Submitted:
16 April 2024
Posted:
17 April 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Study Size
2.2. Approval
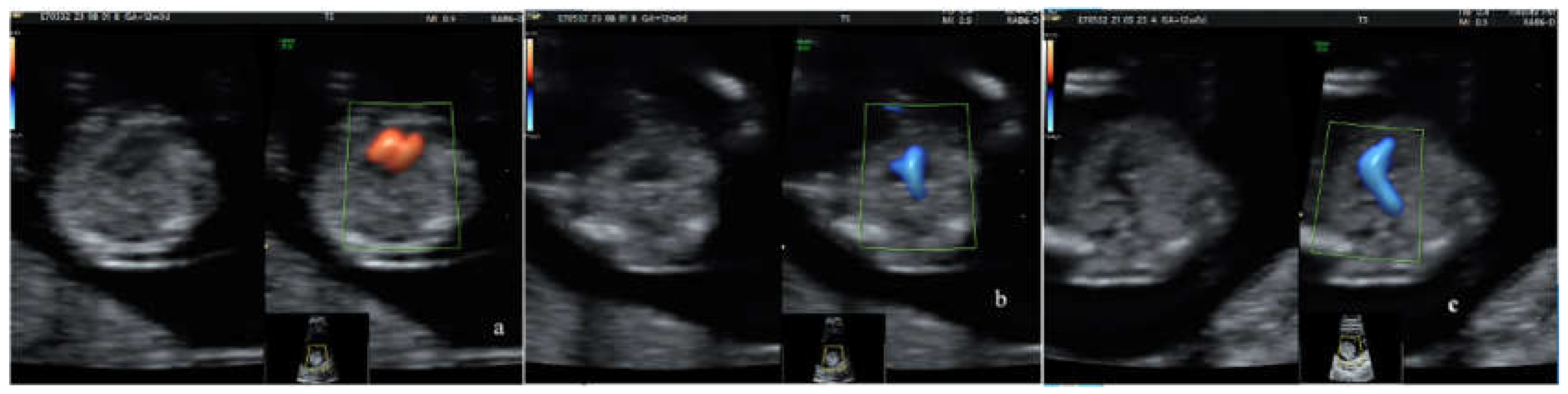
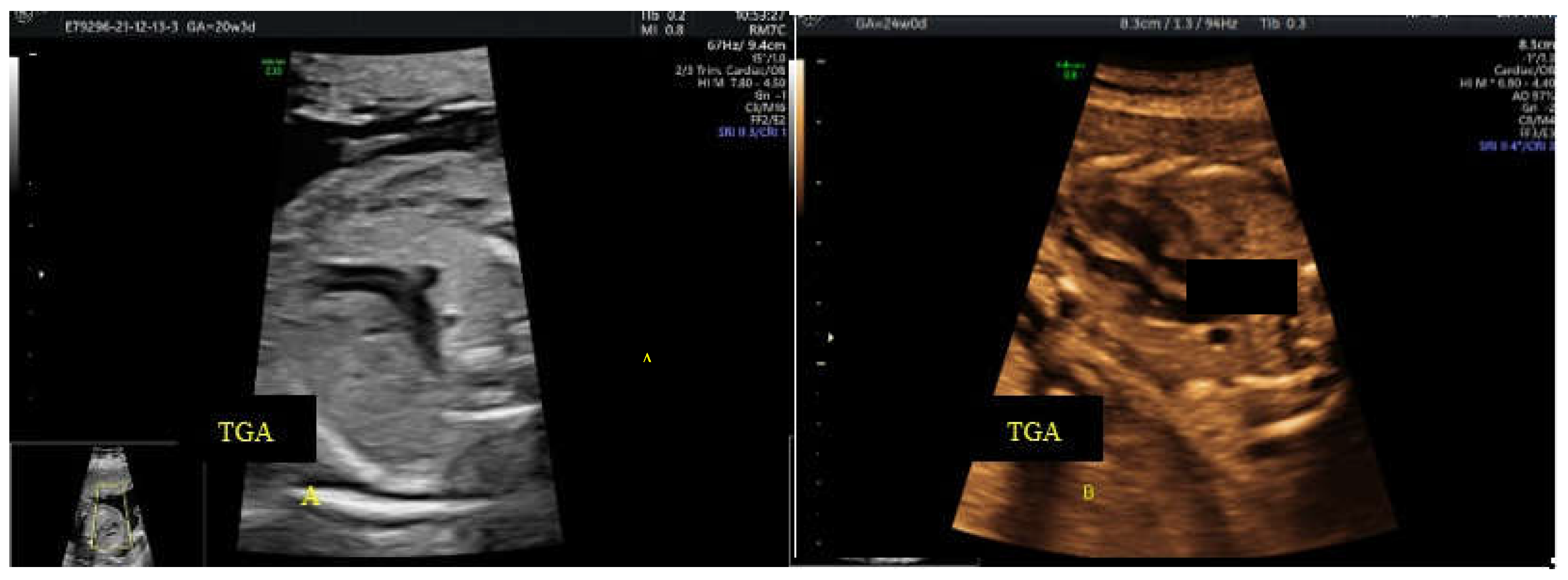
2.3. Screening Ultrasound (US) Protocol
- normal 4-chamber view, normal equal ventricular inflows, in a normal case
- b. confluence of the arches on the left (”the V-sign” in the 3VT view) in a normal case
- c. the ”reverse boomerang sign” - the reverse curvature of right ventricle outflow tract (RVOT) at level of the 3VT view in a TGA case
2.4. Live Births
2.5. Termination of Pregnancy
2.6. Analyses
- incidence of TGA as the number of TGA (live-born and terminated) divided by the total number of fetuses scanned.
- live-birth incidence of TGA as the number of TGA in live-born children divided by the total number of live births.
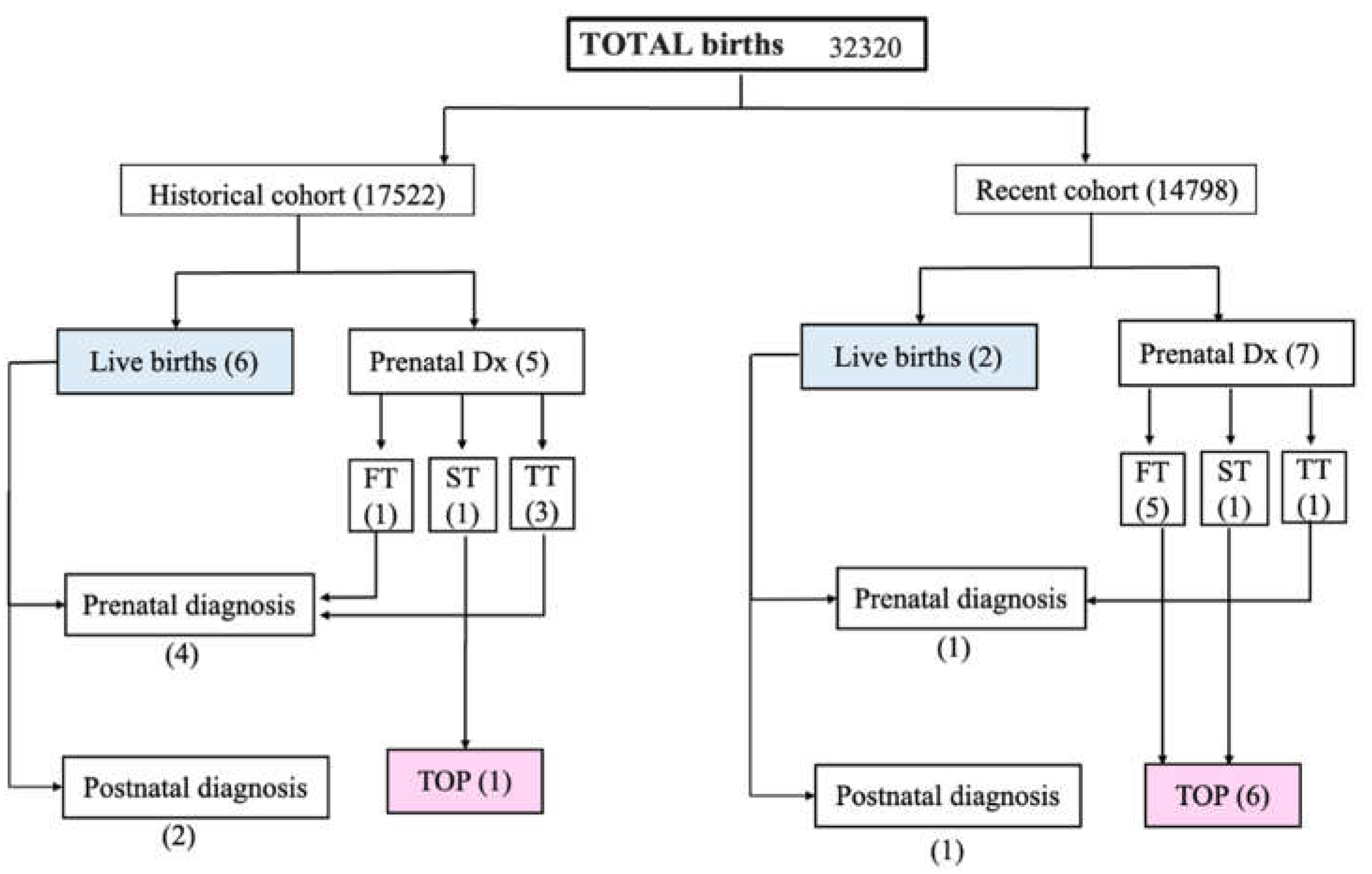
3. Results
3.1. Incidence
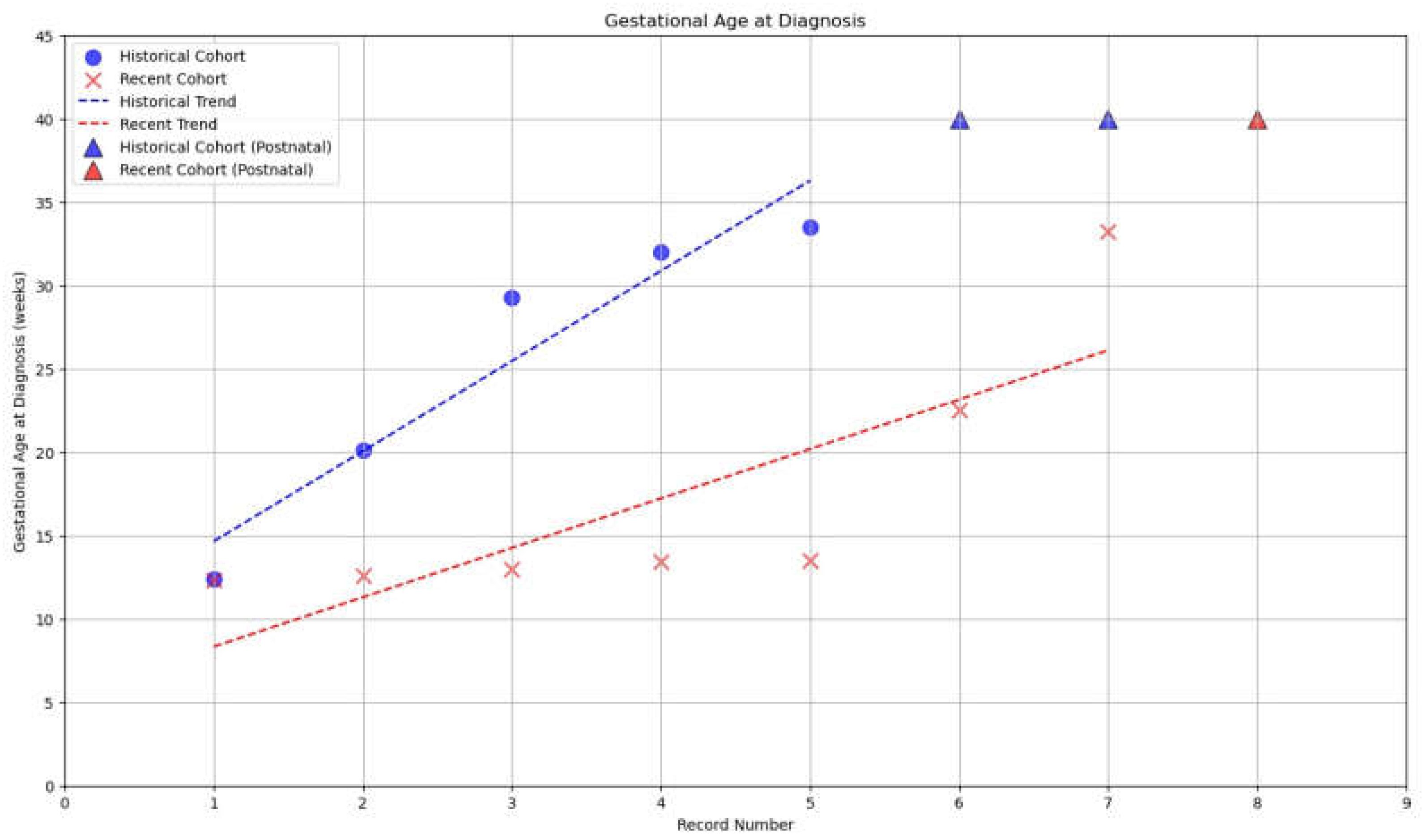
3.2. Detection Rates
3.3. Characteristics of TGA Liveborn in EUCH
| Prenatal vs Postnatal Diagnosis, No./Total No. (%) | P Value | ||
|---|---|---|---|
| 2008 – 2013 (No universal FT Screening) |
2018 – 2023 (universal FT Screening) |
||
| TGA FT | 1/7 (14.28%) | 5/8 (62.5%) | 0.34 |
| TGA ST | 1/7 (14.28%) | 1/8 (12.5%) | 1.0 |
| TGA TT | 3/7 (42.85%) | 1/8 (12.5%) | 0.58 |
| Prenatal Dx TGA overall | 4/7 (57.14%) | 7/8 (87.5%) | 0.7 |
| Postnatal Dx TGA | 2/7 (28.57%) | 1/8 (12.5%) | 1.0 |
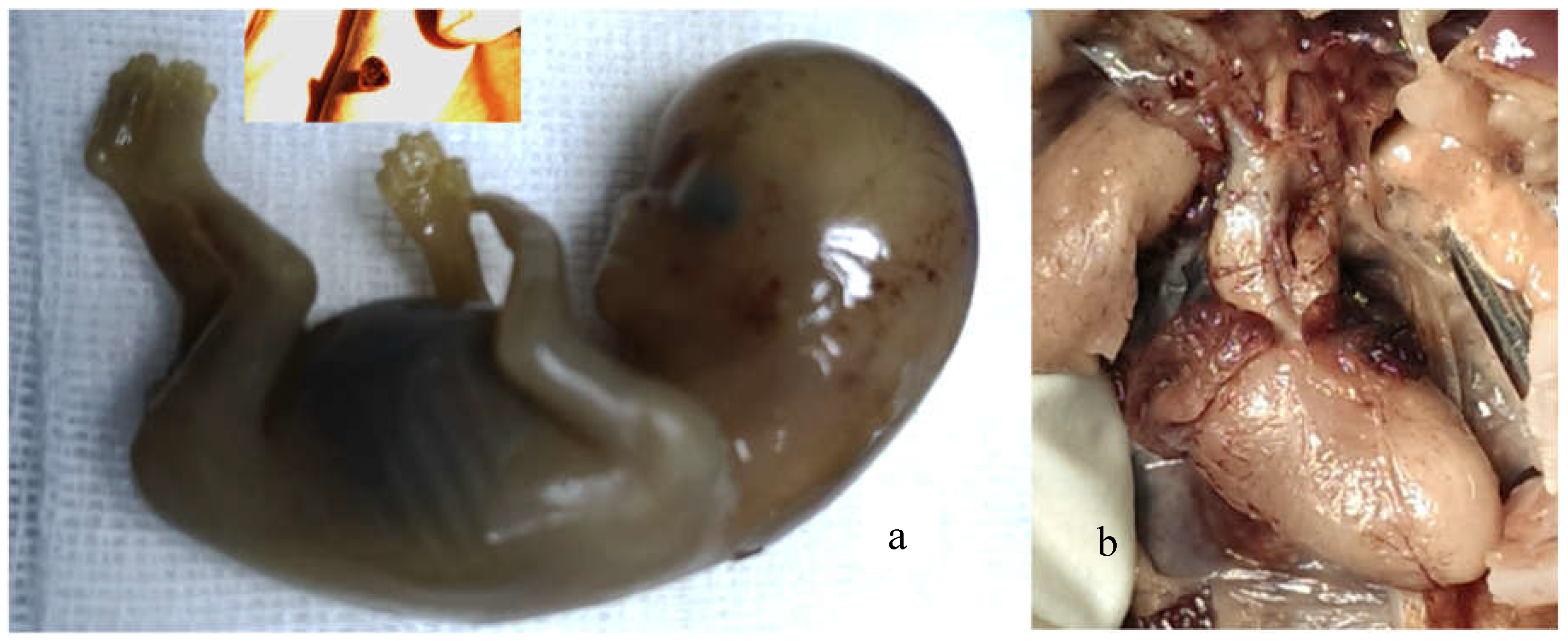
3.4. Termination of Pregnancy
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board and Informed Consent
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sainz, J.A.; Zurita, M.J.; Guillen, I.; Borrero, C.; Garcia-Mejido, J.; Almeida, C.; Turmo, E.; Garrido, R. [Prenatal screening of congenital heart defects in population at low risk of congenital defects. A reality today]. An Pediatr (Barc) 2015, 82, 27–34. [Google Scholar] [CrossRef]
- Tegnander, E.; Williams, W.; Johansen, O.J.; Blaas, H.G.; Eik-Nes, S.H. Prenatal detection of heart defects in a non-selected population of 30,149 fetuses--detection rates and outcome. Ultrasound Obstet Gynecol 2006, 27, 252–265. [Google Scholar] [CrossRef]
- Hu, Q.; Deng, C.; Zhu, Q.; Yang, X.; Liu, H.; Liao, H.; Wang, X.; Yu, H. Dextro-transposition of the great arteries in one twin: Case reports and literature review. Transl Pediatr 2022, 11, 601–609. [Google Scholar] [CrossRef]
- Baumgartner, H.; Bonhoeffer, P.; De Groot, N.M.; de Haan, F.; Deanfield, J.E.; Galie, N.; Gatzoulis, M.A.; Gohlke-Baerwolf, C.; Kaemmerer, H.; Kilner, P.; et al. ESC Guidelines for the management of grown-up congenital heart disease (new version 2010). Eur Heart J 2010, 31, 2915–2957. [Google Scholar] [CrossRef] [PubMed]
- Jatene, A.D.; Fontes, V.F.; Paulista, P.P.; Souza, L.C.; Neger, F.; Galantier, M.; Sousa, J.E. Anatomic correction of transposition of the great vessels. J Thorac Cardiovasc Surg 1976, 72, 364–370. [Google Scholar] [CrossRef] [PubMed]
- Moe, T.G.; Bardo, D.M. E. Long-term Outcomes of the Arterial Switch Operation for d-Transposition of the Great Arteries. Prog Cardiovasc Dis 2018, 61, 360–364. [Google Scholar] [CrossRef]
- Devlin, P.J.; Jegatheeswaran, A.; Williams, W.G.; Blackstone, E.H.; DeCampli, W.M.; Lambert, L.M.; Mussatto, K.A.; Prospero, C.J.; Bondarenko, I.; McCrindle, B.W. Late Survival and Patient-Perceived Health Status of the Congenital Heart Surgeons' Society dextro-Transposition of the Great Arteries Cohort. Ann Thorac Surg 2019, 108, 1447–1455. [Google Scholar] [CrossRef]
- Zhang, X.; Haneishi, H.; Liu, H. Impact of ductus arteriosus constriction and restrictive foramen ovale on global hemodynamics for term fetuses with d-TGA. Int J Numer Method Biomed Eng 2021, 37, e3231. [Google Scholar] [CrossRef]
- Soongswang, J.; Adatia, I.; Newman, C.; Smallhorn, J.F.; Williams, W.G.; Freedom, R.M. Mortality in potential arterial switch candidates with transposition of the great arteries. J Am Coll Cardiol 1998, 32, 753–757. [Google Scholar] [CrossRef] [PubMed]
- Villafane, J.; Lantin-Hermoso, M.R.; Bhatt, A.B.; Tweddell, J.S.; Geva, T.; Nathan, M.; Elliott, M.J.; Vetter, V.L.; Paridon, S.M.; Kochilas, L.; et al. D-transposition of the great arteries: The current era of the arterial switch operation. J Am Coll Cardiol 2014, 64, 498–511. [Google Scholar] [CrossRef]
- Paladini, D.; Alfirevic, Z.; Carvalho, J.S.; Khalil, A.; Malinger, G.; Martinez, J.M.; Rychik, J.; Ville, Y.; Gardiner, H.; Committee, I.C. S. ISUOG consensus statement on current understanding of the association of neurodevelopmental delay and congenital heart disease: Impact on prenatal counseling. Ultrasound Obstet Gynecol 2017, 49, 287–288. [Google Scholar] [CrossRef] [PubMed]
- Peyvandi, S.; Lim, J.M.; Marini, D.; Xu, D.; Reddy, V.M.; Barkovich, A.J.; Miller, S.; McQuillen, P.; Seed, M. Fetal brain growth and risk of postnatal white matter injury in critical congenital heart disease. J Thorac Cardiovasc Surg 2021, 162, 1007–1014. [Google Scholar] [CrossRef] [PubMed]
- Park, C.S.; Lee, J.R.; Lim, H.G.; Kim, W.H.; Kim, Y.J. The long-term result of total repair for tetralogy of Fallot. Eur J Cardiothorac Surg 2010, 38, 311–317. [Google Scholar] [CrossRef] [PubMed]
- Bonnet, D.; Coltri, A.; Butera, G.; Fermont, L.; Le Bidois, J.; Kachaner, J.; Sidi, D. Detection of transposition of the great arteries in fetuses reduces neonatal morbidity and mortality. Circulation 1999, 99, 916–918. [Google Scholar] [CrossRef] [PubMed]
- van Velzen, C.L.; Haak, M.C.; Reijnders, G.; Rijlaarsdam, M.E.; Bax, C.J.; Pajkrt, E.; Hruda, J.; Galindo-Garre, F.; Bilardo, C.M.; de Groot, C.J.; et al. Prenatal detection of transposition of the great arteries reduces mortality and morbidity. Ultrasound Obstet Gynecol 2015, 45, 320–325. [Google Scholar] [CrossRef] [PubMed]
- Raboisson, M.J.; Samson, C.; Ducreux, C.; Rudigoz, R.C.; Gaucherand, P.; Bouvagnet, P.; Bozio, A. Impact of prenatal diagnosis of transposition of the great arteries on obstetric and early postnatal management. Eur J Obstet Gynecol Reprod Biol 2009, 142, 18–22. [Google Scholar] [CrossRef]
- Donofrio, M.T. Images in cardiovascular medicine. Premature closure of the foramen ovale and ductus arteriosus in a fetus with transposition of the great arteries. Circulation 2002, 105, e65–e66. [Google Scholar] [CrossRef] [PubMed]
- Lara, D.A.; Fixler, D.E.; Ethen, M.K.; Canfield, M.A.; Nembhard, W.N.; Morris, S.A. Prenatal diagnosis, hospital characteristics, and mortality in transposition of the great arteries. Birth Defects Res A Clin Mol Teratol 2016, 106, 739–748. [Google Scholar] [CrossRef]
- Slodki, M.; Respondek-Liberska, M.; Pruetz, J.D.; Donofrio, M.T. Fetal cardiology: Changing the definition of critical heart disease in the newborn. J Perinatol 2016, 36, 575–580. [Google Scholar] [CrossRef]
- Bull, C. Current and potential impact of fetal diagnosis on prevalence and spectrum of serious congenital heart disease at term in the UK. British Paediatric Cardiac Association. Lancet 1999, 354, 1242–1247. [Google Scholar] [CrossRef]
- Blyth, M.; Howe, D.; Gnanapragasam, J.; Wellesley, D. The hidden mortality of transposition of the great arteries and survival advantage provided by prenatal diagnosis. BJOG 2008, 115, 1096–1100. [Google Scholar] [CrossRef] [PubMed]
- Skinner, J.; Hornung, T.; Rumball, E. Transposition of the great arteries: From fetus to adult. Heart 2008, 94, 1227–1235. [Google Scholar] [CrossRef] [PubMed]
- Landis, B.J.; Levey, A.; Levasseur, S.M.; Glickstein, J.S.; Kleinman, C.S.; Simpson, L.L.; Williams, I.A. Prenatal diagnosis of congenital heart disease and birth outcomes. Pediatr Cardiol 2013, 34, 597–605. [Google Scholar] [CrossRef] [PubMed]
- Khoshnood, B.; De Vigan, C.; Vodovar, V.; Goujard, J.; Lhomme, A.; Bonnet, D.; Goffinet, F. Trends in prenatal diagnosis, pregnancy termination, and perinatal mortality of newborns with congenital heart disease in France, 1983-2000: A population-based evaluation. Pediatrics 2005, 115, 95–101. [Google Scholar] [CrossRef] [PubMed]
- Escobar-Diaz, M.C.; Freud, L.R.; Bueno, A.; Brown, D.W.; Friedman, K.G.; Schidlow, D.; Emani, S.; Del Nido, P.J.; Tworetzky, W. Prenatal diagnosis of transposition of the great arteries over a 20-year period: Improved but imperfect. Ultrasound Obstet Gynecol 2015, 45, 678–682. [Google Scholar] [CrossRef] [PubMed]
- Slodki, M. Dextro-transposition of great vessels: Difficult to detect prenatally, one of the most dangerous and one of the best prognosed. Transl Pediatr 2022, 11, 783–788. [Google Scholar] [CrossRef] [PubMed]
- Tudorache, S.; Cara, M.; Iliescu, D.G.; Novac, L.; Cernea, N. First trimester two- and four-dimensional cardiac scan: Intra- and interobserver agreement, comparison between methods and benefits of color Doppler technique. Ultrasound Obstet Gynecol 2013, 42, 659–668. [Google Scholar] [CrossRef] [PubMed]
- Iliescu, D.; Tudorache, S.; Comanescu, A.; Antsaklis, P.; Cotarcea, S.; Novac, L.; Cernea, N.; Antsaklis, A. Improved detection rate of structural abnormalities in the first trimester using an extended examination protocol. Ultrasound Obstet Gynecol 2013, 42, 300–309. [Google Scholar] [CrossRef]
- Ştefania Tudorache, A.U. , Roxana Cristina Drăguşin, Florea Maria Şorop, Monica Laura Cara, D.G. Iliescu. FIRST TRIMESTER DIAGNOSTIC ACCURACY OF A TWO- DIMENSIONAL SIMPLIFIED ULTRASOUND TECHNIQUE IN CONGENITAL HEART DISEASES AND GREAT ARTERIES ANOMALIES. Obstetrica & Ginecologia, 2016; LXIV, 165–176. [Google Scholar]
- Tudorache, S.; Florea, M.; Dragusin, R.; Patru, C.; Zorila, L.; Iliescu, D.G. OP15.03: First trimester fetal heart assessment learning curve. Ultrasound in Obstetrics & Gynecology 2016, 48, 97. [Google Scholar]
- Tudorache, S.; M, F.; R, D.; Zorila, L.; Cl, P.; Alexandru, D.; Cara, M.; Iliescu, D. Learning Curve for Ultrasound Assessment of the Fetal Heart at Nuchal Scan. JBR Journal of Clinical Diagnosis and Research 2017, 05. [Google Scholar] [CrossRef]
- Vinals, F.; Medina, L.; Guerra, F.A.; Naveas, R.; Giuliano, A. OC134: Accuracy of prenatal diagnosis of congenital heart defects evaluating the STIC sweep of the acquisition plane. Ultrasound in Obstetrics & Gynecology 2006, 28, 398. [Google Scholar]
- Bravo-Valenzuela, N.J.; Peixoto, A.B.; Araujo Junior, E. Prenatal diagnosis of congenital heart disease: A review of current knowledge. Indian Heart J 2018, 70, 150–164. [Google Scholar] [CrossRef] [PubMed]
- Bravo-Valenzuela, N.J.; Peixoto, A.B.; Araujo Junior, E. Prenatal diagnosis of transposition of the great arteries: An updated review. Ultrasonography 2020, 39, 331–339. [Google Scholar] [CrossRef] [PubMed]
- Bindra, R.; Heath, V.; Liao, A.; Spencer, K.; Nicolaides, K.H. One-stop clinic for assessment of risk for trisomy 21 at 11-14 weeks: A prospective study of 15 030 pregnancies. Ultrasound Obstet Gynecol 2002, 20, 219–225. [Google Scholar] [CrossRef] [PubMed]
- Nicolaides, K.H. Screening for fetal aneuploidies at 11 to 13 weeks. Prenat Diagn 2011, 31, 7–15. [Google Scholar] [CrossRef] [PubMed]
- Cicero, S.; Bindra, R.; Rembouskos, G.; Spencer, K.; Nicolaides, K.H. Integrated ultrasound and biochemical screening for trisomy 21 using fetal nuchal translucency, absent fetal nasal bone, free beta-hCG and PAPP-A at 11 to 14 weeks. Prenat Diagn 2003, 23, 306–310. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, J.S.; Mavrides, E.; Shinebourne, E.A.; Campbell, S.; Thilaganathan, B. Improving the effectiveness of routine prenatal screening for major congenital heart defects. Heart 2002, 88, 387–391. [Google Scholar] [CrossRef] [PubMed]
- Chaoui, R.; McEwing, R. Three cross-sectional planes for fetal color Doppler echocardiography. Ultrasound Obstet Gynecol 2003, 21, 81–93. [Google Scholar] [CrossRef] [PubMed]
- Cardiac screening examination of the fetus: Guidelines for performing the 'basic' and 'extended basic' cardiac scan. Ultrasound Obstet Gynecol 2006, 27, 107–113. [CrossRef]
- Salomon, L.J.; Alfirevic, Z.; Bilardo, C.M.; Chalouhi, G.E.; Ghi, T.; Kagan, K.O.; Lau, T.K.; Papageorghiou, A.T.; Raine-Fenning, N.J.; Stirnemann, J.; et al. ISUOG practice guidelines: Performance of first-trimester fetal ultrasound scan. Ultrasound Obstet Gynecol 2013, 41, 102–113. [Google Scholar] [CrossRef]
- Syngelaki, A.; Chelemen, T.; Dagklis, T.; Allan, L.; Nicolaides, K.H. Challenges in the diagnosis of fetal non-chromosomal abnormalities at 11-13 weeks. Prenat Diagn 2011, 31, 90–102. [Google Scholar] [CrossRef] [PubMed]
- Becker, R.; Wegner, R.D. Detailed screening for fetal anomalies and cardiac defects at the 11-13-week scan. Ultrasound Obstet Gynecol 2006, 27, 613–618. [Google Scholar] [CrossRef] [PubMed]
- Lombardi, C.M.; Bellotti, M.; Fesslova, V.; Cappellini, A. Fetal echocardiography at the time of the nuchal translucency scan. Ultrasound Obstet Gynecol 2007, 29, 249–257. [Google Scholar] [CrossRef] [PubMed]
- Tudorache, S.; Cara, M.; Iliescu, D.G.; Stoica, A.; Simionescu, C.; Novac, L.V.; Cernea, D. Fetal Kidneys Ultrasound Appearance in the First Trimester - Clinical Significance and Limits of Counseling. Curr Health Sci J 2016, 42, 19–30. [Google Scholar] [CrossRef] [PubMed]
- Quarello, E.; Lafouge, A.; Fries, N.; Salomon, L.J.; Cfef. Basic heart examination: Feasibility study of first-trimester systematic simplified fetal echocardiography. Ultrasound Obstet Gynecol 2017, 49, 224–230. [Google Scholar] [CrossRef] [PubMed]
- Vayna, A.M.; Veduta, A.; Duta, S.; Panaitescu, A.M.; Stoica, S.; Buinoiu, N.; Nedelea, F.; Peltecu, G. Diagnosis of Fetal Structural Anomalies at 11 to 14 Weeks. J Ultrasound Med 2018, 37, 2063–2073. [Google Scholar] [CrossRef] [PubMed]
- Menahem, S.; Rotstein, A.; Meagher, S. Rightward convexity of the great vessel arising from the anterior ventricle: A novel fetal marker for transposition of the great arteries. Ultrasound Obstet Gynecol 2013, 41, 168–171. [Google Scholar] [CrossRef] [PubMed]
- Salvesen, K.; Abramowicz, J.; Ter Haar, G.; Miloro, P.; Sinkovskaya, E.; Dall'Asta, A.; Marsal, K.; Lees, C.; Board of the International Society of Ultrasound in, O.; Gynecology. ISUOG statement on the safe use of Doppler for fetal ultrasound examination in the first 13 + 6 weeks of pregnancy (updated). Ultrasound Obstet Gynecol 2021, 57, 1020. [Google Scholar] [CrossRef]
- International Society of Ultrasound in, O.; Gynecology; Bilardo, C. M.; Chaoui, R.; Hyett, J.A.; Kagan, K.O.; Karim, J.N.; Papageorghiou, A.T.; Poon, L.C.; Salomon, L.J.; et al. ISUOG Practice Guidelines (updated): Performance of 11-14-week ultrasound scan. Ultrasound Obstet Gynecol 2023, 61, 127–143. [Google Scholar] [CrossRef] [PubMed]
- Oster, M.E.; Kim, C.H.; Kusano, A.S.; Cragan, J.D.; Dressler, P.; Hales, A.R.; Mahle, W.T.; Correa, A. A population-based study of the association of prenatal diagnosis with survival rate for infants with congenital heart defects. Am J Cardiol 2014, 113, 1036–1040. [Google Scholar] [CrossRef]
- Garne, E.; Stoll, C.; Clementi, M.; Euroscan, G. Evaluation of prenatal diagnosis of congenital heart diseases by ultrasound: Experience from 20 European registries. Ultrasound Obstet Gynecol 2001, 17, 386–391. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, J.I.; Kaplan, S. The incidence of congenital heart disease. J Am Coll Cardiol 2002, 39, 1890–1900. [Google Scholar] [CrossRef] [PubMed]
- Jorgensen, D.E.; Vejlstrup, N.; Jorgensen, C.; Maroun, L.L.; Steensberg, J.; Hessellund, A.; Jorgensen, F.S.; Larsen, T.; Shalmi, A.C.; Skibsted, L.; et al. Prenatal detection of congenital heart disease in a low risk population undergoing first and second trimester screening. Prenat Diagn 2015, 35, 325–330. [Google Scholar] [CrossRef] [PubMed]
- Oyen, N.; Poulsen, G.; Boyd, H.A.; Wohlfahrt, J.; Jensen, P.K.; Melbye, M. National time trends in congenital heart defects, Denmark, 1977-2005. Am Heart J 2009, 157, 467–473. [Google Scholar] [CrossRef] [PubMed]
- Egbe, A.; Uppu, S.; Lee, S.; Ho, D.; Srivastava, S. Changing prevalence of severe congenital heart disease: A population-based study. Pediatr Cardiol 2014, 35, 1232–1238. [Google Scholar] [CrossRef]
- Vedel, C.; Hjortshoj, T.D.; Jorgensen, D.S.; Tabor, A.; Rode, L.; Sundberg, K.; Ekelund, C.K.; Petersen, O.B. Prevalence of chromosomal disorders in cases with congenital heart defect: Registry-based study from Denmark between 2008 and 2018. Ultrasound Obstet Gynecol 2023, 61, 40–48. [Google Scholar] [CrossRef]
- Idorn, L.; Olsen, M.; Jensen, A.S.; Juul, K.; Reimers, J.I.; Sorensen, K.; Johnsen, S.P.; Sondergaard, L. Univentricular hearts in Denmark 1977 to 2009: Incidence and survival. Int J Cardiol 2013, 167, 1311–1316. [Google Scholar] [CrossRef]
- Stumpflen, I.; Stumpflen, A.; Wimmer, M.; Bernaschek, G. Effect of detailed fetal echocardiography as part of routine prenatal ultrasonographic screening on detection of congenital heart disease. Lancet 1996, 348, 854–857. [Google Scholar] [CrossRef]
- Becker, R.; Schmitz, L.; Kilavuz, S.; Stumm, M.; Wegner, R.D.; Bittner, U. 'Normal' nuchal translucency: A justification to refrain from detailed scan? Analysis of 6858 cases with special reference to ethical aspects. Prenat Diagn 2012, 32, 550–556. [Google Scholar] [CrossRef]
- Bonnet, D. Impacts of prenatal diagnosis of congenital heart diseases on outcomes. Transl Pediatr 2021, 10, 2241–2249. [Google Scholar] [CrossRef]
- Jicinska, H.; Vlasin, P.; Jicinsky, M.; Grochova, I.; Tomek, V.; Volaufova, J.; Skovranek, J.; Marek, J. Does First-Trimester Screening Modify the Natural History of Congenital Heart Disease? Analysis of Outcome of Regional Cardiac Screening at 2 Different Time Periods. Circulation 2017, 135, 1045–1055. [Google Scholar] [CrossRef] [PubMed]
- Gardiner, H.M.; Kovacevic, A.; van der Heijden, L.B.; Pfeiffer, P.W.; Franklin, R.C.; Gibbs, J.L.; Averiss, I.E.; Larovere, J.M. Prenatal screening for major congenital heart disease: Assessing performance by combining national cardiac audit with maternity data. Heart 2014, 100, 375–382. [Google Scholar] [CrossRef] [PubMed]
- Galindo, A.; Herraiz, I.; Escribano, D.; Lora, D.; Melchor, J.C.; de la Cruz, J. Prenatal detection of congenital heart defects: A survey on clinical practice in Spain. Fetal Diagn Ther 2011, 29, 287–295. [Google Scholar] [CrossRef] [PubMed]
- Lytzen, R.; Vejlstrup, N.; Bjerre, J.; Petersen, O.B.; Leenskjold, S.; Dodd, J.K.; Jorgensen, F.S.; Sondergaard, L. Live-Born Major Congenital Heart Disease in Denmark: Incidence, Detection Rate, and Termination of Pregnancy Rate From 1996 to 2013. JAMA Cardiol 2018, 3, 829–837. [Google Scholar] [CrossRef] [PubMed]
- Bertagna, F.; Rakza, T.; Vaksmann, G.; Ramdane-Sebbane, N.; Devisme, L.; Storme, L.; Francart, C.; Vaast, P.; Houfflin-Debarge, V. Transposition of the great arteries: Factors influencing prenatal diagnosis. Prenat Diagn 2014, 34, 534–537. [Google Scholar] [CrossRef] [PubMed]
- Han, B.; Li, Y.; Tang, Y.; Qu, X.; Wang, F.; Song, H.; Xu, Y. Clinical analysis of prenatal ultrasound diagnosis of fetal cardiovascular malformations in the first and second trimesters of pregnancy: A CARE-compliant article. Medicine (Baltimore) 2019, 98, e16822. [Google Scholar] [CrossRef] [PubMed]
- Dolk, H.; Loane, M.; Garne, E. ; European Surveillance of Congenital Anomalies Working, G. Congenital heart defects in Europe: Prevalence and perinatal mortality, 2000 to 2005. Circulation 2011, 123, 841–849. [Google Scholar] [CrossRef] [PubMed]
- Qu, Y.; Wen, S.; Liu, X.; Pan, W.; Han, F.; Mai, J.; Ou, Y.; Nie, Z.; Gao, X.; Wu, Y.; et al. Perinatal and early postnatal outcomes for fetuses with prenatally diagnosed d-transposition of the great arteries: A prospective cohort study assessing the effect of standardised prenatal consultation. Cardiol Young 2018, 28, 66–75. [Google Scholar] [CrossRef] [PubMed]
- Termination of pregnancy. Aggression towards the fetus New Penal Code, 2009.
- Thomas, C.; Yu, S.; Lowery, R.; Zampi, J.D. Timing of Balloon Atrial Septostomy in Patients with d-TGA and Association with Birth Location and Patient Outcomes. Pediatr Cardiol 2023, 44, 1333–1341. [Google Scholar] [CrossRef]
- Khairy, P.; Clair, M.; Fernandes, S.M.; Blume, E.D.; Powell, A.J.; Newburger, J.W.; Landzberg, M.J.; Mayer, J.E., Jr. Cardiovascular outcomes after the arterial switch operation for D-transposition of the great arteries. Circulation 2013, 127, 331–339. [Google Scholar] [CrossRef]
- Wernovsky, G.; Mayer, J.E., Jr.; Jonas, R.A.; Hanley, F.L.; Blackstone, E.H.; Kirklin, J.W.; Castaneda, A.R. Factors influencing early and late outcome of the arterial switch operation for transposition of the great arteries. J Thorac Cardiovasc Surg 1995, 109, 289-301; discussion 301-282. [Google Scholar] [CrossRef] [PubMed]
- Maria Sorop-Florea, R.N. C., Mihai Ioana, Alex Emilian Stepan, George Alin Stoica, Florentina Tanase, Maria Cristina Comanescu, Marius-Bogdan Novac, Ioana Dragan, Ciprian Laurentiu Patru, Roxana Cristina Dragusin, George Lucian Zorila, Ovidiu Marian Carbunaru, Nuti Daniela Oprescu, Iuliana Ceausu, Simona Vladareanu, Stefania Tudorache, Dominic Gabriel Iliescu. The importance of perinatal autopsy. Review of the literature and series of cases. Romanian Journal of Morphology and Embryology 2017, 58.
- Ruican, D.; Petrescu, A.M.; Istrate-Ofiteru, A.M.; Iliescu, D.G. Postmortem Evaluation of First Trimester Fetal Heart. Curr Health Sci J 2022, 48, 247–254. [Google Scholar] [CrossRef] [PubMed]
- Staicu, A.; Albu, C.; Popa-Stanila, R.; Chiriac, L.; Boitor-Borza, D.; Bondor, C.; Kovacs, T.; Caracostea, G.; Rotar, I.C.; Turcu, R.V. F.; et al. Potential clinical benefits and limitations of fetal virtopsy using high-field MRI at 7 Tesla versus stereomicroscopic autopsy to assess first trimester fetuses. Prenat Diagn 2019, 39, 505–518. [Google Scholar] [CrossRef] [PubMed]
- Tang, H.; Zhang, Y.; Dai, C.; Ru, T.; Li, J.; Chen, J.; Zhang, B.; Zhou, K.; Lv, P.; Liu, R.; et al. Postmortem 9.4-T MRI for Fetuses With Congenital Heart Defects Diagnosed in the First Trimester. Front Cardiovasc Med 2021, 8, 764587. [Google Scholar] [CrossRef]
- Ushakov, F.; Sacco, A.; Andreeva, E.; Tudorache, S.; Everett, T.; David, A.L.; Pandya, P.P. Crash sign: New first-trimester sonographic marker of spina bifida. Ultrasound Obstet Gynecol 2019, 54, 740–745. [Google Scholar] [CrossRef] [PubMed]
- Ungureanu, D.R.; Dragusin, R.C.; Capitanescu, R.G.; Zorila, L.; Ofiteru, A.M. I.; Marinas, C.; Patru, C.L.; Comanescu, A.C.; Comanescu, M.C.; Sirbu, O.C.; et al. First Trimester Ultrasound Detection of Fetal Central Nervous System Anomalies. Brain Sci 2023, 13. [Google Scholar] [CrossRef] [PubMed]
- Tuo, G.; Paladini, D.; Montobbio, G.; Volpe, P.; Cheli, M.; Calevo, M.G.; Marasini, M. Prenatal Echocardiographic Assessment of Foramen Ovale Appearance in Fetuses with D-Transposition of the Great Arteries and Impact on Neonatal Outcome. Fetal Diagn Ther 2017, 42, 48–56. [Google Scholar] [CrossRef]
- Masci, M.; Pasquini, L.; Alsaied, T.; Di Chiara, L.; Formigari, R.; Galletti, L.; Campanale, C.M.; Romiti, A.; Bonito, M.; Bagolan, P.; et al. Reliability of Fetal Echocardiography in Predicting Postnatal Critical Hypoxia in Patients with Transposition of Great Arteries and Intact Ventricular Septum. Pediatr Cardiol 2021, 42, 1575–1584. [Google Scholar] [CrossRef] [PubMed]
- Buca, D.; Winberg, P.; Rizzo, G.; Khalil, A.; Liberati, M.; Makatsariya, A.; Greco, F.; Nappi, L.; Acharya, G.; D'Antonio, F. Prenatal risk factors for urgent atrial septostomy at birth in fetuses with transposition of the great arteries: A systematic review and meta-analysis. J Matern Fetal Neonatal Med 2022, 35, 598–606. [Google Scholar] [CrossRef]
- Gottschalk, I.; Walter, A.; Menzel, T.; Weber, E.C.; Wendt, S.; Sreeram, N.; Gembruch, U.; Berg, C.; Abel, J.S. D-Transposition of the great arteries with restrictive foramen ovale in the fetus: The dilemma of predicting the need for postnatal urgent balloon atrial septostomy. Arch Gynecol Obstet 2024, 309, 1353–1367. [Google Scholar] [CrossRef] [PubMed]
- Slodki, M.; Axt-Fliedner, R.; Zych-Krekora, K.; Wolter, A.; Kawecki, A.; Enzensberger, C.; Gulczynska, E.; Respondek-Liberska, M.; International Prenatal Cardiology Collaboration, G. New method to predict need for Rashkind procedure in fetuses with dextro-transposition of the great arteries. Ultrasound Obstet Gynecol 2018, 51, 531–536. [Google Scholar] [CrossRef] [PubMed]
- Jouannic, J.M.; Gavard, L.; Fermont, L.; Le Bidois, J.; Parat, S.; Vouhe, P.R.; Dumez, Y.; Sidi, D.; Bonnet, D. Sensitivity and specificity of prenatal features of physiological shunts to predict neonatal clinical status in transposition of the great arteries. Circulation 2004, 110, 1743–1746. [Google Scholar] [CrossRef] [PubMed]
- Rychik, J.; Liu, L.; Tian, Z. OC17.07: Relationship between foramen ovale restriction and flow characteristics of the ductus arteriosus in the fetus with transposition of the great arteries. Ultrasound in Obstetrics & Gynecology 2019, 54, 43–44. [Google Scholar] [CrossRef]
- Zeng, S.; Zhou, Q.C.; Zhou, J.W.; Li, M.; Long, C.; Peng, Q.H. Volume of intracranial structures on three-dimensional ultrasound in fetuses with congenital heart disease. Ultrasound Obstet Gynecol 2015, 46, 174–181. [Google Scholar] [CrossRef] [PubMed]
- Saadeh, M.; Zhao, Y.; Galadima, H.; Chaoui, R.; Sinkovskaya, E.; Abuhamad, A. Relationship Between Cavum Septi Pellucidi Measurements and Fetal Hypoplastic Left Heart Syndrome or Dextro-Transposition of the Great Arteries. J Ultrasound Med 2018, 37, 1673–1680. [Google Scholar] [CrossRef] [PubMed]
- Haligheri, G.; Patel, C.R.; Komarlu, R. Prenatal Delineation of Coronary Anatomy in Dextro-Transposition of Great Arteries. J Cardiovasc Echogr 2021, 31, 171–174. [Google Scholar] [CrossRef] [PubMed]
- Kaji, T.; Hayabuchi, Y.; Maeda, K.; Nakayama, S.; Irahara, M. Prenatal assessment of coronary artery anatomy using color Doppler in cases of D-transposition of the great arteries: Case reports. J Obstet Gynaecol Res 2017, 43, 397–402. [Google Scholar] [CrossRef] [PubMed]
- Pasquini, L.; Sanders, S.P.; Parness, I.A.; Colan, S.D.; Van Praagh, S.; Mayer, J.E., Jr.; Van Praagh, R. Conal anatomy in 119 patients with d-loop transposition of the great arteries and ventricular septal defect: An echocardiographic and pathologic study. J Am Coll Cardiol 1993, 21, 1712–1721. [Google Scholar] [CrossRef]
- Massoudy, P.; Baltalarli, A.; de Leval, M.R.; Cook, A.; Neudorf, U.; Derrick, G.; McCarthy, K.P.; Anderson, R.H. Anatomic variability in coronary arterial distribution with regard to the arterial switch procedure. Circulation 2002, 106, 1980–1984. [Google Scholar] [CrossRef]
- Paladini, D.; Bottelli, L.; Lops, G.; Tuo, G. Color Doppler visualization of fetal coronary arteries is enhanced by high resolution and Radiantflow. Ultrasound Obstet Gynecol 2022, 60, 432–434. [Google Scholar] [CrossRef] [PubMed]
- Wiechec, M.; Nocun, A.; Stettner, D.; Knafel, A. OC02.02: Conotruncal anomalies: How effective is first trimester diagnosis? Ultrasound in Obstetrics & Gynecology 2014, 44, 3. [Google Scholar]
- Nocuń, A.; Wiecheć, M.; Dudzik, M.; Serafin, K. The role of first-trimester single arterial vessel sign instead of a V-sign at the level of the three-vessel and tracheal view in screening for congenital heart diseases. Prenatal Cardiology 2023, 1–12. [Google Scholar] [CrossRef]
- Karim, J.N.; Bradburn, E.; Roberts, N.; Papageorghiou, A.T.; study, A. First-trimester ultrasound detection of fetal heart anomalies: Systematic review and meta-analysis. Ultrasound Obstet Gynecol 2022, 59, 11–25. [Google Scholar] [CrossRef] [PubMed]
- Rotar, I.C.; Muresan, D.; Marginean, C.; Marginean, C.; Iliescu, D.G.; Iliescu, D.G.; Tudorache, S.; Tudorache, S. First trimester fetal heart evaluation. A pictorial essay. Med Ultrason 2020. [CrossRef]
- Lee, M.Y.; Won, H.S.; Han, Y.J.; Ryu, H.M.; Lee, D.E.; Jeong, B.D. Clinical value of chromosomal microarray analysis in prenatally diagnosed dextro-transposition of the great arteries. J Matern Fetal Neonatal Med 2020, 33, 1480–1485. [Google Scholar] [CrossRef] [PubMed]
- Skoric-Milosavljevic, D.; Tadros, R.; Bosada, F.M.; Tessadori, F.; van Weerd, J.H.; Woudstra, O.I.; Tjong, F.V. Y.; Lahrouchi, N.; Bajolle, F.; Cordell, H.J.; et al. Common Genetic Variants Contribute to Risk of Transposition of the Great Arteries. Circ Res 2022, 130, 166–180. [Google Scholar] [CrossRef]
- Peake, L.K.; Draper, E.S.; Budd, J.L.; Field, D. Outcomes when congenital heart disease is diagnosed antenatally versus postnatally in the UK: A retrospective population-based study. BMC Pediatr 2015, 15, 58. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).





