1. Introduction
Meningioma, a primary intracranial tumor originating from the meninges enveloping the brain and spinal cord, predominantly manifests as a benign neoplasm, with only a few exhibiting malignant characteristics [
1,
2]. Currently, they account for 40.8% of all primary intracranial neoplasms [
3]. Surgical resection remains the primary treatment modality for symptomatic or proliferating meningiomas; however, stereotactic radiosurgery (SRS) has emerged as an effective adjuvant or alternative intervention for low-grade meningiomas. This is particularly relevant for tumors close to critical anatomical structures, recurrent lesions, or cases where resection or general anesthesia poses a high risk to patients [
4,
5,
6].
Numerous studies have substantiated the efficacy of SRS in achieving robust tumor control and preserving neurological function over short and long-term durations [
7,
8,
9,
10,
11,
12,
13,
14]. In a comprehensive systematic review by Marchetti et al.[
9], encompassing findings from the International Stereotactic Radiosurgery Society, single-fraction SRS at a prescribed dose of 12–15 Gy for meningiomas exhibited notable efficacy. The results revealed 10-year local control rates (LCR) of 71–100% and progression-free survival (PFS) rates of 55–97%.9 Additional investigations corroborated these findings, demonstrating a LCR of 87–100%, particularly when the administered dose was within the range of 12–16 Gy. Notably, a 10-year LCR >90% has been consistently observed in World Health Organization grade I meningiomas [
8,
15].
SRS is extensively used in treating meningiomas; however, a degree of uncertainty persists regarding the optimal radiation dosage, enduring implications on lesion control, and the potential for radiation-induced complications. A universally accepted guideline for dose selection is absent, compelling practitioners to rely on the amalgamation of empirical data and institutional experience.15 Therefore, it is imperative to continually refine our understanding of the most reasonable radiation dosing for meningiomas, necessitating delicate equilibrium between effective tumor control and the mitigation of treatment-related adverse effects.
The treatment paradigm has undergone a transformative evolution at our institution. Previously, we administered a comparatively elevated dose of ≥14 Gy to patients with meningiomas who underwent SRS. However, guided by our accrued experience and an expanding body of evidence, a deliberate shift in strategy has occurred, leading to a recent reduction in the mean prescribed dose to <14 Gy. This study comprehensively examined the clinical outcomes and associated toxicities of radiosurgery for meningiomas. The study investigated parameters such as LCR and radiation-induced peritumoral edema (PTE) using a comparative analysis between the two cohorts subjected to distinct radiation doses. The overarching objective was to provide contemporary insights into the optimal radiation dose, thereby contributing valuable perspectives for informed clinical decision-making.
2. Materials and Methods
This retrospective study comprehensively examined the medical records and radiology reports of patients subjected to SRS for benign meningioma. Diagnosis involved histopathological findings through open resection or the identifying characteristic imaging features consistent with benign meningioma, validated by a consensus between neurosurgeons and neuroradiologists based on magnetic resonance imaging (MRI) observations. We treated 162 patients with meningiomas at our institution using TrueBeam radiosurgery between March 2014 and December 2022. The inclusion criteria were benign meningioma diagnosis and undergoing single-session SRS, either as a primary intervention or as an adjuvant measure. Individuals who underwent fractionated or repeated radiosurgery for identical lesions were excluded to minimize selection bias. Patients lost to follow-up were also excluded due to the unavailability of treatment outcome data.
Pretreatment high-resolution T1-weighted MRI with a slice thickness of 1 mm and gadolinium enhancement was acquired for treatment planning. In addition, a contrast-enhanced computed tomography (CT) scan, with a slice thickness of 1.5 mm, was conducted with the patient immobilized in a thermoplastic mask using a compatible fiducial localizer. The MRI and CT images were integrated, with subsequent delineation of the target and all organs at risk performed on the MR images, using the iPlan RT Image version 4.1 and iPlan RT Dose version 4.5 planning software (Brainlab, Feldkirchen, Germany). Typically, lesions were subjected to an 85–90% isodose line. Single isocenter treatment plans were executed for all patients employing several static beams or dynamic conformal arcs with three to five gantry positions. The total dose was determined based on the target pathology, lesion size, previous treatments, and proximity to critical structures. The prescribed dose was delivered to each patient in a single fraction through a Varian TrueBeam STx linear accelerator (Varian Medical Systems, Palo Alto, CA). After treatment, clinical examination and imaging follow-up were conducted 6 months after radiosurgery, followed by annual assessments.
The investigated variables included age, sex, meningioma location, prior resection history, histologic subtype, initial target volume, and various irradiation parameters, including prescription dose, conformity index (CI), and coverage. The primary outcome measures for all enrolled patients were LCR, PFS, and radiation-induced toxicity. A secondary analysis compared LCR and PTE between the two groups stratified based on their prescription doses: ≥14 Gy (Group 1) and <14 Gy (Group 2). The period of local tumor control was defined as the time between initial radiosurgery and the date of uncontrolled or recurrent lesion identified on follow-up images. LCR was categorized following the Response Assessment in Neuro-Oncology Working Group criteria as [
16]: 1) complete response (CR), signifying the total disappearance of the target lesion, 2) partial response (PR), indicating a reduction of the sum of the maximal perpendicular diameters by ≥50% relative to baseline, 3) minor response (MR), denoting a decrease between 25% and 50%, encompassing 25%, 4) stable disease (SD), signifying cases that do not fit other classifications, such as <25% decrease but <25% increase in area relative to nadir, 5) progressive disease (PD), encompassing an increasing lesion size >25%. Radiation-induced toxicity was evaluated and categorized according to the Common Terminology Criteria for Adverse Events (CTCAE) version 5.0 [
17].
This study adhered to the guidelines stipulated in the Strengthening the Reporting of Observational Studies in Epidemiology statement. All data acquisition and analysis procedures were approved by the Institutional Review Board (IRB number: #2023-10-016), and the need for written informed consent was waived.
All statistical analyses were performed using SPSS version 20 (IBM, Armonk, NY). The primary outcome measures were assessed by computing the LCR, PFS, and radiation-induced toxicity estimates from the date of the initial treatment using the Kaplan–Meier method. The log-rank test was employed for the significant comparisons of LCR and PTE between the two groups in the secondary analysis. Continuous variables were analyzed using the t-test, whereas categorical variables were examined using the chi-square and Fisher’s exact tests. The Cox proportional hazards method was used to identify predictors of LCR and PTE. Factors with a p-value < 0.05 in univariate analysis were entered into a multivariate analysis. Statistical significance was set at P < 0.05.
3. Results
3.1. Demographics
Between March 2014 and December 2022, 162 patients underwent SRS for 190 meningiomas at our hospital. This study enrolled 147 patients with 164 treated lesions following the exclusion of 15 patients with 26 lesions, comprising 8 meningiomas in 8 patients subjected to fractionated SRS, 7 meningiomas in 7 patients lost to follow-up, and 11 repeatedly treated meningiomas.
The mean age of the cohort was 61 years (range, 37–79 years), with 35 males (23.8%) and 112 females (76.2%). Most patients (55.1%) were asymptomatic, whereas the rest presented with diverse symptoms, including headache, dizziness, visual disturbances, nausea, motor weakness, hearing decline, facial pain, facial palsy, tremors, and seizures. Objective neurological manifestations, including hemiparesis, dysesthesia, visual impairment, and cranial nerve deficits, were observed in 13 patients (8.8%). Diagnostic modalities included MRI in 122 patients, whereas 25 patients (17%) with a history of open resection underwent histopathological confirmation. The lesion distribution included 60 skull-base meningiomas (36.6%) and 104 non-skull-base meningiomas (63.4%). The median follow-up duration was 47 months (range, 12–122 months).
Table 1 presents a detailed overview of the patients’ clinical characteristics.
3.2. Tumor Control
The average target volume for single-session SRS was 4.49 cm
3 (range, 0.33–13.9 cm
3), with a median dose of 14 Gy (range, 12–16 Gy). The mean coverage was 99.32% (range, 90–100%), and the mean CI was 1.80 (range, 1–4.62). Dose parameters exhibited a maximum dose of 16.3 Gy (range, 14.2–23.6 Gy), a minimum dose of 12.3 Gy (range, 5.8–15.2 Gy), and a mean dose of 15.5 Gy (range, 12.8–19.2 Gy). The two groups showed no significant differences in the target volume, lesion location distribution, or treatment parameters, except for the radiation dose, as detailed in
Table 2.
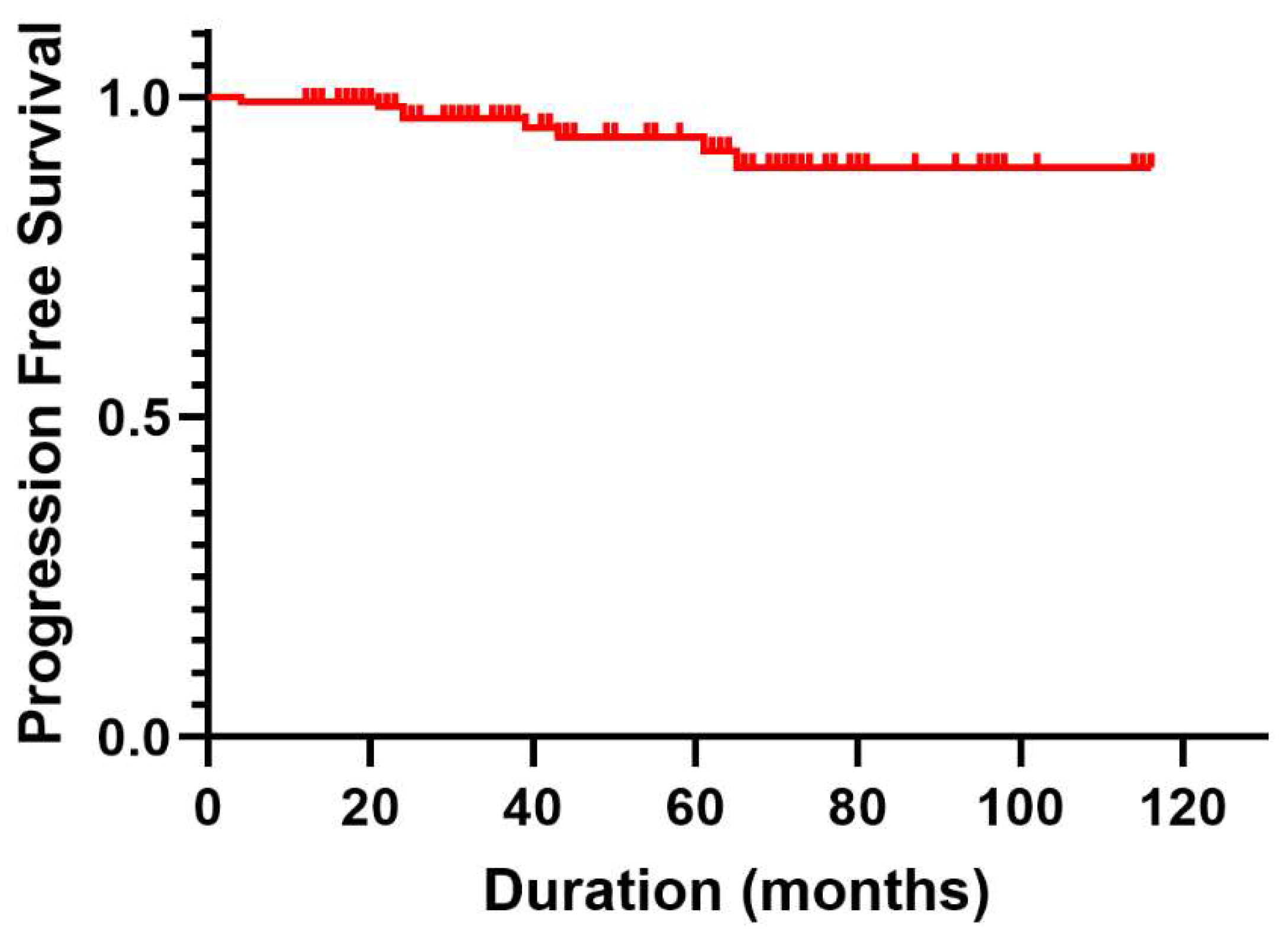
During follow-up period, progression occurred in eight patients (5.4%). Notably, the 1-, 2-, and 5-year PFS rates were 99.3%, 96.7%, and 93.8%, respectively, as illustrated by the Kaplan–Meier curves in
Figure 1. Among the eight patients with recurrent meningioma, three underwent open resection, five underwent repeat SRS, and one who underwent repeat SRS for meningothelial meningioma exhibited malignant progression to atypical meningioma ultimately requiring resection.
The study cohort`s overall crude LCR was 95.1%. No CR was observed; however, PR was observed in 10 lesions (6.1%), MR in 12 lesions (7.3%), and SD in 134 lesions (81.7%). Only eight lesions (4.9%) displayed signs of PD, necessitating additional SRS or resection, as summarized in
Table 3. The LCR over different time intervals were estimated as 99.4%, 97.0%, and 94.5% at 1-, 2-, and 5-year follow-ups, respectively. When comparing the LCR between the two groups (Group 1 and Group 2) with four cases of PD in each group, no significant difference was observed (P = 0.628). The specific LCRs for each group at different time points were as follows: 1-year LCR (Group 1: 98.4% vs. Group 2: 100.0%), 2-year LCR (Group 1: 96.6% vs. Group 2: 97.2%), and 5-year LCR (Group 1: 94.7% vs. Group 2: 93.6%). Univariate and multivariate analysis demonstrated that prior surgery and tumor volume >10 cm
3 were significantly related to local tumor control (
Table 4).
3.3. Radiation Induced Toxicity
Radiation-induced adverse events, categorized according to the CTCAE, were collectively observed in 36 of 147 patients (24.5%). This included 27 patients with CTCAE Grade 1 (20.4%), three with Grade 2 (2.0%), five with Grade 3 (4.1%), and one with Grade 4 (0.7%) toxicity. During the acute phase (within 3 weeks post-SRS), symptoms such as nausea, lethargy, and headache were reported in 11 patients with Grade 1 toxicity. In addition, three patients with Grade 2 toxicity experienced facial numbness and pain 3 months after SRS, which were effectively managed with medication, and one patient with Grade 3 toxicity presented with new-onset generalized seizures, necessitating additional antiseizure medications.
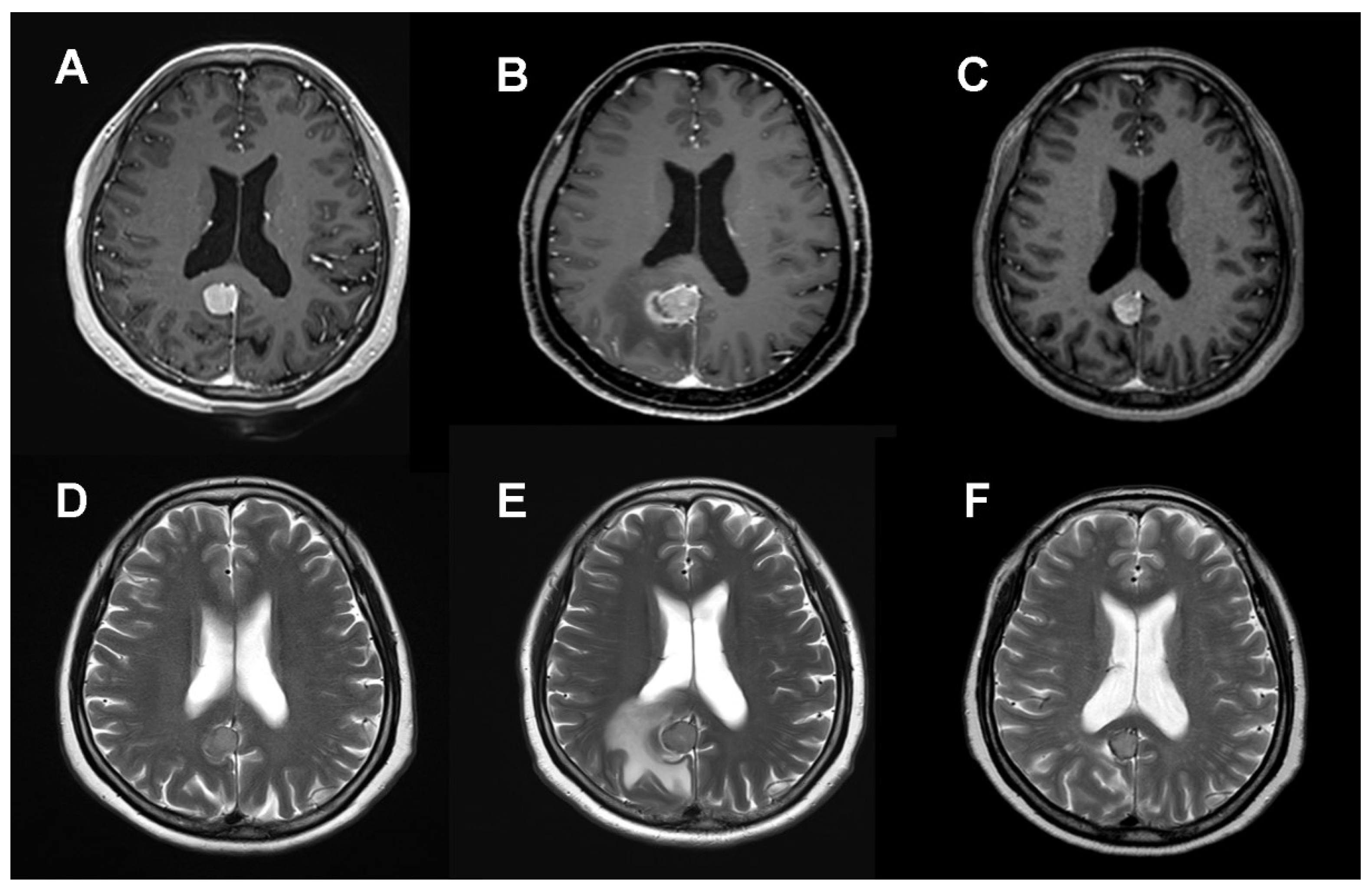
Notably, no instances of clinical or radiological radiation necrosis were identified post-SRS. Radiation-induced PTE directly attributable to SRS was observed in 21 of 164 lesions (12.8%), manifesting approximately 6 months post-treatment (
Figure 2). Among these cases, 16 (9.8%) were classified as CTCAE Grade 1–2, and 5 (3.0%) as CTCAE Grade 3–4. Asymptomatic mild edema, which required no active intervention, was observed in 16 patients. In cases of symptomatic PTE (CTCAE 3), four patients were managed with oral (three cases) and intravenous steroids (one case). Only one case necessitated open resection due to uncontrolled seizures associated with abnormal pachymeningeal thickening around the tumor and PTE (CTCAE 4). A comparison of the PTE incidence between the two groups revealed a significantly higher frequency in Group 1 (12 lesions, 19.7%) than in Group 2 (nine lesions, 8.7%) (P = 0.039). Furthermore, severe edema (CTCAE Grade 3–4) was more prevalent in Group 1 (6.6%) than in Group 2 (1.9%) (P = 0.042). When evaluating the factors related to new-onset or worsened edema after SRS, pre-existing PTE and tumor volume >10 cm
3 were significantly associated both in univariate and multivariate analysis. Marginal dose ≥14 Gy (Group 1) showed meaningful significance in univariate analysis but not in multivariate analysis (
Table 4).
4. Discussion
The primary objective in managing benign meningiomas is to attain sustained, long-term control, achievable through surgical intervention or radiosurgery. Specifically, within the SRS domain, the administered radiation dose is a pivotal determinant in accomplishing effective local control. Commonly reported SRS doses range from 12–18 Gy, meticulously tailored to consider tumor size and its proximity to critical anatomical structures [
15,
18,
19]. Numerous studies have attempted to ascertain the optimal radiation dose for low-grade meningiomas. However, most of these investigations are retrospective, emanating from single-center studies characterized by heterogeneous patient cohorts. Only a few studies have directly compared distinct radiation doses.
Ganz et al. [
20] identified an increased risk of treatment failure in cases where the tumor edge doses were <10 Gy, compared with the group receiving doses >12 Gy, thereby proposing 12 Gy as the minimum threshold for efficacious SRS in meningioma treatment. Conversely, another study demonstrated no discernible advantage in tumor control with marginal doses surpassing 15 Gy compared with doses below this threshold [
21]. Similarly, no significant difference was found in the LCR for benign meningiomas at the 5-year mark when contrasting doses were <16 Gy and >16 Gy, suggesting that higher doses may not uniformly confer additional benefits [
22]. In a long-term retrospective study, elevated recurrence rates were reported in patients receiving doses of ≤13.4 Gy, highlighting the intricate balance required in determining an optimal dose that balances efficacy and safety [
23]. Pollock et al. [
24] corroborated these findings and reported a 10-year LCR of 99.4% with a mean tumor margin dose of 15.8 Gy in an updated study. Collectively, these studies underscore the importance of a personalized approach in radiation therapy, factoring in the minimum effective dose and potential risks associated with higher doses. However, these insights, predominantly derived from single-center investigations, are yet to establish definitive dosing guidelines. Consequently, reliance on recommendations from authoritative bodies such as the Radiation and Oncology Advisory Committee on Radiation Oncology Practice and the National Comprehensive Cancer Network has been advocated, suggesting a dose range of 12–16 Gy [
25,
26].
At our institution, adhering to the recommended radiation dose for SRS for treating low-grade meningiomas is a consistent practice. However, periods of dose reduction have been implemented, allowing us to compare two distinct cohorts subjected to varying radiation doses.
Patients diagnosed with benign meningiomas generally exhibit a favorable long-term prognosis; however, it is imperative to consider the potential toxicity and delayed effects associated with the treatment itself. The occurrence and nature of toxic effects are contingent on variables such as tumor size and location.4 In our study, instances of radiation-induced toxicity were observed in 40 of the 147 patients (27.2%) as assessed using the CTCAE. This observed frequency exceeded the overall rate of 8.1% (range, 2.5–28.2) reported in previous meta-analyses [
4,
13,
27]. This discrepancy may be attributed to the heterogeneity in the definition of radiation-induced toxicity and variations in the evaluation tools employed. Our study scrutinized even mild clinical symptoms using CTCAE following SRS, with more clinically symptomatic events (CTCAE grade 3–4) accounting for only 4.8% (7 of 147 patients), consistent with findings from previous investigations.
In addition, the emergence of new-onset or exacerbated PTE constitutes an objective imaging finding that is pivotal in determining treatment outcomes. Previous studies have reported that the incidence of PTE in patients undergoing SRS for meningiomas ranges from 15% to 28% [
28,
29,
30,
31,
32,
33]. Factors such as larger targeted tumor volume, hemispheric tumor location, pre-existing PTE before SRS, and higher marginal dose or maximum dose have been associated with an elevated risk of PTE [
28,
31,
34]. Our results also showed that large tumor volume >10 cm
3 and pre-existing PTE were significantly related to PTE after SRS. Regarding dose prescription, significant association was observed between a marginal dose >16 Gy and post-SRS PTE [
31]. Similarly, higher frequency of post-SRS complications was reported in cases with a median marginal dose of 17 Gy compared with 14 Gy [
10]. Huang et al. demonstrated that total marginal dose >14 Gy significantly affected the occurrence of peritumoral edema after SRS, similar to our result [
35]. The relative edema indices reach their maximum values at 11 months post-SRS and subsequently decline, with symptom resolution occurring within 24 months in most patients [
29,
30,
34,
36]. In the present study, PTE was observed in 21 of 164 lesions (12.8%), with the majority manifesting at 6 months post-treatment. Comparing the overall incidence of PTE between the two groups showed a disparity, with rates of 19.7% in Group 1 and 8.7% in Group 2.
Collectively, these observations imply that the prescription of radiation doses ≥14 Gy in SRS treatment for benign meningiomas yields no substantial advantages in tumor control while significantly amplifying the incidence of radiation-induced side effects. Consequently, based on the conclusions drawn from this study, radiation doses <14 Gy should be considered in SRS treatment for patients with benign meningiomas.
This study was inherently constrained by its single-center, modest sample size and retrospective design. Furthermore, a substantial proportion of the enrolled patients underwent SRS for radiographically presumed benign meningiomas, potentially encompassing higher-grade tumors, thereby introducing a potential confounding variable that may adversely impact study outcomes. Notably, Group 2 patients receiving radiation doses <14 Gy were treated relatively recently, resulting in a shorter mean follow-up duration than that of Group 1. This temporal discrepancy limits the comparability of outcomes, particularly in long-term follow-up assessments.
Nevertheless, the significance of this study lies in its exclusive consideration of the radiation dose as a variable, with a deliberate effort to mitigate various confounding factors. Future investigations should adopt a structured approach, necessitating large-scale prospective studies with extended follow-up periods to pursue safer and more effective treatments for benign meningiomas.
5. Conclusions
SRS is a highly effective therapeutic modality for benign meningiomas and serves as a complementary intervention to open surgery. A critical determinant of SRS efficacy is the administered radiation dose. Higher radiation doses are traditionally correlated with enhanced tumor control; however, they concurrently increase the likelihood of treatment-related complications, underscoring the necessity for a nuanced equilibrium between LCR and complication rates. This study revealed that a radiation dose <14 Gy did not result in a statistically significant variance in LCR; however, it was associated with a diminished toxicity rate. Therefore, such a dose regimen should be considered in future treatment strategies to balance therapeutic efficacy and safety.
Author Contributions
Conceptualization, H.J.C., J.B.P., and N.Y.J.; methodology, H.J.C., and J.M.L.; software, N.Y.J.; validation, J.B.P. and N.Y.J.; formal analysis, S.H.P. and N.Y.J.; investigation, H.J.C., S.H.P., and N.Y.J.; data curation, S.H.P. and N.Y.J.; writing—original draft preparation, H.J.C.; writing—review and editing, J.B.P. and N.Y.J.; visualization, S.H.P. and N.Y.J.; supervision, N.Y.J. All authors have read and agreed to the published version of the manuscript.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Institutional Review Board Statement
The study was conducted according to the guidelines stipulated in the Strengthening the Reporting of Observational Studies in Epidemiology statement. All data acquisition and analysis procedures were approved by the Institutional Review Board (IRB number: #2023-10-016).
Informed Consent Statement
The need for written informed consent was waived due to retrospective design of this study.
Acknowledgments
The authors thank Ms. Hyang Soon Lee and Mr. Wang Choon Lee for arranging clinical data.
Conflicts of Interest
The authors declare no conflicts of interest concerning the materials used in this study or the findings specified in this paper.
References
- Louis, D.N.; Perry, A.; Reifenberger, G.; von Deimling, A.; Figarella-Branger, D.; Cavenee, W.K.; Ohgaki, H.; Wiestler, O.D.; Kleihues, P.; Ellison, D.W. The 2016 World Health Organization Classification of Tumors of the Central Nervous System: a summary. Acta Neuropathol 2016, 131, 803-820. [CrossRef]
- Cushing, H. The meningiomas (dural endotheliomas). Their source and favoured seats of origin. Brain : a journal of neurology. 1922, 45, 282-316.
- Ostrom, Q.T.; Price, M.; Neff, C.; Cioffi, G.; Waite, K.A.; Kruchko, C.; Barnholtz-Sloan, J.S. CBTRUS Statistical Report: Primary Brain and Other Central Nervous System Tumors Diagnosed in the United States in 2016-2020. Neuro Oncol 2023, 25, iv1-iv99. [CrossRef]
- Pinzi, V.; Biagioli, E.; Roberto, A.; Galli, F.; Rizzi, M.; Chiappa, F.; Brenna, G.; Fariselli, L.; Floriani, I. Radiosurgery for intracranial meningiomas: A systematic review and meta-analysis. Crit Rev Oncol Hematol 2017, 113, 122-134. [CrossRef]
- Goldbrunner, R.; Minniti, G.; Preusser, M.; Jenkinson, M.D.; Sallabanda, K.; Houdart, E.; von Deimling, A.; Stavrinou, P.; Lefranc, F.; Lund-Johansen, M.; et al. EANO guidelines for the diagnosis and treatment of meningiomas. Lancet Oncol 2016, 17, e383-391. [CrossRef]
- Park, C.K.; Jung, N.Y.; Chang, W.S.; Jung, H.H.; Chang, J.W. Gamma Knife Radiosurgery for Postoperative Remnant Meningioma: Analysis of Recurrence Factors According to World Health Organization Grade. World Neurosurg 2019, 132, e399-e402. [CrossRef]
- Kondziolka, D.; Mathieu, D.; Lunsford, L.D.; Martin, J.J.; Madhok, R.; Niranjan, A.; Flickinger, J.C. Radiosurgery as definitive management of intracranial meningiomas. Neurosurgery 2008, 62, 53-58; discussion 58-60. [CrossRef]
- Buerki, R.A.; Horbinski, C.M.; Kruser, T.; Horowitz, P.M.; James, C.D.; Lukas, R.V. An overview of meningiomas. Future Oncol 2018, 14, 2161-2177. [CrossRef]
- Marchetti, M.; Sahgal, A.; De Salles, A.A.F.; Levivier, M.; Ma, L.; Paddick, I.; Pollock, B.E.; Regis, J.; Sheehan, J.; Suh, J.H.; et al. Stereotactic Radiosurgery for Intracranial Noncavernous Sinus Benign Meningioma: International Stereotactic Radiosurgery Society Systematic Review, Meta-Analysis and Practice Guideline. Neurosurgery 2020, 87, 879-890. [CrossRef]
- Flickinger, J.C.; Kondziolka, D.; Maitz, A.H.; Lunsford, L.D. Gamma knife radiosurgery of imaging-diagnosed intracranial meningioma. International journal of radiation oncology, biology, physics 2003, 56, 801-806. [CrossRef]
- Kondziolka, D.; Lunsford, L.D.; Coffey, R.J.; Flickinger, J.C. Stereotactic radiosurgery of meningiomas. J Neurosurg 1991, 74, 552-559. [CrossRef]
- Kondziolka, D.; Patel, A.D.; Kano, H.; Flickinger, J.C.; Lunsford, L.D. Long-term Outcomes After Gamma Knife Radiosurgery for Meningiomas. American journal of clinical oncology 2016, 39, 453-457. [CrossRef]
- Kreil, W.; Luggin, J.; Fuchs, I.; Weigl, V.; Eustacchio, S.; Papaefthymiou, G. Long term experience of gamma knife radiosurgery for benign skull base meningiomas. Journal of neurology, neurosurgery, and psychiatry 2005, 76, 1425-1430. [CrossRef]
- Williams, B.J.; Yen, C.P.; Starke, R.M.; Basina, B.; Nguyen, J.; Rainey, J.; Sherman, J.H.; Schlesinger, D.; Sheehan, J.P. Gamma Knife surgery for parasellar meningiomas: long-term results including complications, predictive factors, and progression-free survival. J Neurosurg 2011, 114, 1571-1577. [CrossRef]
- Rogers, L.; Barani, I.; Chamberlain, M.; Kaley, T.J.; McDermott, M.; Raizer, J.; Schiff, D.; Weber, D.C.; Wen, P.Y.; Vogelbaum, M.A. Meningiomas: knowledge base, treatment outcomes, and uncertainties. A RANO review. J Neurosurg 2015, 122, 4-23. [CrossRef]
- Huang, R.Y.; Bi, W.L.; Weller, M.; Kaley, T.; Blakeley, J.; Dunn, I.; Galanis, E.; Preusser, M.; McDermott, M.; Rogers, L.; et al. Proposed response assessment and endpoints for meningioma clinical trials: report from the Response Assessment in Neuro-Oncology Working Group. Neuro Oncol 2019, 21, 26-36. [CrossRef]
- Common Terminology Criteria for Adverse Events (CTCAE) Version 5. Published: November 27. US Department of Health and Human Services, National Institutes of Health, National Cancer Institute doi:https://view.officeapps.live.com/op/view.aspx?src=https%3A%2F%2Fctep.cancer.gov%2Fprotocoldevelopment%2Felectronic_applications%2Fdocs%2FCTCAE_v5.0.xlsx&wdOrigin=BROWSELINK.
- Shin, M.; Kurita, H.; Sasaki, T.; Kawamoto, S.; Tago, M.; Kawahara, N.; Morita, A.; Ueki, K.; Kirino, T. Analysis of treatment outcome after stereotactic radiosurgery for cavernous sinus meningiomas. J Neurosurg 2001, 95, 435-439. [CrossRef]
- Iwai, Y.; Yamanaka, K.; Ikeda, H. Gamma Knife radiosurgery for skull base meningioma: long-term results of low-dose treatment. J Neurosurg 2008, 109, 804-810. [CrossRef]
- Ganz, J.C.; Backlund, E.O.; Thorsen, F.A. The results of Gamma Knife surgery of meningiomas, related to size of tumor and dose. Stereotact Funct Neurosurg 1993, 61 Suppl 1, 23-29. [CrossRef]
- Kondziolka, D.; Flickinger, J.C.; Perez, B. Judicious resection and/or radiosurgery for parasagittal meningiomas: outcomes from a multicenter review. Gamma Knife Meningioma Study Group. Neurosurgery 1998, 43, 405-413; discussion 413-404. [CrossRef]
- Stafford, S.L.; Pollock, B.E.; Foote, R.L.; Link, M.J.; Gorman, D.A.; Schomberg, P.J.; Leavitt, J.A. Meningioma radiosurgery: tumor control, outcomes, and complications among 190 consecutive patients. Neurosurgery 2001, 49, 1029-1037; discussion 1037-1028. [CrossRef]
- Lippitz, B.E.; Bartek, J., Jr.; Mathiesen, T.; Forander, P. Ten-year follow-up after Gamma Knife radiosurgery of meningioma and review of the literature. Acta Neurochir (Wien) 2020, 162, 2183-2196. [CrossRef]
- Pollock, B.E.; Stafford, S.L.; Link, M.J.; Garces, Y.I.; Foote, R.L. Single-fraction radiosurgery for presumed intracranial meningiomas: efficacy and complications from a 22-year experience. International journal of radiation oncology, biology, physics 2012, 83, 1414-1418. [CrossRef]
- Combs, S.E.; Baumert, B.G.; Bendszus, M.; Bozzao, A.; Brada, M.; Fariselli, L.; Fiorentino, A.; Ganswindt, U.; Grosu, A.L.; Lagerwaard, F.L.; et al. ESTRO ACROP guideline for target volume delineation of skull base tumors. Radiother Oncol 2021, 156, 80-94. [CrossRef]
- Nabors, L.B.; Portnow, J.; Ahluwalia, M. Central Nervous System Cancers, Version 3. 2023. NCCN Clinical Practice Guidelines in Oncology 2023. [CrossRef]
- Hasegawa, T.; Kida, Y.; Yoshimoto, M.; Iizuka, H.; Ishii, D.; Yoshida, K. Gamma Knife surgery for convexity, parasagittal, and falcine meningiomas. J Neurosurg 2011, 114, 1392-1398. [CrossRef]
- Lee, S.R.; Yang, K.A.; Kim, S.K.; Kim, S.H. Radiation-induced intratumoral necrosis and peritumoral edema after gamma knife radiosurgery for intracranial meningiomas. J Korean Neurosurg Soc 2012, 52, 98-102. [CrossRef]
- Cai, R.; Barnett, G.H.; Novak, E.; Chao, S.T.; Suh, J.H. Principal risk of peritumoral edema after stereotactic radiosurgery for intracranial meningioma is tumor-brain contact interface area. Neurosurgery 2010, 66, 513-522. [CrossRef]
- Chang, J.H.; Chang, J.W.; Choi, J.Y.; Park, Y.G.; Chung, S.S. Complications after gamma knife radiosurgery for benign meningiomas. Journal of neurology, neurosurgery, and psychiatry 2003, 74, 226-230. [CrossRef]
- Kollova, A.; Liscak, R.; Novotny, J., Jr.; Vladyka, V.; Simonova, G.; Janouskova, L. Gamma Knife surgery for benign meningioma. J Neurosurg 2007, 107, 325-336. [CrossRef]
- Mansouri, A.; Larjani, S.; Klironomos, G.; Laperriere, N.; Cusimano, M.; Gentili, F.; Schwartz, M.; Zadeh, G. Predictors of response to Gamma Knife radiosurgery for intracranial meningiomas. J Neurosurg 2015, 123, 1294-1300. [CrossRef]
- Novotny, J., Jr.; Kollova, A.; Liscak, R. Prediction of intracranial edema after radiosurgery of meningiomas. J Neurosurg 2006, 105 Suppl, 120-126. [CrossRef]
- Hoe, Y.; Choi, Y.J.; Kim, J.H.; Kwon, D.H.; Kim, C.J.; Cho, Y.H. Peritumoral Brain Edema after Stereotactic Radiosurgery for Asymptomatic Intracranial Meningiomas: Risks and Pattern of Evolution. J Korean Neurosurg Soc 2015, 58, 379-384. [CrossRef]
- Huang, S.H.; Chuang, C.C.; Wang, C.C.; Wei, K.C.; Chen, H.C.; Hsu, P.W. Risk factors for peritumoral edema after radiosurgery for intracranial benign meningiomas: a long-term follow-up in a single institution. Neurosurg Focus 2022, 53, E7. [CrossRef]
- Sheehan, J.P.; Lee, C.C.; Xu, Z.; Przybylowski, C.J.; Melmer, P.D.; Schlesinger, D. Edema following Gamma Knife radiosurgery for parasagittal and parafalcine meningiomas. J Neurosurg 2015, 123, 1287-1293. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).