Submitted:
15 April 2024
Posted:
16 April 2024
You are already at the latest version
Abstract
Keywords:
1. Lupus Remains a Significant Clinical Challenge
2. Insufficient Germinal Center B Cell Tolerance Enables Lupus Autoimmunity
3. PKCδ Is Required to Prevent Lupus Pathogenesis
4. PKCδ Is Involved in B Cell Tolerance
5. PKCδ Regulates GC B Cell Tolerance
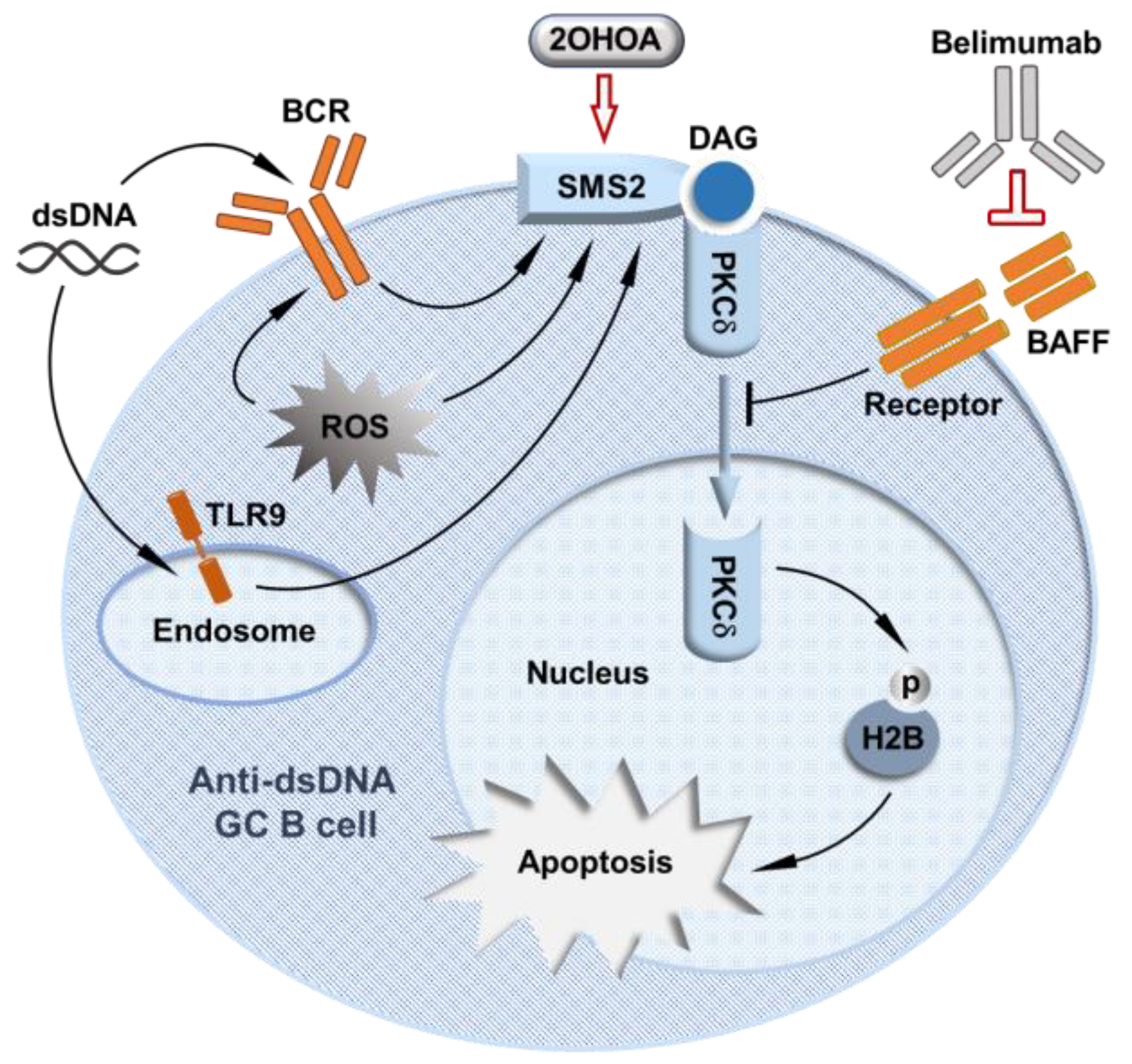
6. PKCδ-Regulated GC B Cell Tolerance Is a Potential Target for Lupus Treatment
References
- Pisetsky, D.S. Anti-DNA antibodies — quintessential biomarkers of SLE. Nat. Rev. Rheumatol. 2015, 12, 102–110. [Google Scholar] [CrossRef] [PubMed]
- Justiz Vaillant AA, Goyal A, Varacallo M. Systemic Lupus Erythematosus. [Updated 2023 Au4]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2023 Jan-. Available from: Https://www.ncbi.nlm.nih.gov/books/NBK535405/.
- Scofield, R.H.; Bruner, G.R.; Namjou, B.; Kimberly, R.P.; Ramsey-Goldman, R.; Petri, M.; Reveille, J.D.; Alarcón, G.S.; Vilá, L.M.; Reid, J.; et al. Klinefelter's syndrome (47,XXY) in male systemic lupus erythematosus patients: Support for the notion of a gene-dose effect from the X chromosome. Arthritis Rheum. 2008, 58, 2511–2517. [Google Scholar] [CrossRef]
- Youness, A.; Miquel, C.-H.; Guéry, J.-C. Escape from X Chromosome Inactivation and the Female Predominance in Autoimmune Diseases. Int. J. Mol. Sci. 2021, 22, 1114. [Google Scholar] [CrossRef] [PubMed]
- Fraenkel, L.; Bathon, J.M.; England, B.R.; St. Clair, E.W.; Arayssi, T.; Carandang, K.; Deane, K.D.; Genovese, M.; Huston, K.K.; Kerr, G.; et al. 2021 American College of Rheumatology Guideline for the Treatment of Rheumatoid Arthritis. Arthritis Care Res. 2021, 73, 924–939. [Google Scholar] [CrossRef] [PubMed]
- Fanouriakis, A.; Kostopoulou, M.; Alunno, A.; Aringer, M.; Bajema, I.; Boletis, J.N.; Cervera, R.; Doria, A.; Gordon, C.; Govoni, M.; et al. 2019 update of the EULAR recommendations for the management of systemic lupus erythematosus. Ann. Rheum. Dis. 2019, 78, 736–745. [Google Scholar] [CrossRef]
- Barber, M.R.; Clarke, A.E. Socioeconomic consequences of systemic lupus erythematosus. Curr. Opin. Rheumatol. 2017, 29, 480–485. [Google Scholar] [CrossRef] [PubMed]
- Carter, E.E.; Barr, S.G.; Clarke, A.E. The global burden of SLE: prevalence, health disparities and socioeconomic impact. Nat. Rev. Rheumatol. 2016, 12, 605–620. [Google Scholar] [CrossRef] [PubMed]
- Meacock, R.; Dale, N.; Harrison, M.J. The Humanistic and Economic Burden of Systemic Lupus Erythematosus. PharmacoEconomics 2012, 31, 49–61. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.-J.; Silverman, E.; Bargman, J.M. The role of antimalarial agents in the treatment of SLE and lupus nephritis. Nat. Rev. Nephrol. 2011, 7, 718–729. [Google Scholar] [CrossRef]
- Almeida-Brasil, C.C.; Hanly, J.G.; Urowitz, M.; Clarke, A.E.; Ruiz-Irastorza, G.; Gordon, C.; Ramsey-Goldman, R.; Petri, M.; Ginzler, E.M.; Wallace, D.J.; et al. Flares after hydroxychloroquine reduction or discontinuation: results from the Systemic Lupus International Collaborating Clinics (SLICC) inception cohort. Ann. Rheum. Dis. 2021, 81, 370–378. [Google Scholar] [CrossRef]
- Schrezenmeier, E.; Dörner, T. Mechanisms of action of hydroxychloroquine and chloroquine: implications for rheumatology. Nat. Rev. Rheumatol. 2020, 16, 155–166. [Google Scholar] [CrossRef] [PubMed]
- Sacre, K.; A Criswell, L.; McCune, J.M. Hydroxychloroquine is associated with impaired interferon-alpha and tumor necrosis factor-alpha production by plasmacytoid dendritic cells in systemic lupus erythematosus. Arthritis Res. Ther. 2012, 14, R155–10. [Google Scholar] [CrossRef]
- Dowdell, K.C. , Pesnicak, Bi, L.L., Hoffmann, V., Rao, V.K., Straus, S.E. Hydroxychloroquine Diminishes Lymphoproliferation in the Fas Deficient MRL/lpr−/− Murine Model of Autoimmune Lymphoproliferative Syndrome (ALPS). Blood, 2007; Volume 110, Issue 11, Page 1385.
- An, N.; Chen, Y.; Wang, C.; Yang, C.; Wu, Z.-H.; Xue, J.; Ye, L.; Wang, S.; Liu, H.-F.; Pan, Q. Chloroquine Autophagic Inhibition Rebalances Th17/Treg-Mediated Immunity and Ameliorates Systemic Lupus Erythematosus. Cell. Physiol. Biochem. 2017, 44, 412–422. [Google Scholar] [CrossRef] [PubMed]
- Pawar, R.D.; Ramanjaneyulu, A.; Kulkarni, O.P.; Lech, M.; Segerer, S.; Anders, H.-J. Inhibition of Toll-Like Receptor-7 (TLR-7) or TLR-7 plus TLR-9 Attenuates Glomerulonephritis and Lung Injury in Experimental Lupus. J. Am. Soc. Nephrol. 2007, 18, 1721–1731. [Google Scholar] [CrossRef]
- Fillatreau, S.; Manfroi, B.; Dörner, T. Toll-like receptor signalling in B cells during systemic lupus erythematosus. Nat. Rev. Rheumatol. 2020, 17, 98–108. [Google Scholar] [CrossRef]
- Furie, R.; Petri, M.; Zamani, O.; Cervera, R.; Wallace, D.J.; Tegzová, D.; Sanchez-Guerrero, J.; Schwarting, A.; Merrill, J.T.; Chatham, W.W.; et al. A phase III, randomized, placebo-controlled study of belimumab, a monoclonal antibody that inhibits B lymphocyte stimulator, in patients with systemic lupus erythematosus. Arthritis Rheum. 2011, 63, 3918–3930. [Google Scholar] [CrossRef]
- Smulski, C.R.; Eibel, H. BAFF and BAFF-Receptor in B Cell Selection and Survival. Front. Immunol. 2018, 9, 2285. [Google Scholar] [CrossRef] [PubMed]
- Stohl, W.; Metyas, S.; Tan, S.; Cheema, G.S.; Oamar, B.; Xu, D.; Roschke, V.; Wu, Y.; Baker, K.P.; Hilbert, D.M. B lymphocyte stimulator overexpression in patients with systemic lupus erythematosus: Longitudinal observations. Arthritis Rheum. 2003, 48, 3475–3486. [Google Scholar] [CrossRef]
- Mackay, F.; Woodcock, S.A.; Lawton, P.; Ambrose, C.; Baetscher, M.; Schneider, P.; Tschopp, J.; Browning, J.L. Mice Transgenic for Baff Develop Lymphocytic Disorders along with Autoimmune Manifestations. J. Exp. Med. 1999, 190, 1697–1710. [Google Scholar] [CrossRef]
- Wallace, D.J.; Ginzler, E.M.; Merrill, J.T.; Furie, R.A.; Stohl, W.; Chatham, W.W.; Weinstein, A.; McKay, J.D.; McCune, W.J.; Petri, M.; et al. Safety and Efficacy of Belimumab Plus Standard Therapy for Up to Thirteen Years in Patients With Systemic Lupus Erythematosus. Arthritis Rheumatol. 2019, 71, 1125–1134. [Google Scholar] [CrossRef]
- Vilas-Boas, A.; Morais, S.A.; Isenberg, D.A. Belimumab in systemic lupus erythematosus. RMD Open 2015, 1, e000011–e000011. [Google Scholar] [CrossRef]
- Furie, R.; Rovin, B.H.; Houssiau, F.; Malvar, A.; Teng, Y.O.; Contreras, G.; Amoura, Z.; Yu, X.; Mok, C.-C.; Santiago, M.B.; et al. Two-Year, Randomized, Controlled Trial of Belimumab in Lupus Nephritis. New Engl. J. Med. 2020, 383, 1117–1128. [Google Scholar] [CrossRef]
- Ginzler, E.M.; Wallace, D.J.; Merrill, J.T.; Furie, R.A.; Stohl, W.; Chatham, W.W.; Weinstein, A.; McKay, J.D.; McCune, W.J.; Zhong, Z.J.; et al. Disease Control and Safety of Belimumab Plus Standard Therapy Over 7 Years in Patients with Systemic Lupus Erythematosus. J. Rheumatol. 2013, 41, 300–309. [Google Scholar] [CrossRef] [PubMed]
- Furie RA, Wallace DJ, Aranow C, et al. Long-Term Safety and Efficacy of Belimumab in Patients with Systemic Lupus Erythematosus: A Continuation of a Seventy-Six-Week Phase III Parent Study in the United States. Arthritis Rheumatol. 2018 Jun;70(6):868-877. 2: PMID, 2940.
- Furie R, Rovin BH, Houssiau F, et al. Safety and Efficacy of Belimumab in Patients with Lupus Nephritis: Open-Label Extension of BLISS-LN Study. Clin J Am Soc Nephrol. 2022 Nov;17(11):1620-1630. 3: PMID, 3630.
- Levy RA, Gonzalez-Rivera T, Khamashta M, et al. 10 Years of belimumab experience: What have we learnt? Lupus. 2021 Oct;30(11):1705-1721. 3: PMID, 3423.
- Sans-Pola, C.; Danés, I.; Bosch, J. .; Marrero-Álvarez, P.; Cortés, J.; Agustí, A. Off-label use of rituximab in patients with systemic lupus erythematosus with extrarenal disease activity: a retrospective study and literature review. Front. Med. 2023, 10, 1159794. [Google Scholar] [CrossRef]
- Wardemann, H. , Nussenzweig M.C. B-cell self-tolerance in humans. Adv. Immunol. 8: 2007; 95, 2007. [Google Scholar]
- Shlomchik, M.J. Sites and Stages of Autoreactive B Cell Activation and Regulation. Immunity 2008, 28, 18–28. [Google Scholar] [CrossRef] [PubMed]
- Melchers, F. Checkpoints that control B cell development. J. Clin. Investig. 2015, 125, 2203–2210. [Google Scholar] [CrossRef]
- Rubin, S.J.S.; Bloom, M.S.; Robinson, W.H. B cell checkpoints in autoimmune rheumatic diseases. Nat. Rev. Rheumatol. 2019, 15, 303–315. [Google Scholar] [CrossRef]
- van Es, J.H.; Meyling, F.H.G.; van de Akker, W.R.; Aanstoot, H.; Derksen, R.H.; Logtenberg, T. Somatic mutations in the variable regions of a human IgG anti-double-stranded DNA autoantibody suggest a role for antigen in the induction of systemic lupus erythematosus. J. Exp. Med. 1991, 173, 461–470. [Google Scholar] [CrossRef]
- Winkler, T.H.; Fehr, H.; Kalden, J.R. Analysis of immunoglobulin variable region genes from human IgG anti-DNA hybridomas. Eur. J. Immunol. 1992, 22, 1719–1728. [Google Scholar] [CrossRef] [PubMed]
- Wellmann, U.; Letz, M.; Herrmann, M.; Angermüller, S.; Kalden, J.R.; Winkler, T.H. The evolution of human anti-double-stranded DNA autoantibodies. Proc. Natl. Acad. Sci. 2005, 102, 9258–9263. [Google Scholar] [CrossRef]
- Mietzner, B.; Tsuiji, M.; Scheid, J.; Velinzon, K.; Tiller, T.; Abraham, K.; Gonzalez, J.B.; Pascual, V.; Stichweh, D.; Wardemann, H.; et al. Autoreactive IgG memory antibodies in patients with systemic lupus erythematosus arise from nonreactive and polyreactive precursors. Proc. Natl. Acad. Sci. 2008, 105, 9727–9732. [Google Scholar] [CrossRef] [PubMed]
- Guo, W.; Smith, D.; Aviszus, K.; Detanico, T.; Heiser, R.A.; Wysocki, L.J. Somatic hypermutation as a generator of antinuclear antibodies in a murine model of systemic autoimmunity. J. Exp. Med. 2010, 207, 2225–2237. [Google Scholar] [CrossRef] [PubMed]
- Shlomchik, M.J.; Marshak-Rothstein, A.; Wolfowicz, C.B.; Rothstein, T.L.; Weigert, M.G. The role of clonal selection and somatic mutation in autoimmunity. Nature 1987, 328, 805–811. [Google Scholar] [CrossRef] [PubMed]
- Detanico, T.; Clair, J.B.S.; Aviszus, K.; Kirchenbaum, G.; Guo, W.; Wysocki, L.J. Somatic mutagenesis in autoimmunity. Autoimmunity 2013, 46, 102–114. [Google Scholar] [CrossRef] [PubMed]
- Schroeder, K.; Herrmann, M.; Winkler, T.H. The role of somatic hypermutation in the generation of pathogenic antibodies in SLE. Autoimmunity 2013, 46, 121–127. [Google Scholar] [CrossRef] [PubMed]
- Spitaler, M.; A Cantrell, D. Protein kinase C and beyond. Nat. Immunol. 2004, 5, 785–790. [Google Scholar] [CrossRef] [PubMed]
- Lim, P.S.; Sutton, C.R.; Rao, S. Protein kinase C in the immune system: from signalling to chromatin regulation. Immunology 2015, 146, 508–522. [Google Scholar] [CrossRef] [PubMed]
- Mochly-Rosen, D.; Das, K.; Grimes, K.V. Protein kinase C, an elusive therapeutic target? Nat. Rev. Drug Discov. 2012, 11, 937–957. [Google Scholar] [CrossRef]
- Altman, A.; Kong, K.-F. Protein kinase C inhibitors for immune disorders. Drug Discov. Today 2014, 19, 1217–1221. [Google Scholar] [CrossRef]
- Hampton, R.Y.; Morand, O.H. Sphingomyelin Synthase and PKC Activation. Science 1989, 246, 1050–1050. [Google Scholar] [CrossRef]
- Huitema, K.; van den Dikkenberg, J.; Brouwers, J.F.H.M.; Holthuis, J.C.M. Identification of a family of animal sphingomyelin synthases. EMBO J. 2004, 23, 33–44. [Google Scholar] [CrossRef] [PubMed]
- Ou, P.; Stanek, A.; Huan, Z.; Roman, C.A.; Huan, C. SMS2 deficiency impairs PKCδ-regulated B cell tolerance in the germinal center. Cell Rep. 2021, 36, 109624. [Google Scholar] [CrossRef] [PubMed]
- Salzer, E.; Santos-Valente, E.; Klaver, S.; Ban, S.A.; Emminger, W.; Prengemann, N.K.; Garncarz, W.; Müllauer, L.; Kain, R.; Boztug, H.; et al. B-cell deficiency and severe autoimmunity caused by deficiency of protein kinase C δ. Blood 2013, 121, 3112–3116. [Google Scholar] [CrossRef]
- Kuehn, H.S.; Niemela, J.E.; Rangel-Santos, A.; Zhang, M.; Pittaluga, S.; Stoddard, J.L.; Hussey, A.A.; Evbuomwan, M.O.; Priel, D.A.L.; Kuhns, D.B.; et al. Loss-of-function of the protein kinase C δ (PKCδ) causes a B-cell lymphoproliferative syndrome in humans. Blood 2013, 121, 3117–3125. [Google Scholar] [CrossRef] [PubMed]
- Belot, A.; Kasher, P.R.; Trotter, E.W.; Foray, A.; Debaud, A.; Rice, G.I.; Szynkiewicz, M.; Zabot, M.; Rouvet, I.; Bhaskar, S.S.; et al. Protein Kinase Cδ Deficiency Causes Mendelian Systemic Lupus Erythematosus With B Cell-Defective Apoptosis and Hyperproliferation. Arthritis Rheum. 2013, 65, 2161–2171. [Google Scholar] [CrossRef] [PubMed]
- Kiykim, A.; Ogulur, I.; Baris, S.; Salzer, E.; Karakoc-Aydiner, E.; Ozen, A.O.; Garncarz, W.; Hirschmugl, T.; Krolo, A.; Yucelten, A.D.; et al. Potentially Beneficial Effect of Hydroxychloroquine in a Patient with a Novel Mutation in Protein Kinase Cδ Deficiency. J. Clin. Immunol. 2015, 35, 523–526. [Google Scholar] [CrossRef]
- Nanthapisal, S.; Omoyinmi, E.; Murphy, C.; Standing, A.; Eisenhut, M.; Eleftheriou, D.; Brogan, P.A. Early-Onset Juvenile SLE Associated With a Novel Mutation in Protein Kinase C δ. PEDIATRICS 2017, 139, e20160781. [Google Scholar] [CrossRef]
- Lei, L.; Muhammad, S.; Al-Obaidi, M.; Sebire, N.; Cheng, I.L.; Eleftheriou, D.; Brogan, P. Successful use of ofatumumab in two cases of early-onset juvenile SLE with thrombocytopenia caused by a mutation in protein kinase C δ. Pediatr. Rheumatol. 2018, 16, 61. [Google Scholar] [CrossRef]
- Sharifinejad, N.; Azizi, G.; Behniafard, N.; Zaki-Dizaji, M.; Jamee, M.; Yazdani, R.; Abolhassani, H.; Aghamohammadi, A. Protein Kinase C-Delta Defect in Autoimmune Lymphoproliferative Syndrome-Like Disease: First Case from the National Iranian Registry and Review of the Literature. Immunol. Investig. 2020, 51, 331–342. [Google Scholar] [CrossRef]
- Salzer, E.; Santos-Valente, E.; Keller, B.; Warnatz, K.; Boztug, K. Protein Kinase C δ: a Gatekeeper of Immune Homeostasis. J. Clin. Immunol. 2016, 36, 631–640. [Google Scholar] [CrossRef]
- Mecklenbräuker, I.; Saijo, K.; Zheng, N.-Y.; Leitges, M.; Tarakhovsky, A. Protein kinase Cδ controls self-antigen-induced B-cell tolerance. Nature 2002, 416, 860–865. [Google Scholar] [CrossRef] [PubMed]
- Miyamoto, A. , Nakayama K., Imaki H., Hirose S., Jiang Y., Abe M., Tsukiyama T., Nagahama H., Ohno S., Hatakeyama S., Nakayama K.I. Increased proliferation of B cells and auto-immunity in mice lacking protein kinase C delta. Nature. 8: 2002; 416, 2002. [Google Scholar]
- Limnander, A.; Depeille, P.; Freedman, T.S.; Liou, J.; Leitges, M.; Kurosaki, T.; Roose, J.P.; Weiss, A. STIM1, PKC-δ and RasGRP set a threshold for proapoptotic Erk signaling during B cell development. Nat. Immunol. 2011, 12, 425–433. [Google Scholar] [CrossRef] [PubMed]
- Limnander, A.; Zikherman, J.; Lau, T.; Leitges, M.; Weiss, A.; Roose, J.P. Protein Kinase Cδ Promotes Transitional B Cell-Negative Selection and Limits Proximal B Cell Receptor Signaling To Enforce Tolerance. Mol. Cell. Biol. 2014, 34, 1474–1485. [Google Scholar] [CrossRef]
- Mecklenbräuker, I.; Kalled, S.L.; Leitges, M.; Mackay, F.; Tarakhovsky, A. Regulation of B-cell survival by BAFF-dependent PKCδ-mediated nuclear signalling. Nature 2004, 431, 456–461. [Google Scholar] [CrossRef] [PubMed]
- Ajiro, K. Histone H2B Phosphorylation in Mammalian Apoptotic Cells. J. Biol. Chem. 2000, 275, 439–443. [Google Scholar] [CrossRef] [PubMed]
- Xie, P.; Stunz, L.L.; Larison, K.D.; Yang, B.; Bishop, G.A. Tumor Necrosis Factor Receptor-Associated Factor 3 Is a Critical Regulator of B Cell Homeostasis in Secondary Lymphoid Organs. Immunity 2007, 27, 253–267. [Google Scholar] [CrossRef] [PubMed]
- Moreews, M.; Mathieu, A.-L.; Pouxvielh, K.; Reuschlé, Q.; Drouillard, A.; Dessay, P.; Meignien, M.; Zhang, J.; Fallone, L.; Rousseaux, N.; et al. mTOR Activation Underlies Enhanced B Cell Proliferation and Autoimmunity in PrkcdG510S/G510S Mice. J. Immunol. 2023, 210, 1209–1221. [Google Scholar] [CrossRef]
- Ersching, J.; Efeyan, A.; Mesin, L.; Jacobsen, J.T.; Pasqual, G.; Grabiner, B.C.; Dominguez-Sola, D.; Sabatini, D.M.; Victora, G.D. Germinal Center Selection and Affinity Maturation Require Dynamic Regulation of mTORC1 Kinase. Immunity 2017, 46, 1045–1058. [Google Scholar] [CrossRef] [PubMed]
- Barceló-Coblijn, G.; Martin, M.L.; de Almeida, R.F.M.; Noguera-Salvà, M.A.; Marcilla-Etxenike, A.; Guardiola-Serrano, F.; Lüth, A.; Kleuser, B.; Halver, J.E.; Escribá, P.V. Sphingomyelin and sphingomyelin synthase (SMS) in the malignant transformation of glioma cells and in 2-hydroxyoleic acid therapy. Proc. Natl. Acad. Sci. USA 2011, 108, 19569–19574. [Google Scholar] [CrossRef]
- Terés, S.; Lladó, V.; Higuera, M.; Barceló-Coblijn, G.; Martin, M.L.; Noguera-Salvà, M.A.; Marcilla-Etxenike, A.; García-Verdugo, J.M.; Soriano-Navarro, M.; Saus, C.; et al. 2-Hydroxyoleate, a nontoxic membrane binding anticancer drug, induces glioma cell differentiation and autophagy. Proc. Natl. Acad. Sci. 2012, 109, 8489–8494. [Google Scholar] [CrossRef]
- Azaro, A.; Plummer, E.R.; Urruticoechea, A.; Rodon, J.; Haris, N.R.M.; Veal, G.; Perier, A.; Tur, V.; Escriba, P.V.; Busquets, X.; et al. Final report of a phase I study of 2-hydroxyoleic acid (2OHOA) a novel sphingomyelin synthase activator in patients (pt) with advanced solid tumors (AST) including recurrent high grade gliomas (rHGG). J. Clin. Oncol. 2017, 35, 2554–2554. [Google Scholar] [CrossRef]
- Huang, W.; Quach, T.D.; Dascalu, C.; Liu, Z.; Leung, T.; Byrne-Steele, M.; Pan, W.; Yang, Q.; Han, J.; Lesser, M.; et al. Belimumab promotes negative selection of activated autoreactive B cells in systemic lupus erythematosus patients. J. Clin. Investig. 2018, 3. [Google Scholar] [CrossRef] [PubMed]
| Reference | Human/Mouse | PKCRD/Prkcd mutations | Relevant Findings |
|---|---|---|---|
| Salzer et al., [49] Blood 2013 | Human | Loss of function splice-site mutation within the catalytic domain of PRKCD c.1352 + 1G>A. No expression of PKCδ. |
Severe autoimmunity with membranous glomerulonephritis, hepatosplenomegaly and generalized lymphadenopathy. Positive for anti-nuclear antibodies and anti-dsDNA antibodies. |
| Kuehn et al. [50], Blood 2013 | Human |
PRKCD c.1840C>T, p.Arg614Trp Reduced PKCδ expression. |
Autoimmunity with chronic lymphadenopathy, splenomegaly, autoantibodies and elevated immunoglobulins, similar to the phenotype observed in PKCδ deficient mice. Strong positive for anti-nuclear antibodies, and negative for anti-dsDNA antibodies. |
| Belot et al. [51], Arthritis & Rheumatism 2013 | Human |
PRKCD c.1258G>A p.Gly510Ser Reduced expression and activity of PKCδ. |
Lupus autoimmunity with lupus nephritis. Patients have increased number of immature B cells in association with increased proliferation and decreased apoptosis. Positive for anti-nuclear antibodies and anti-dsDNA antibodies. |
| Kiykim et al. [52], Journal of Clinical Immunology 2015 | Human | PRKCD c.742G>A p.Gly248Ser | Lupus like disorder with erythematous skin rash. The patient has increased CD19+ B cells and naïve B cells. Positive for anti-nuclear antibodies, and negative for anti-dsDNA antibodies. |
| Nanthapisal et al. [53], Pediatrics 2017 | Human | PRKCD c.1294G>T; p.Gly432Trp | Lupus autoimmunity with scarring alopecia, rash affecting the scalp, a photosensitive malar rash, and hepatosplenomegaly. Positive for anti-nuclear antibodies and anti-dsDNA antibodies. |
| Lei et al. [54], Pediatr Rheumatol Online J. 2018 | Human | PRKCD c.1294G > T; p.Gly432Trp | Lupus autoimmunity with acute cutaneous lupus, non-scarring alopecia, haemolytic anaemia, and thrombocytopenia. Positive for anti-nuclear antibodies and anti-dsDNA antibodies. |
| Sharifinejad et al. [55], Immunol Invest. 2022 |
Human | PRKCD c.1293_1294insA | Autoimmunity with lymphoproliferation, recurrent pneumonia, cardiomyopathy, and dermatological manifestations. |
| Mecklenbrauke et al. [57], Nature 2002 | Mouse | Targeted disruption of prkcd by replacing Exon1 with a Lacz/neo cassette. No expression of PKCδ. |
A lupus-like autoimmune phenotype with splenomegaly and lymphadenopathy. The mice have increased numbers of B cells. Positive for anti-nuclear antibodies and anti-DNA antibodies. |
| Miyamoto et al. [58], Nature 2002 | Mouse | Targeted disruption of prkcd by replacing Exon1 and Exon 2 with a neomycin-resistance cassette. No expression of PKCδ. |
A Lupus-like phenotype with glomerulonephritis. The mice have expanded B cell population and spontaneous formation of numerous GCs. Positive for anti-chromatin antibodies. |
| Limnander et al. [59], Nature Immunology 2011 | Mouse | [43] Targeted disruption of prkcd by replacing Exon1 with a LacZ/neo cassette. No expression of PKCδ. |
Impaired activation of pro-apoptotic Ca2+-ERK pathway during negative selection of immature bone marrow B cells. |
| Limnander et al. [60], MCB 2014 | Mouse | [43] Targeted disruption of prkcd by replacing Exon1 with a LacZ/neo cassette. No expression of PKCδ. |
Impaired antigen-dependent negative selection of splenic transitional B cells. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).





