1. Introduction
The galectin family is made up of 15 proteins and contains the beta-galactoside-binding mammalian lectin galectin-1 that binds cell surface glycoproteins via its carbohydrate recognition domain (CRD) resulting in the modulation of cell-cell and cell matrix interactions [
1,
2]. Galectin-1 has been shown to be a key modulator of the immune system and in progression of cancer that has resulted in it being a focus for therapeutic targeting [
3,
4]. In the context of cancer, the interaction of galectin-1 with glycosylated tumour-associated receptors e.g., growth factor receptors and integrins, has been shown to control tumour cell fitness, migration, stemness and epithelial-to-mesenchymal transition in addition to angiogenic effects on endothelial cells and apoptosis of immunomodulatory T cells [
5]. In addition, increased expression and an association with survival has been demonstrated for galectin-1 across a range of cancer types [
6].
The targeting of galectin-1 from a drug discovery perspective, as is the case for the majority of the galectin family with the exception of galectin-3 [
7] and -9 [
5], has to date been limited. However, there has been some traction with the beginnings of the identification of selective small molecule inhibitors and therapeutic monoclonal antibodies [
8,
9,
10], though none have yet made it to clinical testing.
In previous studies we have used fluorescence polarisation (FP) for assessing small molecule glycomimetics binding galectin-1 and measuring affinity (
KD) and structure activity relationships (SAR) [
11]. As detailed in our recent study, where as part of a lead optimisation project to find small molecule inhibitors of galectin-3 the comparison of SPR assays with FP was investigated [
12], in this study we have endeavoured to explore the same comparisons between assay formats and across species for galectin-1. The aims were therefore similar where we compared our primary FP SAR assay for the project with an SPR counterpart to compare binding affinities and kinetics of novel glycomimetics between human and mouse protein as well as validate for high throughput measurement of
kon and
koff for this galectin.
2. Materials and Methods
2.1. Materials
The small molecule glycomimetics tested in this study had molecular weights ranging from 481 and 676 Da and were synthesized by the Medicinal Chemistry Department at Galecto Biotech AB (Gothenburg, Sweden). Compound screening arrays were made up of disaccharides and monosaccharides (see
Supplementary Tables S1 and S2), with exemplars highlighted for each series in
Figure 1 (disaccharide GB0139 [
13] in
Figure 1A and monosaccharide GB1211 [
14] in
Figure 1B) for comparison across 2 chemical series under SAR investigation. All compound stocks were made up in 100 % DMSO at 10 mM. The maximum concentration tested in the SPR assay (described below) was chosen based on compound solubility or critical aggregation concentration (CAC), where available.
2.2. Protein Expression and Purification
For SPR studies, human (residues M1-D135) and mouse (residues M1-E135) galectin-1 proteins were expressed as His-tagged proteins in E. coli strain BL21 and purified as described previously [
15]. SDS-PAGE and mass spectrometry were used to confirm protein purity with all proteins stored at -80°C until use. Human and mouse proteins for FP were generated as described previously [
14,
16].
2.3. Surface Plasmon Resonance (SPR) Binding Assays
SPR method previously described for galectin-3 proteins was used here for galectin-1 [
12]. Briefly, CM3 sensor chip surfaces (Cytiva, MA, USA) were pre-primed and equilibrated in immobilization buffer (10 mM HEPES, 150 mM NaCl (pH 7.4)) using an Amine Coupling Kit (Cytiva, MA, USA) prior to immobilization of protein (injected at 10 µg/mL in PBS at pH 6.0). Chip surfaces were activated with 1-ethyl-3-[3-dimethylaminopropyl]carbodiimide hydrochloride (EDC)/N-hydroxysuccinimide (NHS) at a flow rate of 5 µL/min for 420 s prior to protein injections (10 µL/min flow-rate) to generate 400-800 response units (RU). Chips surfaces were blocked with ethanolamine HCl (pH 8.5) for 420 s upon reaching the immobilization required then EDC/NHS and ethanolamine used to treat blank surfaces that were protein free. Compounds were diluted in running buffer (RB) (10 mM HEPES, 150 mM NaCl, 1 mM DTT, 0.05 % Tween 20, 5 % DMSO (pH 7.4)), pre-filtered and degassed then injected for 1 min at a flow rate of 30 μL/min. Minimal non-specific binding was observed between compounds and the blank reference surfaces. Following each cycle the chip surface was auto-regenerated aided by fast compound off-rates and a further wash set completed in RB. After 3 min of dissociation baseline returned to the starting level and during runs there was no evidence of surface accumulation or signal loss. H
2O/50% DMSO was used to wash the flow system following each cycle and there were no signs of cross-contamination in the carryover control. At the start and the end of each run solvent correction was completed and included in data analysis. There were no observed mass transport limitation effects. To characterize binding between compounds and galectin-1 protein (all carried out at 25°C) Multi Cycle Kinetics (MCK) and steady-state affinity analyses were completed. A Biacore™ T200 (Cytiva, MA, USA) was used for all SPR experiments.
2.4. Fluorescence Polarisation Binding Assays
Galectin FP binding assays were completed as previously described [
14,
16]. Briefly, FP was detected in black polystyrene 96-well microtiter plates (Costar, Corning, NY) using a PolarStar instrument (BMG, Offenburg, Germany) with 100 μL/well of galectin and probe (detailed in [
10]) at fixed concentration mixed with 100 μL/well of inhibitor solution. For controls, wells containing only fluorescein or fluorescent probe were included. All reagents were diluted in PBS with plates incubated at room temperature for 1 h prior to FP measurement.
2.5. Data Analysis
Biacore™ T200 Evaluation Software (Cytiva, MA, USA) was used to analyze SPR MCK and affinity data applying a simple 1:1 Langmuir interaction model to determine affinities (
KD) and kinetics (data globally fitted to determine association/dissociation rate constants;
kon/
koff) of the compound-protein. Kinetic derived SPR
KD values were determined by dividing
koff by
kon values. To account for background buffer and bulk-shift effects, signals from the reference flow cell were subtracted from each data set. In FP assays,
KD values for inhibitor-galectin interactions were determined by solving the two equations of mass action governing inhibitor-galectin interaction and probe-galectin interaction directly from single data points as previously described [
11]. Affinity fold differences were calculated by dividing the largest
KD by the smallest between assay and species comparisons.
3. Results
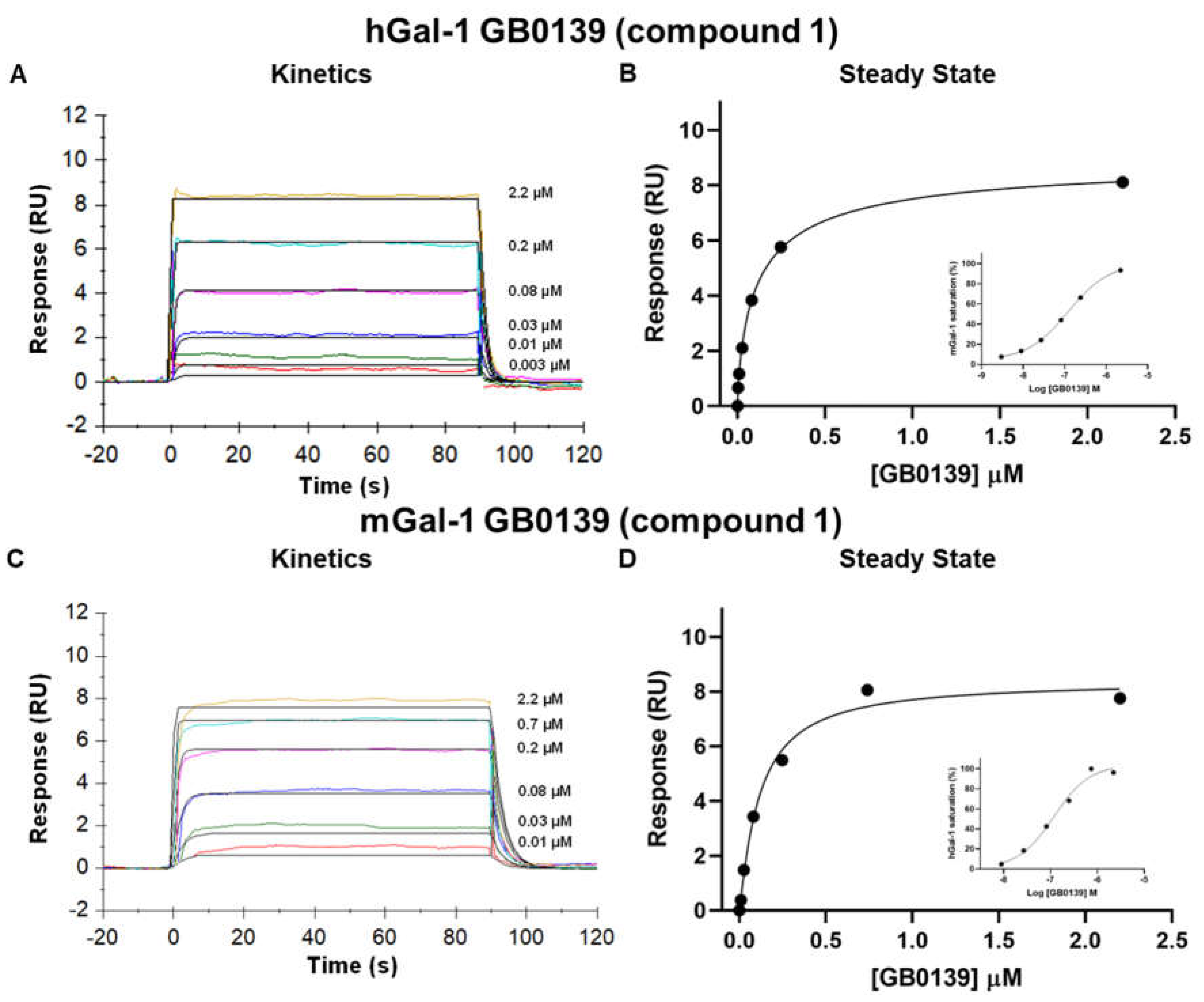
A set of 15 small molecule glycomimetics were screened in a human and mouse galectin-1 protein SPR assay (see Supplementary Results). These consisted of mono- and di-saccharides with 965-fold range determined in FP binding assays. Overall, a higher affinity was observed for disaccharides binding to human and mouse galectin-1, observed in both the SPR and FP assays. Binding to and from the galectin-1 proteins in SPR was demonstrated to be a single phase for all compounds (sensorgram data for all compounds tested in all SPR assays can be found in Supplementary Results), with examples shown for the inhaled clinical candidate GB0139 (compound 1, disaccharide) [
13] (
Figure 2 and
Figure 3A) and the oral clinical candidate GB1211 (compound 3, monosaccharide) [
14] (
Figure 3A). For human and mouse galectin-1, the SPR kinetic
KD values for GB0139 were determined to be 103 nM and 131 nM respectively (
Figure 2 and
Figure 3). The kinetic and steady state SPR K
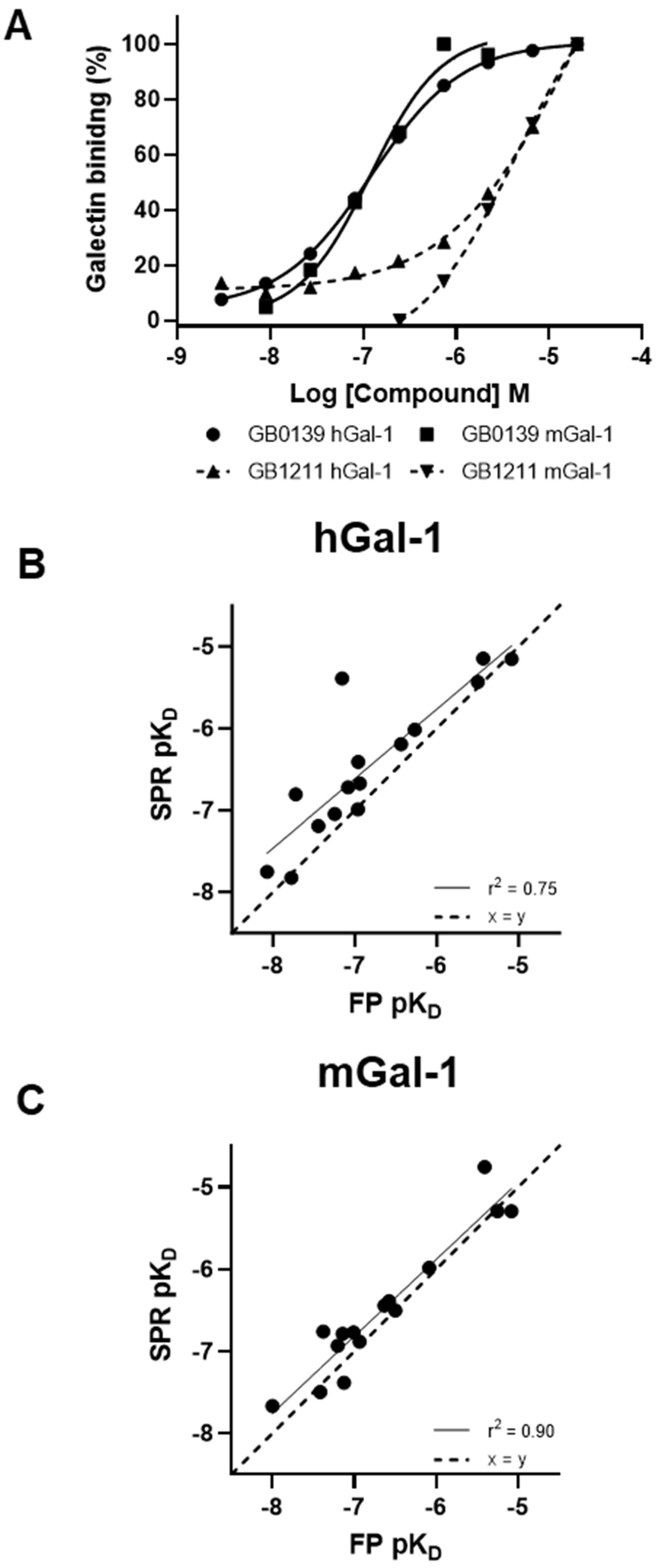
D values for both human (r
2 = 0.88) and mouse (r
2 = 0.98) assays were closely correlated (
Figure 4A,B). Between FP and SPR assays the rank order of compounds were well correlated between formats for both human and mouse galectin-1 with r
2 values of 0.75 and 0.90 respectively (
Figure 3B,C). However, in the human galectin-1 a mean fold shift of
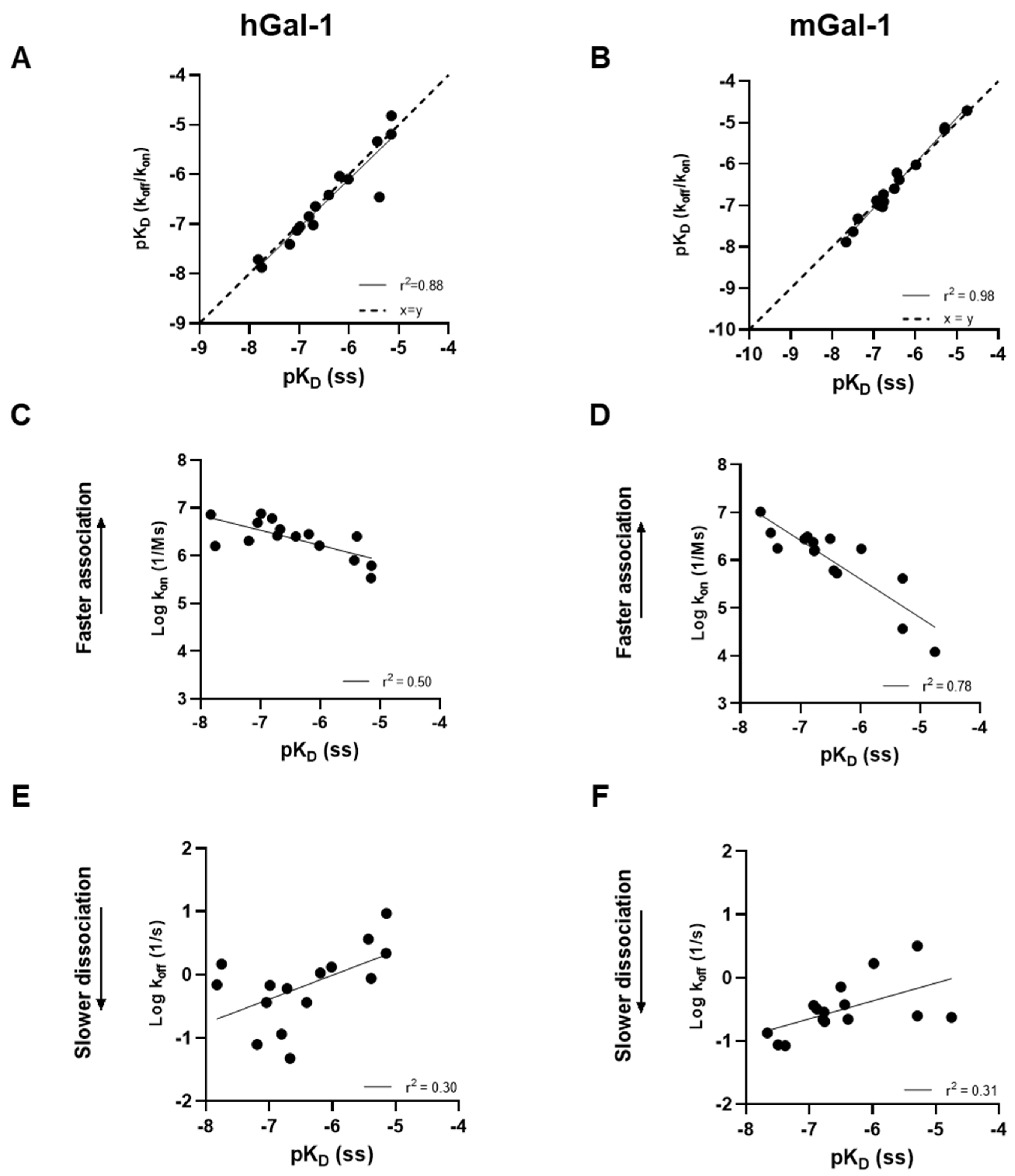
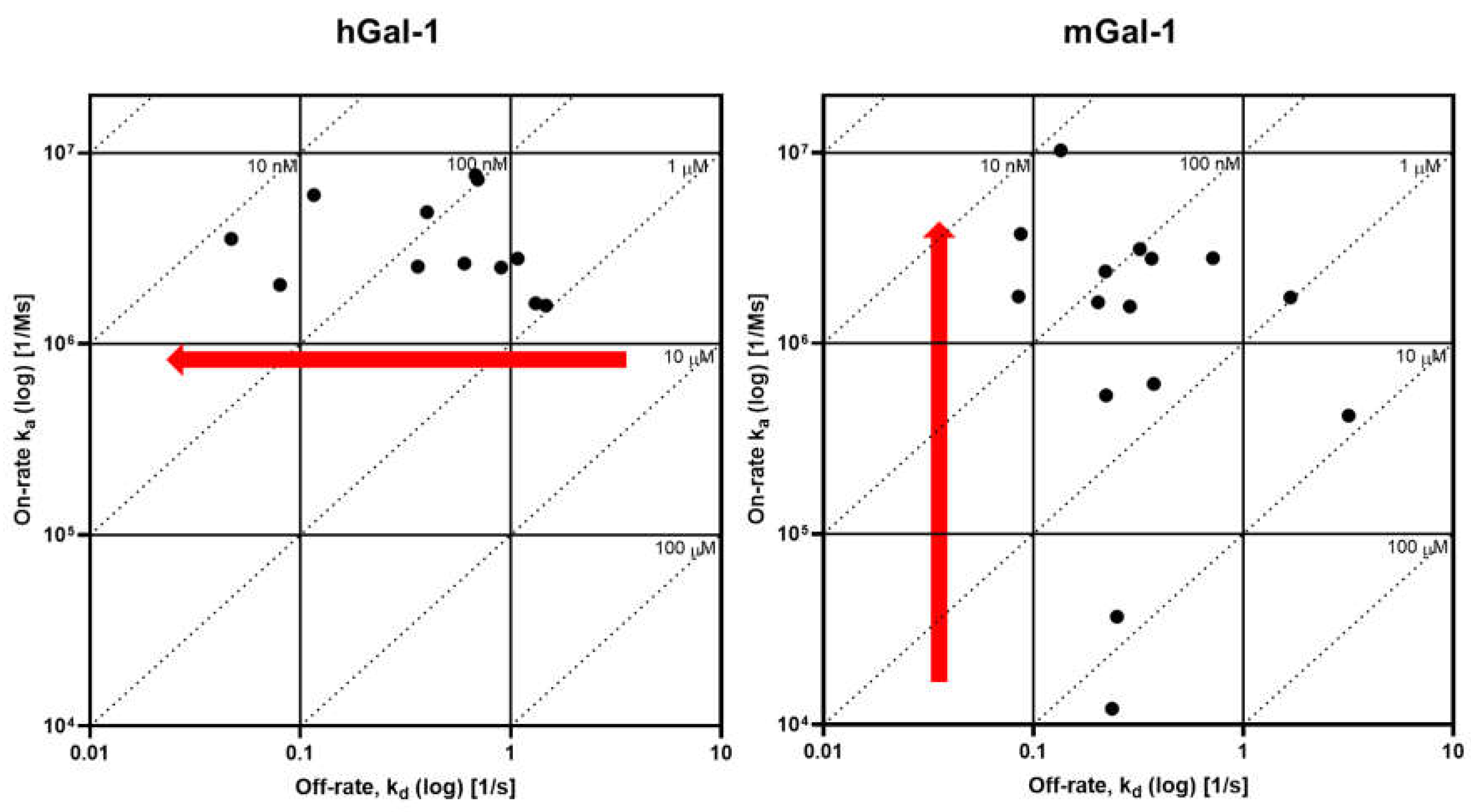
KD of 5.9 to a lower sensitivity in the SPR assay was observed compared to the FP assay, whilst this was a more comparable set of data for the mouse galectin-1 between the two assay formats (mean fold shift of 1.7 from SPR to FP). A decrease in (or slower) dissociation rate was correlated with the increase in affinity for binding of small molecule glycomimetics to human galectin-1 (
Figure 4E). Conversely, an increase in (or faster) association rate but no effect on dissociation rate was correlated with an increase in affinity for binding of small molecule glycomimetics to mouse galectin-1 (
Figure 4D,F). The increase observed in association rate for binding to the human galectin-1 (1 log units) was markedly less than that observed for mouse galectin-1 (3 log unit) (
Figure 4C,D). When
kon and
koff values were correlated for each galectin-1 species (
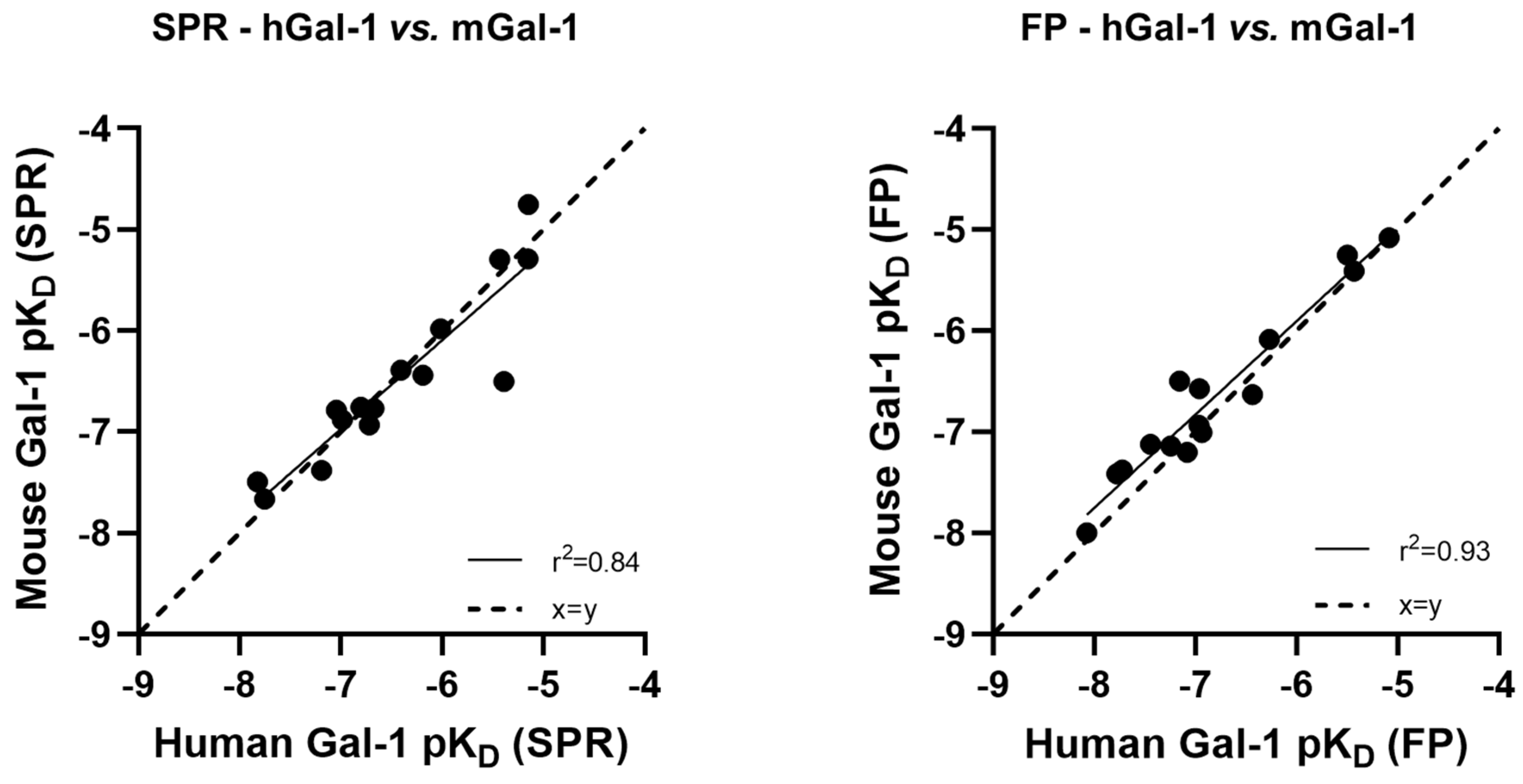
Figure 5A,B) these observations were further highlighted. When comparing glycomimetic affinities in both the SPR and FP assays from human to mouse galectin-1, a good correlation was observed (
Figure 6A,B). When comparing between species, within each assay format, the correlations were also very comparable (SPR assay format r
2 = 0.84; FP assay format r
2 = 0.93).
4. Discussion
The pleotropic role of galectin-1 in a range of cancers has been demonstrated with the lectin inducing detrimental effects across several key mechanisms and cell types [
5] [
6]. As a result it is starting to become a focus for drug targeting in cancer. Even though the weight of scientific evidence is building, no galectin-1 inhibitor has been fully developed for testing in humans. However, recently small and large molecule galectin-1 inhibitors have been identified and have begun to be tested in pre-clinical studies [8-10].
Affinity measurements of galectin-1 small molecule glycomimetics in our studies have been historically completed in FP assays. The application of the SPR method to galectin-1 has been limited and for investigating protein:protein interactions [
17]. The measurement of small molecule interactions has only been limited to galectin-3 and our historical study [
12]. Therefore, in this study we applied the same approach to galectin-1 whereby as a substitute we utilised SPR binding assays to provide a confirmation to FP as well as obtain a high throughput put measure of binding kinetics for a set of galectin-1 inhibitors and allow comparisons between species (human vs. mouse).
As would be expected, when a compound array of 15 compounds made up of mono- and di-saccharides exemplars were tested in SPR assays to determine steady state and kinetic derived KD values, an excellent correlation was observed for both human and mouse galectin-1 systems. Subsequently, when comparing affinity values between SPR and FP steady state KD estimates were used and for both species the SPR values showed a close agreement with those determined in FP.
Although there was no difference in the affinities of compounds for CRD site on the human and the mouse form of galectin-1, a difference in the kinetics between the species was observed. For human galectin-1 a decrease in dissociation rate for small molecule glycomimetics were correlated with an increase in affinity. In contrast, the increase in affinity for binding to mouse galectin-1 was not as a result of a change in dissociation rate but rather an elevation in association rate. In addition, the increase was much more marked for the association rate with the mouse galectin-1 compared with the dissociation rate change at the human form. This is an interesting and novel observation, albeit in a small compound set, and it is not immediately clear what drives this in the set of glycomimetics investigated in this study. Although out of scope of this study, it would be interesting to further investigate this phenomenon with a larger set of glycomimetics small and large molecules in the SPR assay to understand the SAR associated, as well bring in other techniques e.g., X-ray crystallography, computational docking; to elucidate what properties drive this observation.
In summary, for early drug discovery initiatives focussed on the identification of small molecule CRD binders of galectin-1, SPR as a screening approach has been validated and shown to be a solid replacement for, or a confirmation of affinity measures in, FP assays. The added benefit in the use of SPR is that high throughput and kon and koff values can be generated for much earlier kinetic characterisation of galectin-1 small molecule glycomimetics and inhibitors.
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on Preprints.org.
Funding
All studies were funded by Galecto Biotech AB.
Acknowledgments
The authors would like to thank the Department of Structural Biology and the Mass Spectrometry Platform at NovAliX for the production and QC of proteins used in the SPR assays in this study.
Conflicts of Interest
The authors have been, or are currently, employees of Galecto Biotech AB or NovAliX.
References
- S.H. Barondes, V. Castronovo, D.N.W. Cooper, R.D. Cummings, K. Drickamer, T. Felzi, M.A. Gitt, J. Hirabayashi, C. Hughes, K. Kasai, H. Leffler, F.-T. Liu, R. Lotan, A.M. Mercurio, M. Monsigny, S. Pillai, F. Poirer, A. Raz, P.W.J. Rigby, J.M. Rini, J.L. Wang, Galectins: A family of animal β-galactoside-binding lectins, Cell 1994, 76, 597–598. [CrossRef]
- M.F. Troncoso, M.T. Elola, A.G. Blidner, L. Sarrias, M.V. Espelt, G.A. Rabinovich, The universe of galectin-binding partners and their functions in health and disease. J. Biol. Chem. 2023, 299, 105400. [CrossRef]
- R.-Y. Yang, G.A. Rabinovich, F.-T. Liu, Galectins: structure, function and therapeutic potential, Expert Rev. Mol. Med. 2008, 10, e17. [CrossRef]
- F.-T. Liu, G.A. Rabinovich, Galectins as modulators of tumour progression. Nature Reviews Cancer 2005, 5, nrc1527. [CrossRef]
- K.V. Mariño, A.J. Cagnoni, D.O. Croci, G.A. Rabinovich, Targeting galectin-driven regulatory circuits in cancer and fibrosis. Nat Rev Drug Discov. [CrossRef]
- C.-H. Li, Y.-C. Chang, M.-H. Chan, Y.-F. Yang, S.-M. Liang, M. Hsiao, Galectins in Cancer and the Microenvironment: Functional Roles, Therapeutic Developments, and Perspectives. Biomed 2021, 9, 1159. [CrossRef]
- S. Bouffette, I. Botez, F.D. Ceuninck, Targeting galectin-3 in inflammatory and fibrotic diseases. Trends Pharmacol. Sci. [CrossRef]
- N. Bannoud, J.C. Stupirski, A.J. Cagnoni, P.F. Hockl, J.M.P. Sáez, P.A. García, Y.D. Mahmoud, J.G. Tudela, M.A. Scheidegger, A. Marshall, P.G. Corrie, M.R. Middleton, K.V. Mariño, M.R. Girotti, D.O. Croci, G.A. Rabinovich, Circulating galectin-1 delineates response to bevacizumab in melanoma patients and reprograms endothelial cell biology. Proc National Acad Sci 2023, 120, e2214350120. [CrossRef]
- F.R. Zetterberg, C. Diehl, M. Håkansson, B. Kahl-Knutson, H. Leffler, U.J. Nilsson, K. Peterson, J.A. Roper, R.J. Slack, Discovery of Selective and Orally Available Galectin 1 Inhibitors. J. Med. Chem. [CrossRef]
- Zetterberg, Discovery of the selective and orally available galectin-1 inhibitor GB1908 as a potential treatment for lung cancer, J Med Chem (under Review) 2024.
- P. Sörme, B. Kahl-Knutsson, M. Huflejt, U.J. Nilsson, H. Leffler, Fluorescence polarization as an analytical tool to evaluate galectin-ligand interactions. Analytical Biochemistry 2004, 334, 36–47. [CrossRef]
- H. Kim, N. Weidner, C. Ronin, E. Klein, J.A. Roper, B. Kahl-Knutson, K. Peterson, H. Leffler, U.J. Nilsson, A. Pedersen, F.R. Zetterberg, R.J. Slack, Evaluating the affinity and kinetics of small molecule glycomimetics for human and mouse galectin-3 using surface plasmon resonance, Slas Discov 2023. [CrossRef]
- N. Hirani, A.C. MacKinnon, L. Nicol, P. Ford, H. Schambye, A. Pedersen, U.J. Nilsson, H. Leffler, T. Sethi, S. Tantawi, L. Gravelle, R.J. Slack, R. Mills, U. Karmakar, D. Humphries, F. Zetterberg, L. Keeling, L. Paul, P.L. Molyneaux, F. Li, W. Funston, I.A. Forrest, A.J. Simpson, M.A. Gibbons, T.M. Maher, Target inhibition of galectin-3 by inhaled TD139 in patients with idiopathic pulmonary fibrosis. Eur Respir J 2021, 57, 2002559. [CrossRef]
- F.R. Zetterberg, A. MacKinnon, T. Brimert, L. Gravelle, R.E. Johnsson, B. Kahl-Knutson, H. Leffler, U.J. Nilsson, A. Pedersen, K. Peterson, J.A. Roper, H. Schambye, R.J. Slack, S. Tantawi, Discovery and Optimization of the First Highly Effective and Orally Available Galectin 3 Inhibitors for Treatment of Fibrotic Disease. J Med Chem, 2022. [CrossRef]
- C.Atmanene, C. Ronin, S. Téletchéa, F.-M. Gautier, F. Djedaïni-Pilard, F. Ciesielski, V. Vivat, C. Grandjean, Biophysical and structural characterization of mono/di-arylated lactosamine derivatives interaction with human galectin-3, Biochemical and Biophysical Research Communications. 2017, 489, 281–286. [CrossRef]
- E. Salomonsson, M.C. Carlsson, V. Osla, R. Hendus-Altenburger, B. Kahl-Knutson, C.T. Öberg, A. Sundin, R. Nilsson, E. Nordberg-Karlsson, U.J. Nilsson, A. Karlsson, J.M. Rini, H. Leffler, Mutational Tuning of Galectin-3 Specificity and Biological Function*. J Biological Chem 2010, 285, 35079–35091. [CrossRef]
- V. Heine, C. Dey, P. Bojarová, V. Křen, L. Elling, Methods of in vitro study of galectin-glycomaterial interaction. Biotechnol Adv 2022, 58, 107928. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).