY) was used. The assumption of normality was assessed using the Shapiro Wilk test and, when violated, it was complemented with the analysis of the skewness (Sk) and kurtosis (Ku) coefficients. Since the absolute values of these coefficients vary between 2 and 7 (Kim, 2013), parametric tests were always used. The assumption of sphericity was assessed using the Mauchly test, and when this assumption was violated, the Greenhouse-Geisser correction was applied and the value of epsilon (Ɛ) was reported.
3. Results
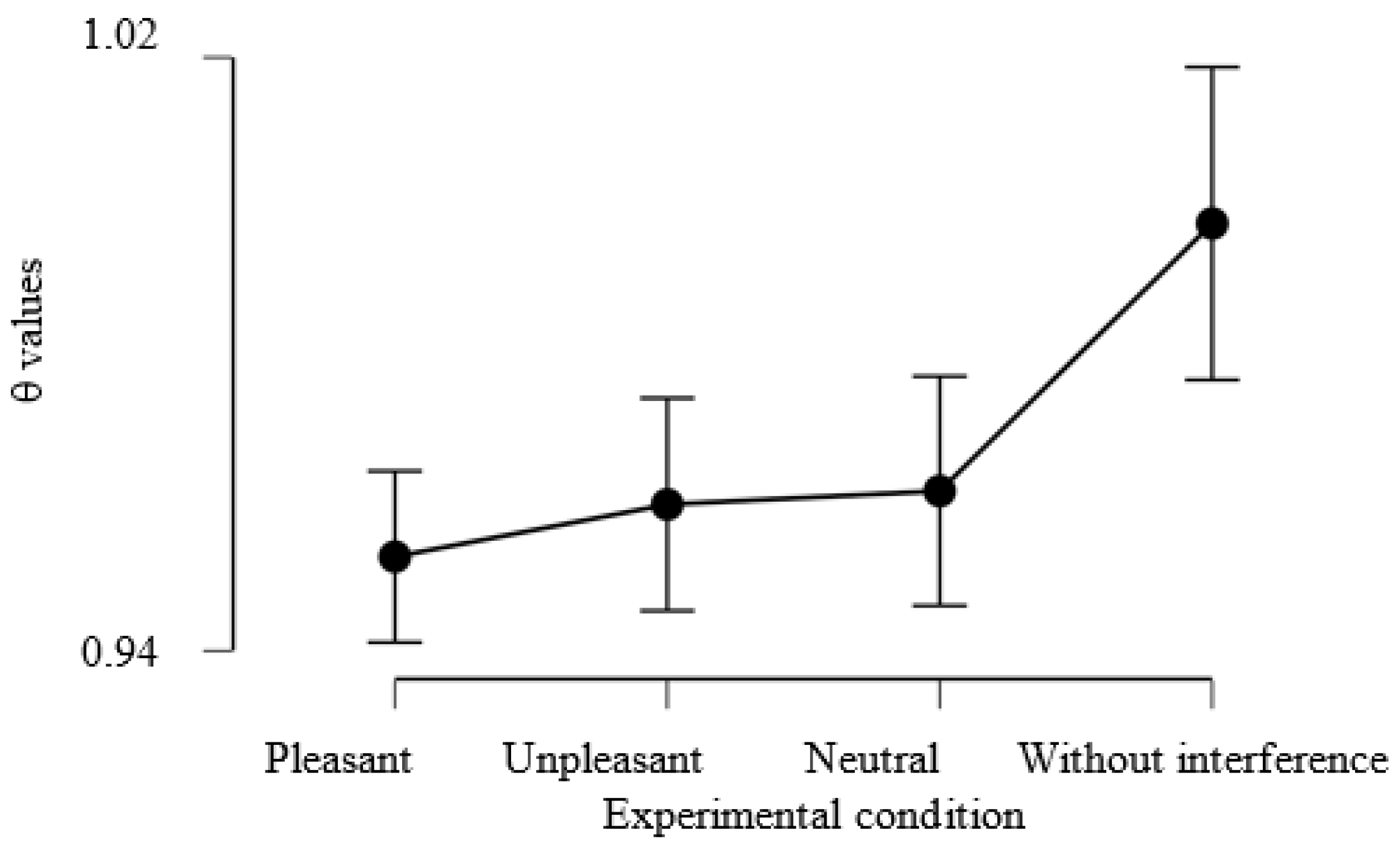
The descriptive analysis of θ values shows that participants underestimated time in the pleasant (M = 0.953, SD = 0.197), unpleasant (M = 0.960, SD = 0.196), and neutral (M = 0.962, SD = 0.203) conditions, as well as in the condition without emotional interference. Although, in this last case, the difference between estimated time and real time is negligible (M = 0.998, SD = 0.200).
The Repeated Measures ANOVA reveals a condition effect, F(2.22, 243.8) = 6.26, p =.002, η² = .054, ε = .739. Post-hoc tests (Holm) revealed a significant difference between the condition without interference and both the pleasant and unpleasant conditions (both t > 2.83, p < .028), as can be seen in
Figure 3. No other significant differences were found.
4. Discussion
There is no clinical condition that can be exclusively defined as a disorder in time perception, but there is knowledge of distortions in time perception among various groups of individuals with disorders, such as impulse control disorders, which can accompany differences in other aspects of sensory processing, as well as in developmental, cognitive, and behavioral profiles (e.g., Ballotta et al., 2018; Montalemberta et al., 2016). Thus, time perception becomes a variable of particular interest for basic research, as well as for clinical research.
The aim of the present study was to examine the effect of emotional stimuli on time estimation. We formulated the hypotheses that (a) individuals overestimate time more markedly when they are under the influence of unpleasant and pleasant images, compared to neutral images and the time estimation condition without emotional interference; (b) individuals overestimate time more when they are under the influence of unpleasant images compared to pleasant, neutral images and the condition without emotional interference.
To test this research hypothesis, 111 participants performed a time estimation experimental protocol with four conditions: in one of the conditions, participants estimated the exposure time of a black screen, wherein duration would vary between 2 to 7 s (estimation task without interference); in the other conditions, participants estimated the exposure time (also varying between 2 to 7 s) of images that induced pleasant, unpleasant or neutral emotions, to which participants were instructed to focus their attention.
The results pointed toward a more precise time estimation in the condition without interference, than in the pleasant and unpleasant conditions, although time was underestimated in all experimental conditions. It should be noted that time estimation under the influence of neutral stimuli did not significantly differ from the estimation observed in the condition without interference. The results found are not consistent with literature. As an example: an image programmed to be exposed for 4000 ms was perceived by participants as being exposed for 3000 ms. Few studies address the influence of emotions on the perception of time. It is known that individuals with predisposition to boredom, patients with depression or cancer patients with high levels of anxiety perceive the passage of time more slowly and overestimate the duration of time estimation tasks (Somov, 2000).
The effects of emotions on the perception of time appear to be different depending on the valence of the stimuli. In time estimation tasks, pleasant and unpleasant images with high activation power have been compared to neutral images and, overall, the participants overestimated (estimated time > real time) the duration of unpleasant images to a greater degree than that of pleasant images, even when the activation levels of pleasant/unpleasant images are controlled (Gil & Droit-Volet, 2009; Gil & Droit-Volet, 2011a, 2011b; Grommet et al., 2011; Tipples, 2008, 2011). Our data do not show this trend. In all conditions (emotional vs. emotional interference) there is an underestimation of time.
It is worth noting that estimation in the condition without interference does not differ from the neutral stimuli condition, legitimizing recourse to the latter as an experimental control condition. The results of the participants in this study did not indicate impulsivity, nor deficits in inhibitory control. This cognitive control was particularly visible in the condition without emotional interference (estimated time close to real time) compared to the pleasant and unpleasant emotional conditions.
Though the results found were not consistent with literature on time overestimation by impulsive individuals, these results may be due to the easiness of the tasks (the participants easily achieved a ceiling effect) or to the existence of only 15 trials for each time interval. This methodological choice was associated with an attempt to reduce the duration of the tasks: 34 minutes for each task described in section Design and procedure. The Method was also constrained by instrumental conditions: the participants answered on a response box with 9 keys (keys from 1 to 9). Therefore, the task was programmed for exposure times between 2 and 7 seconds (the option of 8 s would make it too long) and the response options were limited to varying between 1 and 9 seconds. If a response box that allowed more options had been used, the differences between the participants would have possibly been more expressive. Moreover, the activation induced by stimuli was not controlled. This was a limitation of the study. As a recommendation for future studies, we suggest the stimuli be administered to the participants at the end of the experiment, as to collect valence and activation ratings to confirm that the stimuli produce the expected emotional responses. Otherwise, one should not exclude the possibility that the stimuli are only provoking curiosity, which would explain the atypical results (of underestimation).
5. Conclusions
Although our hypotheses were not verified, the results of this study reveal that participants underestimate time in all the conditions but, in the block without emotional interference, the underestimation was lower than in the pleasant and unpleasant conditions. The results suggest that increased attention to external stimuli, regardless of emotional valence, produces underestimation in time perception.
Author Contributions
Conceptualization, D.M. and F.B.; methodology, D.M.; software, D.M.; validation, D.M. and F.B.; formal analysis, D.M.; investigation, D.M.; resources, D.M.; data curation, D.M.; writing—original draft preparation, D.M.; writing—review and editing, F.B.; visualization, D.M.; supervision, F.B.; project administration, D.M.; funding acquisition, D.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by a PhD Scholarship from the Portuguese Foundation for Science and Technology granted to the first author (SFRH/BD/108216/2015).
Institutional Review Board Statement
The study was approved by the Ethics Committee of the Faculty of Psychology and Educational Sciences of the University of Porto.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data presented in this study are available upon request from the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Ahn, H., Liu, M., & Soman, D. (2009). Memory markers: How consumers recall the duration of experiences. Journal of Consumer Psychology, 19(3), 508–516. [CrossRef]
- Allman, M. (2011). Deficits in temporal processing associated with autistic disorder. Frontiers in Integrative Neuroscience, 5(2), 1–2. [CrossRef]
- Angrilli, A., Cherubini, P., Pavese, A., & Mantredini, S. (1997). The influence of affective factors on time perception. Perception & Psychophysics, 59(6), 972–982. [CrossRef]
- Ballotta, D., Lui, F., Porro, C., Nichelli, P., & Benuzzi, F. (2018). Modulation of neural circuits underlying temporal production by facial expressions of pain. PLoS ONE, 13(2), 1–19. [CrossRef]
- Block, R., Hancock, P., & Zakay, D. (2000). Sex differences in duration judgments: A meta-analytic review. Memory & Cognition, 28(8), 1333–1346. [CrossRef]
- Block, R., Zakay, D., & Hancock, P. (1999). Developmental changes in human duration judgments: A meta-analytic review. Developmental Review, 19(1), 183–211. [CrossRef]
- Campbell, I., & Bryant, R. (2007). How time flies: A study of novice skydivers. Behaviour Research and Therapy, 45(6), 1389–1392. [CrossRef]
- Coull, J., Vidal, F., Nazarian, B., & Macar, F. (2004). Functional anatomy of the attentional modulation of time estimation. Science, 303(5663), 1506–1508. [CrossRef]
- Dirnberger, G., Hesselmann, G., Roiser, J., Preminger, S., Jahanshahi, M., & Paz, P. (2012). Give it time: Neural evidence for distorted time perception and enhanced memory encoding in emotional situations. NeuroImage, 63(1), 591–599. [CrossRef]
- Droit-Volet, S., & Meck, W. (2007). How emotions colour our perception of time. Trends in Cognitive Sciences, 11(12), 504–513. [CrossRef]
- Droit-Volet, S., Brunot, S., & Niedenthal, P. (2004). Perception of the duration of emotional events. Cognition and Emotion, 18(6), 849–858. [CrossRef]
- Faul, F., Erdfelder, E., Buchner, A., & Lang, A. (2009). Statistical power analyses using G*Power 3.1: Tests for correlation and regression analyses. Behavior Research Methods, 41(4), 1149–1160. [CrossRef]
- Faul, F., Erdfelder, E., Lang, A., & Buchner, A. (2007). G*Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behavior Research Methods, 39(2), 175–191. [CrossRef]
- Gil, S., & Droit-Volet, S. (2009). Time perception, depression, and sadness. Behavioural Processes, 80(2), 169–176. [CrossRef]
- Gil, S., & Droit-Volet, S. (2011a). Time perception in response to ashamed faces in children and adults. Scandinavian Journal of Psychology, 52(2), 138–145. [CrossRef]
- Gil, S., & Droit-Volet, S. (2011b). “Time flies in the presence of angry faces”… depending upon the temporal task used! Acta Psychologica, 136(3), 354–362. [CrossRef]
- Harrington, D., Boyd, L., Mayer, A., Sheltraw, D., Lee, R., Huang, M., & Rao, S. (2004). Neural representation of interval encoding and decision making. Brain Research. Cognitive Brain Research, 21(2), 193–205. [CrossRef]
- Hinton, S., & Meck, W. (2004). Frontal-striatal circuitry activated by human peak-interval timing in the supra-seconds range. Brain Research. Cognitive Brain Research, 21(2), 171–182. [CrossRef]
- Ivry, R., & Spencer, R. (2004). The neural representation of time. Current Opinion in Neurobiology, 14(2), 225–232. [CrossRef]
- Kagerer, F., Wittmann, M., Szelag, E., & Steinbüchel, N. (2002). Cortical involvement in temporal reproduction: evidence for differential roles of the hemispheres. Neuropsychologia, 40(3), 357–366. [CrossRef]
- Kim, H. (2013). Statistical notes for clinical researchers: Assessing normal distribution (2) using skewness and kurtosis. Restorative Dentistry & Endodontics, 38(1), 52–54. [CrossRef]
- Klein, L., Corwin, E., & Stine, M. (2003). Smoking abstinence impairs time estimation accuracy in cigarette smokers. Psychopharmacology Bulletin, 37(1), 90–95.
- Lee, K., Seelam, K., & O’Brien, T. (2011). The relativity of time perception produced by facial emotion stimuli. Cognition and Emotion, 25(8), 1471–1480. [CrossRef]
- Marchewka, A., Żurawski, L., Jednoróg, K., & Grabowska, A. (2014). The Nencki Affective Picture System (NAPS): Introduction to a novel, standardized, wide-range, high-quality, realistic picture database. Behavior Research Methods, 46(2), 596–610. [CrossRef]
- Matell, M., & Meck, W. (2004). Cortico-striatal circuits and interval timing: coincidence detection of oscillatory processes. Brain Research. Cognitive Brain Research, 21(2), 139 – 170. [CrossRef]
- Mauk, M., & Buonomano, D. (2004). The neural basis of temporal processing. Annual Review of Neuroscience, 27(1), 307–340. [CrossRef]
- Montalemberta, M., Coulonb, N., Cohen, D., Bonnot, O., & Tordjman, S. (2016). Time perception of simultaneous and sequential events in early-onset schizophrenia. Neurocase, 22(4), 392–399. [CrossRef]
- Moreira, D., Pinto, M., Almeida, F., & Barbosa, F. (2016). Time perception deficits in impulsivity disorders: A systematic review. Aggression and Violent Behavior, 27, 87–92. [CrossRef]
- Myrseth, H., Brunborg, G., & Eidem (2010). Differences in cognitive distortions between pathological and non-pathological gamblers with preferences for chance or skill games. Journal of Gambling Studies, 26(4), 561–569. [CrossRef]
- Noulhiane, M., Mella, N., Samson, S., Ragot, R., & Pouthas, V. (2007). How emotional auditory stimuli modulate time perception. Emotion, 7(4), 697–704. [CrossRef]
- Pande, B., & Pati, A. (2010). Overestimation/underestimation of time: Concept confusion hoodwink conclusion. Biological Rhythm Research, 41(5), 379–390. [CrossRef]
- Pouthas, V., & Perbal, S. (2004). Time perception does not only depend on accurate clock mechanisms but also on unimpaired attention and memory processes. Acta Neurobiologiae Experimentalis, 64(3), 367–385.
- Rubia, K., & Smith, A. (2004). The neural correlates of cognitive time management: A review. Acta Neurobiologiae Experimentalis, 64(3), 329–340.
- Simões, M., Freitas, S., Santana, I., Firmino, H., Martins, C., Nasreddine, Z., & Vilar, M. (2008). Montreal Cognitive Assessment (MoCA): Manual de administração e cotação (versão portuguesa). Coimbra: Serviço de Avaliação Psicológica da Faculdade de Psicologia e de Ciências da Educação da Universidade de Coimbra e Hospitais da Universidade de Coimbra.
- Smith, S., McIver, T., Di Nella, M., & Crease, M. (2011). The effects of valence and arousal on the emotional modulation of time perception: Evidence for multiple stages of processing. Emotion, 11(6), 1305–1313. [CrossRef]
- Somov, P. (2000). Time perception as a measure of pain intensity and pain type. Journal of Back and Musculoskeletal Rehabilitation, 14(3), 111–121. [CrossRef]
- Tipples, J. (2008). Negative emotionality influences the effects of emotion on time perception. Emotion, 8(1), 127–131. [CrossRef]
- Tipples, J. (2011). When time stands still: Fear-specific modulation of temporal bias due to threat. Emotion, 11(1), 74–80. [CrossRef]
- Tse, P., Intriligator, J., Rivest, J., & Cavanagh, P. (2004). Attention and the subjective expansion of time. Perception & Psychophysics, 66(7), 1171–1189. [CrossRef]
- Vohs, K., & Schmeichel, B. (2003). Self-regulation and the extended now: Controlling the self alters the subjective experience of time. Journal of Personality and Social Psychology, 85(2), 217–230. [CrossRef]
- Wearden, J., & Penton-Voak, I. (1995). Feeling the heat: Body temperature and the rate of subjective time, revisited. The Quarterly Journal of Experimental Psychology B: Comparative and Physiological Psychology, 48B(2), 129–141.
- Wittmann, M., & Paulus, M. (2008). Decision-making, impulsivity and time perception. Trends in Cognitive Sciences, 12(1), 7–12. [CrossRef]
- Wittmann, M., & van Wessenhove, V. (2009). The experience of time: Neural mechanisms and the interplay of emotion, cognition, and embodiment. Philosophical Transactions of the Royal Society B: Biological Sciences, 364(1525), 1809–1813. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).