Submitted:
15 April 2024
Posted:
17 April 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Insect Colonies
2.2. Bioassay
2.3. Data Analysis
3. Results
3.1. Predation of M. pygmaeus on Eggs of T. absoluta
3.2. Predation of N. tenuis on Eggs of T. absoluta
3.3. Comparison of Predation Efficacy of M. pygmaeus and N. tenuis
| Source | df | Deviance |
|---|---|---|
|
20 °C |
||
| Predator | 1 | 211.73a |
| Sex | 1 | 591.80a |
| Age | 1 | 104.83b |
| Predator*Sex | 1 | 88.28b |
| Predator*Age | 1 | 51.49c |
| Sex*Age | 1 | 5.82 |
| Predator*Sex*Age | 1 | 11.89 |
|
25 °C |
||
| Predator | 1 | 52.80c |
| Sex | 1 | 578.81a |
| Age | 1 | 136.81b |
| Predator*Sex | 1 | 24.74 |
| Predator*Age | 1 | 24.66 |
| Sex*Age | 1 | 23.53 |
| Predator*Sex*Age | 1 | 5.70 |
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Pérez-Hedo, M.; Urbaneja, A. Prospects for predatory mirid bugs as biocontrol agents of aphids in sweet peppers. J. Pest Sci. 2015, 88, 65–73. [Google Scholar] [CrossRef]
- Sanchez, J.A.; Lopez-Gallego, E.; Pérez-Marcos, M.; Perera-Fernandez, L.G.; Ramirez-Soria, M.J.; 2018. How safe is it to rely on Macrolophus pygmaeus (Hemiptera: Miridae) as a biocontrol agent in tomato crops? Front. Ecol. Evol. 2018, 6, 132. [Google Scholar] [CrossRef]
- Arnó, J.; Castañé, C.; Alomar, O. ; et.al. Forty years of biological control in Mediterranean tomato greenhouses: The story of success. Isr. J. Entomol 2018, 48, 209–226. [Google Scholar]
- Moerkens, R.; Berckmoes, E.; Van Damme, V.; Wittemans, L.; Tirry, L.; Casteels, H. , et al. Inoculative release strategies of Macrolophus pygmaeus Rambur (Hemiptera: Miridae) in tomato crops: population dynamics and dispersal. J. Plant Dis. Prot. 2017, 124, 295–303. [Google Scholar] [CrossRef]
- Sanchez, J.A.; Lacasa, A.; Arnó, J.; Castañé, C.; Alomar, O. Life history parameters for Nesidiocoris tenuis (Reuter) (Het., Miridae) under different temperature regimes. J. Appl. Entomol. 2009, 133, 125–32. [Google Scholar] [CrossRef]
- Pérez-Hedo, M.; Pedroche, V.; Urbaneja, A. Temperature-driven selection of predatory Mirid bugs for improving aphid control in sweet pepper crops. Horticulturae 2023, 9, 572. [Google Scholar] [CrossRef]
- Urbaneja, A.; Montón, H.; Mollá, O. Suitability of the tomato borer Tuta absoluta as prey for Macrolophus pygmaeus and Nesidiocoris tenuis. J. Appl. Entomol. 2009, 133, 292–296. [Google Scholar] [CrossRef]
- Zappalà, L.; Biondi, A.; Alma, A.; Al-Jboory, I.J.; Arnò, J.; Bayram, A.; et al. Natural enemies of the South American moth, Tuta absoluta, in Europe, North Africa and Middle East, and their potential use in pest control strategies. J. Pest Sci. 2013, 86, 635–647. [Google Scholar] [CrossRef]
- Castañé, C.; Arnó, J.; Gabarra, R.; Alomar, O. Plant damage to vegetable crops by zoophytophagous mirid predators. Biol. Control 2011, 59, 22–29. [Google Scholar] [CrossRef]
- Torres, J.B.; Boyd, D.W. Zoophytophagy in predatory Hemiptera. Braz. Arch. Biol. Technol. 2009, 52, 1199–208. [Google Scholar] [CrossRef]
- Urbaneja, A.; Tapia, G.; Stansly, P. Influence of host plant and prey availability on developmental time and survivorship of Nesidiocoris tenius (Het.: Miridae). Biocontrol Sci. Technol. 2005, 15, 513–518. [Google Scholar] [CrossRef]
- Perdikis, D.C.; Lykouressis, D.P. Macrolophus pygmaeus (Hemiptera: Miridae) Population parameters and biological characteristics when feeding on eggplant and tomato without prey. J. Econ. Entomol. 2004, 97, 1291–1298. [Google Scholar] [CrossRef] [PubMed]
- Maselou, D.A.; Perdikis, D.C.; Sabelis, M.W.; Fantinou, A.A. Use of plant resources by an omnivorous predator and the consequences for effective predation. Biol. Control 2014, 79, 92–100. [Google Scholar] [CrossRef]
- De Puysseleyr, V.; De Man, S.; Höfte, M.; De Clercq, P. Plantless rearing of the zoophytophagous bug Nesidiocoris tenuis. BioControl 2013, 58, 205–213. [Google Scholar] [CrossRef]
- Sanchez, J.A. Zoophytophagy in the plantbug Nesidiocoris tenuis. Agric. For. Entomol. 2008, 10, 75–80. [Google Scholar] [CrossRef]
- Lykouressis, D.; Perdikis, D.; Charalampous, P. Plant food effects on prey consumption by the omnivorous predator Macrolophus pygmaeus. Phytoparasitica 2014, 42, 303–309. [Google Scholar] [CrossRef]
- Ingegno, B.L.; Pansa, M.G.; Tavella, L. Plant preference in the zoophytophagous generalist predator Macrolophus pygmaeus (Heteroptera: Miridae). Biol. Control 2011, 58, 174–181. [Google Scholar] [CrossRef]
- Castañé, C; Zapata, R. Rearing the predatory bug Macrolophus caliginosus on a meat-based diet. Biol. Control 2005, 34, 66–72. [Google Scholar] [CrossRef]
- Lalonde, R. G.; McGregor, R. R.; Gillespie, D. R.; Roitberg, B. D. (1999). Plant-feeding by arthropod predators contributes to the stability of predator-prey population dynamics. Oikos 1999, 87, 603–608. [Google Scholar] [CrossRef]
- Castañé, C.; Arnó, J.; Gabarra, R.; Alomar, O. Plant damage to vegetable crops by zoophytophagous mirid predators. Biol. Control 2011, 59, 22–29. [Google Scholar] [CrossRef]
- Perdikis, D.; Lykouressis, D. Effects of various items, host plants, and temperatures on the development and survival of Macrolophus pygmaeus Rambur (Hemiptera: Miridae). Biol. Control 2000, 17, 55–60. [Google Scholar] [CrossRef]
- Biondi, A.; Zappalà, L.; Di Mauro, A.; Tropea Garzia, G.; Russo, A.; Desneux, N.; et al. Can alternative host plant and prey affect phytophagy and biological control by the zoophytophagous mirid Nesidiocoris tenuis? BioControl 2016, 61, 79–90. [Google Scholar] [CrossRef]
- Nakano, R.; Morita, T.; Okamoto, Y.; Fujiwara, A.; Yamanaka, T.; Adachi-Hagimori, T. Cleome hassleriana plants fully support the development and reproduction of Nesidiocoris tenuis. BioControl 2021, 66, 407–418. [Google Scholar] [CrossRef]
- Sánchez, J.A.; Lacasam, A. Impact of the zoophytophagous plant bug Nesidiocoris tenuis (Heteroptera: Miridae) on tomato yield. J. Econ. Entomol 2008, 101, 1864–1870. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, J.A. Density thresholds for Nesidiocoris tenuis (Heteroptera: Miridae) in tomato crops. Biol. Control 2009, 51, 493–498. [Google Scholar] [CrossRef]
- Calvo, J.; Bolckmansm, K.; Stansly, P.A.; Urbaneja, A. Predation by Nesidiocoris tenuis on Bemisia tabaci and injury to tomato. BioControl 2009, 54, 237–246. [Google Scholar] [CrossRef]
- Souto, P.; Abraços-Duarte, G.; da Silva, E.B.; Figueiredo, E. Half Friend, Half Enemy? Comparative phytophagy between two Dicyphini species (Hemiptera: Miridae). Insects 2022, 13, 175. [Google Scholar] [CrossRef] [PubMed]
- Moerkens, R.; Pekas, A.; Bellinkx, S.; Hanssen, I.; Huysmans, M.; Bosmans, L.; et al. Nesidiocoris tenuis as a pest in Northwest Europe: Intervention threshold and influence of Pepino mosaic virus. J. Appl. Entomol. 2020, 144, 566–77. [Google Scholar] [CrossRef]
- Pérez-Hedo, M.; Urbaneja, A. The zoophytophagous predator Nesidiocoris tenuis: a successful but controversial biocontrol agent in tomato crops. In: Advances in Insect Control and Resistance Management, Horowitz, A., Ishaaya, I., Eds.; Springer, Cham, Switzerland, 2016, pp. 121–138.
- Desneux, N.; Wajnberg, E.; Wyckhuys, K.A.G.; Burgio, G.; Arpaia, S.; Narváez-Vasquez, C.A.; et al. Biological invasion of European tomato crops by Tuta absoluta: ecology, geographic expansion and prospects for biological control. J. Pest Sci. 2010, 83, 197–215. [Google Scholar] [CrossRef]
- Biondi, A.; Guedes, R.N.C.; Wan, F.H.; Desneux, N. Ecology, worldwide spread, and management of the invasive South American tomato pinworm, Tuta absoluta: Past, Present, and Future. Annu. Rev. Entomol. 2018, 63, 239–58. [Google Scholar] [CrossRef] [PubMed]
- Siqueira, H.Á.A.; Guedes, R.N.C.; Picanço, M.C. Insecticide resistance in populations of Tuta absoluta (Lepidoptera: Gelechiidae). Agric. For. Entomol. 2000, 2, 147–153. [Google Scholar] [CrossRef]
- Guedes, R.N.C.; Roditakis, E.; Campos, M.R.; Haddi, K.; Bielza, P.; Siqueira, H.A.A.; et al. Insecticide resistance in the tomato pinworm Tuta absoluta: patterns, spread, mechanisms, management and outlook. J. Pest Sci. 2019, 92, 1329–42. [Google Scholar] [CrossRef]
- Silva, G.A.; Picanço, M.C.; Bacci, L.; Crespo, A.L.B.; Rosado, J.F.; Guedes, R.N.C. Control failure likelihood and spatial dependence of insecticide resistance in the tomato pinworm, Tuta absoluta. Pest Manag. Sci. 2011, 67, 913–20. [Google Scholar] [CrossRef] [PubMed]
- Prasannakumar, N.R.; Jyothi, N.; Saroja, S.; Kumar, G.R. Relative toxicity and insecticide resistance of different field population of tomato leaf miner, Tuta absoluta (Meyrick). Int. J. Trop. Insect Sci. 2021, 41, 1397–405. [Google Scholar] [CrossRef]
- Roditakis, E.; Vasakis, E.; García-Vidal, L.; et al. A four-year survey on insecticide resistance and likelihood of chemical control failure for tomato leaf miner Tuta absoluta in the European/Asian region. J. Pest Sci. 2018, 91, 421–35. [Google Scholar] [CrossRef]
- Holling, C.S. The Components of predation as revealed by a study of small-mammal predation of the European pine sawfly. Can Entomol. 1959, 91, 293–320. [Google Scholar] [CrossRef]
- Hassell, M.P.; Lawton, J.H.; Beddington, J.R. Sigmoid functional responses by invertebrate predators and parasitoids. J. Anim. Ecol. 1977, 46, 249–62. [Google Scholar] [CrossRef]
- Holling, CS. The functional response of predators to prey density and its role in mimicry and population regulation. Mem. Ent. Soc. Can. 1965, 97, 5–60. [Google Scholar] [CrossRef]
- Hamdan, A.J.S. Functional and numerical responses of the predatory bug Macrolophus caliginosus Wagner fed on different densities of eggs of the greenhouse whitefly, Trialeurodes vaporariorum (Westwood). J. Biol. Res. 2006, 6, 147–54. [Google Scholar]
- Sharifian, I.; Sabahi, Q.; Khoshabi, J. Functional response of Macrolophus pygmaeus (Rambur) and Nesidiocoris tenuis (Reuter) feeding on two different prey species. Arch. Phytopathol. Plant Prot. 2015, 48, 910–20. [Google Scholar] [CrossRef]
- Michaelides, G.; Sfenthourakis, S.; Pitsillou, M.; Seraphides, N. Functional response and multiple predator effects of two generalist predators preying on Tuta absoluta eggs. Pest Manag. Sci. 2018, 74, 332–9. [Google Scholar] [CrossRef] [PubMed]
- van Alphen, J.J.M; Jervis, M.A. Foraging Behaviour. In: Insect Natural Enemies, Jervis, M., Kidd, N., Eds; Springer, Dordrecht, Netherlands, 1996, pp. 1–62.
- Foglar, H.; Malausa, C. The Functional response and preference of Macrolophus caliginosus [Heteroptera : Miridae] for two of its prey: Myzus persicae and Tetranychus urticae. Entomophaga 1990, 35, 465–74. [Google Scholar] [CrossRef]
- Montserrat, M.; Albajes, R.; Castane, C. Functional response of four Heteropteran predators preying on greenhouse whitefly (Homoptera: Aleyrodidae) and western flower thrips (Thysanoptera: Thripidae). Environ Entomol. 2000, 29, 1075–82. [Google Scholar] [CrossRef]
- Enkegaard, A.; Brødsgaard, H.F.; Hansen, D.L. ; Macrolophus caliginosus: Functional response to whiteflies and preference and switching capacity between whiteflies and spider mites. Entomol. Exp. Appl. 2001, 101, 81–8. [Google Scholar] [CrossRef]
- Panagakis, S.; Perdikis, D.; Fantinou, A. Functional response analysis of predation by an omnivore predator: effect of hunger level and sex. In Proceedings of the IOBC/WPRS Working Group "Integrated Control in Protected Crops", Crete, Greece, 6-11 September 2009. [Google Scholar]
- Maselou, D.; Perdikis, D.; Fantinou, A. Effect of hunger level on prey consumption and functional response of the predator Macrolophus pygmaeus. Bull. Insectology. 2015, 68, 211–8. [Google Scholar]
- Baños, H.L.; Ruiz Gil, T.; del Toro Benitez, M.; Miranda Cabrera, I. Consumption and functional response of Nesidiocoris tenuis Reuter (Hemiptera: Miridae) feeding on immature stages of Bemisia tabaci Gennadius (Hemiptera: Aleyrodidae). Rev. Prot. Veg. 2016, 31, 217–23. [Google Scholar]
- Holling, C.S. Principles of insect predation. Annu. Rev. Entomol. 1961, 6, 163–182. [Google Scholar] [CrossRef]
- Rosenbaum, B.; Rall, B.C. Fitting functional responses: Direct parameter estimation by simulating differential equations. Methods Ecol. Evol. 2018, 9, 2076–2090. [Google Scholar] [CrossRef]
- Real, L.A. Ecological determinants of functional response. Ecology 1979, 60, 481–485. [Google Scholar] [CrossRef]
- Juliano, S. A.; Goughnour, J. A.; &Ower, G. D.; &Ower, G. D. Predation in many dimensions: spatial context is important for meaningful functional response experiments. Front. ecol. evol. 2022, 10, 845560. [Google Scholar] [CrossRef]
- Montserrat, M.; Albajes, R.; Castañé, C. Functional response of four heteropteran predators preying on greenhouse whitefly (Homoptera: Aleyrodidae) and western flower thrips (Thysanoptera: Thripidae). Environ. Entomol. 2000, 29, 1075–1082. [Google Scholar] [CrossRef]
- Enkegaard, A.; Brødsgaard, H.F.; Hansen, D.L. Macrolophus caliginosus: Functional response to whiteflies and preference and switching capacity between whiteflies and spider mites. Entomol. Exp. Appl. 2001, 101, 81–8. [Google Scholar] [CrossRef]
- Hassanpour, M.; Bagheri, M.; Golizadeh, A.; Farrokhi, S. Functional response of Nesidiocoris tenuis (Hemiptera: Miridae) to Trialeurodes vaporariorum (Hemiptera: Aleyrodidae): effect of different host plants. Biocontrol Sci. Technol. 2016, 26, 1489–503. [Google Scholar] [CrossRef]
- Gavkare, O.; Sharma, P.L.; Sanchez, J.A.; Shah, M.A. Functional response of Nesidiocoris tenuis (Hemiptera: Miridae) to the two-spotted spider mite, Tetranychus urticae. Biocontrol Sci. Technol. 2017, 27, 1118–22. [Google Scholar] [CrossRef]
- Fathipour, Y.; Karimi, M.; Farazmand, A.; Talebi, A.A. Age-specific functional response and predation rate of Amblyseius swirskii (Phytoseiidae) on two-spotted spider mite. Syst. Appl. Acarol. 2017, 22, 159–69. [Google Scholar] [CrossRef]
- Nikbin, R.; Sahragard, A.; Hosseini, M. Age-specific functional response of Trichogramma brassicae (Hymenoptera: Trichogrammatidae) parasitizing different egg densities of Ephestia kuehniella (Lepidoptera: Pyralidae). J. Agric. Sci. Technol. 2014, 16, 1217–27. [Google Scholar]
- Ingegno, B.L.; Messelink, G.J.; Bodino, N.; et al. Functional response of the mirid predators Dicyphus bolivari and Dicyphus errans and their efficacy as biological control agents of Tuta absoluta on tomato. J. Pest Sci. 2019, 92, 1457–1466. [Google Scholar] [CrossRef]
- Perdikis, D.C.; Lykouressis, D.P.; Economou, L.P. The influence of temperature, photoperiod and plant type on the predation rate of Macrolophus pygmaeus on Myzus persicae. BioControl 1999, 44, 281–289. [Google Scholar] [CrossRef]
- Hughes, G.E.; Alford, L.; Sterk, G.; Bale, J.S. Thermal activity thresholds of the predatory mirid Nesidiocoris tenuis: Implications for its efficacy as a biological control agent. BioControl 2010, 55, 493–501. [Google Scholar] [CrossRef]
- Sanchez, J.A.; Lacasa, A.; Arnó, J.; Castañé, C.; Alomar, O. Life history parameters for Nesidiocoris tenuis (Reuter) (Het., Miridae) under different temperature regimes. J. Appl. Entomol. 2009, 133, 125–32. [Google Scholar] [CrossRef]
- Ingegno, B.L.; Messelink, G.J.; Leman, A.; Sacco, D.; Tavella, L. Development and thermal activity thresholds of European mirid predatory bugs. Biol. Control 2021, 152. [Google Scholar] [CrossRef]
- Uiterwaal, S.F.; DeLong, J.P. Functional responses are maximized at intermediate temperatures. Ecology 2020, 101. [Google Scholar] [CrossRef] [PubMed]
- Madbouni, M. A. Z.; Samih, M. A.; Namvar, P.; Biondi, A. Temperature-dependent functional response of Nesidiocoris tenuis (Hemiptera: Miridae) to different densities of pupae of cotton whitefly, Bemisia tabaci (Hemiptera: Aleyrodidae). Eur. J. Entomol. 2017, 114, 325. [Google Scholar] [CrossRef]
- Dorsaz, M.; Fischer, S.; Baroffio, C.A. Study of the temperature influence on the functional response of the biological control agent Macrolophus pygmaeus. In Proceedings of the Les Cochenilles: ravageur principal ou secondaire 9ème Conférence Internationale sur les Ravageurs en Agriculture, SupAgro, Montpellier, France, 25-27 October 2011. [Google Scholar]
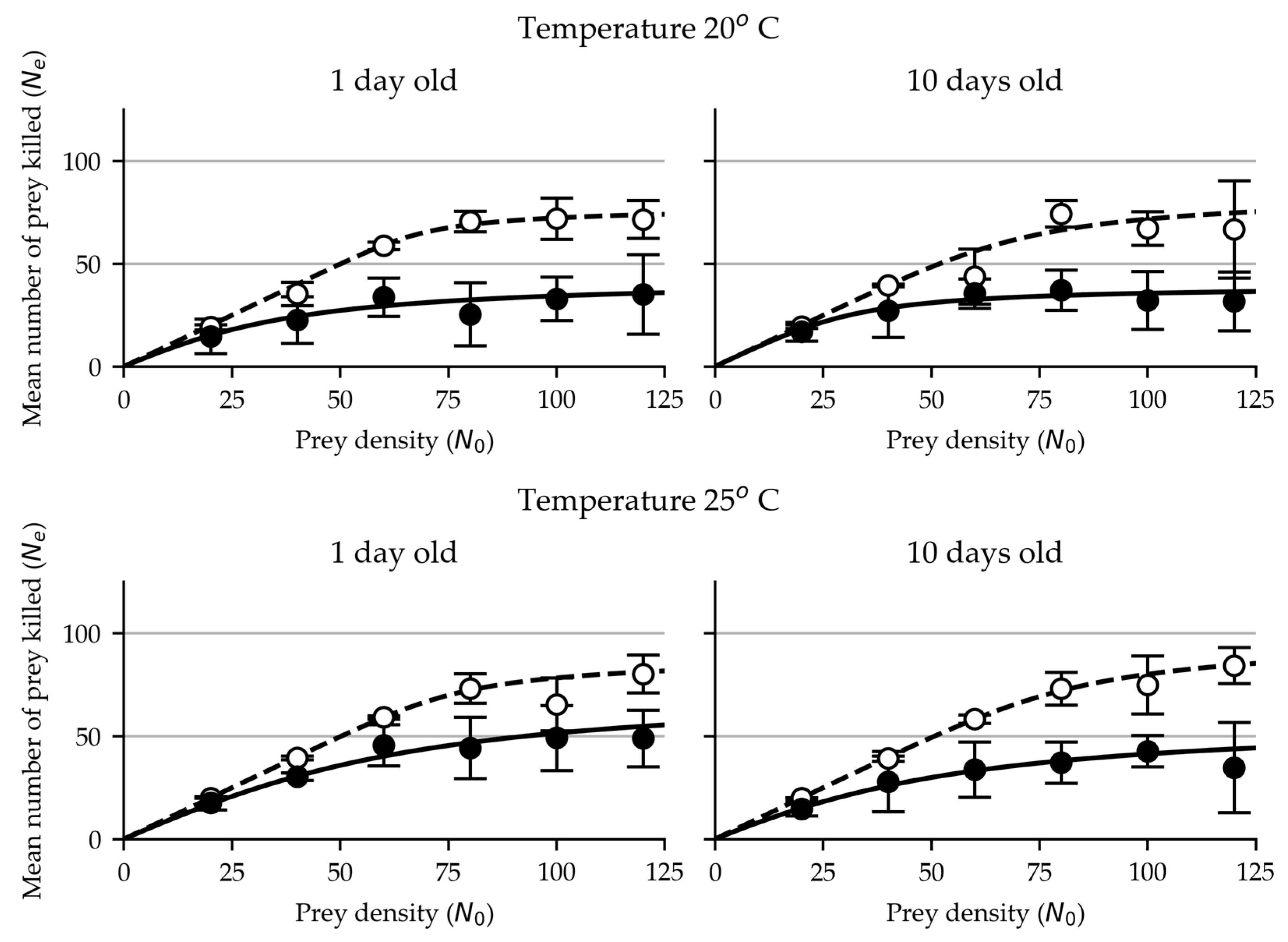
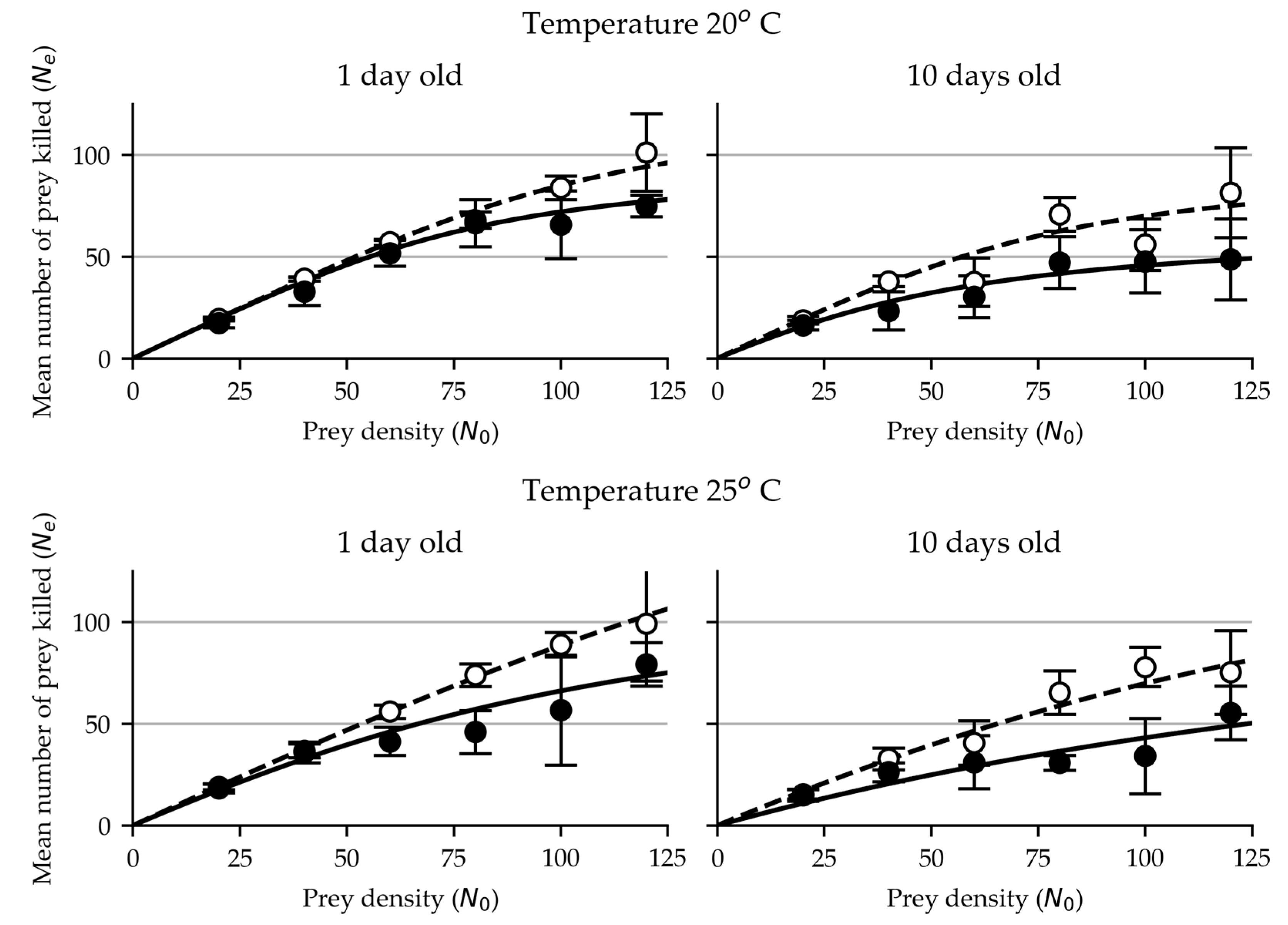
| Species | Sex* | Age (days) | Temperature (°C) |
a (±SE)(hrs-1) |
Th ( ±SE) (hrs) |
R2 |
|---|---|---|---|---|---|---|
| f | 1 | 20 | 0.70±0.26 | 0.31±0.02 | 0.99 | |
| f | 1 | 25 | 0.51±0.12 | 0.27±0.02 | 0.83 | |
| f | 10 | 20 | 0.33±0.09 | 0.28±0.03 | 0.92 | |
| f | 10 | 25 | 0.36±0.04 | 0.24±0.01 | 0.99 | |
| M. pygmaeus | m | 1 | 20 | 0.09±0.04 | 0.57±0.08 | 0.81 |
| m | 1 | 25 | 0.10±0.01 | 0.33±0.03 | 0.93 | |
| m | 10 | 20 | 0.19±0.08 | 0.61±0.05 | 0.71 | |
| m | 10 | 25 | 0.08±0.01 | 0.42±0.03 | 0.88 | |
| f | 1 | 20 | 0.22±0.02 | 0.18±0.02 | 0.99 | |
| f | 1 | 25 | 0.14±0.01 | 0.10±0.01 | 0.99 | |
| f | 10 | 20 | 0.18±0.07 | 0.25±0.06 | 0.87 | |
| N. tenuis | f | 10 | 25 | 0.09±0.01 | 0.14±0.03 | 0.96 |
| m | 1 | 20 | 0.20±0.07 | 0.24±0.03 | 0.98 | |
| m | 1 | 25 | 0.10±0.03 | 0.19±0.06 | 0.88 | |
| m | 10 | 20 | 0.09±0.01 | 0.37±0.05 | 0.93 | |
| m | 10 | 25 | 0.04±0.01 | 0.19±0.11 | 0.88 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





