1. Introduction
Selenium is an essential trace element for pigs. It may have the ability to improve immunity, increase antioxidant activity, and prevent diseases such as muscular dystrophy, exudative diathesis, necrotic degeneration of the liver and mulberry heart pathology (Zarczynska et al., 2013; Cao et al., 2014; Rao et al., 2023). More attention is given in critical periods such as post-weaning piglets, since piglets have a higher selenium requirement in this phase than in the following phases, as suggested by the NRC (2012). In this sense, meeting the selenium requirement is necessary to avoid pathologies and maintain redox balance.
Selenium has one of the narrowest ranges between dietary deficiency (<40 μg/day) and toxic levels (>400 μg/day) of all the elements (WHO, 1996), especially when supplemented through inorganic sources such as sodium selenite. The selenium requirement recommended by the NRC (2012) for piglets is 0.300 mg/kg, and Rostagno et al. (2011) recommend 0.250 mg/kg for all phases only using inorganic sources. However, Rostagno et al. (2017) recommend 0.517 mg/kg of inorganic source and 0.233 mg/kg of organic source for post-weaning piglets. Thus, studies with different selenium concentrations and supply sources are necessary.
Yeast enriched with selenium is the most important organic form to be used in animal feed. Selenomethionine and selenocysteine are recognized as the main forms of selenium present in yeast (EFSA, 2018; Zhang et al., 2020). The selenium supplementation form is crucial for animal nutrition, as organic sources and selenium concentration variations affect muscle deposition (Zoidis et al., 2014; Zhang et al., 2020), and are more bioavailable than inorganic sources (Lyons et al., 2007; Rao et al., 2023).
Therefore, this study was conducted with the objective of investigating the enrichment of diets for weaned piglets using organic (proteinate selenium) and inorganic (sodium selenite) selenium and two addition levels (0.15 and 0.30 mg/kg) on productive performance, diarrhea incidence, digestibility, selenium excretion and retention, selenium concentrations in blood and muscles, serum parameters, antioxidant status and gene expression of selenoproteins in the intestine and liver.
2. Material and methods
The experimental test was carried out at the Pig Farming Laboratory of the Department of Animal Science at the Center for Human, Social and Agrarian Sciences at the Federal University of Paraíba (Bananeiras, Brazil). The experimental procedures were approved by CEUA/UFPB, under experimental protocol number 4328201218.
2.1. Animals and experimental diets
A total of 40 piglets (20 castrated males and 20 females) weaned at 28 days of age, with an average initial weight of 6.60±1.06 kg, and all from the same commercial strain (Topigs®) were used. The pigs were housed in suspended nursery cages with a hollow plastic floor, equipped with pacifier-type drinkers and semi-automatic feeders.
The pigs were distributed in a randomized block design with five treatments and four replications, with the experimental unit consisting of two pigs, one male and one female. The treatments were distributed as follows: Control diet (Control diet); Selenium-enriched control diet proteinate with 0.15 mg/kg of selenium added (SeOrg-0.15); Selenium-enriched control diet proteinate with 0.30 mg/kg of selenium added (SeOrg-0.30); Control diet enriched with sodium selenite with 0.15 mg/kg of selenium added (SeInorg-0.15); and Control diet enriched with sodium selenite with 0.30 mg/kg of selenium added (SeInorg-0.30). The presence of selenium in the control diet came from the ingredients and the mineral supplement (
Table 1).
The experimental diets, with the exception of the selenium concentration, were formulated according to the recommendations of Rostagno et al. (2017) in the following phases: I – from 28 to 35 days of age; II - from 36 to 45 days of age; III – from 46 to 60 days of age (
Table 2).
2.2. Productive performance and fecal score
The pigs and leftover feed were weighed at the beginning and end of each experimental phase, obtaining the average daily feed intake (ADFI), average daily weight gain (ADWG) and Feed Conversion (FC) of the weaned piglets. The zootechnical performance results were analyzed from 28 to 45 days of age and from 28 to 60 days of age. The fecal score was evaluated from 28 to 45 days as an indication of diarrhea incidence. Therefore, the fecal score was recorded daily in the morning and in the late afternoon. Stool consistency was evaluated according to visual criteria, in which: 1, normal; 2, pasty; and 3, liquid; a score of 3 was considered as diarrheal stools following the method proposed by Pascoal et al. (2012).
2.3. Digestibility of the diets
The apparent digestibility of nutrients and energy in the diets was evaluated during the third experimental phase. To do so, 1% of Celite 545
® was added to the diets as an internal marker and an acid-insoluble ash (AIA) source replacing the inert source. The test lasted 7 days, with the first three days for adaptation and the last four days for feces collection. At the end, the feces of each pig were homogenized and dried in an oven at 55°C for 72 hours. Feed and feces samples were ground and passed through a 0.5 mm sieve, then analyzed according to the procedures described by AOAC (2007) for determining dry matter (DM), mineral matter (MM), organic matter (OM) and crude protein. The gross energy was determined using an adiabatic bomb calorimeter (6100 model, Parr Instruments Co., San Francisco, CA, USA). Selenium concentration was analyzed by hydride generation atomic absorption spectrometry (iCE 3500 model, HGAAS ICE 3000 Series Thermo Scientific device, Cambridge, UK) coupled to a UP 100 hydride regenerator. The Solar software program, an air-acetylene flame and a selenium hollow cathode lamp (Photoron-Lamps-Pty-Ltd, Victoria, Australia) were used. The wavelength used for the determination was 196 nm and the analytical curve was from 5 to 40 mg/L. The total tract apparent digestibility coefficients (TTADC) of nutrients and energy were calculated according to Aldeola et al. (2001), in which:
The retained selenium and excreted selenium were calculated as follows:
2.4. Sampling and processing
Blood samples were taken during the experimen, from the jugular vein of a piglet from each experimental unit at 41 and 60 days according to the average weight of the experimental treatment. The serum was separated by centrifugation at 3000 rpm for 10 minutes and transferred to microtubes (Eppendorf®). The piglets were slaughtered at the end of the experimental period after a 12-hour fast by electrical stunning followed by exsanguination in accordance with the procedures approved by CEUA/UFPB. Segments of the first duodenum portion and the middle jejunum portion were collected after slaughter and immediately immersed in metacarn solution for histological and immunohistochemical evaluations. The samples were dehydrated in increasing alcohol solutions, cleared in xylol and embedded in paraffin according to the protocol described by Yoon et al. (2012). Muscle segments (Longissimus dorsi) and blood were collected for selenium analysis following the methodology used for selenium determination in diet and feces. Jejunum and muscle (Longissimus dorsi) segments were collected to analyze Malondialdehyde concentration and jejunum and liver segments to analyze gene expression. Samples destined for malondialdehyde and gene expression analysis were stored in an ultra-freezer at -80ºC for later analysis.
2.5. Intestinal morphometry and integrity
Histological slides were stained with hematoxylin and eosin to assess intestinal morphometry. Villus height (VH), villus width (VW) and crypt depth (CD) were measured, then the villus height/crypt depth ratio (VW/CD). Cell death by apoptosis (Caspase) and nuclear protein proliferation nuclear antigen (PCNA-rate of mitosis) were determined using the protocol used for all antibodies based on the immunohistochemistry technique (Terzian et al., 2007). The apoptosis rate in villous cells of the middle jejunum portion was assessed by anti-neutrophil cytoplasmic antibody positivity. Thus, positivity scores were assigned to the 20 photomicrographs of each treatment according to the following scale: 0 (absence of positivity), 1 (low positivity), 2 (mild positivity) and 3 (intense positivity), with methodology being adapted from Ishak et al. (1995). The crypts were randomly analyzed and measured to assess the cell mitosis rate in the first duodenum portion and the middle jejunum portion, making a total of 10,000 μm of epithelium per treatment. Epithelial cells were quantified according to the number of anti-PCNA+ nuclei. The readings and digitization of the histological slides were performed using an Olympus BX53 light microscope with a 40x objective and a Zeiss Axio camera coupled to a computer equipped with the CellSens Dimension software program for image acquisition.
2.6. Biochemical determinations
The serum concentration determinations of Immunoglobulin A (IgA), Immunoglobulin G (IgG), Gamma Glutamyl Transferase (GGT), Aspartate Aminotransferase (AST) and Creatinine (CRC) were measured in a spectrophotometer using commercial Labtest® kits (Labtest Diagnóstica, Lagoa Santa, Minas Gerais, Brazil), namely: IgA (Turbiquest, ref. 358), IgG (Turbiquest, ref. 359), GGT (Liquiform, ref. 105), AST (Liquiform, ref. 109) and creatinine (CRC). Substances reactive to thiobarbituric acid were determined according to the methodology described by Rosmini et al. (1996). First, 1.0 mL of 0.5% sulfanilamide solution, 10 mL of 10% (v/v) trichloroacetic acid (TCA) solution and 5.0 mL of distilled water were added in a tube containing 5 g of sample. The mixture was then shaken for five minutes to extract malonaldehyde (MDA) and centrifuged for 5 minutes at 3500 rpm. The supernatant was filtered (Whatman qualitative filter paper, Grade 1) and 5 mL of 0.02 M thiobarbituric acid (TBA) was added, and the mixture was heated in a water bath at 100ºC for 35 min. The tubes were subsequently cooled to room temperature (25°C) in an ice bath. Finally, the absorbance reading at 532 nm was performed in a spectrophotometer (UV-V AKSO N6000Plus, USA).
2.7. Gene expression
Middle fraction of the jejunum and liver samples were collected for gene expression analysis by real-time PCR. RNA was extracted from the samples using the Qiagen RNeasy
® Mini kit (Cat. N.74106) and cDNA synthesis was performed using the cDNA High Capacity cDNA Reverse Transcription kit (Applied Biosystems), according to the manufacturers’ recommendations. Relative gene expression was determined by real-time polymerase chain reaction (qPCR) using the SYBR Power SYBR
® green Master Mix (Thermo Fisher Scientific, Applied Biosystems) and specific primers (
Table 3) for glutathione peroxidase 1 (GPX1), glutathione peroxidase 2 (GPX2), glutathione peroxidase 4 (GPX4), selenoprotein P (SePP) and the reference beta-actin (ACTB) and glyceraldehyde 3-phosphate dehydrogenase (GAPDH) genes. The qPCR cycles were performed in a thermocycler and the relative expression calculated based on the 2 ΔΔCt method (Livak and Schmittgen, 2001) using the reference genes for expression normalization.
2.8. Statistical analysis
An analysis of variance (two-way ANOVA) was performed to determine the effects of selenium source (SeOrg or SeInorg) and addition level (0.15 or 0.30 mg/kg) and the interaction between both factors (Source × Level). The significance of the differences between the mean values of the parameters analyzed in the groups was estimated using the Student’s t-test. A contrast was performed between the control diet and the other treatments, being estimated by the Dunnett’s test. Data were processed using the SAS® statistical software program (OnDemand for Academics). Effects were considered significant at P ≤ 0.05.
3. Results
Selenium supplementation influenced feed intake and feed conversion (
Table 4). There was an interaction effect (P=0.018) between selenium source and concentration level for the DFI variable in both periods; in this case, the DFI of the pigs was lower when fed the diet containing 0.30 mg/kg of additional organic selenium. The addition of 0.15 mg/kg of selenium in the diets promoted higher DFI (P=0.006) and DWG (P=0.017) between 28 and 60 days (P<0.05), regardless of the source. Supplementation with organic selenium caused worsening (P=0.052) in FC in the second period from 28 to 60 days of age when compared to inorganic selenium. Fecal score was not influenced by selenium source (P=0.692), addition level (P=0.909) or interaction between factors (P=0.472).
The selenium source influenced the apparent digestibility coefficients of DM (P=0.024), OM (P=0.024) and MM (P=0.021) of the experimental diets, in which organic selenium promoted a higher digestibility coefficient when compared to supplementation through inorganic selenium. When the control diet was compared, the diets with the addition of inorganic selenium and levels of 0.15 and 0.30 mg/kg had lower (P<0.05) DM digestibility. The diet enriched with inorganic selenium at a level of 0.15 mg/kg had lower OM, protein and energy digestibility (P<0.05) compared to the control diet (
Table 5).
The addition of selenium in the diets influenced the retained selenium values, causing an interaction effect (P=0.040), in which the diets supplemented with an organic or inorganic source had greater selenium retention when 0.30 mg/kg was added. When 0.15 mg/kg of selenium was added, the inorganic source of selenium promoted greater retention compared to organic selenium. When performing contrasting the diets, the control diet promoted less selenium retention (P<0.05) compared to the others. Diets with 0.30 mg/kg of selenium promoted greater excretion (P = 0.031) compared with diets added with 0.15 mg/kg. When contrasted with the control diet, diets with organic selenium added at a level of 0.30 mg/kg, and diets with inorganic selenium added with 0.15 and 0.30 mg/kg of selenium had higher excreted values. Muscle selenium concentration was higher in diets supplemented with an inorganic source of selenium (P=0.002), and a concentration level of 0.30 mg/kg (P=0.015). On the other hand, the control diet caused a lower selenium concentration in the muscle when compared with the diet with inorganic selenium added and a level of 0.30 mg/kg (
Table 6).
The addition of 0.30 mg/kg of selenium to diets resulted in lower VH (P=0.001), VW (P=0.001), CD (P<0.0001) and cell mitosis rate (P=0.031) in the duodenum. When contrasted with the control diet, the diets with organic and inorganic sources and a level of 0.30 mg/kg of selenium added caused a lower VW (P<0.05), whereas the diet with organic selenium and 0.30 mg/kg of selenium caused a lower cell mitosis rate (P<0.05) with a lower number of positively stained cells in response to anti-PCNA in the jejunum (
Table 7).
There was an interaction effect (P=0.027) for the jejunum VW, in which only the diets with organic selenium addition with a level of 0.30 mg/kg of selenium reduced VW compared to 0.15 mg/kg. When contrasted with the control diet, the diets with 0.30 mg/kg of selenium addition from both sources promoted lower VW. CD was higher in diets that added an organic selenium source over an inorganic source (P=0.018), and in diets that added 0.30 mg/kg compared to 0.15 mg/kg (P=0.024) (
Table 8).
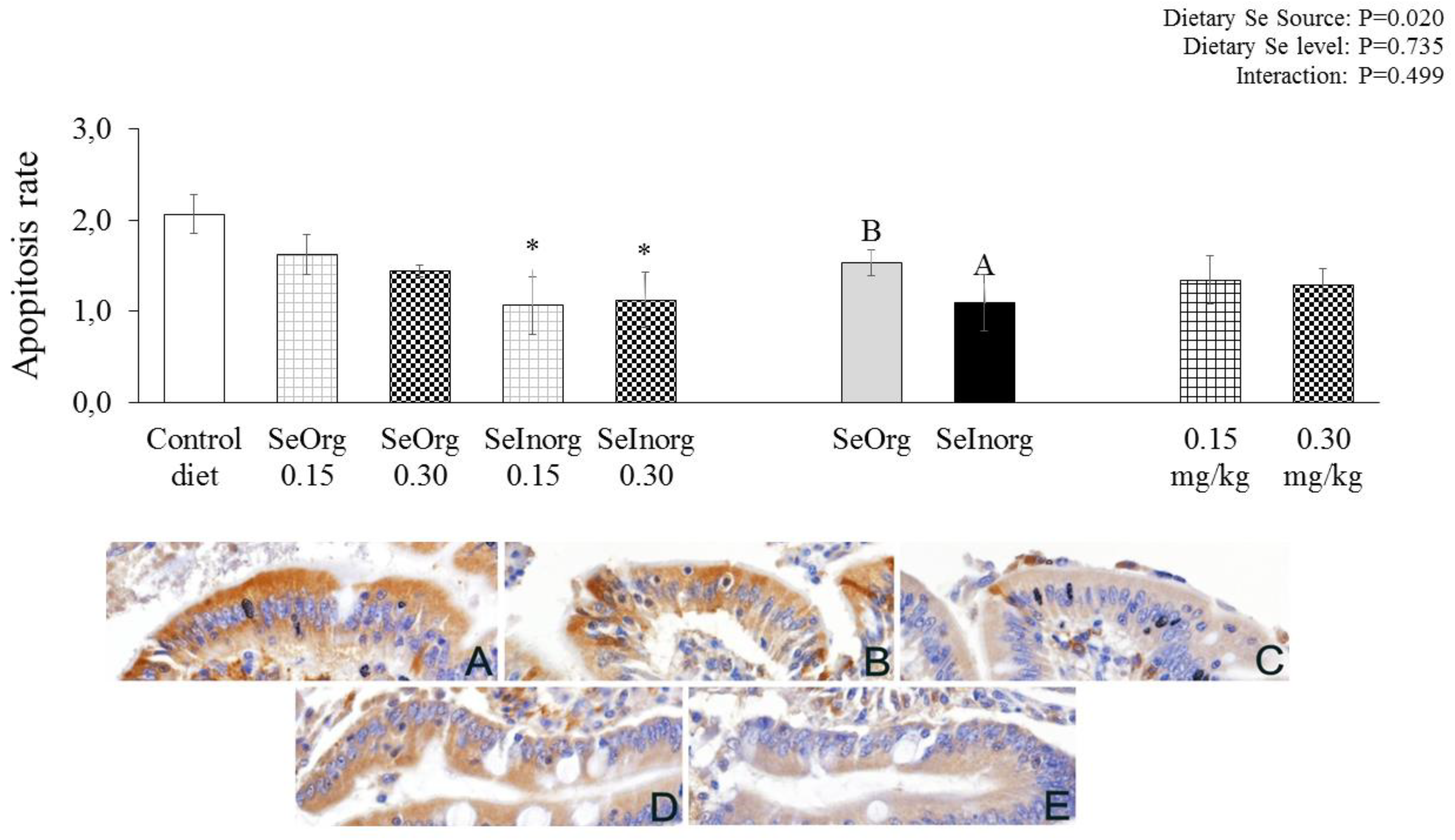
There was an effect (P=0.020) for cellular apoptosis (
Figure 1). Less cytoplasmic positivity to anti-Caspase-3 was observed in the jejunal epithelium of pigs fed a diet with organic selenium in detriment to inorganic selenium. When contrasted with the control diet, the diets with the inorganic selenium addition promoted a lower presence of cell apoptosis (P<0.05).
Among the serum biochemical parameters evaluated at 41 days of age, the GGT concentration was lower (P=0.051) when 0.30 mg/kg was supplemented in the pigs’ diets, regardless of the source. The other parameters were not influenced (P>0.05) by the addition of selenium sources and levels, or even by concentration levels (
Table 9). At the end of the third phase of the experiment at 60 days of age, the addition of 0.30 mg/kg of selenium (regardless of the source used) promoted higher serum CRC (P<0.0001) and IgA (P=0.015) concentrations. The diet with the 0.15 mg/kg of organic selenium addition caused a lower CRC concentration (P<0.05) when compared to the control diet and the diet with the 0.15 mg/kg inorganic selenium addition showed a lower IgA concentration (P<0.05) also when compared with the control diet (
Table 10). The malondialdehyde levels present in the muscle and jejunum of the pigs were not influenced (P>0.05) by the addition of selenium sources or levels.
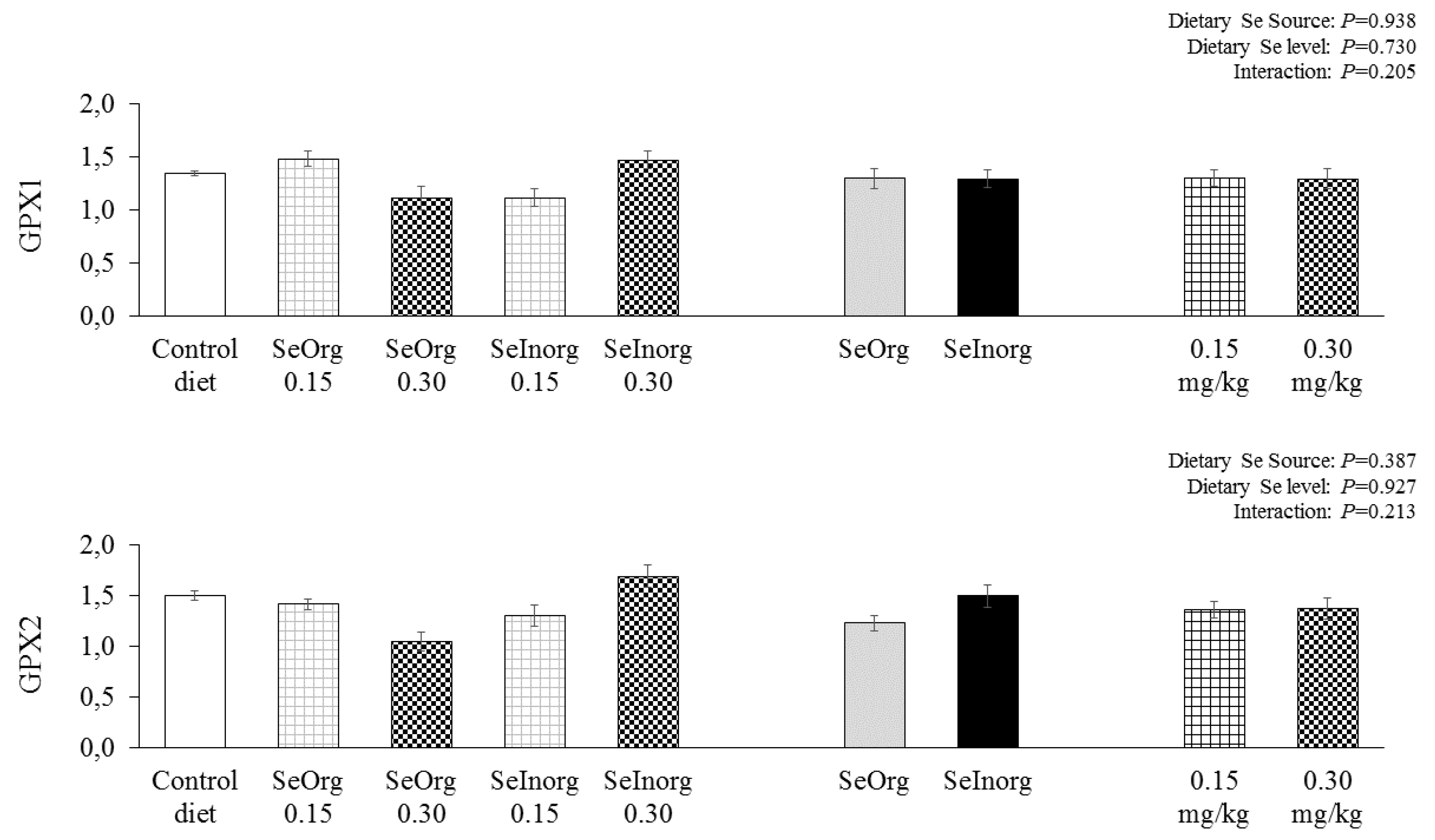
Gene expression in the jejunal epithelium of GPX1 and GPX2 were not influenced (P>0.05) by 0.15 and 0.30 mg/kg organic or inorganic selenium added to diets (
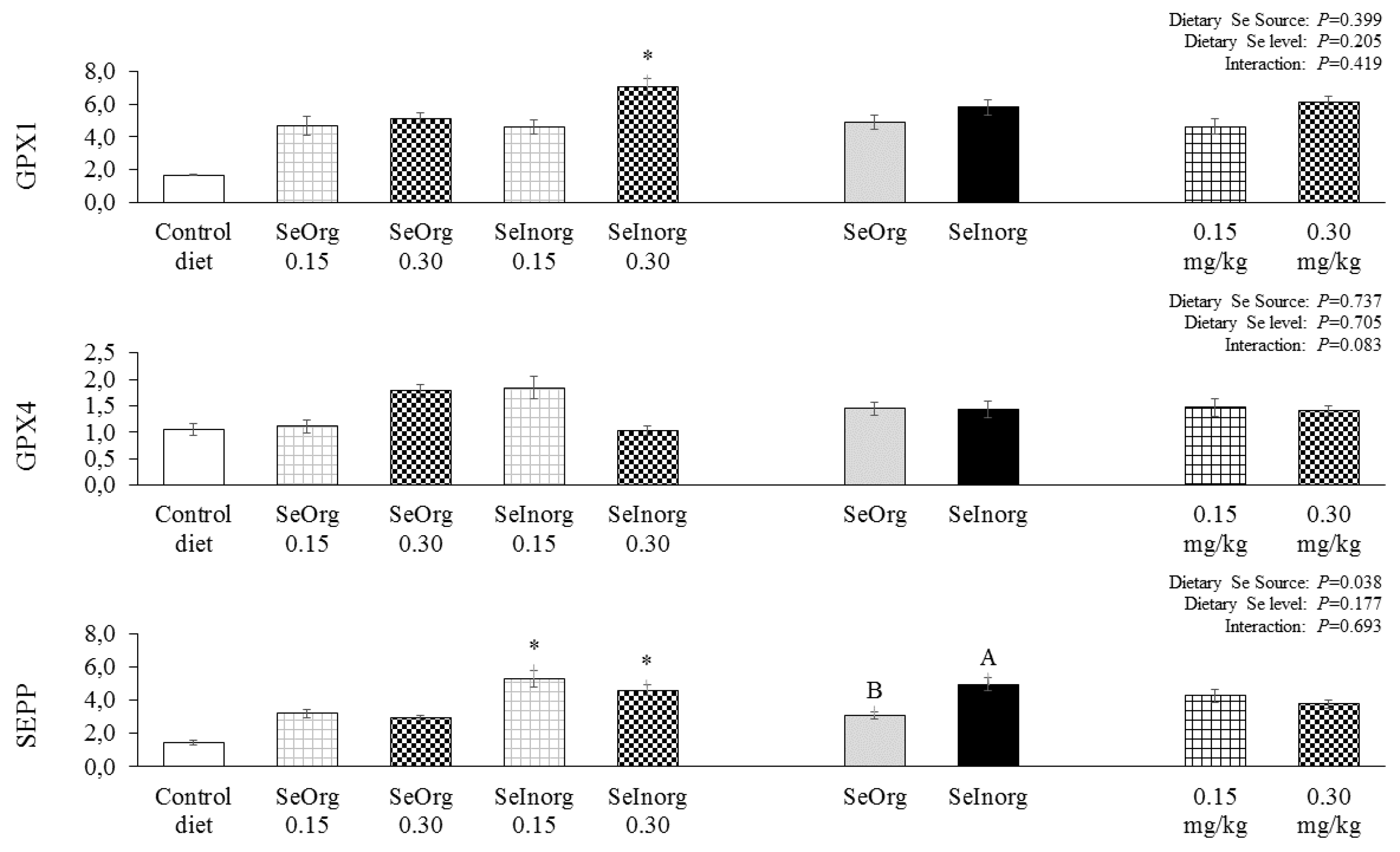
Figure 2). The GPX1 expression was significantly higher in the hepatic tissue (
Figure 3) (P<0.05) in the treatment with 0.30 mg/kg inorganic selenium addition when contrasted with the control diet. SePP expression was significantly higher (P<0.05) in diets with 0.15 and 0.30 mg/kg of inorganic selenium added when contrasted with the control diet. The selenium source influenced (P=0.038) the SePP expression, in which it is possible to observe that the diet with inorganic selenium addition promoted greater gene expression in the hepatic tissue of SePP than organic selenium. GPX4 gene expression in liver tissue was not influenced (P>0.05) by experimental diets.
4. Discussion
The highest selenium level (0.30 mg/kg) caused a reduction in DFI and consequently a lower DWG. Supplementation with 30 mg/kg of organic selenium in particular caused a lower DFI in the two evaluated phases. A similar result was found by Rao et al. (2023) in using selenomethionine instead of organic selenium in piglet diets. Lyons et al. (2007) concluded that organic selenium sources are more bioavailable than inorganic ones, and therefore Rostagno et al. (2017) recommend that 0.233 mg/kg of selenium be provided to newly weaned piglets if the source is organic to the detriment of 0.517 mg/kg of inorganic source. In that study, the diets with the addition of 0.30 mg/kg of selenium had a selenium concentration of 0.576 mg/kg in their composition, constituting values which were too high in relation to what was recommended when supplied from an organic source (0.233 mg/kg), however close to that recommended when supplied via an inorganic source (0.517mg/kg). The diets supplemented with organic selenium worsened the feed conversion of the animals from 28 to 60 days. This is in contrast to Shen et al. (2009), who observed that organic selenium supplementation improves the feed conversion rate of the diet; and Cao et al. (2014), who reported better feed conversion of diets enriched with selenomethionine.
Organic selenium improved dry matter and organic matter digestibility in our study, which is similar to the results found by Shen et al. (2009) in evaluating the digestibility of pigs in the initial phase. They reported that a diet supplemented with selenium and yeast improved the dry matter, crude energy and crude protein digestibility. A reduction in feed intake by pigs fed a diet supplemented with 0.30 mg/kg of selenium may have influenced the digestibility of nutrients, as discussed by Li and Patience (2017). The change in the intestinal microbiota is another factor which may have altered the digestibility of nutrients, since selenium is able to influence the microbiota as reported by Pereira et al. (2020). Nutrients not digested and absorbed in the small intestine are fermented in the colon by the local microbiota (Wernimont et al., 2020), probably affecting the total apparent digestibility, which does not discriminate the contribution of microbial communities to nutrient digestibility.
The selenium concentrations in the diets with the addition of organic and inorganic selenium were higher than the recommendations of the NRC (2012) and by Rostagno et al. (2017) in seeking the effect on immune parameters and expression of selenoproteins related to antioxidant control. Therefore, there was greater selenium retention in all diets with organic or inorganic selenium added; on the other hand, diets with inorganic source addition and the diet with f 0.30 mg/kg of organic selenium added provided greater selenium excretion than the control diet, indicating that there were other mechanisms in addition to the DFI reduction to avoid toxicity due to excess selenium in the diet. The highest selenium retention values resulted in greater muscle deposition, in which the selenium concentration in the tissue was higher with organic selenium in detriment to inorganic selenium, while the 30 mg/kg of selenium addition also increased the muscle concentration of selenium in relation to addition of 0.15 mg/kg. Our results are in line with those found in the literature. Rao et al. (2023) observed that an organic source of selenium resulted in higher selenium deposition in the tissue of piglets in the initial phase; Zhang et al. (2020) reported that sources and levels affect selenium concentrations deposited in the muscle, while Son et al. (2018) found that the selenium concentration level in the diet is directly correlated with concentrations in plasma, hair, liver, kidneys, muscles and urine of pigs.
Intestinal morphology is directly related to the cell mitosis rate. The smaller villi height and width well as the lower crypt depth of the duodenal epithelium of the pigs fed diets with 0.30 mg/kg selenium addition are related to the lower cell mitosis rate in the pigs with the same treatment. The DFI reduction presented by the pigs in the diets with the 0.30 mg/kg selenium addition may have been a factor that affected the morphology and cell mitosis in the epithelium of the pigs, since the intestinal segments need to obtain enough nutrients to meet the demands of mucosal protein synthesis and growth, causing shortening and modification in the villi structure and hyperplasia of the crypt cells (Pluske et al., 1997; Lin et al., 2014).
Supplementation with an inorganic source reduced the CD in the jejunal epithelium compared to organic selenium, whereas supplementation with 0.30 mg/kg of selenium reduced the villi width compared to the control diet. There is a relationship between DFI and intestinal morphology, which is a similar behavior to what occurred in the duodenal epithelium, however this response is also linked to the apoptosis rate, which was lower in the jejunal epithelium of pigs fed a diet supplemented with inorganic selenium in relation to organic selenium and control diet. Gut morphology is related to cell turnover behavior (Pluske et al., 1997), therefore the apoptosis process can influence the mitosis rate in the crypts, and consequently the values of villus height and crypt depth. For this reason, the highest selenium supplementation level can cause exaggerated inhibitory activity of oxidative processes, influencing the normal behavior of cell turnover in the intestinal epithelium.
Metabolic biomarkers are indicative of possible disturbances to the detriment of animal nutrition. AST and GGT enzymes are considered important markers of liver function and for evaluating the enzymatic metabolic profile, but damage to the liver tissue causes these enzymes to leak into the systemic circulation, thus altering their serum levels (Tennant, 1997). It has already been reported in our study that the 0.30 mg/kg selenium addition in the diet resulted in DFI reduction mechanisms and increased selenium excretion, indicating that this may be a very high level in the diet. CRC concentrations within the standard considered normal for pigs, which is 1.0 to 2.7 U/dL (Kaneko et al., 2008); in this study the 0.30 mg/kg selenium addition resulted in 2.83 U/dL, differing from the lower selenium addition levels. Very high creatinine levels in the blood indicate impairment in kidney functionality, which may have been altered with the higher selenium level. The serum IgA concentration at 60 days was higher in pigs fed diets supplemented with 0.30 mg/kg of selenium, which is in line with the GGT and CRC results; however, the levels of antibodies found are below those suggested by Tizard (2014). In a study using selenomethionine at levels of 0.1 to 0.7 mg/kg of selenium, Cao et al. (2014) did not observe an effect on the IGA, IGG and IGM levels; nevertheless, the serum concentrations of these antibodies were higher than those found in our study.
The first selenoprotein identified in mammals was GPX1 (Rotruck et al., 1973). Together with GPX4, it is also the most abundant selenoproteins in various immune cells and tissues (Carlson et al., 2010; Hoffmann et al., 2007). GPX1 and GPX4 are expressed in most tissues, while GPX2 is mainly expressed in the epithelium of the gastrointestinal tract (Koyama et al., 1999). Although there was no difference in the GPX1 and GPX2 expression in the jejunal epithelium of the pigs in relation to the selenium addition sources or levels, GPX1 in the liver was more expressed when the pigs were fed a diet enriched with 0.30 mg/kg of inorganic selenium compared to the control diet; this may have occurred due to GPX1 being more involved in antioxidant processes, being considered an essential selenoprotein. Furthermore, in the case of a change in selenium supplementation, its expression is prioritized to the detriment of other selenoproteins (Schomburg and Schweizer, 2009).
Hepatic SePP was directly influenced by the addition of inorganic selenium in the diets compared to the addition of organic selenium and the control diet. SePP is synthesized in many different tissues, but it serves as a key transporter of selenium in the liver where hepatic SePP is secreted into plasma which does the transport, which in turn influences selenium homeostasis throughout the body (Schomburg and Schweizer, 2009). In our study, it was observed that the addition of inorganic selenium resulted in greater selenium deposition in the muscle, as it is not complexed to an organic molecule; inorganic selenium must be quickly transported to be excreted or deposited in the tissue, and so there is a greater expression of SePP to transport this selenium and maintain body homeostasis. There is also growing evidence that SePP not only transports selenium, but also plays crucial antioxidant roles, which are particularly important for certain immune functions (Huang et al., 2012).
5. Conclusion
Inorganic selenium at high concentrations increases the expression of hepatic selenium transporting selenoproteins and serum creatinine and immunoglobulin A concentrations. Inorganic selenium supplementation in the form of sodium selenite should not be used at 0.30 mg/kg of additional selenium levels in the diet, and supplementation through proteinate selenium is recommended as an organic source with an additional level of up to 0.15 mg/kg.
Author Contributions
Jorge Luiz Santos de Almeida: Conceptualization, Data curation, Formal Analysis, Investigation, Supervision, Validation, Visualization, Writing – original draft and Writing – review & editing. Leonardo Augusto Fonseca Pascoal: Conceptualization, Data curation, Formal Analysis, Funding acquisition, Methodology, Project administration, Resources and Writing – review & editing. Ricardo Romão Guerra: Methodology, Resources and Writing – review & editing. Terezinha Domiciano Dantas Martins: Methodology, Resources and Writing – review & editing. Patrícia Emília Naves Givisiez: Methodology, Resources and Writing – review & editing. Pedro Henrique Watanabe: Methodology and Resources. Jonathan Mádson dos Santos Almeida: Investigation, Validation and Visualization. João Marcos Monteiro Batista: Investigation, Validation and Visualization. Maria Pricila Ferreira Hermínio: Investigation and Validation. Wydemberg José de Araújo: Investigation and Validation.
Funding
This work received support from the Yes Sinergy® company who provided the selenium proteinate used and financed the laboratory analyzes.
Acknowledgments
The authors acknowledge the Federal University of Paraiba (UFPB) for access to the bibliographic collection used and for the access to laboratories during the execution of this work.
Conflicts of Interest
The authors declare that they have no competing interests.
References
- Aldeola, O.; Lewis, A.J.; Southern, L.L. Digestion and balance techniques in pigs. Swine nutrition, 2th ed.; Boca Raton, FL, USA, 2001.
- AOAC. Official Methods of Analysis; Association of Official Analytical Chemists: Washington, DC., USA, 2007. [Google Scholar]
- Cao, J.; Guo, F.; Zhang, L.; Dong, B.; Gong, L. Effects of dietary Selenomethionine supplementation on growth performance, antioxidant status, plasma selenium concentration, and immune function in weaning pigs. J. Anim. Sci. Biotechnol. 2014, 5, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Carlson, B.A.; Yoo, M.-H.; Shrimali, R.K.; Irons, R.; Gladyshev, V.N.; Hatfield, D.L.; Park, J.M. Role of selenium-containing proteins in T-cell and macrophage function. Proc. Nutr. Soc. 2010, 69, 300–310. [Google Scholar] [CrossRef] [PubMed]
- Bampidis, V.; Azimonti, G.; Bastos, M.D.L.; Christensen, H.; Dusemund, B.; Mantovani, A.; EFSA Panel on Additives and Products or Substances used in Animal Feed. Assessment of the application for renewal of authorisation of selenomethionine produced by Saccharomyces cerevisiae CNCM I-3060 (selenised yeast inactivated) for all animal species. EFSA Journal 2018, 16, e05386. [Google Scholar] [PubMed]
- Hoffmann, P.R.; Höge, S.C.; Li, P.-A.; Hoffmann, F.W.; Hashimoto, A.C.; Berry, M.J. The selenoproteome exhibits widely varying, tissue-specific dependence on selenoprotein P for selenium supply. Nucleic Acids Res. 2007, 35, 3963–3973. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Rose, A.H.; Biagi, G.; Hoffmann, P.R. The role of selenium in inflammation and immunity: From molecular mechanisms to therapeutic opportunities. Antioxidants redox signaling 2012, 16, 705–743. [Google Scholar] [CrossRef]
- Ishak, K.; Baptista, A.; Bianchi, L.; Callea, F.; De Groote, J.; Gudat, F.; Denk, H.; Desmet, V.; Korb, G.; MacSween, R.N.; et al. Histological grading and staging of chronic hepatitis. J. Hepatol. 1995, 22, 696–699. [Google Scholar] [CrossRef] [PubMed]
- Kaneko, J.J.; Harvey, J.W.; Bruss, M.L. (Eds.) Clinical biochemistry of domestic animals; Academic press: Cambridge, MA, USA, 2008. [Google Scholar]
- Koyama, H.; Omurab, K.; Ejimab, A.; Kasanumaa, Y.; Watanabea, C.; Satoha, H. Separation of Selenium-Containing Proteins in Human and Mouse Plasma Using Tandem High-Performance Liquid Chromatography Columns Coupled with Inductively Coupled Plasma-Mass Spectrometry. Anal. Biochem. 1999, 267, 84–91. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Patience, J.F. Factors involved in the regulation of feed and energy intake of pigs. Anim. Feed. Sci. Technol. 2016, 233, 22–33. [Google Scholar] [CrossRef]
- Lin, M.; Zhang, B.; Yu, C.; Li, J.; Sun, H.; Gao, F.; Zhou, G. L-Glutamate Supplementation Improves Small Intestinal Architecture and Enhances the Expressions of Jejunal Mucosa Amino Acid Receptors and Transporters in Weaning Piglets. PLOS ONE 2014, 9, e111950. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Lyons, M.P.; Papazyan, T.T.; Surai, P.F. Selenium in Food Chain and Animal Nutrition: Lessons from Nature -Review-. Asian-Australasian J. Anim. Sci. 2007, 20, 1135–1155. [Google Scholar] [CrossRef]
- NRC National Research Council. Nutrient requirements of swine; National Academies Press, 2012.
- Pascoal, L.A.F.; Thomaz, M.C.; Watanabe, P.H.; Ruiz, U.d.S.; Ezequiel, J.M.B.; Amorim, A.B.; Daniel, E.; Masson, G.C.I. Fiber sources in diets for newly weaned piglets. Rev. Bras. de Zootec. 2012, 41, 636–642. [Google Scholar] [CrossRef]
- Pereira, A.M.; Pinna, C.; Biagi, G.; Stefanelli, C.; Maia, M.R.G.; Matos, E.; A Segundo, M.; Fonseca, A.J.M.; Cabrita, A.R.J.; Pereira, A.M.; et al. Supplemental selenium source on gut health: Insights on fecal microbiome and fermentation products of growing puppies. FEMS Microbiol. Ecol. 2020, 96. [Google Scholar] [CrossRef]
- Pluske, J.R.; Hampson, D.J.; Williams, I.H. Factors influencing the structure and function of the small intestine in the weaned pig: A review. Livest. Prod. Sci. 1997, 51, 215–236. [Google Scholar] [CrossRef]
- Rao, Z.-X.; Tokach, M.D.; Woodworth, J.C.; DeRouchey, J.M.; Goodband, R.D.; Gebhardt, J.T. Evaluation of selenium source on nursery pig growth performance, serum and tissue selenium concentrations, and serum antioxidant status. Transl. Anim. Sci. 2023, 7, txad049. [Google Scholar] [CrossRef]
- Rosmini, M.; Perlo, F.; Pérez-Alvarez, J.; Pagán-Moreno, M.; Gago-Gago, A.; López-Santoveña, F.; Aranda-Catalá, V. TBA test by an extractive method applied to ‘paté’. Meat Sci. 1996, 42, 103–110. [Google Scholar] [CrossRef]
- Rostagno, H.S.; Albino, L.F.T.; Donzele, J.L.; Gomes, P.C.; Oliveira, R.D.; Lopes, D.C.; ... & Euclides, R.F., 2011. Tabelas brasileiras para aves e suínos. Composição de alimentos e exigências nutricionais, 3rd ed. Viçosa, MG: UFV, 186p.
- Rostagno, H.S.; Albino, L.F.T.; Hannas, M.I.; Sakomura, N.K.; Perazzo, F.G. ... Brito, c. O., 2017. Tabelas brasileiras para aves e suínos. Composição de alimentos e exigências nutricionais, 4th ed. Viçosa, MG: UFV, 444p.
- Rotruck, J.T.; Pope, A.L.; Ganther, H.E.; Swanson, A.B.; Hafeman, D.G.; Hoekstra, W.G. Selenium: Biochemical Role as a Component of Glutathione Peroxidase. Science 1973, 179, 588–590. [Google Scholar] [CrossRef]
- Schomburg, L.; Schweizer, U. Hierarchical regulation of selenoprotein expression and sex-specific effects of selenium. Biochim. et Biophys. Acta (BBA) - Gen. Subj. 2009, 1790, 1453–1462. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.B.; Piao, X.S.; Kim, S.W.; Wang, L.; Liu, P.; Yoon, I.; Zhen, Y.G. Effects of yeast culture supplementation on growth performance, intestinal health, and immune response of nursery pigs1. J. Anim. Sci. 2009, 87, 2614–2624. [Google Scholar] [CrossRef] [PubMed]
- Son, A.R.; Jeong, J.-Y.; Park, K.R.; Kim, M.; Lee, S.D.; Yoo, J.-H.; Do, Y.-J.; Reddy, K.E.; Lee, H.-J. Effects of graded concentrations of supplemental selenium on selenium concentrations in tissues and prediction equations for estimating dietary selenium intake in pigs. PeerJ 2018, 6, e5791. [Google Scholar] [CrossRef] [PubMed]
- Tennant, B.C. Hepatic function. In Clinical biochemistry of domestic animals; Academic Press: Cambridge, MA, USA, 1997; pp. 327–352. [Google Scholar]
- Terzian, A.C.B.; Zuccari, D.A.P.d.C.; Pereira, R.S.; Pavam, M.V.; Ruiz, C.M.; Sueiro, F.A.R.; Coelho, J. Avaliação da caspase-3 e Ki-67 como marcadores prognósticos nas neoplasias mamárias em cadelas. Braz. J. Veter- Res. Anim. Sci. 2007, 44, 96–102. [Google Scholar] [CrossRef]
- Tizard, I.R., 2014. Imunologia Veterinária. (Tradução da 9ª Edição).
- Wernimont, S.M.; Radosevich, J.; Jackson, M.I.; Ephraim, E.; Badri, D.V.; MacLeay, J.M.; Jewell, D.E.; Suchodolski, J.S. The Effects of Nutrition on the Gastrointestinal Microbiome of Cats and Dogs: Impact on Health and Disease. Front. Microbiol. 2020, 11, 1266. [Google Scholar] [CrossRef] [PubMed]
- WHO World Health Organization. Trace elements in human nutrition and health. World Health Organization, 1996.
- Yoon, J.; Ingale, S.; Kim, J.; Kim, K.; Lee, S.; Park, Y.; Kwon, I.; Chae, B. Effects of dietary supplementation of antimicrobial peptide-A3 on growth performance, nutrient digestibility, intestinal and fecal microflora and intestinal morphology in weanling pigs. Anim. Feed. Sci. Technol. 2012, 177, 98–107. [Google Scholar] [CrossRef]
- Żarczyńska, K.; Sobiech, P.; Radwińska, J.; Rękawek, W. Effects of selenium on animal health. J. Elementology 2012, 18, 329–340. [Google Scholar] [CrossRef]
- Zhang, K.; Guo, X.; Zhao, Q.; Han, Y.; Zhan, T.; Li, Y.; Tang, C.; Zhang, J. Development and application of a HPLC-ICP-MS method to determine selenium speciation in muscle of pigs treated with different selenium supplements. Food Chem. 2019, 302, 125371. [Google Scholar] [CrossRef]
- Zoidis, E.; Demiris, N.; Kominakis, A.; Pappas, A. Meta-analysis of selenium accumulation and expression of antioxidant enzymes in chicken tissues. Animal 2014, 8, 542–554. [Google Scholar] [CrossRef]
Figure 1.
Apopitosis rate in the jejunal epithelium of piglets fed diets enriched with proteinate selenium (SeOrg) or sodium selenite (SeInorg) at two addition levels (0.15 or 0.30 mg/kg). A,B differentiate sources of selenium. * Dietary treatments that differ from the control diet by Dunnett's test (p<0.05). (A) Control diet; (B) SeOrg-0.15; (C) SeInorg-0.15; (D) SeOrg-0.30; (E) SeInorg-0.30.
Figure 1.
Apopitosis rate in the jejunal epithelium of piglets fed diets enriched with proteinate selenium (SeOrg) or sodium selenite (SeInorg) at two addition levels (0.15 or 0.30 mg/kg). A,B differentiate sources of selenium. * Dietary treatments that differ from the control diet by Dunnett's test (p<0.05). (A) Control diet; (B) SeOrg-0.15; (C) SeInorg-0.15; (D) SeOrg-0.30; (E) SeInorg-0.30.
Figure 2.
Expression of glutathione peroxidase 1 (GPX1) and glutathione peroxidase 2 (GPX2) in the jejunal epithelium of piglets fed diets enriched with proteinate selenium (SeOrg) or sodium selenite (SeInorg) at two addition levels (0.15 or 0.30 mg/kg).
Figure 2.
Expression of glutathione peroxidase 1 (GPX1) and glutathione peroxidase 2 (GPX2) in the jejunal epithelium of piglets fed diets enriched with proteinate selenium (SeOrg) or sodium selenite (SeInorg) at two addition levels (0.15 or 0.30 mg/kg).
Figure 3.
Expression of glutathione peroxidase 1 (GPX1), glutathione peroxidase 4 (GPX4) and selenoprotein P (SEPP) in the jejunal epithelium of piglets fed diets enriched with proteinate selenium (SeOrg) or sodium selenite (SeInorg) at two addition levels (0.15 or 0.30 mg/kg). A,B differentiate sources of selenium. * Dietary treatments that differ from the control diet by Dunnett's test (p<0.05).
Figure 3.
Expression of glutathione peroxidase 1 (GPX1), glutathione peroxidase 4 (GPX4) and selenoprotein P (SEPP) in the jejunal epithelium of piglets fed diets enriched with proteinate selenium (SeOrg) or sodium selenite (SeInorg) at two addition levels (0.15 or 0.30 mg/kg). A,B differentiate sources of selenium. * Dietary treatments that differ from the control diet by Dunnett's test (p<0.05).
Table 1.
Selenium concentrations of the diets 1.
Table 1.
Selenium concentrations of the diets 1.
| Treatment |
Selenium mg/kg |
| I (28-35 days) |
II (36-45 days) |
III (46-60 days) |
| Control diet |
0.276 |
0.265 |
0.254 |
| SeOrg-0.15 |
0.426 |
0.415 |
0.404 |
| SeOrg-0.30 |
0.576 |
0.565 |
0.554 |
| SeInorg-0.15 |
0.426 |
0.415 |
0.404 |
| SeInorg-0.30 |
0.579 |
0.568 |
0.557 |
Table 2.
Ingredients and chemical composition (g/kg, as feed) of the diets.
Table 2.
Ingredients and chemical composition (g/kg, as feed) of the diets.
| Ingredientes |
28-35 days |
36-45 days |
46-60 days |
| Grain corn |
457.8 |
529.2 |
700.2 |
| Soybean meal |
327.6 |
291.0 |
239.0 |
| Whey powder |
120.0 |
80.0 |
0.0 |
| Soy oil |
43.4 |
40.9 |
9.5 |
| Dicalcium phosphate |
19.6 |
18.8 |
17.3 |
| Limestone |
9.90 |
8.93 |
7.04 |
| L-lysine HCl |
5.46 |
5.56 |
4.49 |
| DL-methionine |
2.40 |
2.23 |
1.19 |
| L-threonine |
3.06 |
2.94 |
1.72 |
| L-tryptophan |
0.55 |
0.57 |
0.39 |
| L-Arginine |
2.30 |
2.23 |
0.00 |
| L-Valine |
1.37 |
1.31 |
0.41 |
| Vitamin supplement 1
|
2.00 |
2.00 |
2.00 |
| Mineral supplement 2
|
1.00 |
1.00 |
1.00 |
| Salt |
2.73 |
3.48 |
4.72 |
| Inert 3
|
1.00 |
9.95 |
11.00 |
| Calculated values |
|
|
|
| Metabolizable energy (MJ/kg) |
14.23 |
14.12 |
13.60 |
| Crude protein |
214.20 |
198.70 |
176.90 |
| Digestible lysine |
14.51 |
13.46 |
10.97 |
| Digestible methionine + cystine |
8.13 |
7.54 |
6.25 |
| Digestible threonine |
9.72 |
9.02 |
7.13 |
| Digestible tryptophan |
2.76 |
2.56 |
2.08 |
| Digestible Arginine |
14.51 |
13.46 |
10.19 |
| Digestible Valine |
10.01 |
9.29 |
7.60 |
| Limestone |
10.68 |
9.73 |
7.94 |
| Available phosphorous |
5.28 |
4.81 |
3.93 |
| Sodium |
2.24 |
2.19 |
1.99 |
| Potassium |
9.97 |
8.69 |
6.62 |
Table 3.
Genes and sequence of primers used in qPCR.
Table 3.
Genes and sequence of primers used in qPCR.
| Gene |
Forward Primer sequences |
Reverse primers sequences |
PS1
|
Locus 2
|
| GPX1 |
gctcggtgtatgccttctct |
agcgacgctacgttctcaat |
103 |
AF532927 |
| GPX2 |
cgtgaatggtcagaatgagc |
gggatcagtcatgagggaaa |
94 |
DQ898282 |
| GPX4 |
attctcagccaaggacatcg |
cctcattgagaggccacatt |
93 |
NM_214407 |
| SEPP |
gctccttctgtgagcaacct |
gcctgaagaagagcaaccac |
97 |
EF113596 |
| ACTB |
tgttcgagaccttcaacacg |
atccccagagtccatgacaa |
104 |
DQ845171 |
| GAPDH |
acatggcctccaaggagtaaga |
gatcgagttggggctgtgact |
106 |
AF017079 |
Table 4.
Average daily feed intake (ADFI), average daily gain (ADG) and feed:gain (F:G) of piglets fed diets enriched with proteinate selenium (SeOrg) or sodium selenite (SeInorg) at two addition levels (0.15 or 0.30 mg/kg).
Table 4.
Average daily feed intake (ADFI), average daily gain (ADG) and feed:gain (F:G) of piglets fed diets enriched with proteinate selenium (SeOrg) or sodium selenite (SeInorg) at two addition levels (0.15 or 0.30 mg/kg).
| |
28-45 days |
28-60 days |
| ADFI, kg |
ADG, kg |
F:G, kg/kg |
ADFI, kg |
ADG, kg |
F:G, kg/kg |
| Dietary treatment 1
|
|
|
|
|
|
|
| Control diet |
0.353 |
0.199 |
1.780 |
0.611 |
0.396 |
1.545 |
| SeOrg-0.15 |
0.371 Aa |
0.204 |
1.821 |
0.669 Aa |
0.424 |
1.580 |
| SeOrg-0.30 |
0.330 Bb |
0.186 |
1.780 |
0.562 Bb |
0.345 |
1.632 |
| SeInorg-0.15 |
0.349 Aa |
0.198 |
1.759 |
0.620 Aa |
0.410 |
1.512 |
| SeInorg-0.30 |
0.360 Aa |
0.222 |
1.625 |
0.597 Aa |
0.386 |
1.549 |
| Dietary Se Source 2
|
|
|
|
|
|
|
| SeOrg |
0.351 |
0.195 |
1.800 |
0.616 |
0.384 |
1.606 A |
| SeInorg |
0.355 |
0.210 |
1.692 |
0.609 |
0.398 |
1.530 B |
| Dietary Se Level 2
|
|
|
|
|
|
|
| 0.15 mg/kg |
0.360 |
0.201 |
1.790 |
0.645 |
0.417 a |
1.546 |
| 0.30 mg/kg |
0.345 |
0.204 |
1.702 |
0.580 |
0.365 b |
1.590 |
| ANOVA (P value) |
|
|
|
|
|
|
| Dietary Se Source |
0.651 |
0.337 |
0.159 |
0.719 |
0.449 |
0.052 |
| Dietary Se level |
0.132 |
0.854 |
0.855 |
0.006 |
0.017 |
0.216 |
| Interaction |
0.018 |
0.207 |
0.964 |
0.046 |
0.158 |
0.633 |
| Pooled SEM |
0.007 |
0.008 |
0.051 |
0.013 |
0.017 |
0.019 |
Table 5.
Coefficients of apparent total tract digestibility (CATTD) of the nutrients and energy of diets enriched with proteinate selenium (SeOrg) or sodium selenite (SeInorg) at two addition levels (0.15 or 0.30 mg/kg).
Table 5.
Coefficients of apparent total tract digestibility (CATTD) of the nutrients and energy of diets enriched with proteinate selenium (SeOrg) or sodium selenite (SeInorg) at two addition levels (0.15 or 0.30 mg/kg).
| |
Dry matter |
Organic matter |
Mineral matter |
Protein |
Energy |
| Dietary treatment 1
|
|
|
|
|
|
| Control diet |
0.939 |
0.827 |
0.499 |
0.781 |
0.802 |
| SeOrg-0.15 |
0.931 |
0.795 |
0.379 |
0.712 |
0.763 |
| SeOrg-0.30 |
0.935 |
0.814 |
0.409 |
0.742 |
0.784 |
| SeInorg-0.15 |
0.922 * |
0.757 * |
0.543 |
0.667 * |
0.740 * |
| SeInorg-0.30 |
0.923 * |
0.773 |
0.434 |
0.731 |
0.779 |
| Dietary Se Source 2
|
|
|
|
|
|
| SeOrg |
0.933 A |
0.805 A |
0.394 B |
0.727 |
0.774 |
| SeInorg |
0.922 B |
0.765 B |
0.489 A |
0.699 |
0.760 |
| Dietary Se Level 2
|
|
|
|
|
|
| 0.15 mg/kg |
0.926 |
0.776 |
0.461 |
0.689 |
0.751 |
| 0.30 mg/kg |
0.929 |
0.794 |
0.422 |
0.737 |
0.782 |
| ANOVA (P value) |
|
|
|
|
|
| Dietary Se Source |
0.024 |
0.024 |
0.021 |
0.275 |
0.386 |
| Dietary Se level |
0.516 |
0.263 |
0.275 |
0.080 |
0.077 |
| Interaction |
0.676 |
0.946 |
0.073 |
0.507 |
0.571 |
| Pooled SEM |
0.002 |
0.008 |
0.018 |
0.013 |
0.008 |
Table 6.
Selenium retained, selenium excreted in feces, and selenium present in muscle and blood of piglets fed diets enriched with proteinate selenium (SeOrg) or sodium selenite (SeInorg) at two addition levels (0.15 or 0.30 mg/kg).
Table 6.
Selenium retained, selenium excreted in feces, and selenium present in muscle and blood of piglets fed diets enriched with proteinate selenium (SeOrg) or sodium selenite (SeInorg) at two addition levels (0.15 or 0.30 mg/kg).
| |
Selenium, mg/kg |
| |
Retained |
Excreted |
Muscle |
Blood |
| Dietary treatment 1
|
|
|
|
|
| Control diet |
0.232 |
0.033 |
0.139 |
0.117 |
| SeOrg-0.15 |
0.361 Ab * |
0.043 |
0.131 |
0.121 |
| SeOrg-0.30 |
0.469 Aa * |
0.085 * |
0.144 |
0.135 |
| SeInorg-0.15 |
0.326 Bb * |
0.078 * |
0.143 |
0.119 |
| SeInorg-0.30 |
0.469 Aa * |
0.089 * |
0.186 * |
0.147 |
| Dietary Se Source 2
|
|
|
|
|
| SeOrg |
0.415 |
0.064 |
0.138 B |
0.128 |
| SeInorg |
0.397 |
0.083 |
0.165 A |
0.133 |
| Dietary Se Level 2
|
|
|
|
|
| 0.15 mg/kg |
0.344 |
0.061 b |
0.137 b |
0.120 |
| 0.30 mg/kg |
0.469 |
0.087 a |
0.165 a |
0.141 |
| ANOVA (P value) |
|
|
|
|
| Dietary Se Source |
0.023 |
0.074 |
0.002 |
0.353 |
| Dietary Se level |
<0.0001 |
0.031 |
0.015 |
0.331 |
| Interaction |
0.040 |
0.114 |
0.191 |
0.461 |
| Pooled SEM |
0.021 |
0.006 |
0.005 |
0.004 |
Table 7.
Intestinal morphometry and mitosis rate in the duodenal epithelium of piglets fed diets enriched with proteinate selenium (SeOrg) or sodium selenite (SeInorg) at two addition levels (0.15 or 0.30 mg/kg).
Table 7.
Intestinal morphometry and mitosis rate in the duodenal epithelium of piglets fed diets enriched with proteinate selenium (SeOrg) or sodium selenite (SeInorg) at two addition levels (0.15 or 0.30 mg/kg).
| |
Duodenum |
| |
VH, μm |
VW, μm |
CD, μm |
VH/CD |
Mitosis, n° |
| Dietary treatment 1
|
|
|
|
|
|
| Control diet |
293.15 |
135.47 |
96.93 |
3.60 |
767.615 |
| SeOrg-0.15 |
273.46 |
120.98 |
101.06 |
2.98 |
752.802 |
| SeOrg-0.30 |
251.03 |
101.98 * |
92.44 |
2.90 |
619.232 * |
| SeInorg-0.15 |
298.10 |
116.08 |
97.41 |
2.94 |
909.812 |
| SeInorg-0.30 |
261.96 |
101.57 * |
88.25 |
3.12 |
860.389 |
| Dietary Se Source 2
|
|
|
|
|
|
| SeOrg |
262.24 |
111.48 |
96.75 |
2.94 |
686.017 |
| SeInorg |
280.03 |
108.83 |
92.83 |
3.03 |
885.101 |
| Dietary Se Level 2
|
|
|
|
|
|
| 0.15 mg/kg |
285.78 a |
118.53 a |
99.23 a |
2.96 |
831.307 a |
| 0.30 mg/kg |
256.49 b |
101.77 b |
90.34 b |
3.01 |
739.811 b |
| ANOVA (P value) |
|
|
|
|
|
| Dietary Se Source |
0.070 |
0.526 |
0.985 |
0.530 |
<0.0001 |
| Dietary Se level |
0.001 |
0.001 |
<0.0001 |
0.769 |
0.031 |
| Interaction |
0.480 |
0.675 |
0.240 |
0.403 |
0.314 |
| Pooled SEM |
10.08 |
4.91 |
1.99 |
0.10 |
30.843 |
Table 8.
Intestinal morphometry and mitosis rate in the jejunum epithelium of piglets fed diets enriched with proteinate selenium (SeOrg) or sodium selenite (SeInorg) at two addition levels (0.15 or 0.30 mg/kg).
Table 8.
Intestinal morphometry and mitosis rate in the jejunum epithelium of piglets fed diets enriched with proteinate selenium (SeOrg) or sodium selenite (SeInorg) at two addition levels (0.15 or 0.30 mg/kg).
| |
Jejunum |
| |
VH, μm |
VW, μm |
CD, μm |
VH/CD |
Mitosis, n° |
| Dietary treatment 1
|
|
|
|
|
|
| Control diet |
218.69 |
107.60 |
87.97 |
2.68 |
872.645 |
| SeOrg-0.15 |
236.08 |
108.24 Aa |
96.30 |
2.68 |
955.480 |
| SeOrg-0.30 |
235.85 |
86.26 Ab * |
101.81 |
2.46 |
921.157 |
| SeInorg-0.15 |
217.78 |
95.64 Aa |
86.03 |
2.70 |
997.790 |
| SeInorg-0.30 |
229.33 |
90.10 Aa * |
96.67 |
2.51 |
916.568 |
| Dietary Se Source 2
|
|
|
|
|
|
| SeOrg |
235.97 |
97.25 |
99.06 A |
2.57 |
938.319 |
| SeInorg |
223.55 |
92.87 |
91.35 B |
2.60 |
957.179 |
| Dietary Se Level 2
|
|
|
|
|
|
| 0.15 mg/kg |
226.93 |
101.94 |
91.17 b |
2.69 |
976.635 |
| 0.30 mg/kg |
232.59 |
88.18 |
99.24 a |
2.49 |
918.862 |
| ANOVA (P value) |
|
|
|
|
|
| Dietary Se Source |
0.123 |
0.237 |
0.018 |
0.674 |
0.585 |
| Dietary Se level |
0.564 |
<0.0001 |
0.024 |
0.083 |
0.097 |
| Interaction |
0.549 |
0.027 |
0.347 |
0.892 |
0.497 |
| Pooled SEM |
6.80 |
3.27 |
2.71 |
0.10 |
29.008 |
Table 9.
Serum biochemical parameters at 41 days of piglets fed diets enriched with proteinate selenium (SeOrg) or sodium selenite (SeInorg) at two addition levels (0.15 or 0.30 mg/kg).
Table 9.
Serum biochemical parameters at 41 days of piglets fed diets enriched with proteinate selenium (SeOrg) or sodium selenite (SeInorg) at two addition levels (0.15 or 0.30 mg/kg).
| |
41 days |
| GGT. U/dl |
AST. U/dl |
CRC. mg/dl |
IgG. mg/dl |
IgA. mg/dl |
| Dietary treatment 1
|
|
|
|
|
|
| Control diet |
8.11 |
9.17 |
2.15 |
488.3 |
47.73 |
| SeOrg-0.15 |
8.51 |
9.68 |
1.85 |
463.2 |
43.63 |
| SeOrg-0.30 |
7.83 |
9.52 |
2.20 |
483.4 |
46.93 |
| SeInorg-0.15 |
8.37 |
9.51 |
2.45 |
479.9 |
45.03 |
| SeInorg-0.30 |
7.46 |
8.54 |
2.50 |
487.7 |
45.63 |
| Dietary Se Source 2
|
|
|
|
|
|
| SeOrg |
8.17 |
9.60 |
2.03 |
473.3 |
45.28 |
| SeInorg |
7.91 |
9.03 |
2.48 |
483.8 |
45.33 |
| Dietary Se Level 2
|
|
|
|
|
|
| 0.15 mg/kg |
8.44 a |
9.59 |
2.15 |
471.5 |
44.33 |
| 0.30 mg/kg |
7.64 b |
9.03 |
2.35 |
485.6 |
46.28 |
| ANOVA (P value) |
|
|
|
|
|
| Dietary Se Source |
0.489 |
0.352 |
0.603 |
0.468 |
0.987 |
| Dietary Se level |
0.051 |
0.356 |
0.256 |
0.336 |
0.517 |
| Interaction |
0.748 |
0.505 |
0.695 |
0.664 |
0.651 |
| Pooled SEM |
1.689 |
2.235 |
0.154 |
5.245 |
1.035 |
Table 10.
Serum biochemical parameters at 60 days of piglets fed diets enriched with proteinate selenium (SeOrg) or sodium selenite (SeInorg) at two addition levels (0.15 or 0.30 mg/kg).
Table 10.
Serum biochemical parameters at 60 days of piglets fed diets enriched with proteinate selenium (SeOrg) or sodium selenite (SeInorg) at two addition levels (0.15 or 0.30 mg/kg).
| |
60 days |
| GGT. U/dl |
AST. U/dl |
CRC. mg/dl |
IgG. mg/dl |
IgA. mg/dl |
| Dietary treatment 1
|
|
|
|
|
|
| Control diet |
8.66 |
10.13 |
2.73 |
503.5 |
51.10 |
| SeOrg-0.15 |
8.96 |
10.21 |
2.43 * |
497.1 |
46.97 |
| SeOrg-0.30 |
8.89 |
9.90 |
2.80 |
503.6 |
50.05 |
| SeInorg-0.15 |
9.17 |
10.60 |
2.55 |
502.6 |
41.48 * |
| SeInorg-0.30 |
8.69 |
9.65 |
2.85 |
503.1 |
50.40 |
| Dietary Se Source 2
|
|
|
|
|
|
| SeOrg |
8.92 |
10.05 |
2.62 |
500.4 |
48.51 |
| SeInorg |
8.93 |
10.12 |
2.70 |
502.8 |
45.94 |
| Dietary Se Level 2
|
|
|
|
|
|
| 0.15 mg/kg |
9.06 |
10.40 |
2.49 b |
499.9 |
44.22 b |
| 0.30 mg/kg |
8.79 |
9.77 |
2.83 a |
503.3 |
50.23 a |
| ANOVA (P value) |
|
|
|
|
|
| Dietary Se Source |
0.948 |
0.894 |
0.226 |
0.197 |
0.348 |
| Dietary Se level |
0.370 |
0.132 |
<0.0001 |
0.089 |
0.015 |
| Interaction |
0.438 |
0.463 |
0.697 |
0.180 |
0.278 |
| Pooled SEM |
1.045 |
1.690 |
0.045 |
0.793 |
1.185 |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).