Submitted:
13 April 2024
Posted:
16 April 2024
You are already at the latest version
Abstract
Keywords:
Introduction
Experimental
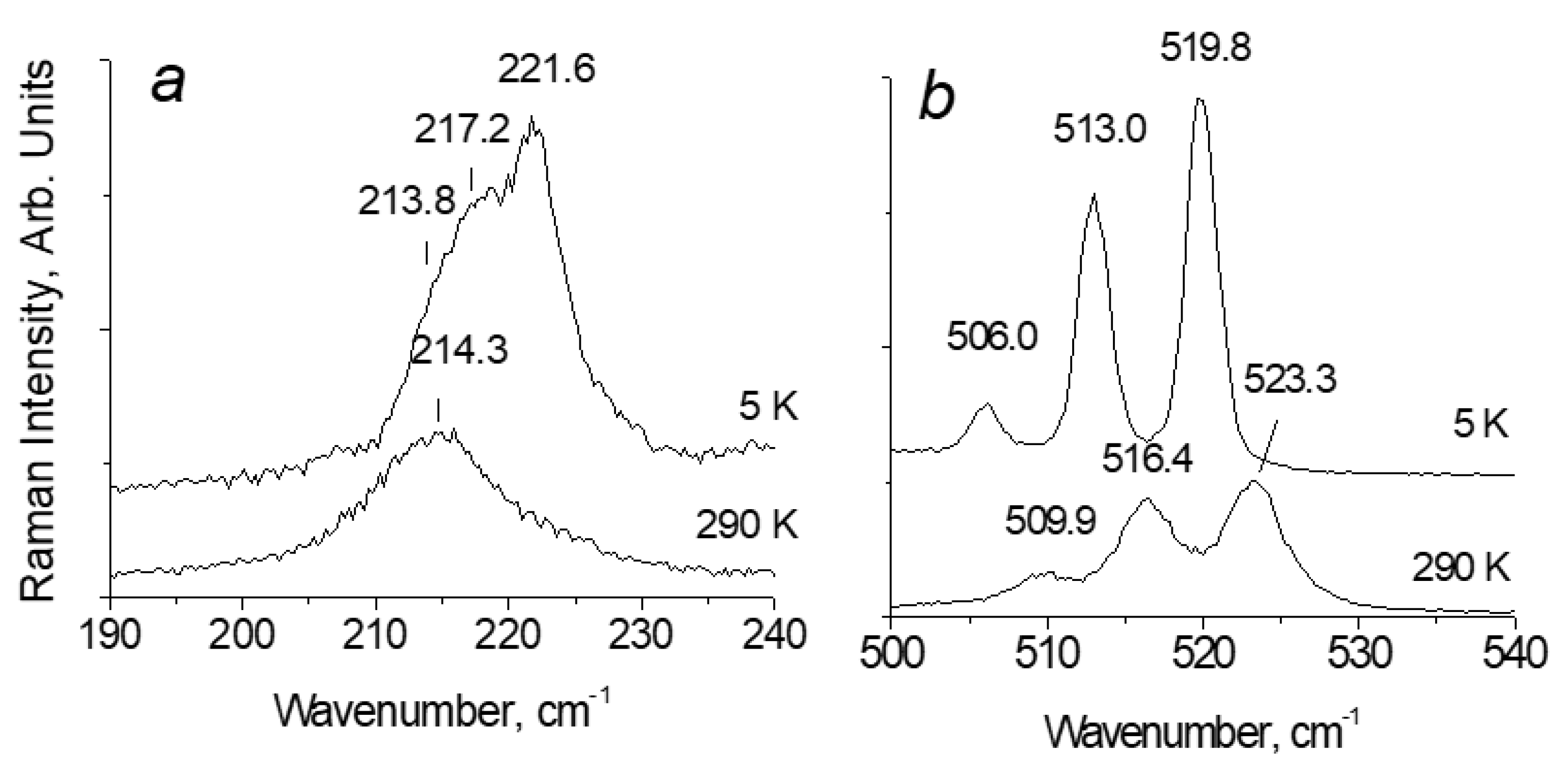
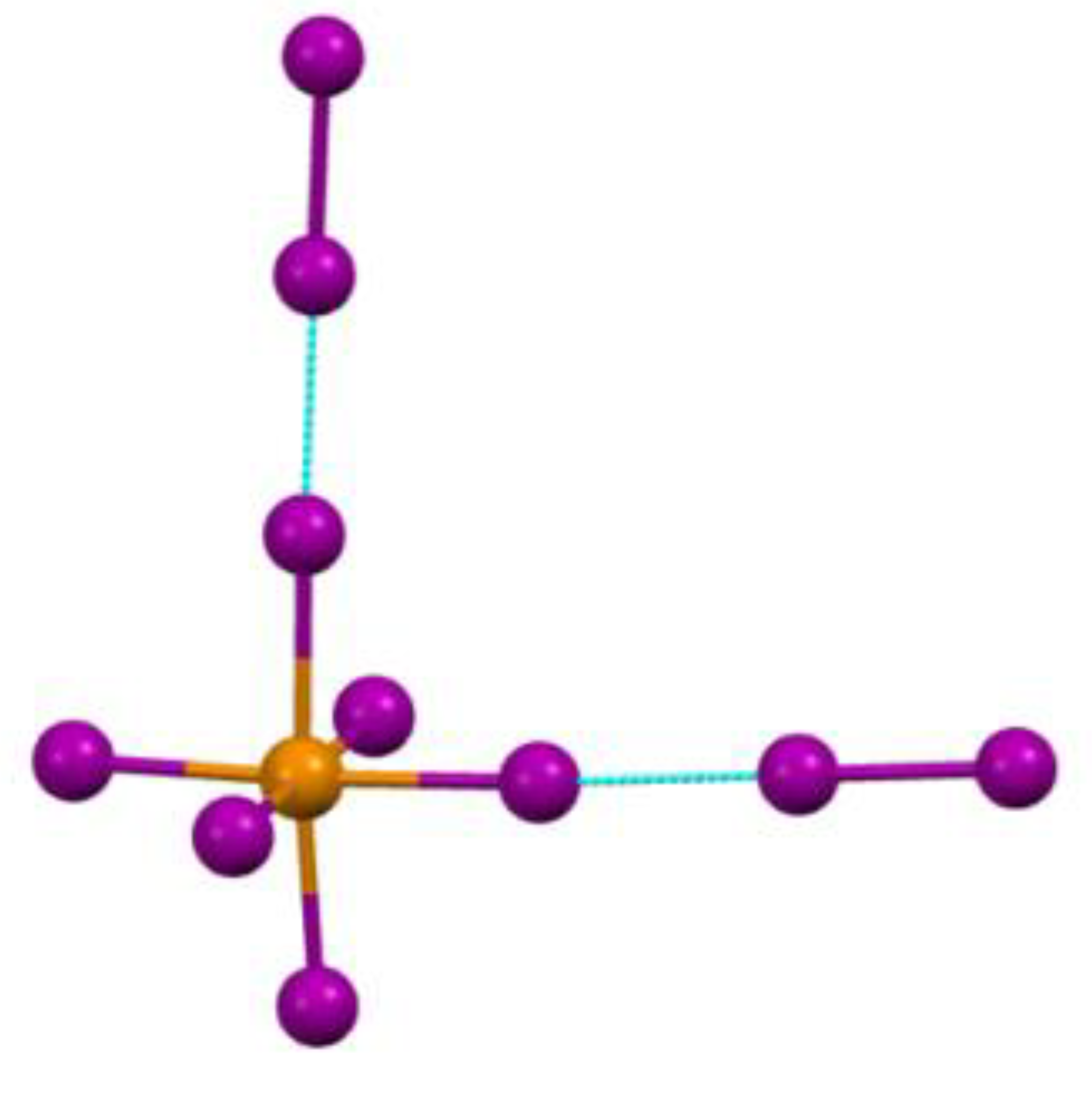
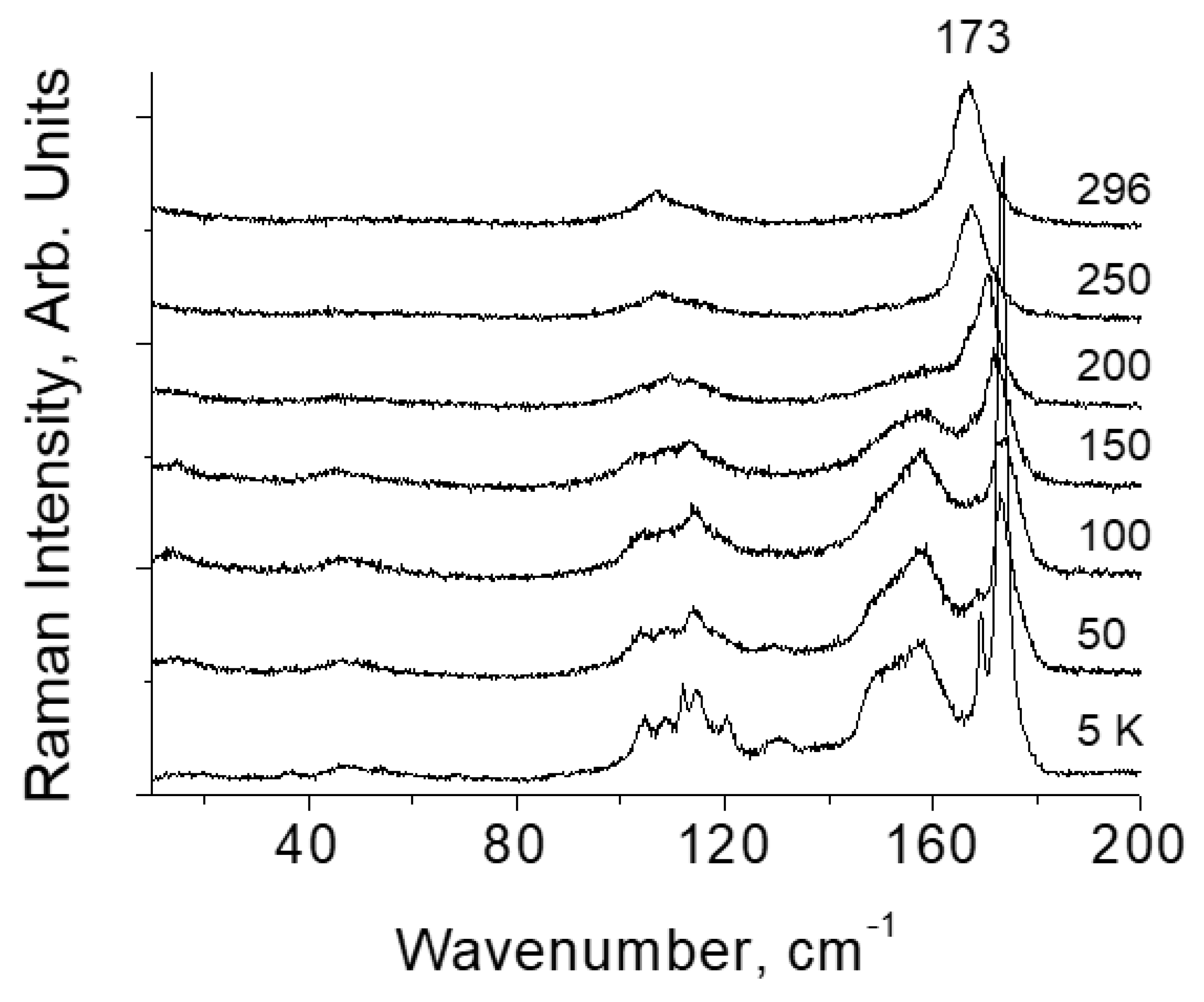
Results and Discussion
Temperature Dependence of ν(Y2) in [TeX6](Y2)
Halogen Crystals Cl2, Br2, I2
Valent or Non-Valent Bonding?
Conclusion
References
- Umeyama, H.; Morokuma, K. The origin of hydrogen bonding. An energy decomposition study. J. Am. Chem. Soc. 1977, 99, 1316–1332. [Google Scholar] [CrossRef]
- Desiraju, G.R.; Ho, P.S.; Kloo, L.; Legon, A.C.; Marquardt, R.; Metrangolo, P.; Politzer, P.; Resnati, G.; Rissanen, K. Pure Appl Chem. 2013, 85, 1711–1713. [Google Scholar] [CrossRef]
- Cavallo, G.; Metrangolo, P.; Milani, R.; Pilati, T.; Priimagi, A.; Resnati, G.; Terraneo, G. The Halogen Bond. Chem. Rev. 2016, 116, 2478–2601. [Google Scholar] [CrossRef]
- Yi Wang; Xinrui Miao; Wenli Deng. Halogen Bonds Fabricate 2D Molecular Self-Assembled Nanostructures by Scanning Tunneling Microscopy. Crystals 2020, 10, 1057. [Google Scholar] [CrossRef]
- Takemura Kenichi; Sato Kyoko; Fujihisa Hiroshi; Onoda Mitsuko. Modulated structure of solid iodine during its molecular dissociation under high pressure. NATURE, 2003, 423, 971–974. [Google Scholar] [CrossRef]
- 6. Min Wu; John S Tse; Yuanming Pan. Anomalous bond length behavior and a new solid phase of bromine under pressure. Scientific Reports. [CrossRef]
- Yushina, I.D.; Kolesov, B.A. Interplay of Intra- and Intermolecular Interactions in Solid Iodine at Low Temperatures: Experimental and Theoretic Spectroscopy Study. J. Phys. Chem. A 2019, 123, 4575–4580. [Google Scholar] [CrossRef]
- Dingdi Wang; Haijing Zhang; William W. Yu; Zikang Tang. Thermal Evolution of One-Dimensional Iodine Chains. J. Phys. Chem. Lett. 2017, 8, 2463–2468. [Google Scholar] [CrossRef]
- Aggarwal, R.L.; Farrar, L.W.; Di Cecca, S.; Jeys, T.H. Raman spectra and cross sections of ammonia, chlorine, hydrogen sulfide, phosgene, and sulfur dioxide toxic gases in the fingerprint region 400-1400 cm−1. AIP Advances 2016, 6, 025310. [Google Scholar] [CrossRef]
- Gill, E.B.; Steele, D. The Raman spectrum of liquid chlorine. MolecularPhysics: An International Journal at the Interface Between Chemistry and Physics. [CrossRef]
- Cahill, J.E.; Leroi, G.E. Raman Spectra of Solid Chlorine and Bromine. J. Chem. Phys. 1969, 51, 4514. [Google Scholar] [CrossRef]
- Dah-Min Hwang; Hua Chang. Observation of Anti-Stokes Resonance Raman Signals of Gaseous Bromine with 5145 Å Excitation. J. Chinese Chem. Soc. 1979, 26, 1–4. [CrossRef]
- Stammreich, H. The Raman Spectrum of Bromine. Phys. Rev. [CrossRef]
- Branigan, E.T.; van Staveren, M.N.; Apkarian, V.A. Janda K. Chem. Phys. 2010, 2010132(4), 044503. [Google Scholar] [CrossRef]
- Branigan, E.T.; Halberstadt, N.; Apkarian, V.A. Solvation dynamics through Raman spectroscopy: Hydration of Br2 and Br3−, and solvation of Br2 in liquid bromine. J. Chem. Phys. 2011, 134, 174503. [Google Scholar] [CrossRef]
- Felmy, H.M.; Clifford, A.J.; Schafer Medina, A.; Cox, R.M.; Wilson, J.M.; Lines, A.M.; Bryan, S.A. On-Line Monitoring of Gas-Phase Molecular Iodine Using Raman and Fluorescence Spectroscopy Paired with Chemometric Analysis. Environ. Sci. Technol. 2021, 55, 3898–3908. [Google Scholar] [CrossRef]
- Huong, P.V. Raman spectra and structure of iodine and bromine intercalated fullerenes C60 and C70. Solid State Comm., 1993, 88, 23–26. [Google Scholar] [CrossRef]
- Wenhao Guo; Dingdi Wang; Juanmei Hu; Z. K. Tang; Shengwang Du. Raman spectroscopy of iodine molecules trapped in zeolite crystals. Appl. Phys. Lett. 2011, 98, 043105. [Google Scholar] [CrossRef]
- Magana, J.R.; Lannin, J.S. Role of density in Raman scattering of iodine. Phys. Rev. B, 1988, 37, 2475–2482. [Google Scholar] [CrossRef]
- Usoltsev, A.N.; Adonin, S.A.; Kolesov, B.A.; Novikov, A.S.; Fedin, V.P.; Sokolov, M.N. Opening the third century of polyhalide chemistry: Thermally stable complex with “trapped” dichlorine. Chem. Eur. J. 2020, 26, 13776–13778. [Google Scholar] [CrossRef]
- Usoltsev, A.N.; Korobeynikov, N.A.; Kolesov, B.A.; Novikov, A.S.; Samsonenko, D.G.; Fedin, V.P.; Sokolov, M.N.; Adonin, S.A. Rule, Not Exclusion: Formation of Dichlorine-Containing Supramolecular Complexes with Chlorometalates(IV). Inorganic Chemistry 2021, 60, 4171–4177. [Google Scholar] [CrossRef]
- Korobeynikov N., A. , Usoltsev A.N. Sokolov M.N., Novikov A.S., Adonin S.A., Polymeric polyiodo-chlorotellurates(IV): new supramolecular hybrids within the halometalate chemistry. [CrossRef]
- Usoltsev, A.N.; Adonin, S.A.; Abramov, P.A.; Novikov, A.S.; Shayapov, V.R.; Plyusnin, P.E.; Korolkov, I.V.; Sokolov, M.N.; Fedin, V.P. 1D and 2D polybromotellurates (IV): structural studies and thermal stabilityEur. Eur. J. Inorg. Chem. 2018, 3264–3269. [Google Scholar] [CrossRef]
- Novikov, A.V.; Usoltsev, A.N.; Adonin, S.A.; Bardin, A.A.; Samsonenko, D.G.; Shilov, G.V.; Sokolov, M.N.; Stevenson, K.J.; Aldoshin, S.M.; Fedin, V.P.; Troshin, P.A. Tellurium complex polyhalides: narrow bandgap photoactive materials for electronic applications. J. Mater. Chem. A, 2020, 8, 21988. [Google Scholar] [CrossRef]
- Kiriyama, H.; Nishizaki, K. Crystal structure and molecular motion of tetramethylammonium hexaiodotellurate(IV)-iodine (1/1) compound. Bull. Chem. Soc. Jpn., 1986, 59, 2415–2419. [Google Scholar] [CrossRef]
- Adonin, S.A.; Usoltsev, A.N.; Novikov, A.S.; Kolesov, B.A.; Fedin, V.P.; Sokolov, M.N. One- and Two-Dimensional Iodine-Rich Iodobismuthate(III) Complexes: Structure, Optical Properties, and Features of Halogen Bonding in the Solid State. Inorg. Chem. 2020, 59, 3290–3296. [Google Scholar] [CrossRef] [PubMed]
- Usoltsev, A.N.; Korobeynikov, N.A.; Novikov, A.S.; Plyusnin, P.E.; Kolesov, B.A.; Fedin, V.P.; Sokolov, M.N.; Adonin, S.A. One-dimensional diiodine–iodobismuthate(III) hybrids cat3{[Bi2I9](I2)3}: Syntheses, stability, and optical properties. Inorganic Chemistry 2020, 59, 17320–17325. [Google Scholar] [CrossRef] [PubMed]
- Usoltsev, A.N.; Korobeynikov, N.A.; Novikov, A.S.; Shayapov, V.R.; Korolkov, I.V.; Samsonenko, D.G.; Fedin, V.P.; Sokolov, M.N.; Adonin, S.A. One-Dimensional Supramolecular Hybrid Iodobismuthate (1-EtPy)3{[Bi2I9](I2)0.75}: Structural Features and Theoretical Studies of I⋅⋅⋅I Non-Covalent Interactions. Journal of Cluster Science 2021, 32, 787–791. [Google Scholar] [CrossRef]
- Kolesov, B.A. , Experimental determination of vibrational anharmonic contributions. J. Raman Spectrosc. 2013, 44, 1786–1788. [Google Scholar] [CrossRef]
- Kolesov B., A. How the vibrational frequency varies with temperature. J. Raman Spectrosc. 2017, 48, 323–326. [Google Scholar] [CrossRef]
- Vazquez-Fernandez, I.; Mariotti, S.; Hutter, O.S.; Birkett, M.; Veal, T.D.; Hobson, T.D.C.; Phillips, L.J.; Danos, L.; Nayak, P.K.; Snaith, H.J.; Wei Xie; Sherburne M. P.; Asta M.; Durose K. Vacancy-Ordered Double Perovskite Cs2TeI6 Thin Films for Optoelectronics. Chem. Mater. 2020, 32, 6676–6684. [Google Scholar] [CrossRef]
- Akahama, Y.; Kawamura, H. High-pressure Raman spectroscopy of solid oxygen. Phys. Rev. B, 1966, 54, R15602–R15605. [Google Scholar] [CrossRef]
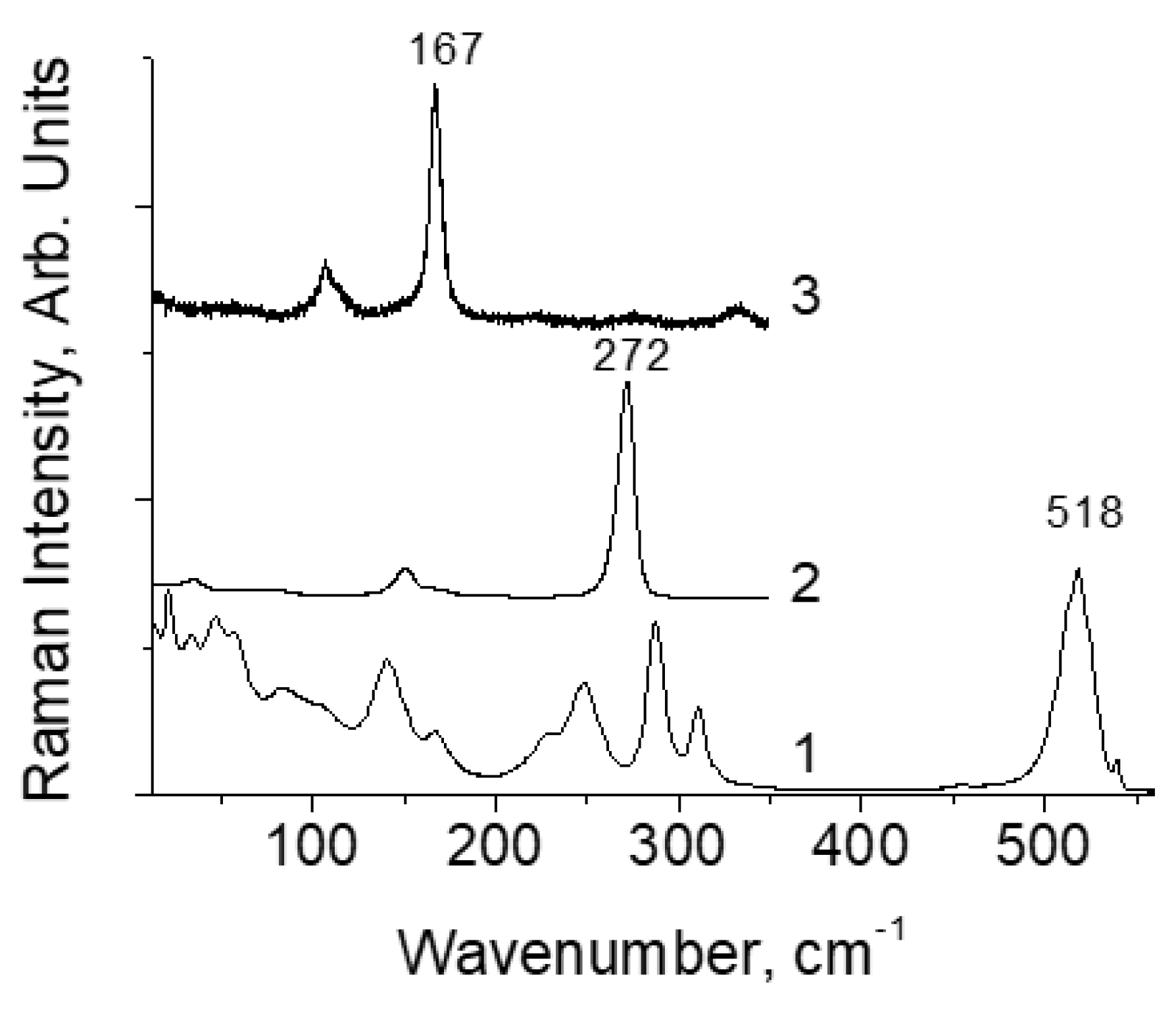
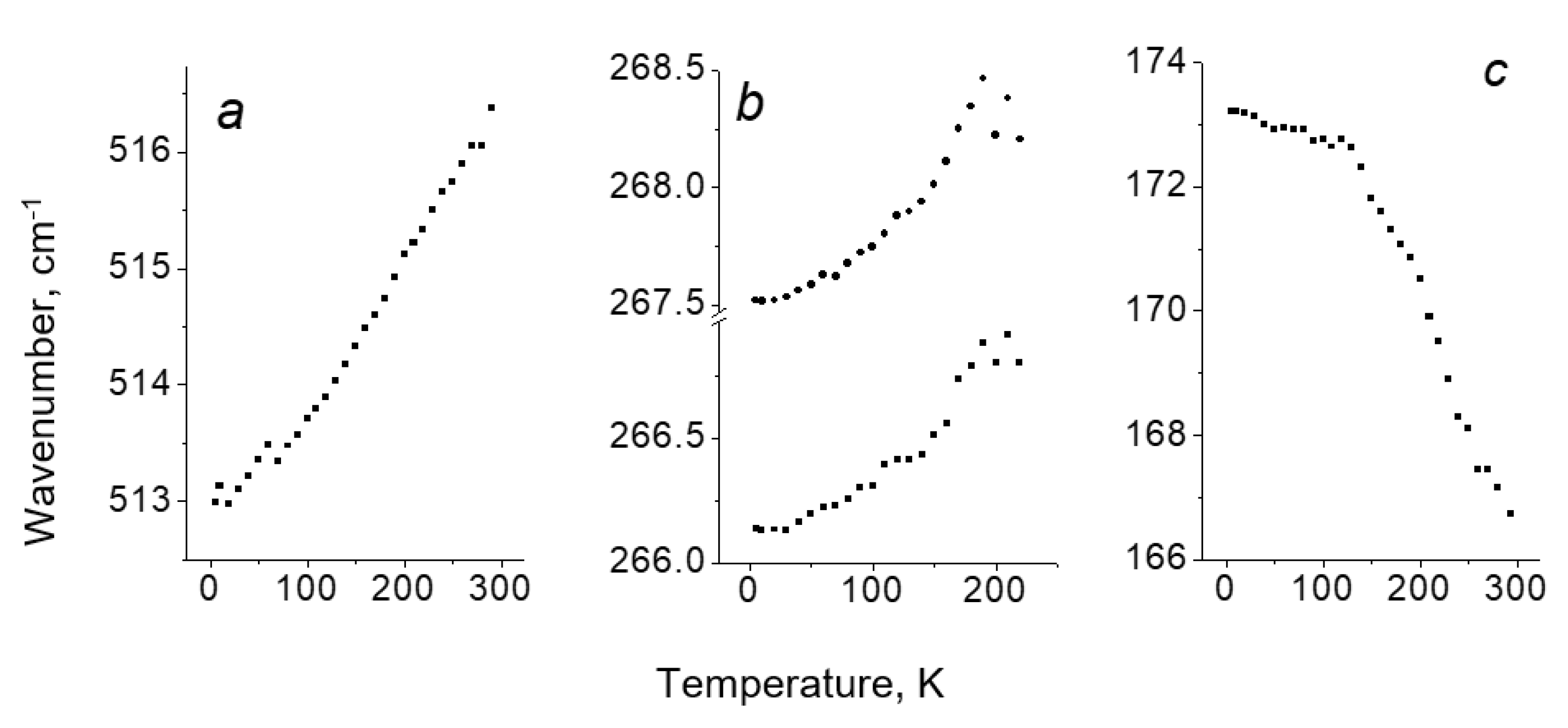
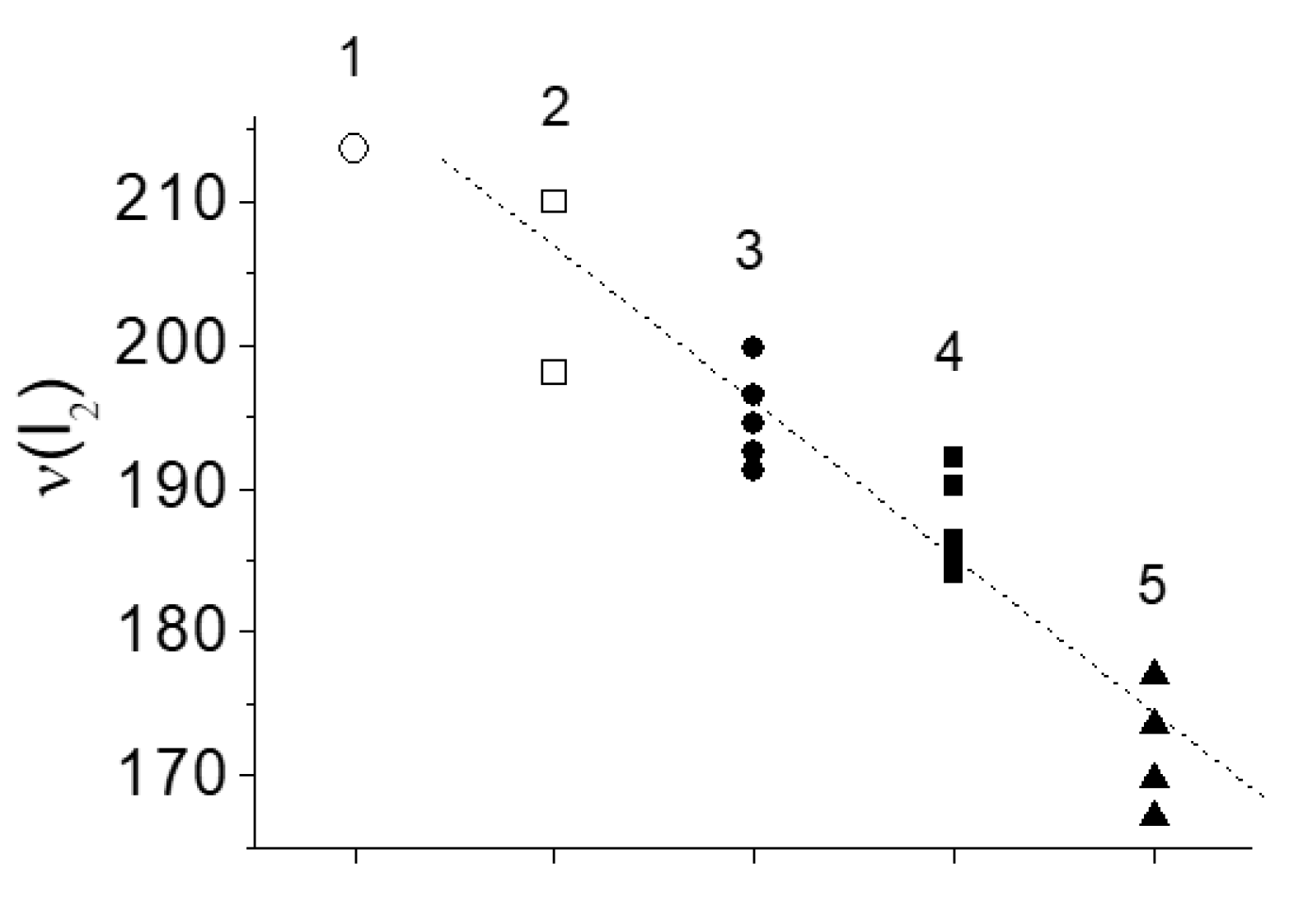
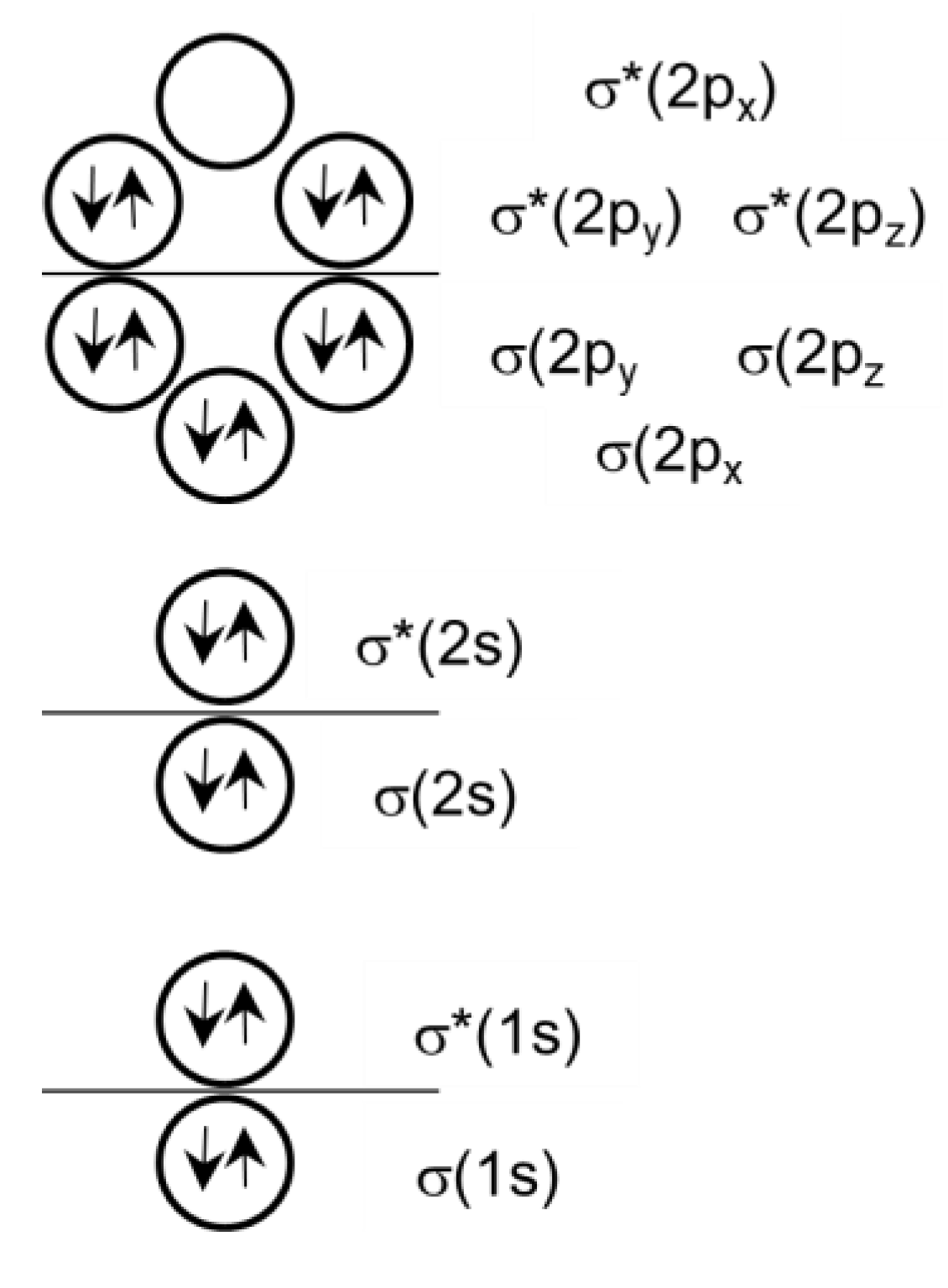
| Compound | ν(Y-Y), cm-1 | d(Y-Y), Å | d(Y-X), Å | Reference |
|---|---|---|---|---|
| Cl2 (Gas) | 554, 547, and 539 | [9] | ||
| Cl2 (Liquid) | 532, 540, 547 | [10] | ||
| Cl2 (Solid) | 540 (15 K) | [11] | ||
| Br2 (Gas) | 323.2, | [12] | ||
| Br2 (Cavity) | 300 in C60 | [13] | ||
| Br2 (Liquid) | 318.6 | [14] | ||
| Br2 (Solid) | 297 (15 K), 302 | [11,15] | ||
| I2 (Gas) | 213.7 | [16] | ||
| I2 (Cavity) | 197-198 in C60, C70, 208-213 in zeolites | [17,18] | ||
| I2 (Liquid) | 204 | [19] | ||
| I2 (Solid) | 180, 189.7 | [7] | ||
| [TeCl6]Cl2 | 505 | 2.007 | 2.951 | [20,21] |
| 513 | 2.002 | 3.155 | ||
| 498 | 2.006 | 2.98 | ||
| [TeCl6]I2 | 191.2 | 2.7 | 3.19 | [22] |
| 196.6 | 2.694 | 3.0-3.2 | ||
| 196.5 | 2.692 | 3.127 | ||
| 192.5 | 2.704 | 3.213 | ||
| 194.5 | 2.695 | 3.177 | ||
| [TeBr6]Br2 | 266.8 | 2.333 | 3.0-3.04 | [23] |
| 271.6 | 2.324 | 3.04 | ||
| [TeBr6]I2 | 192.1 | 2.69 | 3.1-3.18 | [24] |
| 184.0 | 2.715 | 3.201 | ||
| 169.3 | 2.71 | 3.27 | ||
| 192.2 | 2.693 | 3.1 | ||
| 190.2 | 2.73-2.74 | 3.14-3.3 | ||
| 186.5 | 2.707 | 3.32 | ||
| [TeI6](I2) | 167.1 | 2.748 | 3.261 | [25] |
| [BiI6](I2) | 173.5 | 2.749 | 3.527 | [26,27,28] |
| 177.0 | 2.731 | 3.323 | ||
| 169.8 | 2.761 | 3.519 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




