1. Introduction
The COVID-19 pandemic was the first major global health issue for a little over a century in a way not seen since the 1918 H1N1 pandemic, wrongly known as the Spanish Flu. This caused a great effort in biomedical research to obtain a vaccine that could stop or improve the evolution of the infection worldwide [
1].
There was high vaccine hesitancy during COVID-19 pandemic, close to 30% due, among other reasons, to the doubts about the efficacy of the vaccine [
2]. The effectiveness for full vaccination against SARS-CoV-2 infection was estimated to be from 70.4% of the ChAdOx1 nCoV-19 vaccine (AZD1222; Oxford-AstraZeneca) to 95% of the BNT162b2 mRNA COVID-19 vaccine (Pfizer- BioNTech). When prevention of hospitalization and Intensive Care Unit admission and severe disease where assessed, the effectiveness of vaccine ranged between 93%-89% [
3]. WHO suggested that a clear demonstration of efficacy should be a minimum criterion for any acceptable COVID-19 vaccine. The efficacy can be assessed against disease, severe disease, and/or shedding/transmission and the standardization of quantifiable endpoints is still a challenge today [
4].
Several studies identified a significant correlation between the dynamics of antibodies and the effectiveness of COVID-19 vaccination. Likewise, there is a correlation between anti-SARS-CoV-2 antibodies and protection from infection as well as antibody levels and the likelihood of transmission. In a general manner, peak humoral responses reached at 3–4 weeks post second dose of messenger RNA (mRNA) vaccines such as Pfizer-BioNTech (mRNA BNT161b2) and Moderna (mRNA-1273), after which antibody levels progressively decreased at 120–180 days postvaccination. The significant decline in anti-SARS-CoV-2 antibodies over time shows the progressive decline in the vaccine efficacy for preventing SARS-CoV-2 infections although with a reduction of the risk of developing severe COVID-19. This decay of specific antibodies supports the need for boosters [
5]. Protective and efficient humoral immune responses, with SARS-CoV-2 neutralisation antibodies, developed after the second or third dose of COVID-19 vaccination with a later a decrease [
6]. There are still many unknowns to be resolved about COVID-19 vaccines such as the duration of humoral immunity post-primary infection or vaccination. Likewise, the protection over time conferred by booster shots is also unknown [
7].
Cellular immunity plays an important role in limiting disease severity and the resolution of SARS-CoV-2 virus infection. T cells have an important role in protection against SARS-CoV-2 infection and vaccination leads to the development of both humoral and cellular immunity against the spike protein. The presence of SARS-CoV-2-specific CD4+ and CD8+ T cells were associated with reduced disease severity. Moreover, the immunogenicity of SARS-CoV-2 vaccines involves the cellular response but the phenotypic and functional diversity of T cell subsets involved in vaccine protection and infection control is not well understood [
8]. Inactivated vaccines with peptides from SARS-CoV-2 proteins elicited specific humoral and cellular responses. The frequencies of CD4+ T cells producing IFN-γ, IL-2, and TNF-α in response to SARS-CoV-2 spike, nucleocapsid or membrane proteins were significantly higher in double-vaccinated subjects [
9].
T cell differentiation in memory T cell subsets plays a major role in the effectiveness of vaccines. For these reasons, in this work we have studied naïve T cells –TN-, central memory T cells –TCM, peripheral effector memory T cells -TEM- and terminal effector memory T cells -TEMRA- [
10].
The main objective of this work was the study of humoral and cellular immunity after the administration of the Pfizer–BioNTech and Oxford AstraZeneca vaccines against SARS-CoV-2. For this, the specific IgG and IgA antibodies and the αβ and γδ T cells were studied. The study was conducted over a period of one year and after three doses of vaccines. Subsequently, the relationship of the results obtained with the appearance of post-vaccination COVID-19 was studied.
2. Materials and Methods
2.1. Ethics Statement
The Research Ethics Committee of Arnau de Vilanova hospital, Valencia (Spain) approved the study (10/2021- March 24). Each volunteer participant signed an informed consent document. This study was conducted following the recommendations of the Spanish Bioethics Committee, the Spanish legislation on Biomedical Research (Law 14/2007) and Personal Data Protection (Spanish Law 3/2018 and European Law UE676/2018). The anonymity of the subjects participating in the study has been ensured.
2.2. Study Population
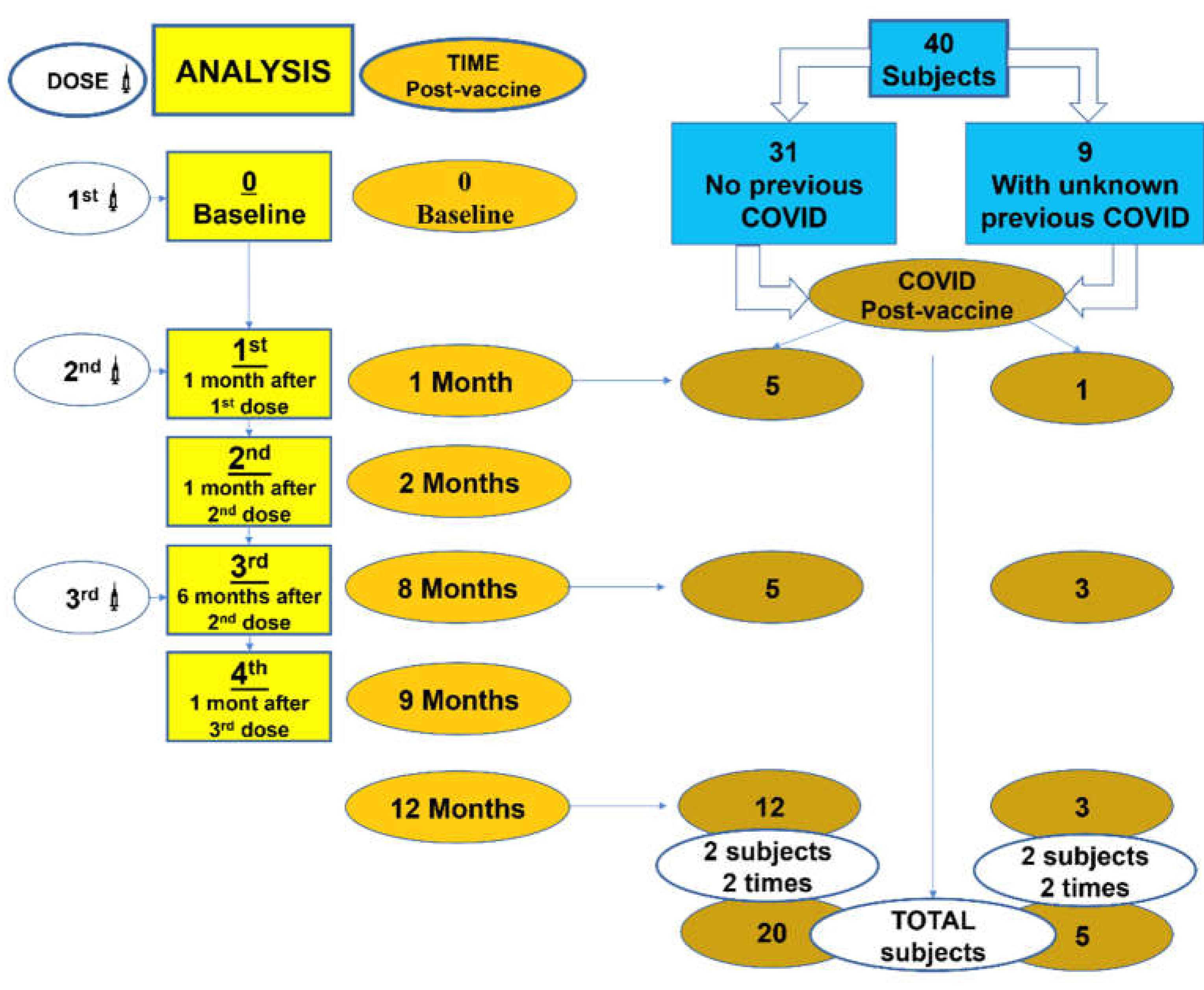
In this prospective follow-up study, we have recruited a total of 40 volunteers, followed up over time (1 year), before and after vaccination. Pfizer–BioNTech (BNT162b2) COVID-19 vaccine (mRNA) and Oxford AstraZeneca (ChAdOx1) (adenovirus modified) were used for vaccination. Serum samples were taken at five time points. SARS-CoV-2 IgG and IgA antibodies, αβ and γδ T cell subsets and their differentiation stages were analyzed at different post-vaccination times: 0 (Baseline): pre-vaccine, 1st: 1 month after 1st dose; 2nd: 1 month after 2nd dose; 3rd: 6 months after 2nd dose; 4th: 1 month after 3rd dose (Box 1). Blood samples were obtained just prior to administration of the vaccines. Study subjects should meet a series of criteria: not suffer from or have previously suffered from COVID-19, not have had suggestive symptoms, not suffer from an infectious disease at the time of vaccination, not have known immunodeficiency or autoimmune or neoplastic disease, not carry immunosuppressive treatment, as well as not having received another vaccine in the previous six months. All subjects who presented symptoms related to SARS-CoV-2 infection after vaccination were assessed using an antigen test. If the result was negative, a PCR test was performed to confirm or exclude infection.
Box 1.
Evolution time of vaccination and post-vaccination COVID-19 infection. Analysis: 0 (Baseline)= pre-vaccine, 1st: 1 month after 1st dose, 2nd: 1 month after 2nd dose, 3rd: 6 months after 2nd dose, 4th: 1 month after 3rd dose.
Box 1.
Evolution time of vaccination and post-vaccination COVID-19 infection. Analysis: 0 (Baseline)= pre-vaccine, 1st: 1 month after 1st dose, 2nd: 1 month after 2nd dose, 3rd: 6 months after 2nd dose, 4th: 1 month after 3rd dose.
2.3. Methods of Blood Simple Analysis
2.3.1. Cell Isolation for Analysis of γδ and αβ T Cells
Blood cell counts were obtained in a cell counter (LH750 Beckman Coulter, Inc, Fullerton, CA).
We obtained by centrifugation on density gradient from EDTA anticoagulated blood sample, an enrichment of the sample in mononuclear cells (MNCs). We have used Lymphoprep™ (Palex Mediacal SA), spinning at 3.500 rpm for 20 minutes. After two washes in phosphate buffered saline (PBS), the cells obtained were resuspended in 200 ml of PBS.
2.3.2. Functional Analysis of γδ and αβ T Cells
To evaluate the functional analysis of γδ and αβ T cells the MNCs were firstly incubated in two tubes with anti-TCR PAN αβ-PE and anti-TCR PAN γδ-PE antibodies during 10 minutes at room temperature, and then labelled with the following monoclonal antibodies conjugated with fluorochromes were used (Beckman Coulter, Inc, Miami, USA):
Tube1: anti-TCR PANαβ-PE (clon: IP26A), CD19- PE (clon: J3-119), CD45RA- ECD (clon: 2H4LDH11LDB9 (2H4)), CD56-PC7 (clon N901 (NKH-1)), CD62L- APC (clon: DREG56), CD4-APC A750 (clon 13B8.2), CD3-APC A700 (clon UCHT1), CD8-PB (clon B9.11), CD45-KRO (clon J33).
Tube 2: anti-TCR PANγδ-PE (clon: IMMU 510), CD19- PE (clon: J3-119), CD45RA- ECD (clon: 2H4LDH11LDB9 (2H4)), CD56-PC7 (clon N901 (NKH-1)), CD62L- APC (clon: DREG56), CD4-APC A750 (clon 13B8.2), CD3-APC A700 (clon UCHT1), CD8-PB (clon B9.11), CD45-KRO (clon J33).
After incubation for 10 more minutes at room temperature, we then used the VersaLyse lysis agent (Beckman Coulter, Inc). We add 1 ml of the “Fix-and-Lyse” mixture prepared at that time to the mononuclear cells (MNCs). Mix and incubate for 20 minutes at room temperature, protecting from light. We add 2 mL of PBS. We centrifuged for 5 minutes at 1500 rpm and at room temperature. We remove the supernatant by aspiration and resuspend the cell button in 0.5 mL of PBS.
Acquisition and analysis were done on a Navios flow cytometer (Beckman Coulter, Inc) and analyzed with Kaluza Software. A total of 100,000 events were acquired. Absolute counts of circulating cell subsets were calculated using a dual-platform counting technology.
2.3.3. Apoptosis Evaluation
The apoptosis detection was performed with ANNEXIN V-FITC/7-AAD Kit (Beckman Coulter, Inc), based on the binding properties of annexin V to phosphatidylserine and on the specificity of 7-amino-actinomycin D (7-AAD) for DNA guanine-cytosine base pair, following instructions of the manufacturer. The results described in this work refer to early apoptosis (Annexin V +, 7-AAD -), which accounts for 90–95% of total apoptosis.
2.3.4. Detection of IgG and IgA Antibodies against SARS-CoV2
Detection of IgG antibodies against SARS-CoV-2 was performed using the SARS-CoV-2 IgM and SARS-CoV-2 IgG II Quant assays, with the corresponding calibrators and controls for the ARCHITECT i2000SR analyzer (Abbott Diagnostics). The ARCHITECT System uses chemiluminescent microparticle immunoassay (CMIA) technology as a detection method to measure and quantify the concentration of antibody present in the serum. The SARS-CoV-2 IgG II Quant assay quantifies IgG in BAU/mL, since it is standardized with the WHO standard. We have correlated the IgG values with the WHO international standard 20/136, thus having the units in BAU/ml (BAU: Binding Antibody Units). The cut-off level of a positive IgG result was ≥ 7.1 BAU/mL. The levels of IgA against SARS-CoV-2 were analyzed in serum, using the DIAPRO COVID19 IgA Elisa KIT (Diagnostic Bioprobes Srl) according to the manufacturer’s recommendations. The microplates are coated with immunodominant and nucleocapsid extender recombinant spike glycoproteins specific for COVID-19. The cut-off level of a positive IgA result was ≥ 1.1 AU/mL
2.4. Statistical Analysis
Non-parametric Wilcoxon test was used to compare the evolution of antibodies mean values and αβ-γδ T cell subsets. Mann-Whitney U test was used was used to compare differences between Preinfection -antibodies against SARS-CoV-2 previous vaccination- (PI) and No Infection. Fisher’s Exact Test in Contingency Tables (Odds ratio with CI95%) was used for qualitative variables. P value < 0.05 was considered statistically significant.
The graph and statistical analyses were performed using GraphPad Prism software version 6.0 for Windows (GraphPad Software, San Diego, California, USA).
3. Results
Highlights
SARS-CoV-2 antibodies are higher in subjects with COVID-19 previous to vaccination.
The increase of antibodies is very high after the 3rd dose.
The vaccine does not fully protect against infection, but lessens its severity.
COVID-19 post-vaccine is related with low number γδ T cell pre-vaccine.
3.1. Subjects Studied
Forty healthy volunteers were enrolled in the present study, 29 female (72.5%) and 11 male (27.5%). The mean age was 48.9 ± 10.7 years old (range 26-64). Box 1 shows the evolution of vaccination time and post-vaccination COVID-19 infection throughout the study. According to vaccine type, 21 subjects (52.5%) received mRNA vaccine and 19 subjects (47.5%) received adenovirus-based vaccine.
3.2. IgG and IgA SARS-CoV-2 Specific Antibodies Responses and CD19+ B Cells after Vaccine
When we analyzed IgG and IgA antibodies against SARS-CoV-2 prior to the first vaccine dose, we found that 5 (12.5%) and 9 (22.5%) patients had IgG and IgA antibodies against SARS-CoV-2, respectively. Those positive subjects were asymptomatic.
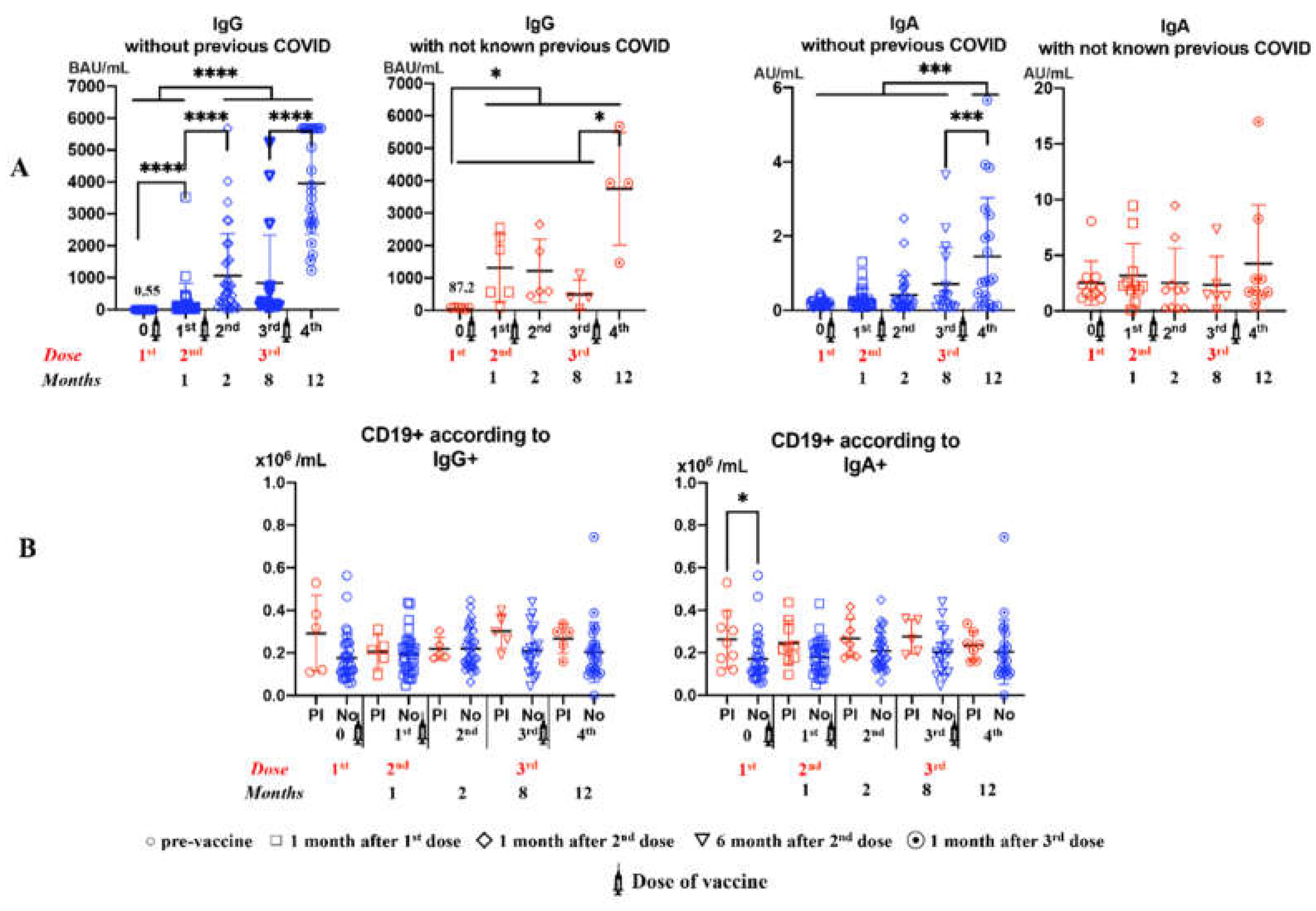
Figure 1A shows the dynamics of IgG and IgA antibodies against SARS-CoV-2 during the vaccination period (1st to 4th analysis) depending on whether or not patients presented previous antibodies against SARS-CoV-2. In summary, a progressive increase in anti-SARS-CoV-2 antibodies was observed, which was greater after the third dose of the vaccine. IgG levels one month after the first dose were higher in subjects who had pre-vaccination anti-SARS-CoV-2 antibodies. Specific antibody levels tended to decrease 8 months post-vaccination, that is, 6 months from the second dose. In the case of IgG, subjects without prior antibodies showed significant increases after the first dose. Subjects who did have prior antibodies only showed significant increases in IgG after the third dose. In the case of IgA, the vaccine only produced significant increases in subjects who did not have previous antibodies. The vaccine did not produce any change in IgA levels in the subjects who had antibodies prior to the administration of the first dose. In addition, these subjects showed the highest IgA levels throughout the entire follow-up.
Figure 1B shows the number of B cells during the vaccination period depending on whether or not the subjects had prior antibodies. Basal CD19+ cell levels were higher in subjects who had anti-SARS-CoV-2 antibodies prior to vaccination. In the case of IgA, this difference was statistically significant. Any change in CD19+ cell levels was not observed throughout the vaccination period.
3.3. Evolution of αβ and γδ T Cell Subsets Number and Their Apoptosis during the Vaccination Period
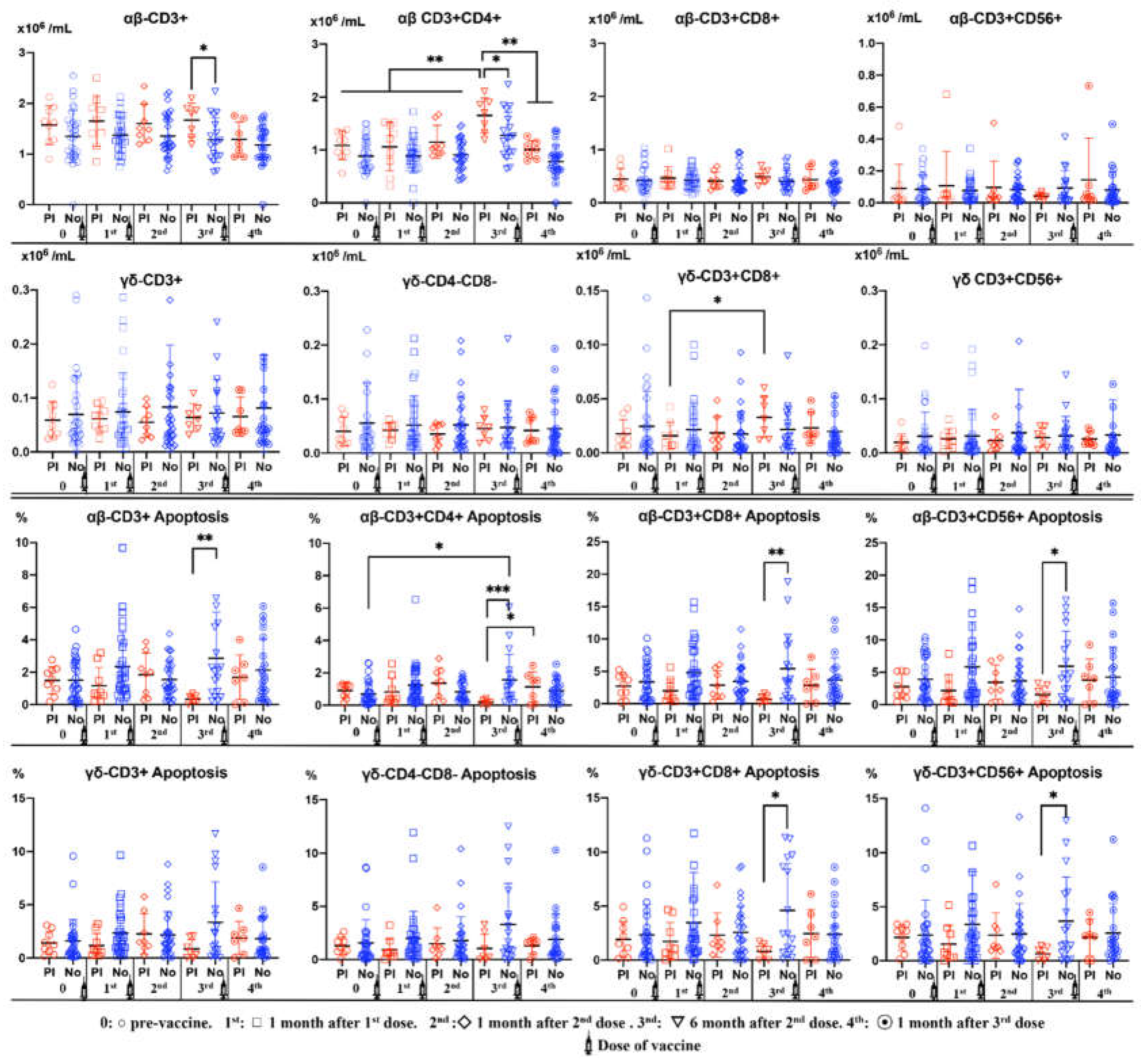
Figure 2 shows a significant increase in the number of CD3+CD4+αβ and CD3+CD8+γδ T cells 6 months after administering the second dose of vaccine in subjects with previous anti-SARS-CoV-2 antibodies. In these subjects, CD3+CD4+αβ T cells decreased significantly from the third dose.
A decrease in apoptosis of CD3+, CD3+CD4+, CD3+CD8+ and CD3+CD56+ αβ T cells was observed in subjects with prior anti-SARS-CoV-2 antibodies. These differences were highly significant at 6 months after the second dose. In the case of γδ T cells this fact was observed with CD3+CD8+ and CD3+CD56+ subsets. In subjects without previous antibodies, the vaccine induced an increase in apoptosis over time that was significant 6 months after the second dose.
3.4. Dynamics of αβ T cell Differentiation Stages during the Vaccination Period
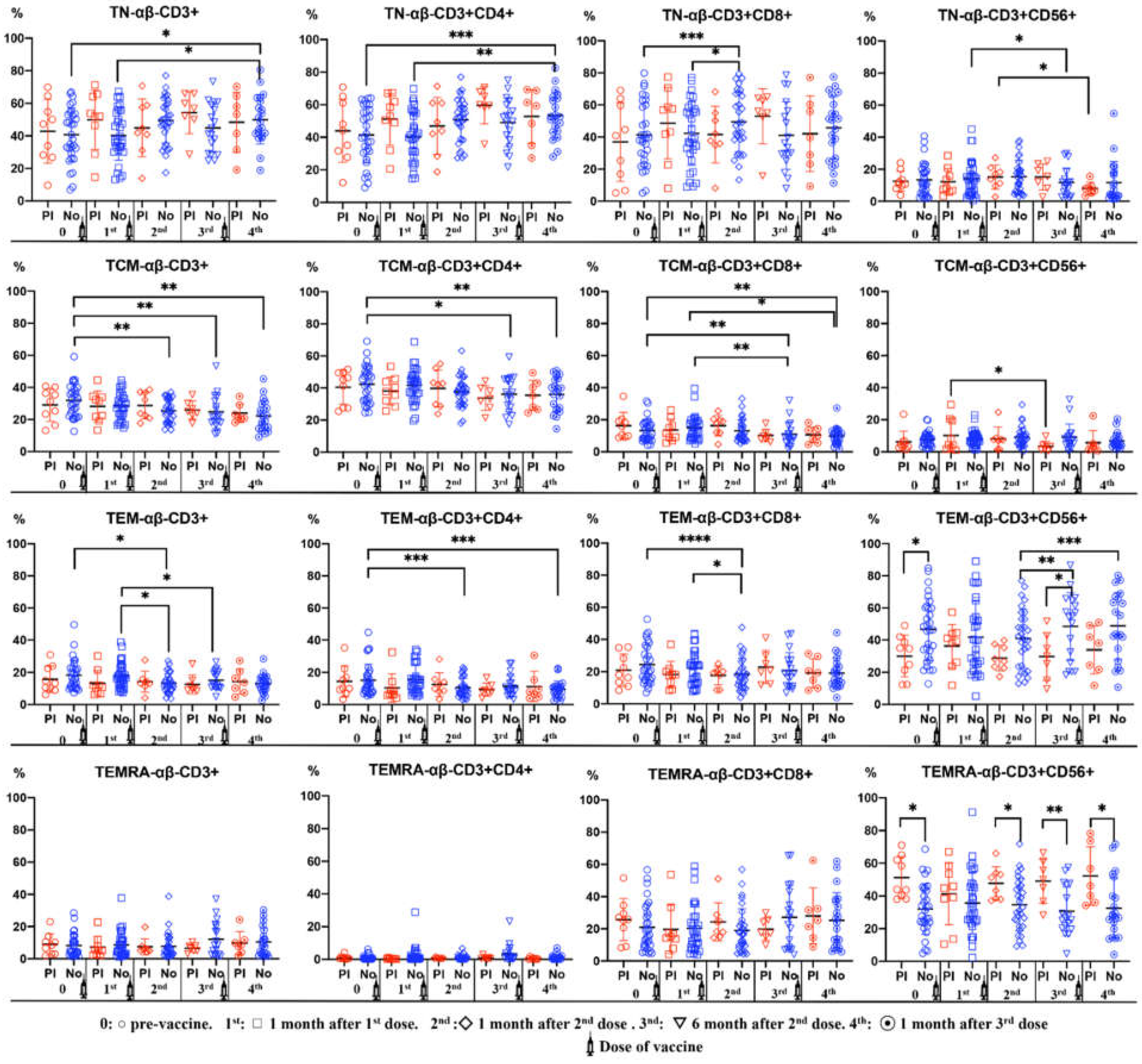
A significant increase in naïve CD4+ and CD8+ αβ T cell counts was observed throughout the vaccination period in subjects without previous anti-SARS-CoV-2 antibodies. On the other hand, there was a decrease in naïve CD56+ αβ T cells after the third dose in subjects with previous anti-SARS-CoV-2 antibodies.
However, TCM CD4+ and CD8+ αβ T cells showed a decreasing trend after successive vaccine doses in subjects without previous anti-SARS-CoV-2 antibodies.
In a general manner, TEM αβ T cells were significantly decreased in subjects without previous anti-SARS-CoV-2 antibodies. In contrast, TEM CD56+ αβ T cells increased at 6- and 12-months post-vaccination in subjects without previous anti-SARS-CoV-2 antibodies.
The percentages of TEMRA CD56+ αβ T cells were significantly higher in subjects with previous anti-SARS-CoV-2 antibodies. This difference was observed before the administration of the first vaccine dose (
Figure 3).
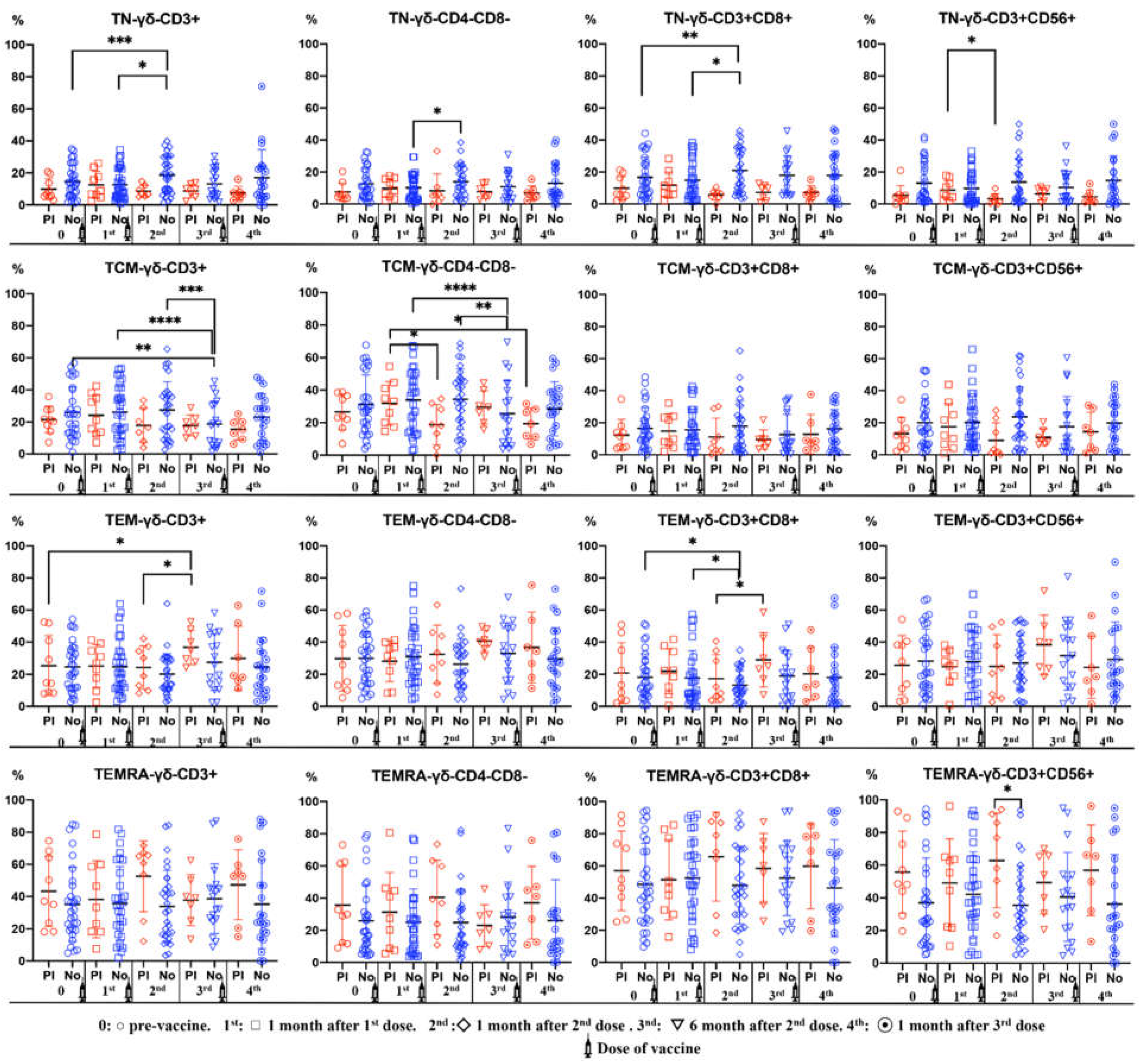
3.5. Dynamics of γδ T Cell Differentiation Stages during the Vaccination Period
The percentage of naïve γδ T cell subsets was higher in subjects without previous anti-SARS-CoV-2 antibodies. We observe and incresase of naïve double-negative and CD8+ γδ T cell after the second dose. There was a decrease in the percentages of TCM CD3+ and double-negative γδ T cells 6 months after the second dose in subjects with previous anti-SARS-CoV-2 antibodies.
TEMRA CD56+ γδ T cell percentages were significantly higher in subjects with previous anti-SARS-CoV-2 antibodies (
Figure 4).
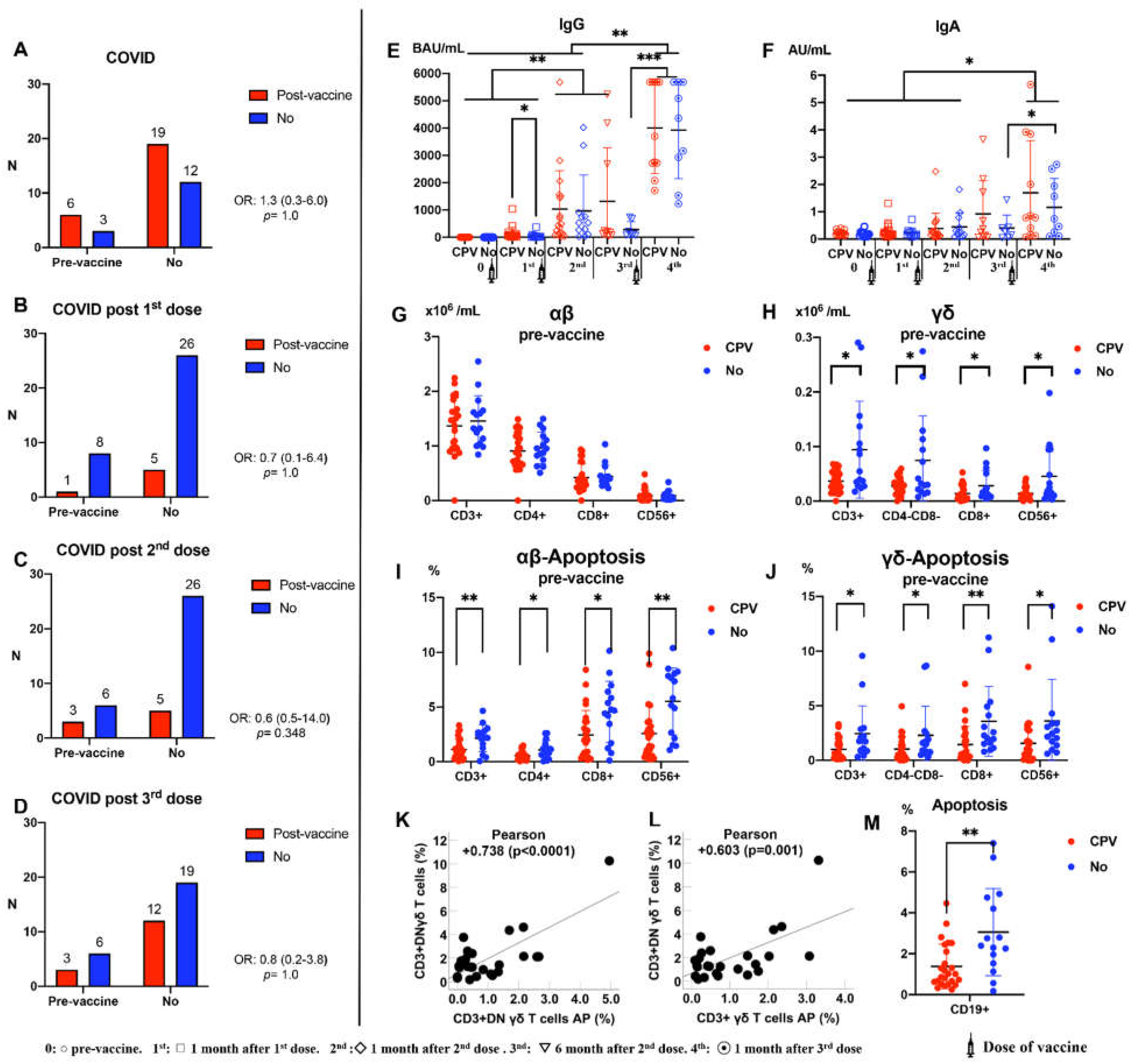
3.6. COVID Post-Vaccination and Its Relationship with Immunity
Figure 5 shows the number of subjects who suffered from COVID-19 over the 12 months post-vaccination. Of the 40 subjects recruited in the study, 25 (62.5%) suffered from COVID-19 throughout the study period. Four subjects had COVID-19 twice. No relationship was found between subjects with anti-SARS-CoV-2 antibodies prior to vaccination (n=9) and those with COVID-19 after vaccination (Panels A-D). We study the evolution of IgG and IgA antibody levels throughout the vaccination period according to COVID-19 post-vaccine (CPV) or not (
Figure 5E,F, respectively). A significant increase in IgG was observed after the first dose in subjects with CPV. This increase was much greater after the third dose. IgA began to increase after the 2st dose, reaching significantly higher levels after the third dose in subjects with previous anti-SARS-CoV-2 antibodies.
Figure 5G and
Figure 5H show the number of αβ and γδ T cells prior to vaccination according to CPV or not. Subjects who developed COVID-19 after vaccination had significantly lower baseline γδ T cell levels before vaccination. Subjects who developed COVID-19 after vaccination had significantly lower pre-vaccination levels of αβ and γδ T cell apoptosis (
Figure 5I,J). Likewise, pre-vaccination apoptosis levels of CD19+ cells were also significantly lower in patients who suffered from COVID-19 after vaccination (
Figure 5M). No differences in these results were observed when comparing subjects who had pre-vaccine anti-SARS-CoV-2 antibodies (n=9) with those who did not have pre-vaccine antibodies (n=31). On the other hand, a statistically significant positive correlation was observed between the percentage of γδ T cells and their levels of apoptosis in patients who suffered from COVID-19 after vaccination (
Figure 5K,L).
The effectiveness of the vaccines throughout the 12 months of the study was 37.5%. None of the patients who became infected with SARS-CoV-2 after the vaccine had severe infection or pneumonia. None of the patients was hospitalized and none died.
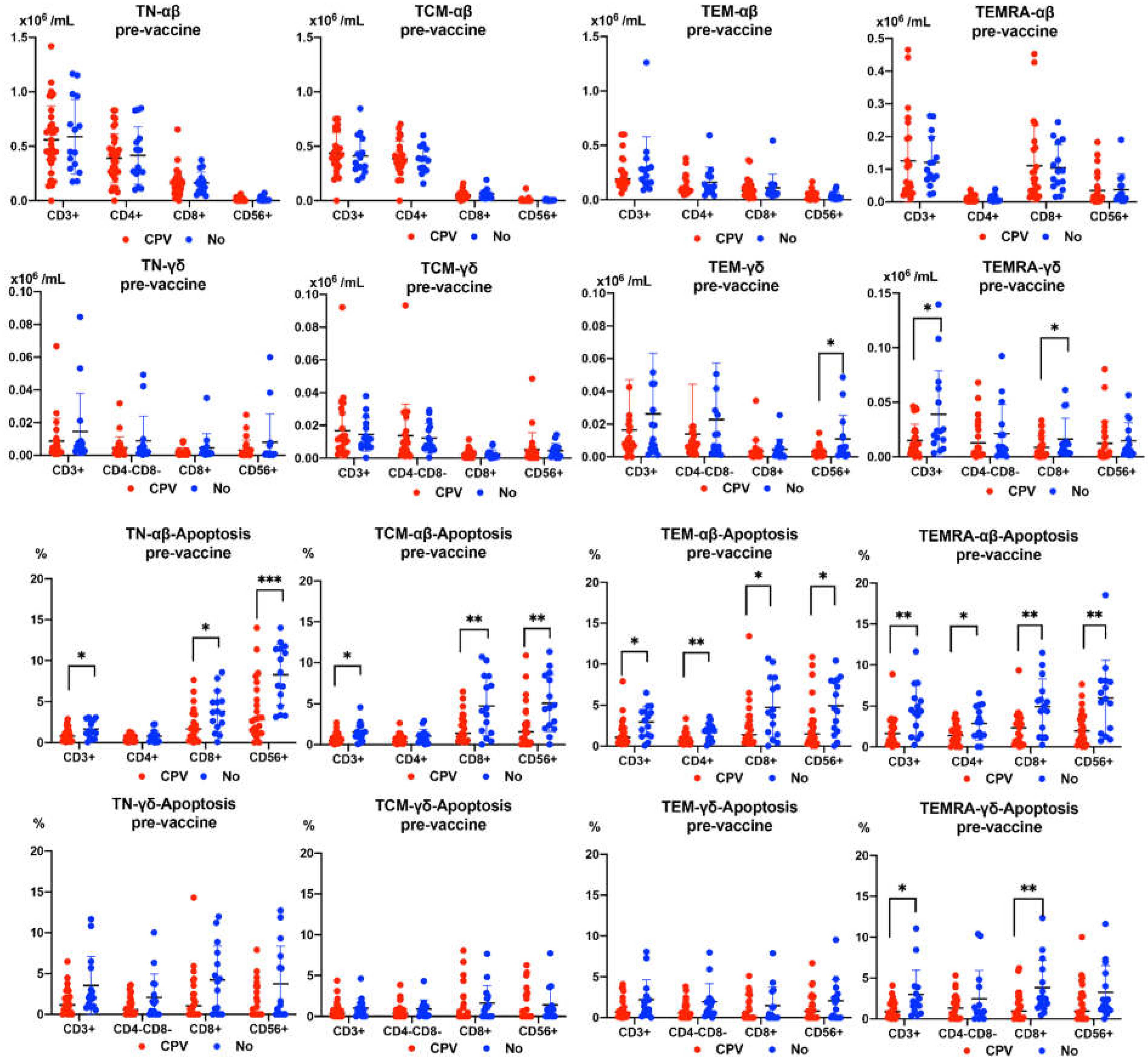
3.7. Number and Apoptosis in Pre-Vaccine Differentiation Stages of αβ and γδ T Cell Subsets according to COVID-19 Post-Vaccination
Subjects suffering from CPV had significantly lower pre-vaccine levels of TEM CD56+ and TEMRA CD8+ γδ T cells. However, no differences were observed in the pre-vaccine values of the differentiation stages of αβ T cells. On the other hand, the subjects who suffered from CPV presented significantly lower values in the levels of pre-vaccine apoptosis of all stages of differentiation of αβ T cells. In the case of γδ T cells, pre-vaccine apoptosis was also lower in CPV subjects, with significant differences in the TEMRA CD3+CD8+ subset (
Figure 6).
4. Discussion
In the present work, we analyzed the immune responses induced by the administration of three doses of the Pfizer–BioNTech (BNT162b2) (mRNA) and Oxford AstraZeneca (ChAdOx1) (adenovirus modified) vaccines. Likewise, the appearance of COVID-19 in the study subjects was evaluated for one year from the first dose of vaccine (Box 1). It was confirmed, as in other studies, that IgG anti-SARS-CoV-2 antibodies showed a further increase from the second vaccine dose [
9,
11]. However, subjects without previous antibodies showed significant increases before the second dose while subjects with previous antibodies needed three vaccine doses. This has been verified in very few studies and only with assessment of IgG anti SARS-CoV-2 especific antibodies [
12]. In our study, subjects without prior specific IgG showed significant increases after the first dose, while subjects with prior antibodies only showed significant increases in IgG after the third dose. This could suggest that the vaccine does not stimulate memory cells induced after natural infection, that is, the vaccine only stimulates the response of memory cells induced by its own antigens.
Previously, it was shown that early responses to SARS-CoV-2 were dominated by IgA antibodies. IgA remains detectable in saliva for 10 weeks while in serum it disappears one month after infection [
13]. In our study, the vaccine only produced changes in IgA leves in the subjects without anti-SARS-CoV-2 pre-vaccine antibodies. They were the ones that presented the highest levels throughout the entire follow-up. The highest IgA responses were observed after 8-12 months post-vaccine. This confirms that the highest levels of anti-SARS-CoV-2 antibodies, both IgG and IgA, are achieved after the third vaccination dose. When the dynamics of specific IgA production over time were studied, no significant differences were observed between subjects who suffered from post-vaccination COVID-19 and those who did not. However, an increase in the dynamics of specific IgG production was observed over time, after the first dose, in the subjects who suffered from post-vaccination COVID-19. Garcia-Beltran WF et al. observed that the highest levels of IgG and IgA antibodies directed against receptor binding domain and spike were present in critically ill patients who were intubated or died due to COVID-19 [
14]. Patients with higher levels of specific IgG could have a higher viral load that increases the risk of symptomatic infection despite vaccination. It has been shown that subjects with previous SARS-CoV-2 infection generate strong humoral and cellular responses -very strong T cell responses- compared to subjects who had no prior contact with the virus [
15,
16,
17].
It has been shown that CD8 T cells display a protective function from COVID-19 acute disease caused by natural SARS-CoV-2 infection improving survival. These cytotoxic cells remain acting in the tissues for a minimum period of two months [
18,
19]. Likewise, CD8+ T cells are detectable and functional 7 days after the first vaccine dose when circulating CD4+ T cells and neutralizing antibodies cannot yet be detected [
20]. In our study, in addition to the increase in specific IgG in subjects who had anti-SARS-CoV-2 antibodies prior to the vaccine, a significant increase in the number of αβ CD3+CD4+ and CD3+CD8+γδ T cells was observed at six months after the second dose of vaccine. In contrast, apoptosis was decreased in both αβ CD3+CD4+ T cells and CD3+CD8+ and CD3+CD56+ γδ T cells. This demonstrates the important role that γδ T cells play in vaccinal immunization of previously naturally infected subjects.
However, there was a significant increase in the number and apoptosis of CD3+CD4+ αβ T cells in subjects who did not have previous SARS-CoV-2-specific antibodies. These results demonstrate that subjects naturally exposed to SARS-CoV-2 antigens prior to vaccination behave differently from those who receive the vaccine without prior infection. The decrease in IgG observed six months after the second dose of vaccine corresponds to an increase in the number of CD4+ αβ and CD8+ γδ T cells and a decrease in their apoptosis. This fact could be due to an attempt by the immune system to compensate for this IgG deficit, a fact that does not occur in subjects who did not have previous immunity. This shows that natural immunity acts in a more robust way, probably due to stimulation by all the virus antigens, while the vaccine only induces immunity against the spike protein. This could again indicate that the vaccine does not stimulate memory cells induced after natural infection. In other words, the vaccine would only stimulate the response of memory cells that induce their own antigens.
Knowledge of the differentiation stages of the different T cell subsets is essential to know the situation of activity and reserve that the immune system has in relation to these cells [
10]. The study of the different states of activation and memory of T cells have been the subject of review [
21,
22,
23]. Specific CD4
+ T central memory (TCM), CD4
+ effector memory (TEM), CD8
+ TEM, and CD8
+ terminal effector (TE) cells were all detectable and functional up to 12 months after the second dose of COVID-19 vaccines [
24]. In our study, there was a significant increase in naïve CD4+ and CD8+ αβ T cells and naïve CD8+ γδ T cells throughout the vaccination period. In addition, TCM and TEM αβ CD4+ and CD8+ T cells also decreased throughout the study. Similar findings were found in a recent study [
25]. However, TEM αβ CD56+ T cells increased at the end of the vaccination process. TEM γδ CD8+ T cells were significantly increased after the second vaccine dose (3rd analysis) in patients who had pre-vaccination anti-SARS-CoV-2 antibodies. As will be discussed later, the important role of cytotoxic γδ T cells in maintaining immunity against SARS-CoV-2 is evident. This fact would be demonstrated by the great activity and importance of this subset in the mucosa, the entry site of SARS-CoV-2.
Different scenarios of mass COVID-19 vaccination have been studied. While in the best scenario was estimated a 95% of vaccine efficacy and three years of protection, in the worse scenario these data only reached a 50% of vaccine efficacy with 45 weeks of protection. These situations can produce dramatical health and economic beneficts or damages in the different countries [
26,
27]. The immunity conferred by primary infection vs hybrid immunity (immunity developed through a combination of SARS-CoV-2 infection and vaccination) have been studied, demonstrating the greater effectiveness of the latter. In our study, more than 60% of the recruited subjects suffered from COVID-19 infection throughout the 12-month follow-up, although the symptoms were very mild. No patient suffered pneumonia, nor required hospital admission. In addition, no subject died throughout the study. This confirms that the vaccine does not fully protect against infection, but it does extraordinarily minimize its severity. The effectiveness at 12 months against reinfection in subjects with hybrid immunity is slightly higher than that found in our study in patients with only post-vaccine immunity [
28].
In a previous study, we demonstrated a relationship between the decrease in γδ T cells with severity and mortality in sepsis [
29]. Likewise, in hospitalized patients with COVID-19 the most severity is related with lowest amount of γδ T cells [
30].
In the current study, we demonstrated that there was a relationship between the number of γδ T cells with the occurrence of SARS-CoV-2 infection after vaccination. The subjects who had COVID-19 post-vaccine had low frequency of pre-vaccine γδ T cell subsets compared to those who did not have COVID-19 or do not develop symptoms after infection. To our knowledge, this finding had not been described until now. We demonstrated that the stages related to this COVID-19 resistance were TEM and TEMRA cytotoxic γδ T cells. This finding was not observed in the αβ T cell subsets, which demonstrates the relationship of γδ T cells with the development of immunity against SARS-CoV-2, as well as with the immunization mechanisms of the vaccines. In addition, the lymphopenia found in COVID-19 patients has been attributed to SARS-CoV-2 induced activation of apoptosis and P53 signalling pathway [
31]. In the present study, patients who suffered post-vaccination COVID-19 had lower levels of T cell apoptosis. Likewise, we observed a direct correlation between apoptosis and CD3+CD4-CD8- γδ T cells. It therefore seems that in some way the immune system, given the lower number of γδ T cells in subjects susceptible to subsequent infection by SARS-CoV-2, reduces the apoptosis of these subsets to try to prevent a dramatic decrease in the number of γδ T cells. Further studies are necessary to investigate this assumption.
5. Conclusions
Anti-SARS-CoV-2 IgG and IgA antibodies showed a progressive increase throughout the vaccination period. This increase was greatest after the third dose. The highest levels were observed in subjects who had anti-SARS-CoV-2 antibodies prior to vaccination.
A decrease in specific IgG was observed 6 months after the second dose, at which time there was an increase in CD3+CD4+ αβ, CD3+CD8+ γδ and TEM CD8+ γδ T cells, and a decrease in apoptosis in CD4+CD8+ and CD56+ αβ and γδ T cells. This fact was manifested in subjects who had anti-SARS-CoV-2 antibodies prior to vaccination.
Post-vaccination symptomatic COVID-19 infection was greater than 60%. Despite this, the symptoms of the disease were very mild and were related to a pre-vaccination deficiency of γδ T cells, specifically CD8+ TEMRA and CD56+ γδ TEM, as well as lower levels of pre-vaccine apoptosis of αβ and γδ T cells subsets.
Those results demonstrate the important role that γδ T cells play in SARS-CoV-2 vaccine immunization.
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on Preprints.org
Author Contributions
The conception and design of the study: Andreu-Ballester JC. Acquisition of data: Galindo-Regal L. Citometry analysis: Galindo-Regal L, García-Ballesteros C and López-Chuliá F. Antibodies analysis: Domínguez-Márquez MV, Galindo-Regal L. Analysis and interpretation of data: Cuellar C and Andreu-Ballester JC. Drafting the article or revising it critically for important intellectual content: Andreu-Ballester JC, Llombart-Cussac A, Domínguez-Márquez MV, Fernandez L and Cuellar C. Final approval of the version to be summited: Cuellar C, and Andreu-Ballester JC.
Funding
Project/Equipment funded by Consellería de Sanitat Universal i Salut Pública (Generalitat Valenciana, Spain and the EU Operational Program of the European Regional Development Fund (ERDF) for the Valencian Community 2014-2020, within the framework of the REACT-EU programme, as the Union’s response to the COVID-19 pandemic.
Institutional Review Board Statement
The Research Ethics Committee of Arnau de Vilanova hospital, Valencia (Spain) approved the study (10/2021- March 24). Each volunteer participant signed an informed consent document. This study was conducted following the recommendations of the Spanish Bioethics Committee, the Spanish legislation on Biomedical Research (Law 14/2007) and Personal Data Protection (Spanish Law 3/2018 and European Law UE676/2018). The anonymity of the subjects participating in the study has been ensured.
Informed Consent Statement
“Written informed consent has been obtained from the patient(s) to publish this paper”
Data Availability Statement
We encourage all authors of articles published in MDPI journals to share their research data. In this section, please provide details regarding where data supporting reported results can be found, including links to publicly archived datasets analyzed or generated during the study. Where no new data were created, or where data is unavailable due to privacy or ethical restrictions, a statement is still required. Suggested Data Availability Statements are available in section “MDPI Research Data Policies” at
https://www.mdpi.com/ethics.
Acknowledgments
In this section, you can acknowledge any support given which is not covered by the author contribution or funding sections. This may include administrative and technical support, or donations in kind (e.g., materials used for experiments).
Conflicts of Interest
“The authors declare no conflicts of interest.”
References
- Blann AD, Heitmar R. SARS-CoV-2 and COVID-19: A Narrative Review. Br J Biomed Sci 2022, 7, 10426. [Google Scholar] [CrossRef]
- Troiano G, Nardi A. Vaccine hesitancy in the era of COVID-19. Public Health 2021, 194, 245–251. [Google Scholar] [CrossRef] [PubMed]
- Zheng C, Shao W, Chen X, Zhang B, Wang G, Zhang W. Real-world effectiveness of COVID-19 vaccines: A literature review and meta-analysis. Int J Infect Dis 2022, 114, 252–260. [Google Scholar] [CrossRef] [PubMed]
- Hodgson SH, Mansatta K, Mallett G, Harris V, Emary KRW, Pollard AJ. What defines an efficacious COVID-19 vaccine? A review of the challenges assessing the clinical efficacy of vaccines against SARS-CoV-2. Lancet Infect Dis 2021, 21, e26–e35. [Google Scholar] [CrossRef] [PubMed]
- Notarte KI, Guerrero-Arguero I, Velasco JV, Ver AT, Santos de Oliveira MH, Catahay JA, Khan MSR, Pastrana A, Juszczyk G, Torrelles JB, Lippi G, Martinez-Sobrido L, Henry BM. Characterization of the significant decline in humoral immune response six months post-SARS-CoV-2 mRNA vaccination: A systematic review. J Med Virol 2022, 94, 2939–2961. [Google Scholar] [CrossRef] [PubMed]
- Sadeghalvad M, Mansourabadi AH, Noori M, Nejadghaderi SA, Masoomikarimi M, Alimohammadi M, Rezaei N. Recent developments in SARS-CoV-2 vaccines: A systematic review of the current studies. Rev Med Virol 2023, 33, e2359. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, D. Instructing durable humoral immunity for COVID-19 and other vaccinable diseases. Immunity 2022, 55, 945–964. [Google Scholar] [CrossRef] [PubMed]
- Moga E, Lynton-Pons E, Domingo P. The Robustness of Cellular Immunity Determines the Fate of SARS-CoV-2 Infection. Front Immunol 2022, 13, 904686. [Google Scholar] [CrossRef] [PubMed]
- Li Z, Xiang T, Liang B, Deng H, Wang H, Feng X, Quan X, Wang X, Li S, Lu S, Yang X, Wang B, Zelinskyy G, Trilling M, Sutter K, Lu M, Dittmer U, Yang D, Zheng X, Liu J. Characterization of SARS-CoV-2-Specific Humoral and Cellular Immune Responses Induced by Inactivated COVID-19 Vaccines in a Real-World Setting. Front Immunol 2021, 12, 802858. [Google Scholar] [CrossRef]
- Sallusto F, Geginat J, Lanzavecchia A. Central memory and effector memory T cell subsets: Function, generation, and maintenance. Annu Rev Immunol 2004, 22, 745–763. [Google Scholar] [CrossRef]
- Guiomar R, Santos AJ, Melo AM, Costa I, Matos R, Rodrigues AP, Kislaya I, Silva AS, Roque C, Nunes C, Aguiar J, Graça F, Silva Graça A, Machado A. Monitoring of SARS-CoV-2 Specific Antibodies after Vaccination. Vaccines (Basel) 2022, 10, 154. [Google Scholar] [CrossRef] [PubMed]
- Yavuz E, Günal Ö, Başbulut E, Şen A. SARS-CoV-2 specific antibody responses in healthcare workers after a third booster dose of CoronaVac or BNT162b2 vaccine. J Med Virol 2022, 94, 3768–3775. [Google Scholar] [CrossRef] [PubMed]
- Sterlin D, Mathian A, Miyara M, Mohr A, Anna F, Claër L, Quentric P, Fadlallah J, Devilliers H, Ghillani P, Gunn C, Hockett R, Mudumba S, Guihot A, Luyt CE, Mayaux J, Beurton A, Fourati S, Bruel T, Schwartz O, Lacorte JM, Yssel H, Parizot C, Dorgham K, Charneau P, Amoura Z, Gorochov G. IgA dominates the early neutralizing antibody response to SARS-CoV-2. Sci Transl Med 2021, 13, eabd2223. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Beltran WF, Lam EC, Astudillo MG, Yang D, Miller TE, Feldman J, Hauser BM, Caradonna TM, Clayton KL, Nitido AD, Murali MR, Alter G, Charles RC, Dighe A, Branda JA, Lennerz JK, Lingwood D, Schmidt AG, Iafrate AJ, Balazs AB. COVID-19-neutralizing antibodies predict disease severity and survival. Cell 2021, 184, 476–488.e11. [Google Scholar] [CrossRef] [PubMed]
- Prendecki M, Clarke C, Brown J, Cox A, Gleeson S, Guckian M, Randell P, Pria AD, Lightstone L, Xu XN, Barclay W, McAdoo SP, Kelleher P, Willicombe M. Effect of previous SARS-CoV-2 infection on humoral and T-cell responses to single-dose BNT162b2 vaccine. Lancet 2021, 397, 1178–1181. [CrossRef]
- Hansen CB, Jarlhelt I, Hasselbalch RB, Hamm SR, Fogh K, Pries-Heje MM, Møller DL, Heftdal LD, Pérez-Alós L, Sørensen E, Larsen MAH, Skjoedt MO, Ostrowski SR, Frikke-Schmidt R, Bayarri-Olmos R, Hilsted LM, Bundgaard H, Nielsen SD, Iversen KK, Garred P. Antibody-dependent neutralizing capacity of the SARS-CoV-2 vaccine BNT162b2 with and without previous COVID-19 priming. J Intern Med 2021, 290, 1272–1274. [Google Scholar] [CrossRef] [PubMed]
- Ebinger JE, Fert-Bober J, Printsev I, Wu M, Sun N, Prostko JC, Frias EC, Stewart JL, Van Eyk JE, Braun JG, Cheng S, Sobhani K. Antibody responses to the BNT162b2 mRNA vaccine in individuals previously infected with SARS-CoV-2. Nat Med 2021, 27, 981–984. [Google Scholar] [CrossRef] [PubMed]
- Adam L, Rosenbaum P, Quentric P, Parizot C, Bonduelle O, Guillou N, Corneau A, Dorgham K, Miyara M, Luyt CE, Guihot A, Gorochov G, Combadière C, Combadière B. CD8+PD-L1+CXCR3+ polyfunctional T cell abundances are associated with survival in critical SARS-CoV-2-infected patients. JCI Insight. 2021, 6, e151571. [Google Scholar] [CrossRef] [PubMed]
- Roukens AHE, Pothast CR, König M, Huisman W, Dalebout T, Tak T, Azimi S, Kruize Y, Hagedoorn RS, Zlei M, Staal FJT, de Bie FJ, van Dongen JJM, Arbous SM, Zhang JLH, Verheij M, Prins C, van der Does AM, Hiemstra PS, de Vries JJC, Janse JJ, Roestenberg M, Myeni SK, Kikkert M, Yazdanbakhsh M, Heemskerk MHM, Smits HH, Jochems SP; in collaboration with BEAT-COVID group; in collaboration with COVID-19 LUMC group. Prolonged activation of nasal immune cell populations and development of tissue-resident SARS-CoV-2-specific CD8+ T cell responses following COVID-19. Nat Immunol 2022, 23, 23–32. [Google Scholar] [CrossRef]
- Oberhardt V, Luxenburger H, Kemming J, Schulien I, Ciminski K, Giese S, Csernalabics B, Lang-Meli J, Janowska I, Staniek J, Wild K, Basho K, Marinescu MS, Fuchs J, Topfstedt F, Janda A, Sogukpinar O, Hilger H, Stete K, Emmerich F, Bengsch B, Waller CF, Rieg S, Sagar, Boettler T, Zoldan K, Kochs G, Schwemmle M, Rizzi M, Thimme R, Neumann-Haefelin C, Hofmann M. Rapid and stable mobilization of CD8+ T cells by SARS-CoV-2 mRNA vaccine. Nature 2021, 597, 268–273. [Google Scholar] [CrossRef]
- Natalini A, Simonetti S, Sher C, D’Oro U, Hayday AC, Di Rosa F. Durable CD8 T Cell. Int J Mol Sci 2022, 23, 14367. [Google Scholar] [CrossRef]
- Sette A, Crotty S. Immunological memory to SARS-CoV-2 infection and COVID-19 vaccines. Immunol Rev 2022, 310, 27–46. [Google Scholar] [CrossRef]
- Goldblatt D, Alter G, Crotty S, Plotkin SA. Correlates of protection against SARS-CoV-2 infection and COVID-19 disease. Immunol Rev 2022, 310, 6–26. [Google Scholar] [CrossRef]
- Zhao W, Chen W, Li J, Chen M, Li Q, Lv M, Zhou S, Bai S, Wang Y, Zhang L, Zhang P, Wang J, Zheng Q, Wu J. Status of Humoral and Cellular Immune Responses within 12 Months following CoronaVac Vaccination against COVID-19. mBio 2022, 13, e0018122. [Google Scholar] [CrossRef]
- Odak I, Barros-Martins J, Bošnjak B, Stahl K, David S, Wiesner O, Busch M, Hoeper MM, Pink I, Welte T, Cornberg M, Stoll M, Goudeva L, Blasczyk R, Ganser A, Prinz I, Förster R, Koenecke C, Schultze-Florey CR. Reappearance of effector T cells is associated with recovery from COVID-19. EBioMedicine 2020, 57, 102885. [Google Scholar] [CrossRef]
- MacIntyre, CR. Navigating post-vaccine COVID-19 futures in the health and economic context. Lancet Infect Dis 2021, 21, 893–894. [Google Scholar] [CrossRef] [PubMed]
- Sandmann FG, Davies NG, Vassall A, Edmunds WJ, Jit M; Centre for the Mathematical Modelling of Infectious Diseases COVID-19 working group. The potential health and economic value of SARS-CoV-2 vaccination alongside physical distancing in the UK: A transmission model-based future scenario analysis and economic evaluation. Lancet Infect Dis 2021, 21, 962–974. [Google Scholar] [CrossRef] [PubMed]
- Bobrovitz N, Ware H, Ma X, Li Z, Hosseini R, Cao C, Selemon A, Whelan M, Premji Z, Issa H, Cheng B, Abu Raddad LJ, Buckeridge DL, Van Kerkhove MD, Piechotta V, Higdon MM, Wilder-Smith A, Bergeri I, Feikin DR, Arora RK, Patel MK, Subissi L. Protective effectiveness of previous SARS-CoV-2 infection and hybrid immunity against the omicron variant and severe disease: A systematic review and meta-regression. Lancet Infect Dis 2023, 23, 556–567. [Google Scholar] [CrossRef] [PubMed]
- Andreu-Ballester JC, Tormo-Calandín C, Garcia-Ballesteros C, Pérez-Griera J, Amigó V, Almela-Quilis A, Ruiz del Castillo J, Peñarroja-Otero C, Ballester F. Association of γδ T cells with disease severity and mortality in septic patients. Clin Vaccine Immunol 2013, 20, 738–746. [Google Scholar] [CrossRef]
- Rijkers G, Vervenne T, van der Pol P. More bricks in the wall against SARS-CoV-2 infection: Involvement of γ9δ2 T cells. Cell Mol Immunol. 2020, 17, 771–772. [CrossRef]
- Xiong Y, Liu Y, Cao L, Wang D, Guo M, Jiang A, Guo D, Hu W, Yang J, Tang Z, Wu H, Lin Y, Zhang M, Zhang Q, Shi M, Liu Y, Zhou Y, Lan K, Chen Y. Transcriptomic characteristics of bronchoalveolar lavage fluid and peripheral blood mononuclear cells in COVID-19 patients. Emerg Microbes Infect 2020, 9, 761–770. [Google Scholar] [CrossRef] [PubMed]
Figure 1.
Panel A: SARS-CoV-2 IgG and IgA antibodies after vaccination. 0= pre-vaccine, 1st: 1 month after 1st dose, 2nd: 1 month after 2nd dose, 3rd: 6 months after 2nd dose, 4th: 1 month after 3rd dose. Panel B: CD19+ B cells according to IgG and IgA positives pre-vaccination (PI) or not (No). Number B cells are expressed as means, and double T bars denote standard deviation (*p <0.05). Differences between five analysis, Wilcoxon matched-pairs signed rank test was used. Differences between Pre-infection (PI) and No infection (No), Mann-Whitney U test was used.
Figure 1.
Panel A: SARS-CoV-2 IgG and IgA antibodies after vaccination. 0= pre-vaccine, 1st: 1 month after 1st dose, 2nd: 1 month after 2nd dose, 3rd: 6 months after 2nd dose, 4th: 1 month after 3rd dose. Panel B: CD19+ B cells according to IgG and IgA positives pre-vaccination (PI) or not (No). Number B cells are expressed as means, and double T bars denote standard deviation (*p <0.05). Differences between five analysis, Wilcoxon matched-pairs signed rank test was used. Differences between Pre-infection (PI) and No infection (No), Mann-Whitney U test was used.
Figure 2.
Number and Percentages (Apoptosis) of αβ and γδ T-cell subsets (CD3+, CD3+CD4+, CD3+CD4-CD8-, CD3+CD56+) in peripheral blood of subjects vaccinated against SARS-CoV-2, according to Pre-anti-SARS-CoV-2 antibodies (PI) (Red) or No (Blue). Analysis during vaccination: 0= pre-vaccine, 1st: 1 month after 1st dose, 2nd: 1 month after 2nd dose, 3rd: 6 months after 2nd dose, 4th: 1 month after 3rd dose. Non-parametric Wilcoxon test was used to compare the evolution of antibodies of γδ T cells subsets. Mann-Whitney U test was used was used to compare differences between Pre-infection (PI) and No infection (No). Significance: *p<0,05, **p<0.001.
Figure 2.
Number and Percentages (Apoptosis) of αβ and γδ T-cell subsets (CD3+, CD3+CD4+, CD3+CD4-CD8-, CD3+CD56+) in peripheral blood of subjects vaccinated against SARS-CoV-2, according to Pre-anti-SARS-CoV-2 antibodies (PI) (Red) or No (Blue). Analysis during vaccination: 0= pre-vaccine, 1st: 1 month after 1st dose, 2nd: 1 month after 2nd dose, 3rd: 6 months after 2nd dose, 4th: 1 month after 3rd dose. Non-parametric Wilcoxon test was used to compare the evolution of antibodies of γδ T cells subsets. Mann-Whitney U test was used was used to compare differences between Pre-infection (PI) and No infection (No). Significance: *p<0,05, **p<0.001.
Figure 3.
Percentages of αβ T-cell subsets (CD3+, CD3+CD4+, CD3+CD4-CD8-, CD3+CD56+) in differentiation stages (TN: Naïve, TCM: Central Memory, TEM: Effector Memory TEMRA: Terminal Effector Memory) in peripheral blood of subjects vaccinated against SARS-CoV-2, according to Pre-infection (PI) (Red) or No infection (Blue). Analysis during vaccination: 0= pre-vaccine, 1st: 1 month after 1st dose, 2nd: 1 month after 2nd dose, 3rd: 6 months after 2nd dose, 4th: 1 month after 3rd dose. Non-parametric Wilcoxon test was used to compare the evolution of antibodies mean of αβ T cells subsets. Mann-Whitney U test was used was used to compare differences between Pre-infection (PI) and No Infection (No). Significance: *p<0,05, **p<0.001.
Figure 3.
Percentages of αβ T-cell subsets (CD3+, CD3+CD4+, CD3+CD4-CD8-, CD3+CD56+) in differentiation stages (TN: Naïve, TCM: Central Memory, TEM: Effector Memory TEMRA: Terminal Effector Memory) in peripheral blood of subjects vaccinated against SARS-CoV-2, according to Pre-infection (PI) (Red) or No infection (Blue). Analysis during vaccination: 0= pre-vaccine, 1st: 1 month after 1st dose, 2nd: 1 month after 2nd dose, 3rd: 6 months after 2nd dose, 4th: 1 month after 3rd dose. Non-parametric Wilcoxon test was used to compare the evolution of antibodies mean of αβ T cells subsets. Mann-Whitney U test was used was used to compare differences between Pre-infection (PI) and No Infection (No). Significance: *p<0,05, **p<0.001.
Figure 4.
Percentages of γδ T cell subsets (CD3+, CD3+CD4+, CD3+CD4-CD8-, CD3+CD56+) in differentiation stages (TN: Naïve, TCM: Central Memory, TEM: Effector Memory, TEMRA: Terminal Effector Memory) in peripheral blood of subjects vaccinated against SARS-CoV-2, according to Pre-infection (PI) (Red) or No infection (Blue). Analysis during vaccination: 0= pre-vaccine, 1st: 1 month after 1st dose, 2nd: 1 month after 2nd dose, 3rd: 6 months after 2nd dose, 4th: 1 month after 3rd dose. Non-parametric Wilcoxon test was used to compare the evolution of antibodies of γδ T cell subsets. Mann-Whitney U test was used was used to compare differences between Pre-infection (PI) and No infection (No). Significance: *p<0,05, **p<0.01.
Figure 4.
Percentages of γδ T cell subsets (CD3+, CD3+CD4+, CD3+CD4-CD8-, CD3+CD56+) in differentiation stages (TN: Naïve, TCM: Central Memory, TEM: Effector Memory, TEMRA: Terminal Effector Memory) in peripheral blood of subjects vaccinated against SARS-CoV-2, according to Pre-infection (PI) (Red) or No infection (Blue). Analysis during vaccination: 0= pre-vaccine, 1st: 1 month after 1st dose, 2nd: 1 month after 2nd dose, 3rd: 6 months after 2nd dose, 4th: 1 month after 3rd dose. Non-parametric Wilcoxon test was used to compare the evolution of antibodies of γδ T cell subsets. Mann-Whitney U test was used was used to compare differences between Pre-infection (PI) and No infection (No). Significance: *p<0,05, **p<0.01.
Figure 5.
A: Relation between COVID-19 at some point in the vaccination period (COVID-19 Post-vaccine –CPV-) (N=25) and pre-vaccination anti-SARS-CoV-2. B: Post-vaccine COVID-19 after 1st dose. C: Post-vaccine COVID-19 after 2nd dose. D: Post-vaccine COVID-19 after 3nd dose. E: IgG according to CPV (COVID-19 Post-vaccine). G and H: Pre-vaccine αβ-γδ T cell subsets number according to CPV (COVID-19 Post-vaccine). I and H: αβ-γδ T cells subsets APOPTOSIS pre-vaccine according to CPV (COVID-19 Post-vaccine). U test was used to compare differences between CPV and No. Significance: *p<0.05, **p<0.01. K and L: Relation between γδ T cells and apoptosis (n=25) (Pearson Test was used). DN=Double Negative (CD4-CD8-).
Figure 5.
A: Relation between COVID-19 at some point in the vaccination period (COVID-19 Post-vaccine –CPV-) (N=25) and pre-vaccination anti-SARS-CoV-2. B: Post-vaccine COVID-19 after 1st dose. C: Post-vaccine COVID-19 after 2nd dose. D: Post-vaccine COVID-19 after 3nd dose. E: IgG according to CPV (COVID-19 Post-vaccine). G and H: Pre-vaccine αβ-γδ T cell subsets number according to CPV (COVID-19 Post-vaccine). I and H: αβ-γδ T cells subsets APOPTOSIS pre-vaccine according to CPV (COVID-19 Post-vaccine). U test was used to compare differences between CPV and No. Significance: *p<0.05, **p<0.01. K and L: Relation between γδ T cells and apoptosis (n=25) (Pearson Test was used). DN=Double Negative (CD4-CD8-).
Figure 6.
Diferentiation stages of αβ-γδ T cell subsets APOPTOSIS pre-vaccine evolution according to CPV (COVID-19 Post-vaccine).
Figure 6.
Diferentiation stages of αβ-γδ T cell subsets APOPTOSIS pre-vaccine evolution according to CPV (COVID-19 Post-vaccine).
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).