Submitted:
15 April 2024
Posted:
17 April 2024
You are already at the latest version
Abstract
Keywords:
Introduction
Rotaviruses
Other RNA Viruses
Acknowledgements
References
- Baek YB, Kwon HJ, Sharif M, Lim J, Lee IC, Ryu YB, Lee JI, Kim JS, Lee YS, Kim DH, Park SI, Kim DK, Kim JS, Choy HE, Lee S, Choi HS, Osborne TF, Jeon TI, Cho KO. Therapeutic strategy targeting host lipolysis limits infection by SARS-CoV-2 and influenza A virus. Signal Transduct Target Ther. 2022 Oct 17;7(1):367. [CrossRef] [PubMed] [PubMed Central]
- Belov GA, van Kuppeveld FJM. Lipid droplets grease enterovirus replication. Cell Host Microbe. 2019 Aug 14;26(2):149-151. [CrossRef] [PubMed] [PubMed Central]
- Bergman H, Henschke N, Hungerford D, Pitan F, Ndwandwe D, Cunliffe N, Soares-Weiser K. Vaccines for preventing rotavirus diarrhoea: vaccines in use. Cochrane Database Syst Rev. 2021 Nov 17;11(11):CD008521. [CrossRef] [PubMed] [PubMed Central]
- Boulant S, Targett-Adams P, McLauchlan J. Disrupting the association of hepatitis C virus core protein with lipid droplets correlates with a loss in production of infectious virus. J Gen Virol. 2007 Aug;88(Pt 8):2204-2213. [CrossRef] [PubMed]
- Cesar-Silva D, Pereira-Dutra FS, Giannini ALM, Maya-Monteiro CM, de Almeida CJG. Lipid compartments and lipid metabolism as therapeutic targets against coronavirus. Front Immunol. 2023 Dec 1;14:1268854. [CrossRef] [PubMed] [PubMed Central]
- Chatel-Chaix L, Bartenschlager R. Dengue virus- and hepatitis C virus-induced replication and assembly compartments: the enemy inside--caught in the web. J Virol. 2014 Jun;88(11):5907-11. [CrossRef] [PubMed] [PubMed Central]
- Chen Q, Gouilly J, Ferrat YJ, Espino A, Glaziou Q, Cartron G, El Costa H, Al-Daccak R, Jabrane-Ferrat N. Metabolic reprogramming by Zika virus provokes inflammation in human placenta. Nat Commun. 2020 Jun 11;11(1):2967. [CrossRef] [PubMed] [PubMed Central]
- Chen X, Wang S, Gan P, Zhang J, Tong G, Liu S. Comprehensive analysis of lipid metabolism in influenza virus infection. Microb Pathog. 2023 Feb;175:106002. [CrossRef] [PubMed]
- Cheung W, Gaunt E, Lever A, Desselberger U. Rotavirus replication: The role of lipid droplets. In: Viral Gastroenteritis, L Svensson, U Desselberger, HB Greenberg, MK Estes, eds, pp 175-187. Elsevier-Academic Press, Amsterdam, 2016.
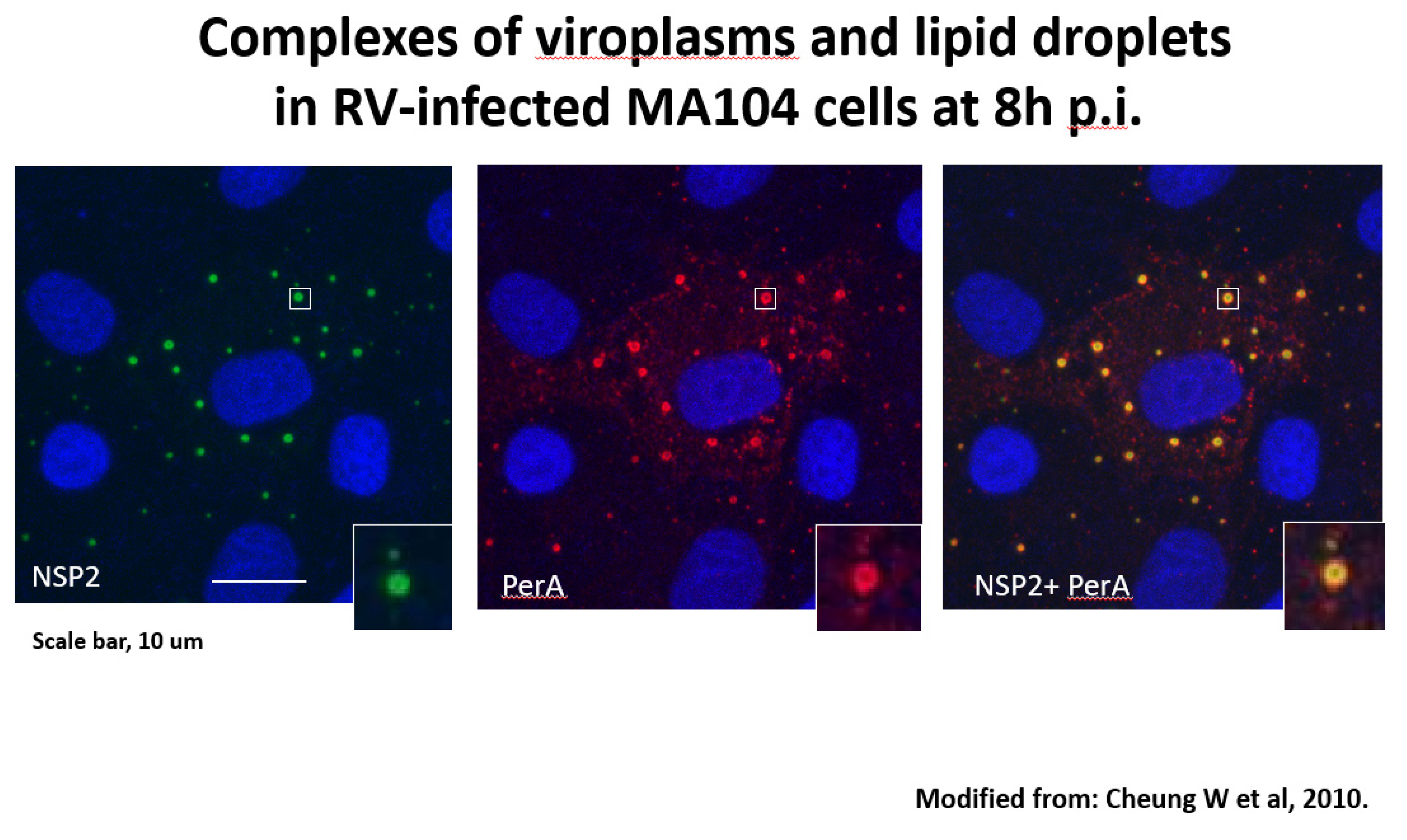
- Cheung W, Gill M, Esposito A, Kaminski CF, Courousse N, Chwetzoff S, Trugnan G, Keshavan N, Lever A, Desselberger U. Rotaviruses associate with cellular lipid droplet components to replicate in viroplasms, and compounds disrupting or blocking lipid droplets inhibit viroplasm formation and viral replication. J Virol. 2010 Jul;84(13):6782-98. [CrossRef] [PubMed] [PubMed Central]
- Crawford SE, Desselberger U. Lipid droplets form complexes with viroplasms and are crucial for rotavirus replication. Curr Opin Virol. 2016 Aug;19:11-5. [CrossRef] [PubMed] [PubMed Central]
- Crawford SE, Ramani S, Tate JE, Parashar UD, Svensson L, Hagbom M, Franco MA, Greenberg HB, O'Ryan M, Kang G, Desselberger U, Estes MK. Rotavirus infection. Nat Rev Dis Primers. 2017 Nov 9;3:17083. [CrossRef] [PubMed] [PubMed Central]
- Criglar JM, Estes MK, Crawford SE. Rotavirus-induced lipid droplet biogenesis is critical for virus replication. Front Physiol. 2022 Mar 23;13:836870. [CrossRef] [PubMed] [PubMed Central]
- D'Avila H, Lima CNR, Rampinelli PG, Mateus LCO, Sousa Silva RV, Correa JR, Almeida PE. Lipid metabolism modulation during SARS-CoV-2 infection: A spotlight on extracellular vesicles and therapeutic prospects. Int J Mol Sci. 2024 Jan 4;25(1):640. [CrossRef] [PubMed] [PubMed Central]
- Desselberger U. Differences of rotavirus vaccine effectiveness by country: Likely causes and contributing factors. Pathogens. 2017 Dec 12;6(4):65. [CrossRef] [PubMed] [PubMed Central]
- Dias SSG, Soares VC, Ferreira AC, Sacramento CQ, Fintelman-Rodrigues N, Temerozo JR, Teixeira L, Nunes da Silva MA, Barreto E, Mattos M, de Freitas CS, Azevedo-Quintanilha IG, Manso PPA, Miranda MD, Siqueira MM, Hottz ED, Pão CRR, Bou-Habib DC, Barreto-Vieira DF, Bozza FA, Souza TML, Bozza PT. Lipid droplets fuel SARS-CoV-2 replication and production of inflammatory mediators. PLoS Pathog. 2020 Dec 16;16(12):e1009127. [CrossRef] [PubMed] [PubMed Central]
- Doerflinger SY, Cortese M, Romero-Brey I, Menne Z, Tubiana T, Schenk C, White PA, Bartenschlager R, Bressanelli S, Hansman GS, Lohmann V. Membrane alterations induced by nonstructural proteins of human norovirus. PLoS Pathog. 2017 Oct 27;13(10):e1006705. [CrossRef] [PubMed] [PubMed Central]
- Fahy E, Cotter D, Sud M, Subramaniam S. Lipid classification, structures and tools. Biochim Biophys Acta. 2011 Nov;1811(11):637-47. [CrossRef] [PubMed] [PubMed Central]
- Farese RV Jr, Walther TC. Lipid droplets finally get a little R-E-S-P-E-C-T. Cell. 2009 Nov 25;139(5):855-60. [CrossRef] [PubMed] [PubMed Central]
- Farías MA, Diethelm-Varela B, Kalergis AM, González PA. Interplay between lipid metabolism, lipid droplets and RNA virus replication. Crit Rev Microbiol. 2023 Jun 22:1-25. [CrossRef] [PubMed]
- Farías MA, Diethelm-Varela B, Navarro AJ, Kalergis AM, González PA. Interplay between Lipid Metabolism, Lipid Droplets, and DNA Virus Infections. Cells. 2022 Jul 17;11(14):2224. [CrossRef] [PubMed] [PubMed Central]
- Gaunt ER, Cheung W, Richards JE, Lever A, Desselberger U. Inhibition of rotavirus replication by downregulation of fatty acid synthesis. J Gen Virol. 2013a Jun;94(Pt 6):1310-1317. Epub 2013 Mar 13. Erratum in: J Gen Virol. 2013 Sep;94(Pt 9):2140. [CrossRef] [PubMed]
- Gaunt ER, Zhang Q, Cheung W, Wakelam MJO, Lever AML, Desselberger U. Lipidome analysis of rotavirus-infected cells confirms the close interaction of lipid droplets with viroplasms. J Gen Virol. 2013b Jul;94(Pt 7):1576-1586. [CrossRef] [PubMed] [PubMed Central]
- Geiger F, Acker J, Papa G, Wang X, Arter WE, Saar KL, Erkamp NA, Qi R, Bravo JP, Strauss S, Krainer G, Burrone OR, Jungmann R, Knowles TP, Engelke H, Borodavka A. Liquid-liquid phase separation underpins the formation of replication factories in rotaviruses. EMBO J. 2021 Nov 2;40(21):e107711. [CrossRef] [PubMed] [PubMed Central]
- Heaton NS, Randall G. Multifaceted roles for lipids in viral infection. Trends Microbiol. 2011 Jul;19(7):368-75. [CrossRef] [PubMed] [PubMed Central]
- Herker E. Lipid droplets in virus replication. FEBS Lett. 2024 Feb 13. [CrossRef] [PubMed]
- Hope RG, Murphy DJ, McLauchlan J. The domains required to direct core proteins of hepatitis C virus and GB virus-B to lipid droplets share common features with plant oleosin proteins. J Biol Chem. 2002 Feb 8;277(6):4261-70. [CrossRef] [PubMed]
- Husby ML, Stahelin RV. Negative-sense RNA viruses: An underexplored platform for examining virus-host lipid interactions. Mol Biol Cell. 2021 Oct 1;32(20):pe1. [CrossRef] [PubMed] [PubMed Central]
- Islam KU, Anwar S, Patel AA, Mirdad MT, Mirdad MT, Azmi MI, Ahmad T, Fatima Z, Iqbal J. Global lipidome profiling revealed multifaceted role of lipid species in hepatitis C virus replication, assembly, and host antiviral gesponse. Viruses. 2023 Feb 7;15(2):464. [CrossRef] [PubMed] [PubMed Central]
- Kanai Y, Komoto S, Kawagishi T, Nouda R, Nagasawa N, Onishi M, Matsuura Y, Taniguchi K, Kobayashi T. Entirely plasmid-based reverse genetics system for rotaviruses. Proc Natl Acad Sci U S A. 2017 Feb 28;114(9):2349-2354. [CrossRef] [PubMed] [PubMed Central]
- Kim Y, George D, Prior AM, Prasain K, Hao S, Le DD, Hua DH, Chang KO. Novel triacsin C analogs as potential antivirals against rotavirus infections. Eur J Med Chem. 2012 Apr;50:311-8. [CrossRef] [PubMed] [PubMed Central]
- Laufman O, Perrino J, Andino R. Viral generated inter-organelle contacts redirect lipid flux for genome replication. Cell. 2019 Jul 11;178(2):275-289.e16. [CrossRef] [PubMed] [PubMed Central]
- Li YJ, Chen CY, Yang JH, Chiu YF. Modulating cholesterol-rich lipid rafts to disrupt influenza A virus infection. Front Immunol. 2022 Sep 13;13:982264. [CrossRef] [PubMed] [PubMed Central]
- Liu Z, Smith H, Criglar JM, Valentin AJ, Karandikar U, Zeng XL, Estes MK, Crawford SE. Rotavirus-mediated DGAT1 degradation: A pathophysiological mechanism of viral-induced malabsorptive diarrhea. Proc Natl Acad Sci U S A. 2023 Dec 19;120(51):e2302161120. [CrossRef] [PubMed] [PubMed Central]
- Madsen JJ, Rossman JS. Cholesterol and M2 rendezvous in budding and scission of influenza A virus. Subcell Biochem. 2023;106:441-459. [CrossRef] [PubMed]
- Marcinkiewicz A, Gauthier D, Garcia A, Brasaemle DL. The phosphorylation of serine 492 of perilipin a directs lipid droplet fragmentation and dispersion. J Biol Chem. 2006 Apr 28;281(17):11901-9. [CrossRef] [PubMed]
- Mejhert N, Kuruvilla L, Gabriel KR, Elliott SD, Guie MA, Wang H, Lai ZW, Lane EA, Christiano R, Danial NN, Farese RV Jr, Walther TC. Partitioning of MLX-family transcription factors to lipid droplets regulates metabolic gene expression. Mol Cell. 2020 Mar 19;77(6):1251-1264.e9. [CrossRef] [PubMed] [PubMed Central]
- Miyanari Y, Atsuzawa K, Usuda N, Watashi K, Hishiki T, Zayas M, Bartenschlager R, Wakita T, Hijikata M, Shimotohno K. The lipid droplet is an important organelle for hepatitis C virus production. Nat Cell Biol. 2007 Sep;9(9):1089-97. Epub 2007 Aug 26. Erratum in: Nat Cell Biol. 2007 Oct;9(10):1216. [CrossRef] [PubMed]
- Nejmeddine M, Trugnan G, Sapin C, Kohli E, Svensson L, Lopez S, Cohen J. Rotavirus spike protein VP4 is present at the plasma membrane and is associated with microtubules in infected cells. J Virol. 2000 Apr;74(7):3313-20. [CrossRef] [PubMed] [PubMed Central]
- Papa G, Borodavka A, Desselberger U. Viroplasms: Assembly and functions of rotavirus replication factories. Viruses. 2021 Jul 12;13(7):1349. [CrossRef] [PubMed] [PubMed Central]
- Parker EP, Ramani S, Lopman BA, Church JA, Iturriza-Gómara M, Prendergast AJ, Grassly NC. Causes of impaired oral vaccine efficacy in developing countries. Future Microbiol. 2018 Jan;13(1):97-118. [CrossRef] [PubMed] [PubMed Central]
- Qu Y, Wang W, Xiao MZX, Zheng Y, Liang Q. The interplay between lipid droplets and virus infection. J Med Virol. 2023 Jul;95(7):e28967. [CrossRef] [PubMed]
- Roingeard P, Eymieux S, Burlaud-Gaillard J, Hourioux C, Patient R, Blanchard E. The double-membrane vesicle (DMV): a virus-induced organelle dedicated to the replication of SARS-CoV-2 and other positive-sense single-stranded RNA viruses. Cell Mol Life Sci. 2022 Jul 16;79(8):425. [CrossRef] [PubMed] [PubMed Central]
- Samsa MM, Mondotte JA, Iglesias NG, Assunção-Miranda I, Barbosa-Lima G, Da Poian AT, Bozza PT, Gamarnik AV. Dengue virus capsid protein usurps lipid droplets for viral particle formation. PLoS Pathog. 2009 Oct;5(10):e1000632. [CrossRef] [PubMed] [PubMed Central]
- Shavinskaya A, Boulant S, Penin F, McLauchlan J, Bartenschlager R. The lipid droplet binding domain of hepatitis C virus core protein is a major determinant for efficient virus assembly. J Biol Chem. 2007 Dec 21;282(51):37158-69. [CrossRef] [PubMed]
- Tabata K, Prasad V, Paul D, Lee JY, Pham MT, Twu WI, Neufeldt CJ, Cortese M, Cerikan B, Stahl Y, Joecks S, Tran CS, Lüchtenborg C, V'kovski P, Hörmann K, Müller AC, Zitzmann C, Haselmann U, Beneke J, Kaderali L, Erfle H, Thiel V, Lohmann V, Superti-Furga G, Brügger B, Bartenschlager R. Convergent use of phosphatidic acid for hepatitis C virus and SARS-CoV-2 replication organelle formation. Nat Commun. 2021 Dec 14;12(1):7276. [CrossRef] [PubMed] [PubMed Central]
- Tansey JT, Huml AM, Vogt R, Davis KE, Jones JM, Fraser KA, Brasaemle DL, Kimmel AR, Londos C. Functional studies on native and mutated forms of perilipins. A role in protein kinase A-mediated lipolysis of triacylglycerols. J Biol Chem. 2003 Mar 7;278(10):8401-6. [CrossRef] [PubMed]
- Theken KN, Tang SY, Sengupta S, FitzGerald GA. The roles of lipids in SARS-CoV-2 viral replication and the host immune response. J Lipid Res. 2021;62:100129. [CrossRef] [PubMed] [PubMed Central]
- Troeger C, Khalil IA, Rao PC, Cao S, Blacker BF, Ahmed T, Armah G, Bines JE, Brewer TG, Colombara DV, Kang G, Kirkpatrick BD, Kirkwood CD, Mwenda JM, Parashar UD, Petri WA Jr, Riddle MS, Steele AD, Thompson RL, Walson JL, Sanders JW, Mokdad AH, Murray CJL, Hay SI, Reiner RC Jr. Rotavirus vaccination and the burden of rotavirus diarrhea among children younger than 5 years. JAMA Pediatr. 2018 Oct 1;172(10):958-965. Erratum in: JAMA Pediatr. 2022 Feb 1;176(2):208. [CrossRef] [PubMed] [PubMed Central]
- Vieyres G, Pietschmann T. HCV pit stop at the lipid droplet: Refuel lipids and put on a lipoprotein coat before exit. Cells. 2019 Mar 12;8(3):233. [CrossRef] [PubMed] [PubMed Central]
- Viktorova EG, Nchoutmboube JA, Ford-Siltz LA, Iverson E, Belov GA. Phospholipid synthesis fueled by lipid droplets drives the structural development of poliovirus replication organelles. PLoS Pathog. 2018 Aug 27;14(8):e1007280. [CrossRef] [PubMed] [PubMed Central]
- Waheed AA, Zhu Y, Agostino E, Naing L, Hikichi Y, Soheilian F, Yoo SW, Song Y, Zhang P, Slusher BS, Haughey NJ, Freed EO. Neutral sphingomyelinase 2 is required for HIV-1 maturation. Proc Natl Acad Sci U S A. 2023 Jul 11;120(28):e2219475120. [CrossRef] [PubMed] [PubMed Central]
- Yoo SW, Waheed AA, Deme P, Tohumeken S, Rais R, Smith MD, DeMarino C, Calabresi PA, Kashanchi F, Freed EO, Slusher BS, Haughey NJ. Inhibition of neutral sphingomyelinase 2 impairs HIV-1 envelope formation and substantially delays or eliminates viral rebound. Proc Natl Acad Sci U S A. 2023 Jul 11;120(28):e2219543120. [CrossRef] [PubMed] [PubMed Central]
- Zhang J, Lan Y, Li MY, Lamers MM, Fusade-Boyer M, Klemm E, Thiele C, Ashour J, Sanyal S. Flaviviruses exploit the lipid droplet protein AUP1 to trigger lipophagy and drive virus production. Cell Host Microbe. 2018 Jun 13;23(6):819-831.e5. [CrossRef] [PubMed]
- Zhao J, Zeng Z, Chen Y, Liu W, Chen H, Fu ZF, Zhao L, Zhou M. Lipid droplets are beneficial for rabies virus replication by facilitating viral budding. J Virol. 2022 Jan 26;96(2):e0147321. [CrossRef] [PubMed] [PubMed Central]
| Treatment of cells | Viral dsRNA | Infectivity of progeny | |||||
|---|---|---|---|---|---|---|---|
|
Relative Valuea |
Differenceb |
log TCID50/ml ± S.E. (n) |
Differenceb | ||||
| Isoproterenol+IBMXc | - | 1.00 | 8.2 ± 0.3 (3) | ||||
| + | 0.25 | 4.0-fold | 6.5 ± 0.1 (3) | 50-fold | |||
| Triacsin Cc | - | 1.00 | 7.5 ± 0.1 (3) | ||||
| + | 0.26 | 3.8-fold | 6.2 ± 0.2 (3) | 20-fold | |||
| TOFAd | - | 1.00 | 8.4 ± 0.5 (6) | ||||
| + | 0.17 | 5.9-fold | 6.7 ± 0.5 (6) | 50-fold | |||
| Virus | Mechanism | References |
|---|---|---|
| Hepatitis C virus (HCV) | Formation of double membrane vesicles (DMVs) for viral replication, interacting with LDs via viral core protein D2 domain and NS5A; role of apolipoproteins; dynamic profile of lipidome-DMV complexes during HCV infection | Miyanari et al, 2007; Shavinskaya et al, 2007; Boulant et al, 2007; Chatel-Chaix and Bartenschlager,2014; Vieyres and Pietschmann, 2019; Islam et al, 2023 |
| GB virus-B | similar to HCV | Hope et al, 2002 |
| Dengue virus | Viral capsid protein-LD interactions; similar to HCV | Samsa et al, 2009; Chatel-Chaix and Bartenschlager, 2014 |
| Zika virus | Disturbance of lipid homeostasis; induction of mitochondrial dysfunction | Chen Q et al, 2020 |
| Picornaviruses | Phosphatidylcholine synthesis to support membranous replication complexes; hydrolysis of TAGs in LDs, lipolysis | Victorova et al, 2018; Laufman et al, 2019; Belov and von Kuppeveld, 2019 |
| Norovirus | Multimembrane vesicle clusters replication organelles, as in picornaviruses, containing NoV NS proteins; | Doerflinger et al, 2017 |
| SARS-CoV-2 | DMVs as replication complexes; viral reprograming of lipid synthesis pathways; LDs as assembly platforms? | Dias et al, 2020; Roingeard et al, 2022; Cesar-Silva et al, 2023 |
| HIV-1 | Requirement of ceramide for viral capsid maturation and full infectivity | Waheed et al, 2023; Yoo et al, 2023 |
| Influenza virus | Upregulation of LDs upon infection; viral replication depending on cellular lipase activity; elevation of lipid metabolites in IV-infected cells | Baek et al, 2022; Chen X et al, 2023 |
| Virus | Compound |
| Rotavirus | TOFA; [isoproterenol + IBMX] |
| Hepatitis C virus | Lipid-specific compounds targeting the interaction of viral core proteins with LDs |
| Zikavirus | DGAT-1 inhibitor A922500 |
| Enterovirus | Adipose triglyceride lipase inhibitor atglistatin |
| SARS-CoV-2 | Inhibitors of sphingolipid metabolism; inhibitors of inflammatory compounds; lipase inhibitors; statins |
| HIV-1 | Neutral sphingomyelinase 2 inhibitor PDDC, preventing ceramide biosynthesis |
| Influenza A virus | methyl-beta-cytodextrin; sphingomyelinase inhibitors; lipase inhibitors; statins |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





