1. Introduction
In 2023, about 44,000 new cases of lung cancer are expected in Italy: it is the second most common neoplasm in men (15%) and the third in women (6%). In 2022, about 35,700 deaths from lung cancer were recorded in Italy, 23,600 in men and 12,100 in women. Lung cancer is the first cause of cancer death in men and the second in women. The 5-year survival rate of lung cancer patients in Italy is 16% for men and 23% for women, which is negatively affected by the high proportion of patients diagnosed with advanced stage disease [
1].
Nevertheless, there have been significant advancements in therapeutic options for patients with metastatic Non Small Cell Lung Cancer (NSCLC) in recent years, which provides hope for improving survival rates. Developments in molecular testing and the availability of new therapies targeting specific molecular alterations have significantly impacted patient management. Clinicians are now able to select therapies that are most likely to improve individual clinical outcomes, taking into account patients’ characteristics and tumor histology.
According to several studies, it has been found that a significant percentage of NSCLC patients, ranging from 28-44%, possess biomarkers that can be targeted by first-line (1L) or subsequent therapies approved for metastatic NSCLC [
2,
3,
4,
5,
6]. Currently, targetable genetic alterations for NSCLC include epidermal growth factor receptor (
EGFR), anaplastic lymphoma kinase (
ALK), ROS proto-oncogene 1 (
ROS1), and B-Raf proto-oncogene (
BRAF), Erbb2 receptor tyrosine-protein kinase 2 (
ERBB2), Kirsten rat sarcoma virus (
KRAS), MET proto-oncogene (
MET), neurotrophic tyrosine receptor kinase (
NTRK), and rearranged during transfection (
RET) [
7]. In addition to molecularly targeted therapies, immunotherapies have also improved treatment for non-small cell lung cancer (NSCLC), providing survival benefits for patients with metastatic NSCLC regardless of their PD-L1 expression level [
8].
For patients with metastatic NSCLC without driver mutation of
EGFR,
ALK and
ROS1, drug active on PD-1/PDL-1 axis are predominantly used in the first-line setting with or without platinum chemotherapy. This happened since Pembrolizumab monotherapy proved his superiority in term of Progression Free Survival (10.3 mo. vs 6.0 mo., HR 0.50; 95% CI, 0.37 to 0.68; P<0.001) and Overall Survival (30.0 mo. vs 14.2 mo.; HR, 0.63; 95% CI, 0.47 to 0.86; P-value=0.002) against platinum based chemotherapy in
Keynote-042 and become available and reimbursed by national health system (NHS) in June 2017 for patients with NSCLC with Tumor Proportional Score (TPS) of PDL-1 ≥ 50 % [
9,
10]. Following the results of Keynote-189 and Keynote-407, Pembrolizumab has become available in combination with chemotherapy selected based on tumor histology. [
11,
12] However the Italian Regulatory Agency (AIFA) limited reimbursement for chemo-immunotherapy only for patients with PD-L1 expression < 50 %, even though superiority over chemotherapy alone was demonstrated regardless of PD-L1 expression. Considering this limitation, single agent immunotherapy is still the best available option for previously untreated patients with metastatic NSCLC with Tumor Proportional Score (TPS) of PDL-1 ≥ 50% and without
EGFR,
ALK and
ROS1 mutation [
13].
The survival benefit shown by Pembrolizumab is also confirmed by long-term data, which are not yet available for the other two alternatives, Atezolizumab and Cemiplimab, which have been reimbursed by NHS for the same therapeutic indication since June and August 2022 respectively. Nevertheless, the role of real-world observational studies is very important to confirm that the benefits seen in clinical trials are also seen in the population treated in everyday clinical practice.
Velcheti et al. have recently published the 3-year follow-up real-world results of their observational study in US oncology practice. The study has confirmed the results of the Keynote-024 clinical trials in terms of real-world time on treatment (rwToT), which is a surrogate indicator associated with survival in NSCLC studies [
14]
However, given the differences in the population that could be treated in Italy for regulatory reasons, the availability of different therapeutic options for subsequent lines that could change patient survival, and the importance of outcome indicators such as progression and death of patients treated in real – life, we design a multicenter observational study to evaluate the long-term effectiveness and safety outcome of Pembrolizumab monotherapy (PEMBROREAL study).
This work aims to show 3 years effectiveness and safety results from a cohort of patients treated in sixteen institutions in Italy following the standard clinical practice.
2. Materials and Methods
2.1. Study Design
PEMBROREAL is a retrospective, multicenter study conducted in Italy. The study involved a cohort of patients who received at least one dose of Pembrolizumab monotherapy in the first-line treatment of metastatic NSCLC without EGFR and ALK driver mutation. The medical records of adult patients with NSCLC who were consecutively treated at one of the sixteen study centers were reviewed by study investigators from each participating center, according to the eligibility criteria established by AIFA. Chart extractions were performed on two pre-specified dates for each enrolled patient: one in January 2021 and the other in February 2022. The first data extraction aimed to ensure that participating centers met high standards of data collection. In contrast to the inclusion and exclusion criteria for Keynote-024, it has been decided that patients with NSCLC who have not received prior treatment for metastatic disease will also have access to pembrolizumab monotherapy if they have ECOG PS 2, active central nervous system (CNS) metastases and a life expectancy of less than three months. To be considered for inclusion, patients who began treatment with Pembrolizumab between June 2017 and December 2020 were required to have given informed consent for their medical records to be accessed. Patients who could not be reached despite all reasonable efforts or who had passed away were included in accordance with national regulations. Patients who received Pembrolizumab through an interventional clinical trial, compassionate-use program, or off-label were not eligible for inclusion. The study protocol (protocol approval ID: 6346/2020 I.5/187) was approved by the Ethics Committees of all participating institutions. The study was conducted in accordance with the principles of the 1964 Declaration of Helsinki and its subsequent amendments.
2.2. Assessment
The study aimed to evaluate the effectiveness, safety, and activity of Pembrolizumab monotherapy in previously untreated metastatic NSCLC patients, based on the reimbursement criteria set by AIFA. The aim was to assess the consistency of the results obtained in Keynote-042 in the real world clinical practice. The study evaluated two main outcomes: real-world Progression Free Survival (PFS) and Overall Survival (OS). PFS was calculated from the index date (the first administration of Pembrolizumab) until the date of progression as determined by treating physicians or death (if no progression), or the end of follow-up, whichever occurred first. OS was calculated from the index date until death, or the end of follow-up, whichever occurred first. Additionally we assessed the overall response rate (ORR), calculated as the proportion of patients who have achieved a complete or partial response out of the total number of patients. Similarly, the disease control rate (DCR) is determined by the proportion of patients who have achieved a complete or partial response or have stable disease out of the total number of patients. The duration of response (DoR) is measured from the date of the first response documented in medical records until disease progression or death (if no progression). During the follow-up period, patients who did not exhibit any signs of progression were censored. The identification and management of adverse events (AEs) were carried out by the treating healthcare practitioner. The grade of AE was determined by the reporter using the Common Terminology Criteria for Adverse Events, version 4. Due to the real-world nature of PEMBROREAL, physicians may determine progression and response to therapy through assessments or by following the Response Evaluation Criteria in Solid Tumours (RECIST) version 1.1, depending on local practice.
In addition, key secondary endpoints include rwPFS and OS for subgroups of interest, as well as demographic, patient, and disease characteristics.
2.3. Statistical Analyses
No formal sample size calculation was performed. Categorical variables were presented as absolute and relative frequency , whereas for continuous variables, data were presented as median with minimum and maximum values. The rwPFS and rwOS data were censored for patients lost to follow-up (i.e., alive at last visit or contact before database cut-off). Median and landmark rates were calculated using the Kaplan-Meier method and the corresponding 95% confidence interval was calculated using Greenwood’s method. The log-rank test was used to assess the potential prognostic role of investigator identified factors. A multivariable Cox regression model was used to assess independent prognostic factors and the hazard ratios (HR) were calculated. Proportional hazard assumptions was tested using Schoenfeld residuals. In case of violation, a time dependent effect was also calculated and added to the main effect.
Analyses were based on the total population involved in the study; subgroup analyses (i.e., stratified by disease, therapy, or other demographic or prognostic variables) were performed as indicated by the investigators at the participating centres. A p-value <0.05 was considered statistically significant. Statistical analysis were performed using STATA/MP 15.0 for Windows (StataCorpLP, College Station, TX, USA).
3. Results
3.1. Patients and Treatment Characteristics
Between 25 June 2017 and 31 December 2020, a total of 880 eligible patients received their first Pembrolizumab dose and were included in the analysis. The median follow-up duration in the full analysis set was 35.1 months (ranging from 0.1 to 56.0). Only 39 patients (4.4%) were lost to follow-up, and none of the enrolled patients were excluded from the final analysis. The median age of patients in the full analysis set was 69.9 years (ranging from 37.9 to 90.2) at their first administration of Pembrolizumab.
Until February 28, 2022 (the end date of the second chart extraction), we identified 880 eligible patients who received their first Pembrolizumab dose between 25 June 2017 and 31 December 2020. All 880 were included in the analysis. The median follow-up duration in the full analysis set was 35.1 months (range from 0.1 to 56.0); 39 patients (4,4%) were lost to follow-up. No one of the enrolled patients was excluded from the final analysis. The median age of the patients in the full analysis set was 69.9 years (minimum-maximum: 37.9 – 90.2) at first Pembrolizumab administration; 229 (26.0 %) were aged above 75 years. Among these patients, 229 (26.0%) were aged above 75 years. The majority of patients (70.2%) were male and had a PS of 0 or 1 (91.9%) at their first administration of Pembrolizumab. The most common histological type was lung adenocarcinoma, with 673 (76.5%) patients enrolled, while 158 (18.0%) patients had squamous cell carcinoma. In addition to the two most prevalent histological types, our study also included a small number of patients with NSCLC Not Otherwise Specified (NOS), adenosquamous carcinoma, and large-cell lung cancer. For one patient, the tumor histology was unknown.
TPS of PD-L1 were immunohistochemically evaluated by a validated 22C3 IHC laboratory test for all patients. All patients enrolled had PD-L1% expression ≥ 50%. For most of them, 527 of the 880 patients (59.9 %), the exact expression of PD-L1 was available in the clinical documentation. Of these 527 patients, 120 (22,8 %) had high expression of PD-1 (≥ 90%). One hundred thirty-for patients were diagnosed with brain metastasis (15.7%). For 29 patients included in the study, the presence or absence of brain metastasis was not documented. Out of the 880 patients included in the study, 542 (61.6%) had known smoker status, with the majority being either current (n=186; 34.3%) or former smokers (n=251; 46.3%).
EGFR and
ALK mutations were tested for 788 and 790 of 880 included patients respectively using Next Generation Sequencing (NGS) for the first mentioned mutation and immunohistochemical validated Ventana
ALK (D5F3) and Rabbit Monoclonal for the second one. The result was negative for 100.0% of the tested patients. Of note, all patients with unknown
EFGR and
ALK status, 92 and 90 respectively, had a squamous NSCLC (
Table 1). Giving the rarity of these two mutations and the low efficacy of target treatments in this histological subtype cell tumor, searching for these driver-gene alterations is not mandatory considering current guidelines and the eligibility criteria for NHS reimbursement.
The study did not collect data on other driver mutations, such as KRAS, BRAF, RET, and ROS1, which may have affected OS and PFS. It is worth noting that participating centers evaluated these mutations at their discretion.
Additionally, the median total treatment duration, including dose interruptions, was 189 days. Notably, 34.7% of patients received Pembrolizumab for over 12 months. Patients received a median of 9.0 infusions of Pembrolizumab (range: 1-75). It is worth noting that 8.1% of patients experienced a temporary treatment interruption lasting more than three weeks for any reason.
3.2. Reasons for Discontinuing Pembrolizumab
The most common reasons for discontinuing Pembrolizumab treatment were disease progression (53.9%) and death (13.6%). A small percentage of patients (6.3%) stopped treatment due to unacceptable toxicity. At the time of data cut-off, 16.0% of patients were still receiving Pembrolizumab treatment (refer to
Supplementary Table S1).
3.3. Analysis of Real-World PFS
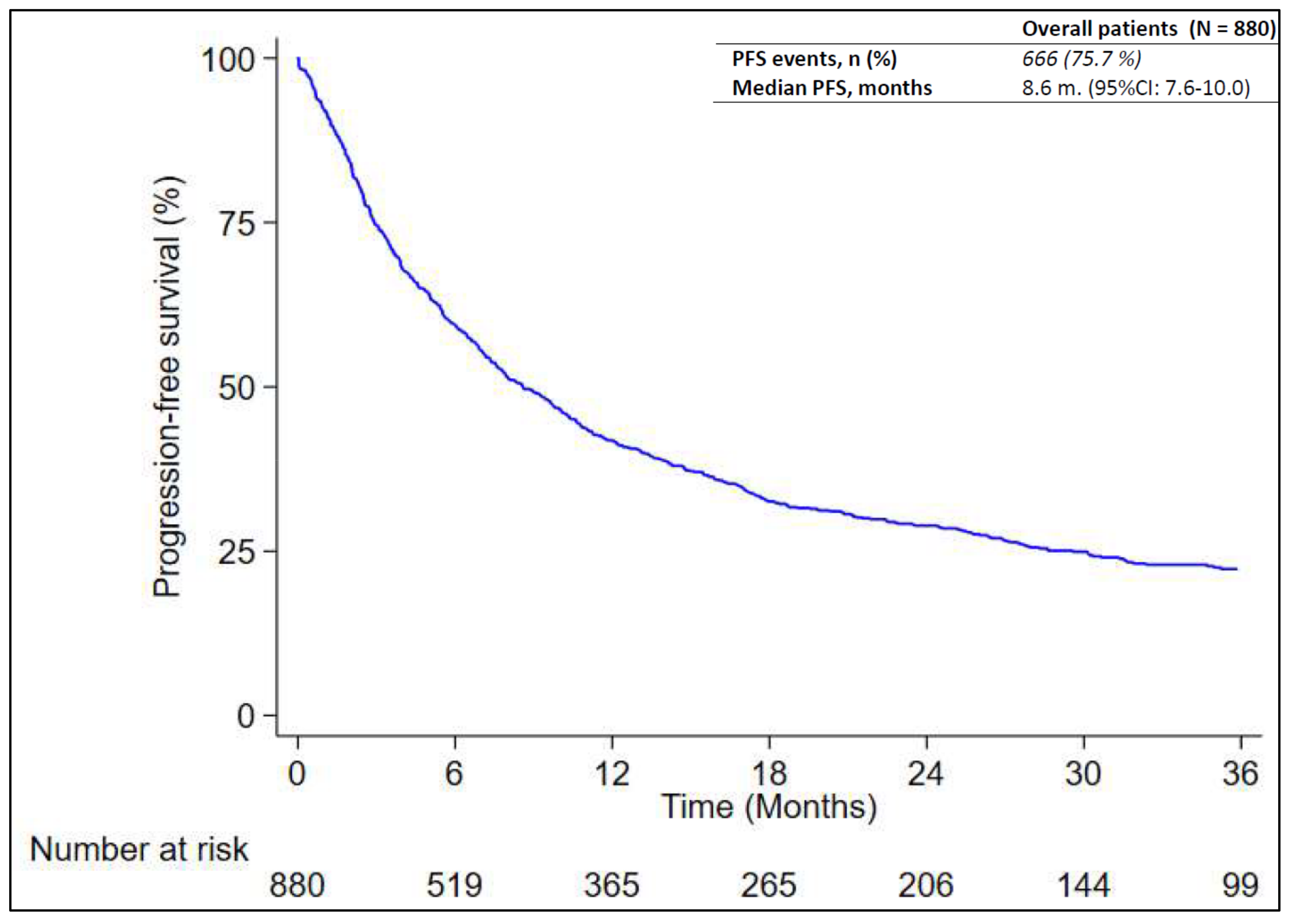
At the time of data cut-off, 666 out of 880 patients (75.7%) experienced disease progression or died without documentation of disease progression. Progression was determined per RECIST or clinical assessment. A radiological evaluation was made in local clinical practice, usually every three months, but this was not always possible for logistic or clinical reasons. The median RwPFS was 8.6 months (95% CI: 7.6 – 10.0) as reported in
Figure 1. According to
Table 2, patients with PD-L1 greater than or equal to 90% had a significantly longer RwPFS (median: 13.9 months; 95% CI: 8.9-19.0) compared to those with PD-L1 between 50% and 69% (median: 6.9 months; 95% CI: 5.4-8.9) and patients with PD-L1 level between 70% and 89% (median: 7.0 months; 95% CI: 5.6-10.0; p-value = 0.032) (
Figure S1A in Supplementary Data). The study found that patients with ECOG performance status 0 had a median survival time of 11.1 months (95% CI: 9.8-14.8), which was longer than those with performance status 1 and 2, who had median survival times of 7.8 months (95% CI: 6.4-9.5) and 2.8 months (95% CI: 1.7-5.1), respectively (p = 0.013) (refer to
Figure S1B in Supplementary Data). Another significant difference in rwPFS was registered between smoker/former-smoker patients (median: 10.8 months; 95% CI: 8.9-13.1) versus never-smoker patients (median: 5.9 months; 95% CI: 3.9-8.6; p – value = 0.007) (
Figure S1C Supplementary Data)
Meanwhile, RwPFS was not different among patients aged less than 75 (median: 8.5 months; 95% CI: 7.1-10.1) versus greater or equal to 75 years (median 9.1 months; 95% CI: 7.4-10.8; p - value = 0.640) but also among patient with squamous (median: 8.4 months; 95% CI: 6.2-12.1) and non-squamous (median: 8.6 months; 95% CI: 7.5-10.4; p-value: 0.092) cancer type. Patient’s sex also appears to be unrelated to RwPFS, although it was slightly longer in female patients (median: 9.2; 95% CI: 7.2-12.1) than in male patients (median: 8.3 months; CI 95%: 7.2-10.0; p-value 0.108). Central nervous system involvement did not have a significant effect on rwPFS, but it was shorter in patients with brain metastases (median: 6.3; 95% CI: 4.2-8.9) than in those without brain metastases (median: 9.3 months; CI 95%: 7.7-10.4; p = 0.053).
The multivariable analysis in
Supplementary Table S4, confirms that differences in rwPFS between subgroups stratified by PD-L1 level were mainly driven by differences between patients with PD-L1 ≥ 90%, who had a lower risk of progression compared to patients with PD-L1 levels greater than 50% and less than or equal to 69% (HR. 0.62; 95%CI: 0.46-0.83; p-value = 0.001) than between the patients in this latter group and patient with PD-L1 level greater than 70% and less than or equal to 89% (HR. 0.82; 95% 0.64-1.05; p-value = 0.125). Patients with PS ECOG 1 and with PS ECOG 2 had a higher risk of progression compared to patients with PS ECOG 0, with an HR equal to 1.51 (95%CI:1.20-1.89; p-value< 0.001) and 2.36 (95%CI: 1.50-3.69; p-value < 0.001) respectively. The difference between smoker or former smoker and current smoker in rwPFS was not confirmed by the multivariable model (HR 0.81; 95%CI: 0.61-1.07; p = 0.144).
3.4. Analysis of Real-World OS
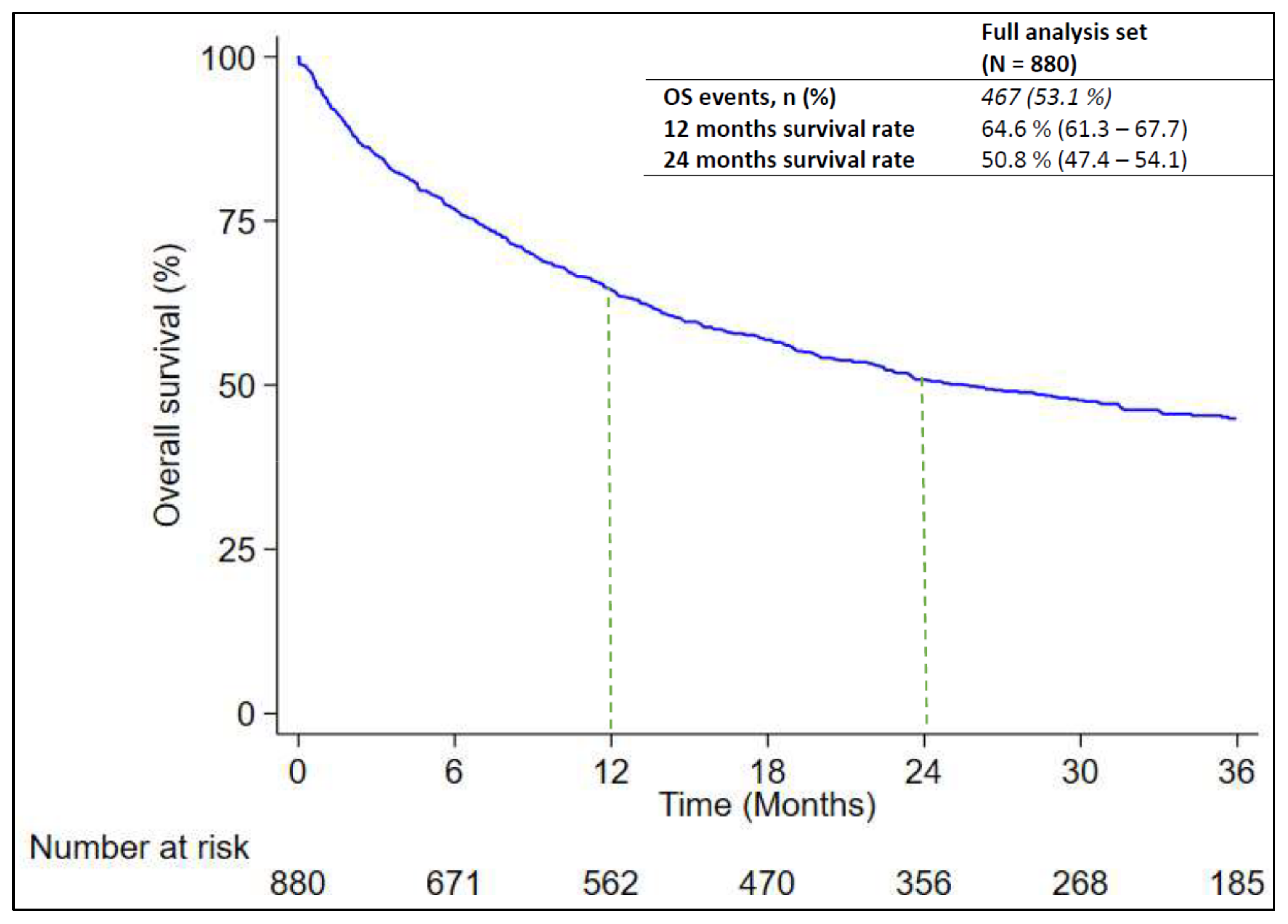
At the time of the data cut-off, 467 out of 880 patients (53.1%) died. The median RwOS was 25.5 months (95% CI: .21.8 – 31.6) as reported in
Figure 2. The RwOS was analyzed in subgroups of interest to evaluate any possible associations with prognostic factors. As found in
Table 3, RwOS was significantly longer among female patients (median: 39.1 months; 95% CI: 25.5-NE) versus male patients (median: 22.3 months; 95% CI: 17.8-28.9; p-value = 0.014) and among patients with non-squamous NSCLC (median: 18.5 months; 95% CI: 15.5-22.8) than in patients with squamous NSCLC (median: 29.9 months; 95% CI: 23.5 - 39.1; p-value = 0.010). However the effect of the last variables seems to be time dependent and resulted only considering the survival rate after 24 month of observation with non-squamous NSCLC versus squamous NSCLC (53.2%; CI: 49.4-56.8 and 40.4%; CI: 32.5-48.2 respectively) as compared to the difference in survival rate after 12 month observation (64.7%; CI: 61.1-68.1 and 64.6%; CI: 56.6-61.5 respectively). Moreover a significant difference in RwOS was found among patients ≥ 75 years (median: 15.4 months; 95% CI: 12.4 - 22.5) and patients < 75 years (median: 30.7 months; 95% CI: 23.6 - 48.7; p-value = 0.002) and among patients current or former smoker (median: 22.4 months; 95% CI: 18.7 - 28.9) and never-smoker (median: N.R.; p-value= 0.044). Finally, survival also appears to be influenced by ECOG PS, considering the difference found among patients with ECOG PS 0 (median: 38.5 months; 95% CI: 29.5-N.E.), ECOG PS 1 (median: 18.6 months; 95% CI: 14.1-18.6) and ECOG PS 2 (median: 5.9 months; 95% CI: 2.8-15.5; p < 0.001) (
Figure S2A–E in Supplementary Data).
PD-L1 expression and presence of brain metastases appear to be unrelated with RwOS considering that no differences were found between patient with (median: 23.5 months; 95% CI: 13.5-29.9) and without brain metastases (median: 25.5 months; 95% CI: 20.0-35.7; p-value= 0.291) and among patients with PD-L1 greater than or equal to 90% (median: 22.5 months; 95% CI: 14.1-40.4) versus patients with PD-L1 between 50% and 69% (median: 15.5 months; 95% CI: 10.7-19.8) and patients with PD-L1 level between 70 % e 89% (median: 15.3 months; 95% CI: 11.6-21.3, p-value= 0.117).
The multivariate analysis is shown in
Supplementary Table S5: among all the prognostic factor that affected RwOS, the only variable that confirmed this effect was ECOG PS end the effect was time dependent.
3.5. Analysis of Treatment Response
In the study, it was found that out of 880 patients, 611 (69.4%) reported a response to treatment. Among these patients, 179 (20.3%) had a partial (17.6%) or complete response rate (2.7%) as the best response to treatment (refer to
Supplementary Table S2). At the first evaluation, 25.9% of patients had experienced disease progression. The disease control rate (DCR) for the included patients was 79.2%. The median duration of response (DoR) for patients with a documented complete or partial response was 27.1 months (95% CI: 22.0 – 33.8).
3.6. Safety
Three hundred and fifty-one out of 880 eligible patients (39.9%) reported at least one AE. AEs reported for at least 15% of the eligible patients include: gastrointestinal disorders (23.8%); general disorders and administration site conditions (23.4%); skin and subcutaneous tissue disorders (22.4%); respiratory, thoracic and mediastinal disorders (15.9 %).
Sixty-seven patients reported grade 3-4 AEs of which 14 were gastrointestinal disease, 13 were skin and subcutaneous tissue disorders; 13 were general disorders and administration site conditions; 13 were related to alteration of diagnostic exams (
Table 4).
It is worth noting that most of the reported adverse events did not require any management measures such as drug administration suspension, reduction, definitive interruption, hospitalization, or specific pharmacologic treatment. Among the patients who experienced an adverse event (AE) that required specific measures, it was observed that 20.1% (177 cases) were managed with pharmacologic treatment, while 8.1% (71 cases) required temporary treatment interruption. It was found that only 5.3% (47 patients) definitively discontinued treatment due to toxicity (see
Supplementary Table S3).
4. Discussion
In this large multicenter, real-life observational study, we considered 880 patients with metastatic NSCLC and a PD-L1 expression ≥ 50%, EGFR and ALK wild type treated with first-line Pembrolizumab monotherapy. With a median follow-up time of 35.1 months, we reported a median PFS of 8.6 months and a median OS of 25.5 months. These results are consistent with findings from Keynote-024 in which PFS was 7.7 months (CI 95%: 6.1-10.2) and OS was 26.3 months (CI 95%: 18.3-40.4). However, we observed that patients were somewhat older in our cohort of patients than in the experimental arm of Keynote-024 (median age 69.9 vs 65.5) and included proportionately more women (40.3% vs 29.8 %).
In addition, we also included 8.1% of patients with ECOG PS 2, with less than three months of life expectancy and with active brain metastases who were treated in routine clinical practice but would be excluded from the enrollment in clinical trial. Moreover, one of the limitations of Keynote-024 was that only a few never-smokers were enrolled (3.2%), whereas in the real-world setting, this rate was higher among patients for whose smoking habits were known (19.4%).
To our best knowledge, despite many other real-world studies were published, they had shorter follow-ups, focused on surrogate endpoints, or with results referred to few patients. We have compiled a table of all previously published real-world studies of the same treatment for similar populations. (
Table 5) [
14,
15,
16,
17,
18,
19,
20,
21,
22,
23,
24,
25,
26,
27,
28,
29,
30]. Compared with the results of other previously published real-world studies in the same setting, OS in the PEMBROREAL study was longer, based on a longer follow-up, and derived from the observation of more patients than the majority of other reported studies. PFS was similar to that previously observed in the reported studies, except for that of Jiménez Galán [
16] in which it should be noted that almost one third of the patients enrolled had an ECOG PS ≥ 2. A possible explanation of the longer OS in our recent study could be the availability of newer second-line therapies, especially for patients with mutations of other driver genes (e.g.,
KRAS,
BRAF,
RET,
MET), that could be responsible for a longer survival for these patients. On the other hand, the consistency of the PFS data could be explained by the fact that the availability of this outcome takes less time if compared to OS, and therefore data from other studies are sufficiently robust, despite the limited follow-up of some of them.
Univariate Cox regression confirm that PD-L1 expression, ECOG PS and smoker status significantly influence PFS. Other variables studied, such as age ≥ 75 years at diagnosis, sex, histological type and presence of brain metastases, were not statistically significant in determining PFS. However a slightly favourable trend for patients without brain metastases was observed for this latter variable.
According with previously published paper [
15,
16,
31,
32], clinical outcomes are significantly improved in NSCLCs with a PD-L1 expression of ≥90% and ECOG PS < 2. The prognostic role of ECOG PS was explored in many other studies and despite the limitations due to the subjective nature of this parameter, considering that two physicians may classify the same patient with different performance status, it is an important variables influencing patients outcome. In addition, we know from the work of Facchinetti et al. that the worst outcome came from patients for whom a high ECOG PS value was determined by disease burden rather than from patients for whom it was determined by comorbidities [
33]. However, it was not possible to differentiate between these two types of patients in our study.
Smoking status also was already found to be related to clinical outcome of NSCLC patients receiving immunotherapy [
29]. A recent study of patients with NSCLC treated with different immune checkpoint inhibitors across multiple lines, showed a non-statically significant trend towards an improved PFS in heavy and light smokers compared to never smokers [
34]. The authors identified a higher tumour mutational burden (TMB) among heavy smokers, which makes cancer cells easier for the immune system to recognize, as an explanation for the difference in the clinical outcome. Notably, the difference between patients never and former or current smokers was not confirmed by the multivariable model, so this finding on the prognostic role of smoking habit should be considered with caution.
Interesting results were obtained from the univariate Cox regression analysis of the variables that influence OS. Not surprisingly a significantly better OS was found for patients with age at diagnosis < 75 years and for patients with lower value of ECOG PS. We considered more interesting the role of sex in determining OS, since resulted that female patients had a better prognosis than male patients. Another interesting variable of prognostic significance was tumor histology since patients with non- squamous tumor type had a better outcome than patients with squamous tumor type. At last the prognostic value determined for smoker status was inverted if we considered OS compared to PFS. If the outcome for PFS was better for patients who were former or current smokers, the outcome for OS was better for patients who had never smoked. It is important to note that these findings are exploratory and unadjusted, therefore they should be interpreted with caution.
Research has consistently shown that sex is a prognostic factor in lung cancer, even before the availability of immune checkpoint inhibitors. A recent study conducted on a large Australian cohort confirmed that women have a significantly longer survival rate after a lung cancer diagnosis compared to men [
35]. The study also found that men were typically older at diagnosis, less likely to be non-smokers, and had more comorbidities. Some authors have suggested that lung cancer in women may have a different natural history, which could be related to immunological differences as well as patient factors [
36,
37].
Regarding results about the influence of cancer histology on OS, this was consistent with findings from the real-world study conducted by Tambo et al. [
26]. In this study patients with squamous NSCLC had a greater number of metastatic sites compared with patients with adenocarcinoma and these findings was correlated with a longer OS. Unfortunately, there are no published data relative to the effect of histologic type on the outcome of patients treated with immuno-therapy, however squamous cell carcinoma is a well-known poor prognostic factor.
Considering the opposite effect of smoker status on the OS, these results are consistent with findings of the meta-analyses conducted by Popat et al. [
38] and could be explained considering that according to the previously published report, Never-smokers were more likely to be women and be diagnosed with non-squamous tumor histology [
39].
Treatment response seems to be inconsistent across different studies. In our cohort of patients, ORR results were lower if compared with results from Keynote-024 (ORR: 44.8% vs 20.3 %). However, these differences may be explained by differences in response assessment between an experimental clinical trial, where the response is rigorously assessed and centrally reviewed, and retrospective-observational studies, where reports of tumor response are individually reported by a single clinician and collected by chart extraction, with concerns about missing reports.
Safety assessment revealed that Pembrolizumab monotherapy was generally well tolerated. Comparing the results of our observational study with those of patients treated in the experimental arm of Keynote-024, the rate of patients with at least one adverse event was halved in patients treated in current clinical practice (39.9 vs 73.4). Grade 3 and 4 AE were uncommon in current clinical practice, occurring only in 67 out of 880 (7.6%). Interestingly, in the majority of cases, toxicity management does not require specific intervention, and situations that do require specific management can be easily managed with pharmacological therapy or temporary withholding of Pembrolizumab.
Most concerns regarding safety evaluation in our study are related to the phenomenon of under reporting of toxicity by treating physicians, especially if resulted in low grade AEs or considered as not clinically relevant.
Strenghts of this study include the large multicenter cohort with the longest follow-up ever published before. These consideration give consistence to our outcome results compared with other previously published with a limit follow-up or with few patient.
However, it is important to note that retrospective data evaluation has limitations, such as the potential for missing or inaccurately recorded data. For example, smoking status was unknown in more than 23% of patients, and for 40% of patients we only know that they had a TPS of PD-L1 ≥ 50%, but we didn't know the exact value of their score.
Another limit are related to outcome assessments. Patient included in our study were treated within the valid standard of care outside a clinical trial with varying imaging intervals, thereby potentially biasing PFS. Additionally, progression could be determined based on radiological or clinical evidence. It may be worthwhile to conduct further analysis to explore the potential impact of this limitation on PFS
Finally, considering that 13 of the 16 institutions selected to participate in our study had an internationally recognized role in cancer research and treatment, this could be considered a selection bias in the choice of patients, as these patients are more likely to receive the best care than patients followed at less relevant hospital sites.
Despite these limitations, our results are consistent with other retrospective studies and with the results of Keynote-024, and help to identify patients who may best benefit from treatment with Pembrolizumab monotherapy.
5. Conclusions
In conclusion, the results of our retrospective observational study of Pembrolizumab monotherapy in first-line metastatic NSCLC with PD-L1 ≥ 50% are consistent with previously reported real-world studies and the results of Keynote-024.
This is true even though the distribution of patient characteristics in routine clinical practice is slightly different from that in the clinical trial, and even though patients in clinical trials are selected by strict selection criteria, including some related to performance status, presence of active brain metastases and life expectancy.
In addition, we identified several prognostic factors that influence patient outcomes. This is of interest for patients that should be informed about the benefit of a particular therapy for patients with their characteristics, to physicians who can identify which patients will benefit most from a particular treatment and which will not, and to health care administrators who want to understand whether a new technology is cost effective or not.
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on Preprints.org, Table S1: Reasons for Pembrolizumab Treatment discontinuation, Table S2: Response Rate, Table S3: Adverse reaction management, Table S4: univariables and multivariable model for PFS; Table S5: univariables and multivariable model for OS; Figure S1: Kaplan – Meier curves of real world PFS in subgroup of interest. A) PD-L1 level (50-69%; 70-89%; ≥ 90%) B) ECOG PS 0, 1 and 2 C) Smoking status (former/current smoker vs ever smoker); Figure S2: Kaplan – Meier curves of real world OS in subgroup of interest. A) age (<75 years vs ≥ 75 years) . B) Sex (male vs female patients) C) Histologyc type (squamous NSCLC vs non-squamous NSCLC) D) smoking status (former/current smoker vs ever smoker E) ECOG PS 0, 1 and 2
Author Contributions
Conceptualization, A.C.; L.C.; and C.M.; methodology, A.C.; and F.F.; software, F.F.; validation, L.C. and C.M. formal analysis, F.F.; investigation, A.C.; M.C.; P.B.; S.O.; F.E.; F.F.; D.P.; V.L.; R.L.; P.N.; S.F.; P.M.; F.G.; A.R.G.; G.C; R.P.; P.C.C.; A.P.; S.V.; M.V.; A.E.D.; C.L.P.; A.R. and E.S.; resources, M.C.; and C.M.; data curation, A.C.; writing—original draft preparation, A.C.; writing—review and editing, A.C; L.C.; visualization, M.C.; L.C; A.D.; supervision, M.C. and C.M.; project administration, C.M.; funding acquisition, M.C. All authors have read and agreed to the published version of the manuscript.
Funding
The author(s) declare that no finantial support was received for the research, authorship and publication of this article.
Institutional Review Board Statement
The study was conducted according to the guidelines of the declaration of Helsinki, and ethical approval of the study protocol (number 1.0; titled: Retrospective observational study of Pembrolizumab as monotherapy or in combination with chemotherapy in the first-line treatment of patients with EGFR and ALK mutation-negative metastatic NSCLC) was obtained from the CEROM Institutional review Board 6346/2020 I.5/187).
Informed Consent Statement
Informed consent was obtained for all available patients. Patients who could not be contacted, despite all reasonable efforts or who died, were included following national law.
Data Availability Statement
The raw data supporting the conclusions of this article will be mada available by the authors, without undue reservation.
Acknowledgments
Under the direction of the first author, data collection was carried out by Eleonora Ferretti, Giada Toscano, Lisa Faoro, Guidoni Francesco, Sara Simonetta, Benedetta Versari, Ilaria Corbucci, Alessandra Ferraiuolo, Giulio Rocca, Alfredo Tartarone, and Emanuela Zifarone.
Conflicts of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationship that could be constructed as a potential conflict of interest.
References
- AIOM, Airtum. I numeri del cancro in Italia 2023. Online.
- Melosky B, Wheatley-Price P, Juergens RA, Sacher A, Leighl NB, Tsao MS, Cheema P, Snow S, Liu G, Card PB, Chu Q. The rapidly evolving landscape of novel targeted therapies in advanced non-small cell lung cancer. Lung Cancer. 2021 Oct;160:136-151. [CrossRef] [PubMed]
- Kerr KM, Bibeau F, Thunnissen E, Botling J, Ryška A, Wolf J, Öhrling K, Burdon P, Malapelle U, Büttner R. The evolving landscape of biomarker testing for non-small cell lung cancer in Europe. Lung Cancer. 2021 Apr;154:161-175. [CrossRef] [PubMed]
- Gregg JP, Li T, Yoneda KY. Molecular testing strategies in non-small cell lung cancer: Optimizing the diagnostic journey. Transl Lung Cancer Res. 2019;8:286–301.
- Arbour KC, Riely GJ. Systemic therapy for locally advanced and metastatic non–small cell lung cancer: A review. JAMA. 2019;322:764–774.
- Guo H, Zhang J, Qin C, Yan H, Liu T, Hu H, Tang S, Tang S, Zhou H. Biomarker-Targeted Therapies in Non-Small Cell Lung Cancer: Current Status and Perspectives. Cells. 2022 Oct 12;11(20):3200. [CrossRef] [PubMed] [PubMed Central]
- Hendriks LE, Kerr KM, Menis J, Mok TS, Nestle U, Passaro A, Peters S, Planchard D, Smit EF, Solomon BJ, Veronesi G, Reck M; ESMO Guidelines Committee. Electronic address: clinicalguidelines@esmo.org. Oncogene-addicted metastatic non-small-cell lung cancer: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann Oncol. 2023 Apr;34(4):339-357. [CrossRef] [PubMed]
- Bodor JN, Boumber Y, Borghaei H. Biomarkers for immune checkpoint inhibition in non–small cell lung cancer (NSCLC). Cancer. 2020;126:260–270.
- Reck M, Rodríguez-Abreu D, Robinson AG, Hui R, Csőszi T, Fülöp A, Gottfried M, Peled N, Tafreshi A, Cuffe S, O'Brien M, Rao S, Hotta K, Leiby MA, Lubiniecki GM, Shentu Y, Rangwala R, Brahmer JR; KEYNOTE-024 Investigators. Pembrolizumab versus Chemotherapy for PD-L1-Positive Non-Small-Cell Lung Cancer. N Engl J Med. 2016 Nov 10;375(19):1823-1833. [CrossRef] [PubMed]
- Reck M, Rodríguez-Abreu D, Robinson AG, Hui R, Csőszi T, Fülöp A, Gottfried M, Peled N, Tafreshi A, Cuffe S, O'Brien M, Rao S, Hotta K, Vandormael K, Riccio A, Yang J, Pietanza MC, Brahmer JR. Updated Analysis of KEYNOTE-024: Pembrolizumab Versus Platinum-Based Chemotherapy for Advanced Non-Small-Cell Lung Cancer With PD-L1 Tumor Proportion Score of 50% or Greater. J Clin Oncol. 2019 Mar 1;37(7):537-546. [CrossRef] [PubMed]
- Garassino MC, Gadgeel S, Speranza G, Felip E, Esteban E, Dómine M, Hochmair MJ, Powell SF, Bischoff HG, Peled N, Grossi F, Jennens RR, Reck M, Hui R, Garon EB, Kurata T, Gray JE, Schwarzenberger P, Jensen E, Pietanza MC, Rodríguez-Abreu D. Pembrolizumab Plus Pemetrexed and Platinum in Nonsquamous Non-Small-Cell Lung Cancer: 5-Year Outcomes From the Phase 3 KEYNOTE-189 Study. J Clin Oncol. 2023 Apr 10;41(11):1992-1998. [CrossRef] [PubMed] [PubMed Central]
- Novello S, Kowalski DM, Luft A, Gümüş M, Vicente D, Mazières J, Rodríguez-Cid J, Tafreshi A, Cheng Y, Lee KH, Golf A, Sugawara S, Robinson AG, Halmos B, Jensen E, Schwarzenberger P, Pietanza MC, Paz-Ares L. Pembrolizumab Plus Chemotherapy in Squamous Non-Small-Cell Lung Cancer: 5-Year Update of the Phase III KEYNOTE-407 Study. J Clin Oncol. 2023 Apr 10;41(11):1999-2006. [CrossRef] [PubMed] [PubMed Central]
- Cafaro A, Foca F, Nanni O, Chiumente M, Coppola M, Baldo P, Orzetti S, Enrico F, Ladisa V, Lerose R, Nardulli P, Maiolino P, Gradellini F, Gasbarro AR, Carrucciu G, Provasi R, Cappelletto PC, Pasqualini A, Vecchia S, Veraldi M, De Francesco AE, Crinò L, Delmonte A and Masini C (2024) A real-world retrospective, observational study of first-line pembrolizumab plus chemotherapy for metastatic non-squamous non-small cell lung cancer with PD-L1 tumor proportion score <50% (PEMBROREAL). Front. Oncol. 14:1351995. [CrossRef]
- Velcheti V, Hu X, Li Y, El-Osta H, Pietanza MC, Burke T. Real-World Time on Treatment with First-Line Pembrolizumab Monotherapy for Advanced NSCLC with PD-L1 Expression ≥ 50%: 3-Year Follow-Up Data. Cancers (Basel). 2022 Feb 18;14(4):1041. [CrossRef] [PubMed] [PubMed Central]
- Bérard G, Guévremont C, Marcotte N, Schroeder C, Bouchard N, Rajan R; Programme de Gestion Thérapeutique des Médicaments (PGTM). Descriptive Analysis of First-Line Non-Small Cell Lung Cancer Treatment with Pembrolizumab in Tumors Expressing PD-L1 ≥ 50% in Patients Treated in Quebec's University Teaching Hospitals (DALP-First Study). Curr Oncol. 2023 Mar 11;30(3):3251-3262. [CrossRef] [PubMed] [PubMed Central]
- Jiménez Galán R, Prado-Mel E, Pérez-Moreno MA, Caballano-Infantes E, Flores Moreno S. Influence of Performance Status on the Effectiveness of Pembrolizumab Monotherapy in First-Line for Advanced Non-Small-Cell Lung Cancer: Results in a Real-World Population. Biology (Basel). 2021 Sep 9;10(9):890. [CrossRef] [PubMed] [PubMed Central]
- Pons-Tostivint E, Hulo P, Guardiolle V, Bodot L, Rabeau A, Porte M, Hiret S, Demontrond P, Curcio H, Boudoussier A, Veillon R, Mayenga M, Dumenil C, Chatellier T, Gourraud PA, Mazieres J, Bennouna J. Real-world multicentre cohort of first-line pembrolizumab alone or in combination with platinum-based chemotherapy in non-small cell lung cancer PD-L1 ≥ 50. Cancer Immunol Immunother. 2023 Jun;72(6):1881-1890. Erratum in: Cancer Immunol Immunother. 2023 Feb 27. [CrossRef] [PubMed] [PubMed Central]
- Izano MA, Sweetnam C, Zhang C, Weese JL, Reding D, Treisman J, Patel A, Potugari B, Stafford A, Wolf FM, Tran M, Brown TD, Gadgeel SM. Brief Report on Use of Pembrolizumab With or Without Chemotherapy for Advanced Lung Cancer: A Real-World Analysis. Clin Lung Cancer. 2023 Jun;24(4):362-365. [CrossRef] [PubMed]
- Descourt R, Chouaid C, Pérol M, Besse B, Greillier L, Bylicki O, Ricordel C, Guisier F, Gervais R, Schott R, Auliac JB, Robinet G, Decroisette C. First-line pembrolizumab with or without platinum doublet chemotherapy in non-small-cell lung cancer patients with PD-L1 expression ≥50. Future Oncol. 2021 Aug;17(23):3007-3016. [CrossRef] [PubMed]
- Amrane K, Geier M, Corre R, Léna H, Léveiller G, Gadby F, Lamy R, Bizec JL, Goarant E, Robinet G, Gouva S, Quere G, Abgral R, Schick U, Bernier C, Chouaid C, Descourt R. First-line pembrolizumab for non-small cell lung cancer patients with PD-L1 ≥50% in a multicenter real-life cohort: The PEMBREIZH study. Cancer Med. 2020 Apr;9(7):2309-2316. [CrossRef] [PubMed] [PubMed Central]
- Velcheti V, Hu X, Yang L, Pietanza MC, Burke T. Long-Term Real-World Outcomes of First-Line Pembrolizumab Monotherapy for Metastatic Non-Small Cell Lung Cancer With ≥50% Expression of Programmed Cell Death-Ligand 1. Front Oncol. 2022 Mar 25;12:834761. [CrossRef] [PubMed]
- Tamiya M, Tamiya A, Hosoya K, Taniguchi Y, Yokoyama T, Fukuda Y, Hirano K, Matsumoto H, Kominami R, Suzuki H, Hirashima T, Uchida J, Morita M, Kanazu M, Sawa N, Kinoshita Y, Hara S, Kumagai T, Fujimoto D. Efficacy and safety of pembrolizumab as first-line therapy in advanced non-small cell lung cancer with at least 50% PD-L1 positivity: A multicenter retrospective cohort study (HOPE-001). Invest New Drugs. 2019 Dec;37(6):1266-1273. [CrossRef] [PubMed]
- Goto Y, Tamura A, Matsumoto H, Isobe K, Ozaki T, Santorelli ML, Taniguchi K, Kamitani T, Irisawa M, Kanda K, Abe M, Burke T, Nokihara H. First-Line Pembrolizumab Monotherapy for Advanced NSCLC With Programmed Death-Ligand 1 Expression Greater Than or Equal to 50%: Real-World Study Including Older Patients in Japan. JTO Clin Res Rep. 2022 Aug 5;3(9):100397. [CrossRef] [PubMed] [PubMed Central]
- Mountzios G, de Toma A, Economopoulou P, Friedlaender A, Banini M, Lo Russo G, Baxevanos P, Roila F, Banna GL, Christopoulou A, Jimenez B, Collazo-Lorduy A, Linardou H, Calles A, Galetta D, Addeo A, Camerini A, Pizzutilo P, Kosmidis P, Garassino MC, Proto C, Signorelli D, Metro G. Steroid Use Independently Predicts for Poor Outcomes in Patients With Advanced NSCLC and High PD-L1 Expression Receiving First-Line Pembrolizumab Monotherapy. Clin Lung Cancer. 2021 Mar;22(2):e180-e192. [CrossRef] [PubMed]
- Dudnik E, Moskovitz M, Rottenberg Y, Lobachov A, Mandelboim R, Shochat T, Urban D, Wollner M, Nechushtan H, Rotem O, Zer A, Daher S, Bar J; Israel Lung Cancer Group. Pembrolizumab as a monotherapy or in combination with platinum-based chemotherapy in advanced non-small cell lung cancer with PD-L1 tumor proportion score (TPS) ≥50%: Real-world data. Oncoimmunology. 2021 Jan 28;10(1):1865653. [CrossRef] [PubMed] [PubMed Central]
- Tambo Y, Sone T, Shibata K, Nishi K, Shirasaki H, Yoneda T, Araya T, Kase K, Nishikawa S, Kimura H, Kasahara K. Real-World Efficacy of First-Line Pembrolizumab in Patients With Advanced or Recurrent Non-Small-Cell Lung Cancer and High PD-L1 Tumor Expression. Clin Lung Cancer. 2020 Sep;21(5):e366-e379. [CrossRef] [PubMed]
- Cavaille F, Peretti M, Garcia ME, Giorgi R, Ausias N, Vanelle P, Barlesi F, Montana M. Real-world efficacy and safety of pembrolizumab in patients with non-small cell lung cancer: A retrospective observational study. Tumori. 2021 Feb;107(1):32-38. [CrossRef] [PubMed]
- Frost N, Kollmeier J, Misch D, Vollbrecht C, Grah C, Matthes B, Pultermann D, Olive E, Raspe M, Ochsenreither S, von Laffert M, Suttorp N, Witzenrath M, Grohé C. Pembrolizumab as First-Line Palliative Therapy in PD-L1 Overexpressing (≥ 50%) NSCLC: Real-world Results with Special Focus on PS ≥ 2, Brain Metastases, and Steroids. Clin Lung Cancer. 2021 Sep;22(5):411-422. [CrossRef] [PubMed]
- Cortellini A, Tiseo M, Banna GL, Cappuzzo F, Aerts JGJV, Barbieri F, Giusti R, Bria E, Cortinovis D, Grossi F, et al. Clinicopathologic correlates of first-line pembrolizumab effectiveness in patients with advanced NSCLC and a PD-L1 expression of ≥ 50. Cancer Immunol Immunother. 2020 Nov;69(11):2209-2221. [CrossRef] [PubMed]
- Velcheti V, Chandwani S, Chen X, Pietanza MC, Piperdi B, Burke T. Outcomes of first-line pembrolizumab monotherapy for PD-L1-positive (TPS ≥50%) metastatic NSCLC at US oncology practices. Immunotherapy. 2019 Dec;11(18):1541-1554. [CrossRef] [PubMed]
- Aguilar EJ, Ricciuti B, Gainor JF, Kehl KL, Kravets S, Dahlberg S, Nishino M, Sholl LM, Adeni A, Subegdjo S, Khosrowjerdi S, Peterson RM, Digumarthy S, Liu C, Sauter J, Rizvi H, Arbour KC, Carter BW, Heymach JV, Altan M, Hellmann MD, Awad MM. Outcomes to first-line pembrolizumab in patients with non-small-cell lung cancer and very high PD-L1 expression. Ann Oncol. 2019 Oct 1;30(10):1653-1659. [CrossRef] [PubMed]
- Shah M, Hubbard RA, Mamtani R, Marmarelis ME, Hennessy S. Very high PD-L1 expression as a prognostic indicator of overall survival among patients with advanced non-small cell lung cancer receiving anti-PD-(L)1 monotherapies in routine practice. Pharmacoepidemiol Drug Saf. 2022 Oct;31(10):1121-1126. [CrossRef] [PubMed] [PubMed Central]
- Facchinetti F, Mazzaschi G, Barbieri F, Passiglia F, Mazzoni F, Berardi R, Proto C, Cecere FL, Pilotto S, Scotti V, Rossi S, Del Conte A, Vita E, Bennati C, Ardizzoni A, Cerea G, Migliorino MR, Sala E, Camerini A, Bearz A, De Carlo E, Zanelli F, Guaitoli G, Garassino MC, Ciccone LP, Sartori G, Toschi L, Dall'Olio FG, Landi L, Pizzutilo EG, Bartoli G, Baldessari C, Novello S, Bria E, Cortinovis DL, Rossi G, Rossi A, Banna GL, Camisa R, Di Maio M, Tiseo M. First-line pembrolizumab in advanced non-small cell lung cancer patients with poor performance status. Eur J Cancer. 2020 May;130:155-167. [CrossRef] [PubMed]
- Gainor JF, Rizvi H, Jimenez Aguilar E, Skoulidis F, Yeap BY, Naidoo J, Khosrowjerdi S, Mooradian M, Lydon C, Illei P, Zhang J, Peterson R, Ricciuti B, Nishino M, Zhang J, Roth JA, Grishman J, Anderson D, Little BP, Carter BW, Arbour K, Sauter JL, Mino-Kenudson M, Heymach JV, Digumarthy S, Shaw AT, Awad MM, Hellmann MD. Clinical activity of programmed cell death 1 (PD-1) blockade in never, light, and heavy smokers with non-small-cell lung cancer and PD-L1 expression ≥50. Ann Oncol. 2020 Mar;31(3):404-411. [CrossRef] [PubMed] [PubMed Central]
- Yu XQ, Yap ML, Cheng ES, Ngo PJ, Vaneckova P, Karikios D, Canfell K, Weber MF. Evaluating Prognostic Factors for Sex Differences in Lung Cancer Survival: Findings From a Large Australian Cohort. J Thorac Oncol. 2022 May;17(5):688-699. [CrossRef] [PubMed]
- Klein SL, Flanagan KL (2016) Sex differences in immune responses. Nat Rev Immunol 16:626–638. [CrossRef]
- Conforti F, Pala L, Pagan E, Bagnardi V, De Pas T, Queirolo P, Pennacchioli E, Catania C, Cocorocchio E, Ferrucci PF.; et al. Sex-Based Dimorphism of Anticancer Immune Response and Molecular Mechanisms of Immune Evasion. Clin Cancer Res. 2021 Aug 1;27(15):4311-4324. [CrossRef] [PubMed] [PubMed Central]
- Popat S, Liu SV, Scheuer N, Gupta A, Hsu GG, Ramagopalan SV, Griesinger F, Subbiah V. Association Between Smoking History and Overall Survival in Patients Receiving Pembrolizumab for First-Line Treatment of Advanced Non-Small Cell Lung Cancer. JAMA Netw Open. 2022 ;5(5):e2214046. [CrossRef] [PubMed] [PubMed Central]
- Cho J, Choi SM, Lee J, Lee CH, Lee SM, Kim DW, Yim JJ, Kim YT, Yoo CG, Kim YW, Han SK, Park YS. Proportion and clinical features of never-smokers with non-small cell lung cancer. Chin J Cancer. 2017 Feb 8;36(1):20. [CrossRef] [PubMed] [PubMed Central]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).