1. Introduction
Affinity constants are one of the most crucial characteristics of antibodies since many performance parameters of immunoassays and even in therapeutics are strongly linked to this value [
1,
2]. Furthermore, in the context of the reproducibility crisis, antibody characterization came into focus [
3]. Affinity constants are often listed as the most crucial parameters for antibody validation [
4] and hence, experimental protocols [
5] seem to be incomplete, as long as affinity constants are not provided. Surprisingly, affinity constants are nevertheless neglected in many papers. And even when an affinity constant is given, the value is often unreliable or ambiguous. Many researchers are not aware of the relevance and complexity of affinity determinations and of the vast number of pitfalls in this context. It is not possible to discuss all these issues in this paper. Here, we want to focus on a practical solution, which makes it easier for the researcher to determine useful affinity constants without excessive efforts. In addition, this paper cannot give a comprehensive review of all methods based on commercial or academic devices. The reader is referred to excellent reviews [
6,
7,
8], which have been published previously. It also has to be noted that we do not claim to determine a “true” thermodynamic equilibrium constant, which is very difficult to measure in the strict sense [
9]. The determination of “true” binding constants would often require research projects on their own. To assume that these efforts can be spent in a typical biomedical and biochemical lab seems to be unrealistic. However, we think that the deviation from our values to this “true” constant is not critical in most cases, particularly if the broad range of values is considered, which is obtained by different methods and replicated measurements.
Today, only a few methods for affinity determinations seem to have some practical relevance. In a limited field, the application of isothermal titration microcalorimetry (ITC) is considered the gold standard. A significant advantage is its universality and the homogeneous and label-free reaction [
10,
11]. This avoids many of the problems encountered in heterogeneous systems. Additional thermodynamic data can be extracted from these measurements. Unfortunately, this approach is not very sensitive and hence consumes large amounts of reagents, which is prohibitive for many applications. Therefore, it seems to be not very popular for the characterization of expensive antibodies and their antigens.
During the last decades, surface plasmon resonance (SPR) has developed into the most dominant method for the determination of affinity constants [
12,
13]. This method is also known as the
BiacoreTM method, based on the former name of a leading company offering such systems. As the term suggests, SPR is a surface method using a gold layer with attached reagents. Many different types of pre-coated SPR chips are commercially available, which makes their application quite accessible. However, most users greatly underestimate the pitfalls associated with this technology. The main drawback is the heterogenous assay, which may lead to severe dissociation limitations, particularly if thicker layers (“3D”) are used to achieve higher signals. The main advantage of the method is the fact that it can be considered label-free, and hence it is assumed that the undisturbed affinity constant could be obtained in contrast to other methods, which rely on labeled reagents. However, from our point of view, this is only half of the story. In all cases, one of the reagents needs to be immobilized on the SPR chip, which is often even more disturbing as the attachment of a radioisotope, dye, or enzyme. With SPR, not only thermodynamic equilibrium constants can be determined, but also kinetic binding constants, such as the on-rate and off-rate constant. This is definitely a big plus of SPR. Unfortunately, these data also can be distorted by unwanted surface- or diffusion effects. In addition, label-free methods are severely hampered by any non-specific binding of irrelevant materials and, in the case of SPR, by even minimal changes of the refractive index. Reference channels can only partially compensate for these interferences. Finally, if antigen-coated chips are used, often multivalence effects [
14] may lead to tremendous overestimations of the monovalent binding strength of an antibody to its antigen. The functional affinity, including multivalence [
15] and other complex mechanisms, is known as avidity. This number may have some relevance in a special situations, but it should not be mixed up with the traditional affinity constant because this can lead to severe misinterpretations, as seen in many papers. Anyway, SPR is the most frequently used method for the determination of affinity constants today, irrespective of the repeated critical discussions [
16,
17]. Also, a similar technique known as bio-layer interferometry (BLI), is commercialized in Octet systems. These and other devices have some similarity to SPR, but are claimed to be more robust in terms of refractive index changes.
A very traditional method for affinity determination is the use of the quenching of the intrinsic tryptophan fluorescence of proteins [
18,
19]. However, this method also seems to be not trivial to apply and hence many of the results might be not reliable [
20].
Another well-established method is known as equilibrium dialysis [
19]. It was introduced by Marrack and Smith in 1932 [
21]. Commercial dialysis chambers of different formats are available. Also, a 3D printed device was published [
22]. This method is useful for the binding analysis of small molecules and ions to proteins and other substances of high molecular mass.
Recently, a new technique termed microscale thermophoresis (MST) marketed by Nanotemper seems to gain some popularity [
23]. MST is a homogeneous method and does not need any immobilized reagents. Hence, surface or diffusion issues are much less relevant here. For MST, however, a reagent usually has to be fluorescently labeled, and kinetic data cannot be obtained directly in a similar way to SPR. However, the method is a good addition to the portfolio of methods, which can be used for the technically complementary determination of affinity constants.
Furthermore, many other different methods have been used for this purpose, such as nuclear magnetic resonance (NMR) [
24], fluorescence anisotropy, also known as fluorescence polarization [
25,
26], and many others [
27,
28,
29,
30]. Also, quite popular is the use of immunoassays of different formats for the “estimation” of affinity constants or the determination of relative affinities. However, in many cases, it remains unclear how different these results are from the thermodynamic equilibrium constant and often only relative values are given. The most well-known method seems to be the approach of Friguet [
31]. It can be considered as label-free, and a solid phase is only used to trap some of the antibodies. Although this method is occasionally used, some limitations seem to prevent its widespread use [
32].
In this paper, we propose a simple method to use immunoassays of different formats to determine equilibrium constants, which seems to be able to obtain a value, which is quite close to the numbers obtained with other, more complex methods. This approach is based on the idea that the IC
50 values (fitted midpoint of the 4-parameter equation) of competitive assays converge towards the reciprocal affinity constant when the reagents are diluted to infinity [
33,
34]. Of course, this is not possible in practice; however, the convergence trend shows how closely the experimental value approached the affinity constant. This convergence is tested by further dilution of the reagents. In most cases, two reagents are relevant in this context. Hence a 2-dimensional dilution array is necessary. In the best case, you only need four (or nine) calibration curves for a final result. If no convergence is reached yet, the experiment can be repeated with lower concentrations of the reagents until convergence is achieved. There are several advantages of this approach: The experiment is very simple and fast; different formats can be used, only one (absolute) concentration needs to be known, the molar concentration of the analyte, and the transparent evaluation of the data. Most other methods need complicated calculations and corrections, which might lead to unintended errors, or the user has to trust blindly in the values generated by a commercial software.
2. Experimental Design
In this paper a model system was used to test different ways to determine affinity constants. For this purpose, the monoclonal antibody against the target FLAG, a peptide-based label [
35], was chosen. This mouse antibody (IgG
1) excreted from the clone M2 [
36,
37,
38,
39,
40,
41] is commercially available and was used quite frequently. However, not too many efforts have been made to determine its affinity to its antigen, FLAG. In this work, two different immunoassay formats have been tested, a direct competitive immunoassay (antibody immobilized, enzyme-labelled FLAG) and an indirect competitive immunoassay (FLAG immobilized, antibody unlabeled, secondary antibody enzyme-labeled). Since these assays are competitive ones, they are preferentially used for small molecules (haptens). In our case, the target (and competitor) was the 8-amino acid peptide FLAG with the sequence Asp-Tyr-Lys-Asp-Asp-Asp-Asp-Lys (DYKDDDDK).
A typical procedure would be as follows:
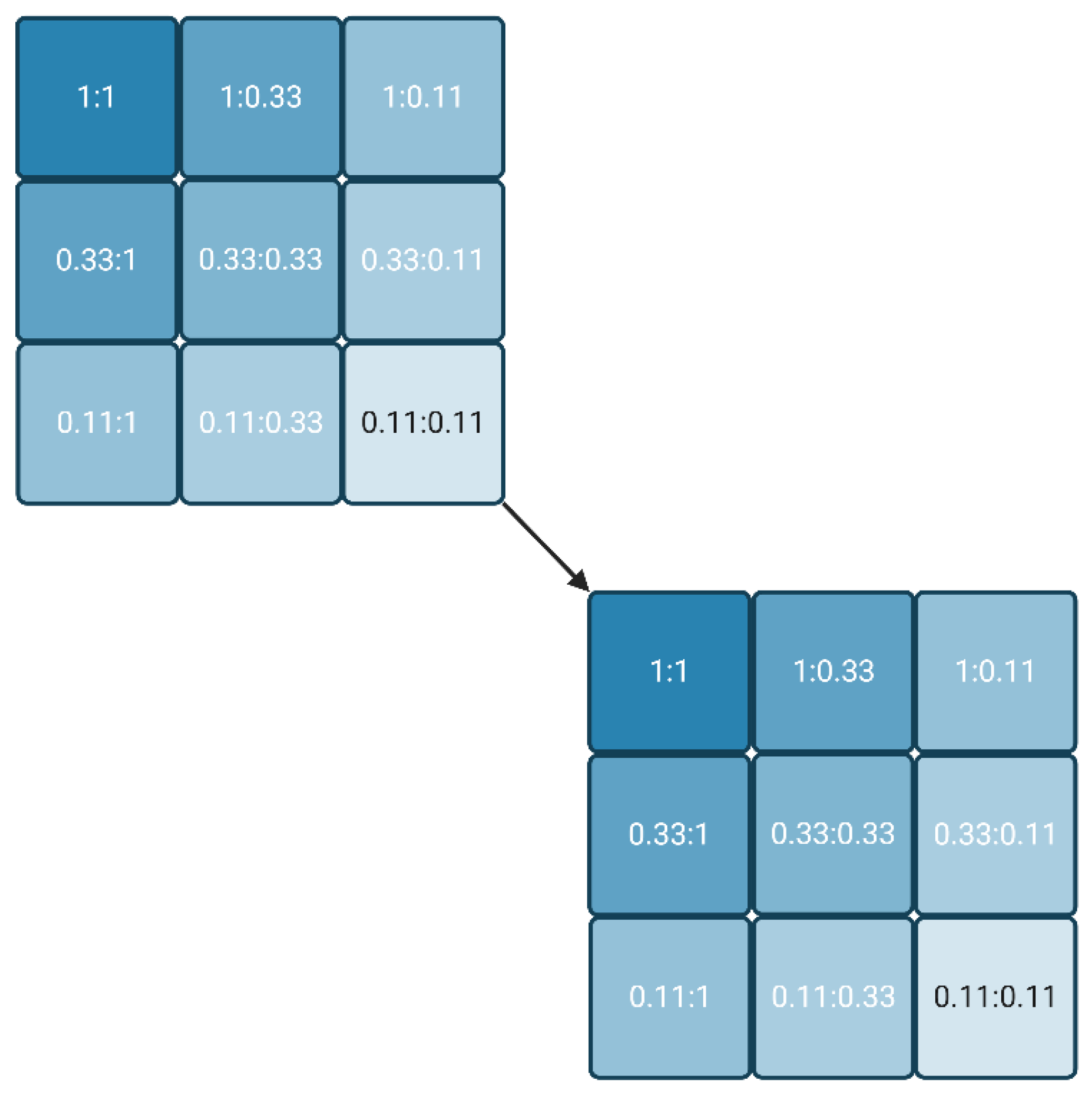
Nine IC
50 values need to be determined on three ELISA 96-well microplates. Each measurement would be obtained in quadruplicates, which seems to be a good compromise between effort and reliability. A calibration curve consisting of eight different concentrations of the analyte would be prepared (usually including the blank value). In most cases, dilution steps of a factor of ten would be adequate to cover a large concentration range. Otherwise, preliminary experiments to determine the optimal concentration steps would be required. In many cases, the development of an assay already delivered useful starting concentrations for the calibration curve. However, some steps in the lower concentration range should be planned, because the sigmoidal curve will shift to lower concentrations during this process. In
Table 1 a typical plate setup is shown. Three of these plates would be required to vary the second reagent, too.
The lowest reagent concentrations are the most interesting, as long as a significant signal could be measured. If a converging IC
50 was obtained, the lowest IC
50 already delivers the Kd value, or at least a good estimate. If the IC
50 values did not achieve convergence, the whole approach can be repeated (cascaded) with another series of three plates, usually starting with the lowest reagent combinations of the first round (
Figure 1). The process stops when either a convergent IC
50 is obtained or the signal is not detectable anymore. The latter condition underlines that the most sensitive labels are preferable for this approach.
Protocol direct enzyme immunoassay (antibody immobilized):
High-binding, flat bottom microtitration plates (MTP, Greiner) are first coated with donkey anti-mouse antibodies diluted 1:1000 in PBS overnight (100 µL per well). After washing the plate with PBS-Tween 20 three times (300 µL per well), the surface is blocked with 1% of BSA in PBS for 1 hour (200 µL per well). After a repeating washing step, different dilutions of the primary antibody M2 are added in PBS/BSA/Tween 20 (100 µL per well, pH 7.45, 0.1% BSA, 0.01% Tween 20) and incubated for 1 hour under shaking. After a subsequent washing step, 100 µL per well of FLAG peptide solutions (calibration curve) in PBS/BSA/Tween 20, directly followed by 100 µL per well of FLAG-HRP conjugate were incubated for one hour. After the next washing step, 100 µL of TMB substrate (Seramun) was added and incubated for up to 45 min. Then 100 µL of 0.25 M H2SO4 is added to stop the reaction. The plate is measured at 450 nm in an MTP reader. All buffers are filtered though a syringe filter (0.45 µm) or equivalent.
Protocol indirect enzyme immunoassay (antigen immobilized):
High-binding, flat bottom microtitration plates (MTP, Greiner) are first coated with FLAG-BSA conjugate diluted 1:1500, 1:4500 and 1:13,500 in PBS overnight (100 µL per well). After washing the plate with PBS-Tween 20 three times (300 µL per well) different dilutions of the FLAG peptide in PBS/BSA/Tween 20 (100 µL per well, pH 7.45, 0.1% BSA, 0.01% Tween 20) and subsequently the primary antibody M2 are added in PBS/BSA/Tween 20 (100 µL) and incubated for 1 hour under shaking. After the next washing step, 100 µL of anti-mouse-HRP (1:40,000 in PBS/BSA/Tween 20) is incubated for 1 hour, followed by the next washing step. 100 µL of TMB substrate (Seramun) was added and incubated for up to 45 min. Then 100 µL of 0.25 M H2SO4 is added to stop the reaction. The plate is measured at 450 nm in an MTP reader. All buffers are filtered though a syringe filter (0.45 µm) or equivalent.
Synthesis of FLAG conjugates:
The conjugates were prepared based on Cys-FLAG (H-CDYKDDDDK-NH2). Maleimide-activated HRP (Thermo) was dissolved in PBS to obtain a concentration of 1 mg/mL and mixed with Cys-FLAG in PBS (1 mg/mL) and incubated three hours at room temperature and overnight at 4°C. The conjugate was purified on a PD Spin Trap GD-25 column. MALDI-MS measurements showed a conjugation ratio of about 1:1 for the FLAG-HRP product.
The BSA conjugate was prepared in PBS adjusted to pH 8. First a BSA solution of about 1 mg/mL was prepared. Then, SBAP (succinimidyl 3-(bromoacetamido)propionate) was dissolved in DMSO to obtain a solution of 1 mg/mL. 150 µL of the BSA solution was slowly mixed with 15 µL of the SBAP solution and incubated at 4° C for one hour. The conjugate was purified on a PD Spin Trap GD-25 column. Subsequently, a 1 mg/mL concentration of Cys-FLAG was prepared in purified water. 150 µl of the BSA-SBAB conjugate was mixed with 55 µl of the Cys-FLAG solution and incubated for two hours at room temperature and overnight at 4° C. Then the BSA-SBAP-Cys-FLAG conjugate was purified on a PD Spin Trap GD-25 column equilibrated with PBS (pH 7.4). MALDI-MS measurements showed a conjugation ratio of about 3:1 for the FLAG-BSA product.
3. Results and Discussion
The test midpoints (IC50) of two different formats have been determined according to the approach described above.
For direct (antibody-immobilized) assays, the concentration of antibody and the concentration of conjugate (hapten-label) was diluted in 3 steps respectively, which leads to 9 different reagent combinations. Please note that only relative concentrations of the reagents are needed. We suggest a dilution of a factor of 3, which is large enough to be significant, but small enough not end up in extremely low signals. The starting concentrations of the antibody/conjugate pair is somewhat arbitrary and should be chosen according to previous experience with the respective assay. An optimized ELISA might be obtained with the help of a checkerboard titration, if performed anyway. As an orientation, in our assays the concentration of the antibodies and of the conjugate were 11 ng/mL, repectively. If a biointeraction is used for the oriented immobilization of the antibodies or other reagents (e.g., streptavidin/biotinylated antibody, protein A or G, anti-IgG), these reagents are not subject of this approach and can be chosen more or less arbitrarily, since they are not directly involved in the competitive step.
For indirect (antigen-immobilized) assays, the concentration of antigen (or hapten) and the concentration of antibody was also diluted in 3 steps respectively, which also leads to 9 different reagent combinations. Similar to the direct immunoassays, any secondary reagents, such as enzyme-labeled antibodies, are not subject of the dilution steps, only reagents directly involved in the competition are relevant here. As an orientation: In our assays, the starting concentration of the hapten-BSA (conjugation density: ≈ 3.5) conjugate was 0.044 ng/mL and 33.3 ng/mL for the antibody.
The concentration of the FLAG peptide was determined gravimetrically, whereby no impurities from water, salts or counterions such as trifluoroacetates were taken into account. Since the concentration of the antigen is crucial for the accuracy of the affinity constant, as it is directly reflected in deviations from the "true" affinity constant, the impurities should be taken into account if a high accuracy of the result is important. The most common method for a reliable determination of the peptide concentration seems to be amino acid analysis, e.g., [
44,
45,
46,
47], which is sometimes offered by companies performing custom peptide synthesis. If an identical calibration substance of known purity is available, many other methods might be suitable [
48], such as HPLC with UV detection.
In
Table 2, molar IC
50 values of nine calibration curves of the direct, competitive immunoassays are shown. Usually, the IC
50 decreases with the dilution of the reagents. However, by approaching the strictly affinity-controlled regime, the IC
50 converges and finally remains constant. The final IC
50 is the basis for the calculation of K
d. There is a small issue with this method. In both assay formats, the analyte is diluted with the reagent by a known factor, based on the volumes of the reagents. Due to pre-incubation effects (“cold start”) and different diffusion coefficients of the analyte and the reagent, their kinetic behavior might be different. Usually, it is not known whether the equilibrium is achieved or not. Therefore, there is a small uncertainty, to which extent the dilution of the analyte has to be corrected. To minimize this effect, a relatively large volume of the analyte (e.g., 200 µL) and a small volume of the reagent (e.g., 20 µL) might be used. Furthermore, relatively long incubation times should be used for the competitive step (at least 1 hour). In this case, a correction factor for the IC
50 of 0.91 would be adequate. The effect of incubation time was previously discussed [
49]. However, considering the huge discrepancies of reported affinity constants in different papers, this small difference seems to be of minor relevance. It has to be also considered that affinity constants are dependent on the environment of the complex and particularly on the temperature. Therefore, only affinity constants determined under standardized conditions can be expected to be identical.
In
Table 3, the nine calibration curves of the indirect, competitive immunoassays delivered also nine molar IC
50 values. Perhaps surprisingly, the converged IC
50 values of both methods are essentially the same, although the assay format is quite different. This is a strong indication that these IC
50 values really represent an affinity constant, which is a characteristic of the respective system. We do not claim that these values are identical to a strict thermodynamic derivation but are “fit for purpose” in research and commercial quality control. We are confident that these values are practically useful and facilitate to compare antibodies and their properties, not only in a relative way. In addition, this approach is so fast and simple that essentially all antibodies could be characterized this way without excessive investments and workload.
Table 4 compares the results of our work with the available literature data. These figures appear to be sufficiently consistent in view of the large deviations found in the literature.
According to model calculations [
33], the IC
50 drops proportional to the dilution of the respective reagent, as long the assay is governed by the concentration regime. This means that in this case, large dilution steps are required to reach the signal-limited range. In this regime, the IC
50 converges to a constant value, however the signal drops proportional to the dilution, until the limit of detection of the label is reached.
If both dilution steps of reagent A and B and their combination, lead to the same IC50, the affinity limited range is reached, and the affinity constant has been determined. In this work, it should be examined, whether this approach leads to the same affinity constant, irrespective of the starting point and the immunoassay format, which was indeed the case.
In rare cases, particularly with antibodies of extremely high affinity, the IC50 values might not converge sufficiently, when the signal reached the limit of detection. In these cases, the lowest IC50 obtained in these experiments determines the minimum affinity constant (maximal Kd). This situation is not uncommon; most methods used for the determination of affinities show a limit to high affinities (or very low Kd) values. This is caused by the fact that the relevant analyte concentrations become lower and lower and hence cannot be quantified precisely anymore. This also means that more powerful (sensitive and accurate) methods for the determination of the respective conjugates will lead to an improvement for this approach, too. A final word of encouragement: Very low signal intensities do not mean that the measurement is useless or even failed. Experimental reproducibility, as measured by the variability of replicates, is often surprisingly good, even at very low signal intensities.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Data Availability Statement
Some data are given in the Supplementary Materials.
Acknowledgments
We would like to thank Belyntic GmbH for providing some of the FLAG derivatives.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Azimzadeh, A.; Van Regenmortel, M.H. Antibody affinity measurements. J Mol Recognition 1990, 3, 108–116. [Google Scholar] [CrossRef] [PubMed]
- Steward, M.W.; Lew, A.M. The Importance of Antibody-Affinity in the Performance of Immunoassays for Antibody. J Immunol Methods 1985, 78, 173–190. [Google Scholar] [CrossRef] [PubMed]
- Weller, M.G. Quality Issues of Research Antibodies. Anal Chem Insights 2016, 11, 21–27. [Google Scholar] [CrossRef] [PubMed]
- Weller, M.G. Ten Basic Rules of Antibody Validation. Anal Chem Insights 2018, 13. [Google Scholar] [CrossRef] [PubMed]
- Weller, M.G. The Protocol Gap. Method Protocol 2021, 4. [Google Scholar] [CrossRef] [PubMed]
- van Regenmortel, M.H.V.; Azimzadeh, A. Determination of antibody affinity (Reprinted from Immunochemistry, pg 805-828, 1994). J Immunoassay 2000, 21, 211–234. [Google Scholar] [CrossRef] [PubMed]
- Goldberg, M.E.; Djavadiohaniance, L. Methods for Measurement of Antibody Antigen Affinity Based on ELISA and RIA. Curr Opin Immunol 1993, 5, 278–281. [Google Scholar] [CrossRef] [PubMed]
- Jarmoskaite, I.; AlSadhan, I.; Vaidyanathan, P.P.; Herschlag, D. How to measure and evaluate binding affinities. Elife 2020, 9. [Google Scholar] [CrossRef] [PubMed]
- Barbet, J.; Huclier-Markai, S. Equilibrium, affinity, dissociation constants, IC5O: Facts and fantasies. Pharm Stat 2019, 18, 513–525. [Google Scholar] [CrossRef]
- Jelesarov, I.; Leder, B. Probing the Energetics of Antigen-Antibody Recognition by Titration Microcalorimetry. Methods 1996, 9, 533–541. [Google Scholar] [CrossRef]
- Pierce, M.M.; Raman, C.S.; Nall, B.T. Isothermal titration calorimetry of protein-protein interactions. Methods 1999, 19, 213–221. [Google Scholar] [CrossRef] [PubMed]
- Schuck, P. Reliable determination of binding affinity and kinetics using surface plasmon resonance biosensors. Curr Opin Biotech 1997, 8, 498–502. [Google Scholar] [CrossRef] [PubMed]
- Schasfoort, R.B.M.; de Lau, W.; van der Kooi, A.; Clevers, H.; Engbers, G.H.M. Method for estimating the single molecular affinity. Anal Biochem 2012, 421, 794–796. [Google Scholar] [CrossRef]
- Wang, J.H.; Jiang, P.J.; Qiu, L.; Wang, C.L.; Xia, J. Resolving antibody-peptide complexes with different ligand stoichiometries reveals a marked affinity enhancement through multivalency. Talanta 2013, 115, 394–400. [Google Scholar] [CrossRef] [PubMed]
- Hornick, C.L.K., F. Antibody Affinity - III The Role of Multivalence. Immunochemistry 1972, 9, 325–340. [Google Scholar] [CrossRef] [PubMed]
- Rich, R.L.; Myszka, D.G. Grading the commercial optical biosensor literature-Class of 2008: 'The Mighty Binders'. Journal of Molecular Recognition 2010, 23, 1–64. [Google Scholar] [CrossRef] [PubMed]
- Rich, R.L.; Myszka, D.G. Survey of the 2009 commercial optical biosensor literature. Journal of Molecular Recognition 2011, 24, 892–914. [Google Scholar] [CrossRef] [PubMed]
- Epps, D.E.; Raub, T.J.; Caiolfa, V.; Chiari, A.; Zamai, M. Determination of the affinity of drugs toward serum albumin by measurement of the quenching of the intrinsic tryptophan fluorescence of the protein. J Pharm Pharmacol 1999, 51, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Eisen, H.N.; Siskind, G.W. Variations in Affinities of Antibodies during the Immune Response. Biochemistry-Us 1964, 3, 996–1008. [Google Scholar] [CrossRef]
- Bakar, K.A.; Feroz, S.R. A critical view on the analysis of fluorescence quenching data for determining ligand-protein binding affinity. Spectrochim Acta A 2019, 223. [Google Scholar] [CrossRef]
- Marrack, J.; Smith, F.C. Quantitative aspects of immunity reactions: The combination of anitbodies with simple haptenes. Brit J Exp Pathol 1932, 13, 394–402. [Google Scholar]
- Pinger, C.W.; Heller, A.A.; Spence, D.M. A Printed Equilibrium Dialysis Device with Integrated Membranes for Improved Binding Affinity Measurements. Anal Chem 2017, 89, 7302–7306. [Google Scholar] [CrossRef] [PubMed]
- Lippok, S.; Seidel, S.A.I.; Duhr, S.; Uhland, K.; Holthoff, H.P.; Jenne, D.; Braun, D. Direct Detection of Antibody Concentration and Affinity in Human Serum Using Microscale Thermophoresis. Anal Chem 2012, 84, 3523–3530. [Google Scholar] [CrossRef] [PubMed]
- Nakayama, T.; Arata, Y.; Shimada, I. A Multinuclear NMR-Study of the Affinity Maturation of Anti-Np Mouse Monoclonal-Antibodies - Comparison of Antibody Combining Sites of Primary Response Antibody N1g9 and Secondary Response Antibody-3b62. Biochemistry-Us 1993, 32, 13961–13968. [Google Scholar] [CrossRef]
- Jiskoot, W.; Hoogerhout, P.; Beuvery, E.C.; Herron, J.N.; Crommelin, D.J.A. Preparation and Application of a Fluorescein-Labeled Peptide for Determining the Affinity Constant of a Monoclonal-Antibody Hapten Complex by Fluorescence Polarization. Anal Biochem 1991, 196, 421–426. [Google Scholar] [CrossRef] [PubMed]
- Portmann, A.J.; Levison, S.A.; Dandliker, W.B. Anti-Fluorescein Antibody of High Affinity and Restricted Heterogeneity as Characterized by Fluorescence Polarization and Quenching Equilibrium Techniques. Biochem Bioph Res Co 1971, 43, 207. [Google Scholar] [CrossRef] [PubMed]
- Müller, R. Calculation of Average Antibody-Affinity in Anti-Hapten Sera from Data Obtained by Competitive Radioimmunoassay. J Immunol Methods 1980, 34, 345–352. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.W.; Li, Z.; Podariu, M.I.; Hage, D.S. Determination of Rate Constants and Equilibrium Constants for Solution-Phase Drug-Protein Interactions by Ultrafast Affinity Extraction. Anal Chem 2014, 86, 6454–6460. [Google Scholar] [CrossRef] [PubMed]
- Landry, J.P.; Ke, Y.H.; Yu, G.L.; Zhu, X.D. Measuring affinity constants of 1450 monoclonal antibodies to peptide targets with a microarray-based label-free assay platform. J Immunol Methods 2015, 417, 86–96. [Google Scholar] [CrossRef]
- Dong, C.; Liu, Z.; Wang, F. Radioligand saturation binding for quantitative analysis of ligand-receptor interactions. Biophys Rep 2015, 1, 148–155. [Google Scholar] [CrossRef]
- Friguet, B.; Chaffotte, A.F.; Djavadiohaniance, L.; Goldberg, M.E. Measurements of the True Affinity Constant in Solution of Antigen-Antibody Complexes by Enzyme-Linked Immunosorbent-Assay. J Immunol Methods 1985, 77, 305–319. [Google Scholar] [CrossRef] [PubMed]
- Bobrovnik, S.A. Determination of antibody affinity by ELISA. Theory. J Biochem Bioph Meth 2003, 57, 213–236. [Google Scholar] [CrossRef] [PubMed]
- Weller, M.G. Strukturelle und kinetische Untersuchungen zur Entwicklung und Optimierung von Hapten-Enzymimmunoassays (ELISAs) am Beispiel der Bestimmung von Triazinherbiziden. Dissertation, Technische Universität München, München, 1992.
- Winklmair, M.; Weller, M.G.; Mangler, J.; Schlosshauer, B.; Niessner, R. Development of a highly sensitive enzyme-immunoassay for the determination of triazine herbicides. Fresen J Anal Chem 1997, 358, 614–622. [Google Scholar] [CrossRef]
- Hopp, T.P.; Prickett, K.S.; Price, V.L.; Libby, R.T.; March, C.J.; Cerretti, D.P.; Urdal, D.L.; Conlon, P.J. A Short Polypeptide Marker Sequence Useful for Recombinant Protein Identification and Purification. Bio-Technol 1988, 6, 1204–1210. [Google Scholar] [CrossRef]
- Slootstra, J.W.; Kuperus, D.; Pluckthun, A.; Meloen, R.H. Identification of new tag sequences with differential and selective recognition properties for the anti-FLAG monoclonal antibodies M1, M2 and M5. Mol Divers 1997, 2, 156–164. [Google Scholar] [CrossRef] [PubMed]
- Einhauer, A.; Jungbauer, A. Affinity of the monoclonal antibody M1 directed against the FLAG peptide. J Chromatogr A 2001, 921, 25–30. [Google Scholar] [CrossRef] [PubMed]
- Srila, W.; Yamabhai, M. Identification of Amino Acid Residues Responsible for the Binding to Anti-FLAG™ M2 Antibody Using a Phage Display Combinatorial Peptide Library. Appl Biochem Biotech 2013, 171, 583–589. [Google Scholar] [CrossRef] [PubMed]
- Einhauer, A.; Jungbauer, A. The FLAG™ peptide, a versatile fusion tag for the purification of recombinant proteins. J Biochem Bioph Meth 2001, 49, 455–465. [Google Scholar] [CrossRef] [PubMed]
- Roosild, T.P.; Castronovo, S.; Choe, S. Structure of anti-FLAG M2 Fab domain and its use in the stabilization of engineered membrane proteins. Acta Crystallogr F 2006, 62, 835–839. [Google Scholar] [CrossRef]
- Knappik, A.; Plückthun, A. An Improved Affinity Tag Based on the Flag(R) Peptide for the Detection and Purification of Recombinant Antibody Fragments. Biotechniques 1994, 17, 754–761. [Google Scholar]
- Hanaoka, K.; Lubag, A.J.M.; Castillo-Muzquiz, A.; Kodadek, T.; Sherry, A.D. The detection limit of a Gd-based agent is substantially reduced when targeted to a protein microdomain. Magn Reson Imaging 2008, 26, 608–617. [Google Scholar] [CrossRef] [PubMed]
- Schwaar, T.; Lettow, M.; Remmler, D.; Borner, H.G.; Weller, M.G. Efficient Screening of Combinatorial Peptide Libraries by Spatially Ordered Beads Immobilized on Conventional Glass Slides. High Throughput 2019, 8. [Google Scholar] [CrossRef] [PubMed]
- Hesse, A.; Weller, M.G. Protein Quantification by Derivatization-Free High-Performance Liquid Chromatography of Aromatic Amino Acids. J Amino Acids 2016, 2016, 7374316. [Google Scholar] [CrossRef] [PubMed]
- Tchipilov, T.; Meyer, K.; Weller, M.G. Quantitative (1)H Nuclear Magnetic Resonance (qNMR) of Aromatic Amino Acids for Protein Quantification. Methods Protoc 2023, 6. [Google Scholar] [CrossRef] [PubMed]
- Jones, B.N.; Paabo, S.; Stein, S. Amino-Acid-Analysis and Enzymatic Sequence Determination of Peptides by an Improved Ortho-Phthaldialdehyde Pre-Column Labeling Procedure. J Liq Chromatogr 1981, 4, 565–586. [Google Scholar] [CrossRef]
- Fountoulakis, M.; Lahm, H.W. Hydrolysis and amino acid composition analysis of proteins. J Chromatogr A 1998, 826, 109–134. [Google Scholar] [CrossRef] [PubMed]
- Reinmuth-Selzle, K.; Tchipilov, T.; Backes, A.T.; Tscheuschner, G.; Tang, K.; Ziegler, K.; Lucas, K.; Pöschl, U.; Fröhlich-Nowoisky, J.; Weller, M.G. Determination of the protein content of complex samples by aromatic amino acid analysis, liquid chromatography-UV absorbance, and colorimetry. Anal Bioanal Chem 2022, 414, 4457–4470. [Google Scholar] [CrossRef]
- Weller, M.G.; Weil, L.; Niessner, R. Increased Sensitivity of an Enzyme-Immunoassay (Elisa) for the Determination of Triazine Herbicides by Variation of Tracer Incubation-Time. Mikrochim Acta 1992, 108, 29–40. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).