Submitted:
17 April 2024
Posted:
17 April 2024
You are already at the latest version
Abstract
Keywords:
Introduction
Prevalence of Cancer
Current Treatments and Gaps
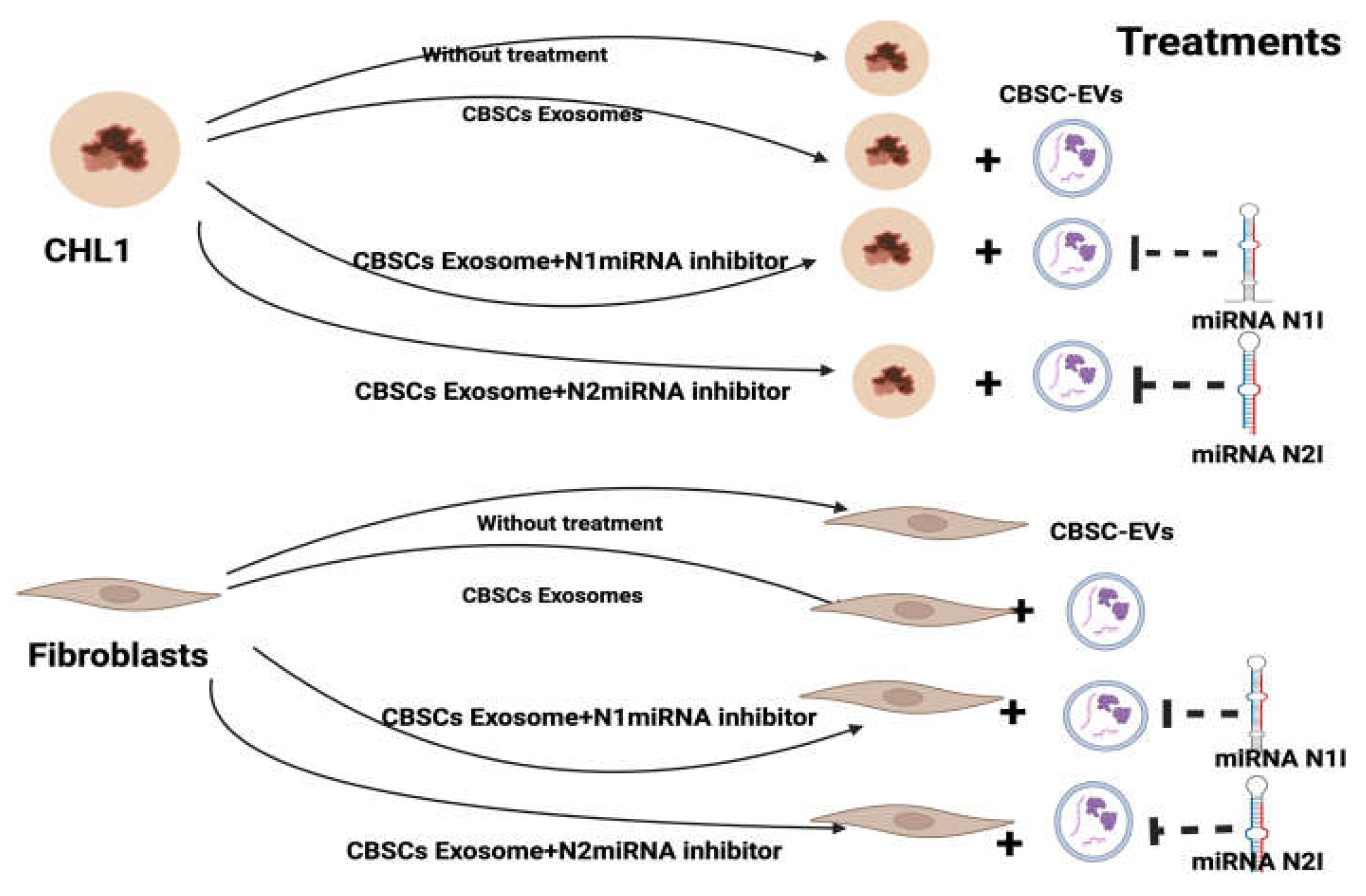
Materials and Methods
Cell Culturing and Treatment
RNA Extraction
RNA/Transcriptome Sequencing
Library Preparation for Transcriptome Sequencing
Clustering and Sequencing
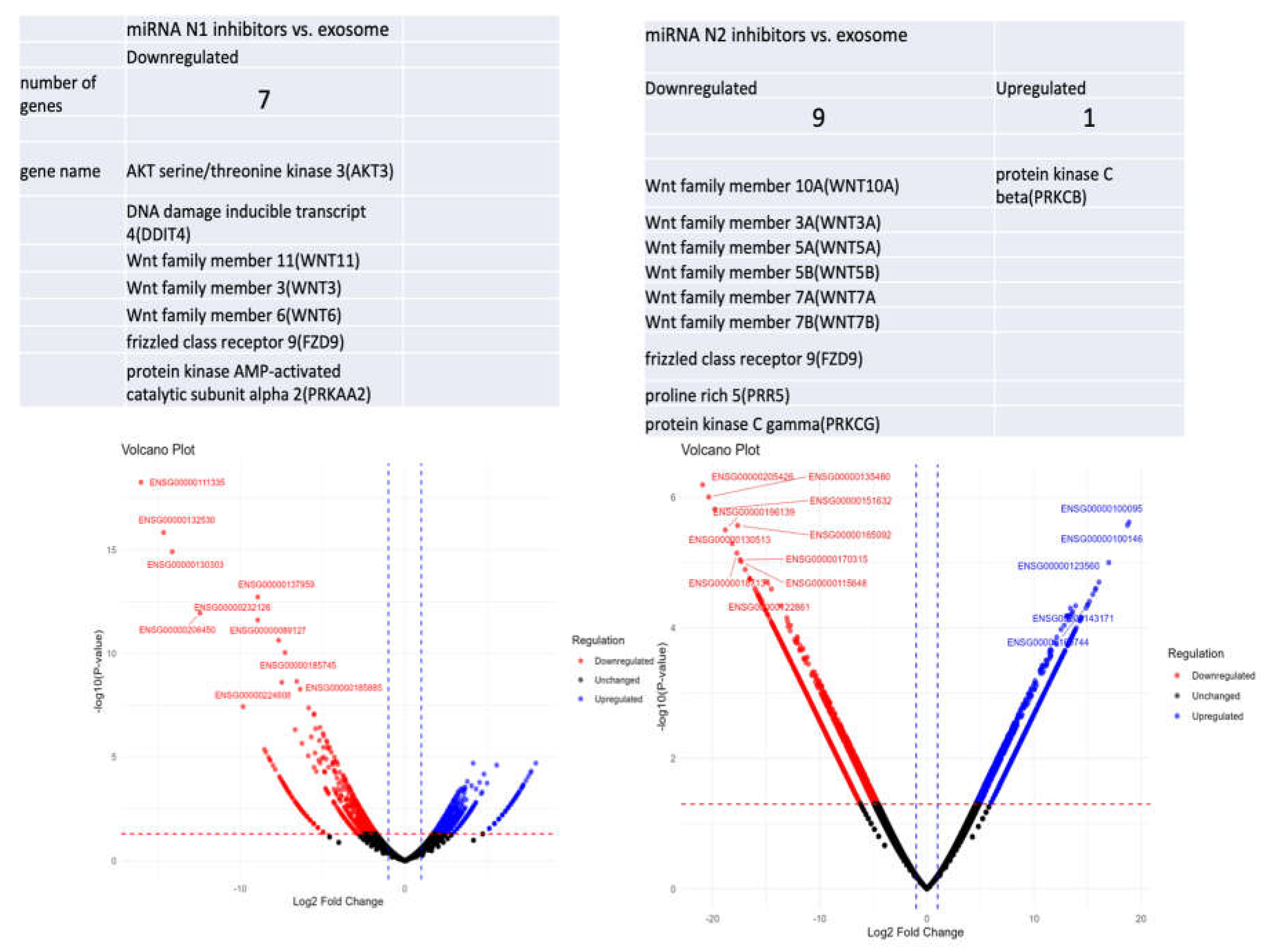
Results
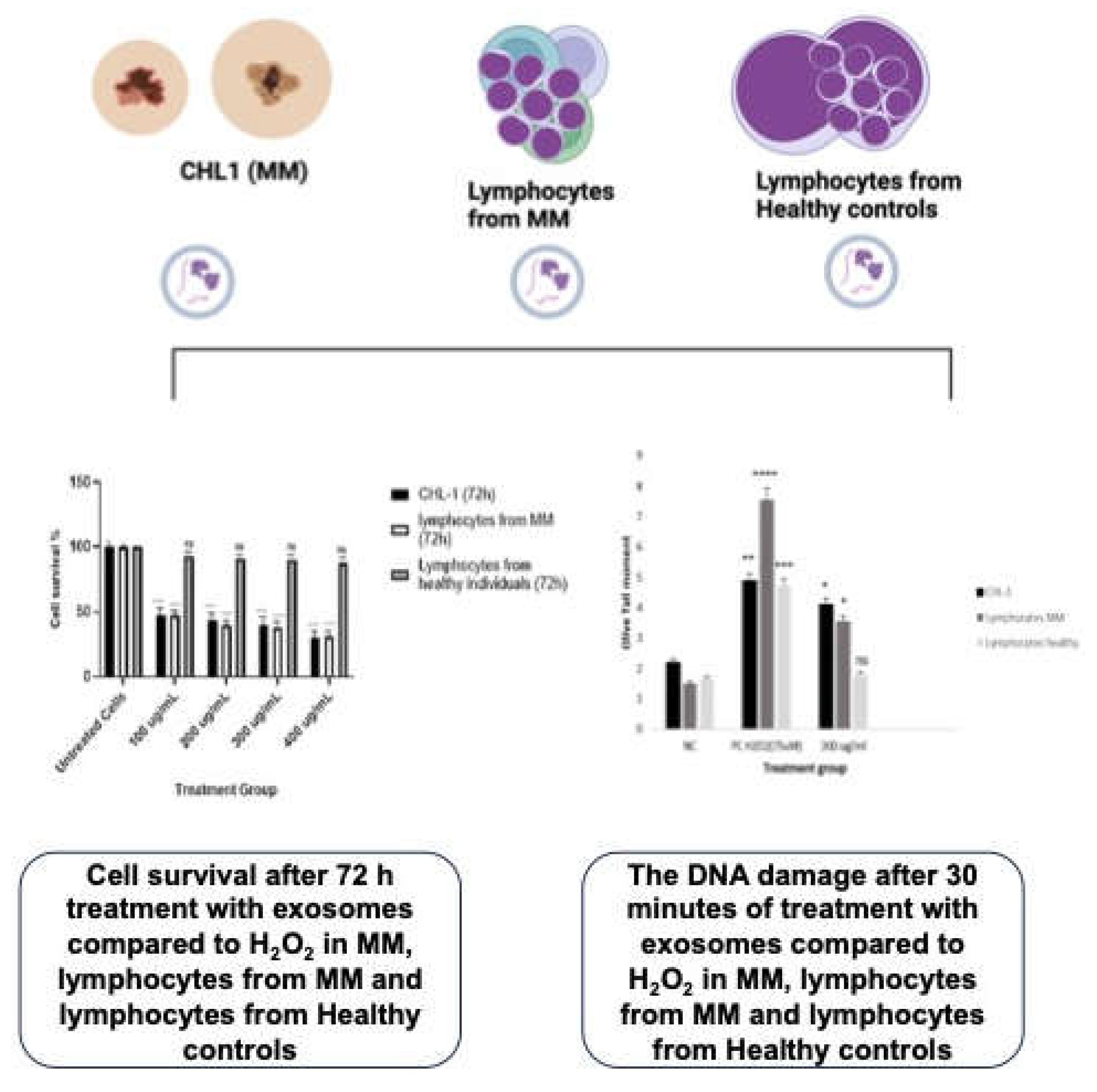
Cell Survival Rate (CCK8)
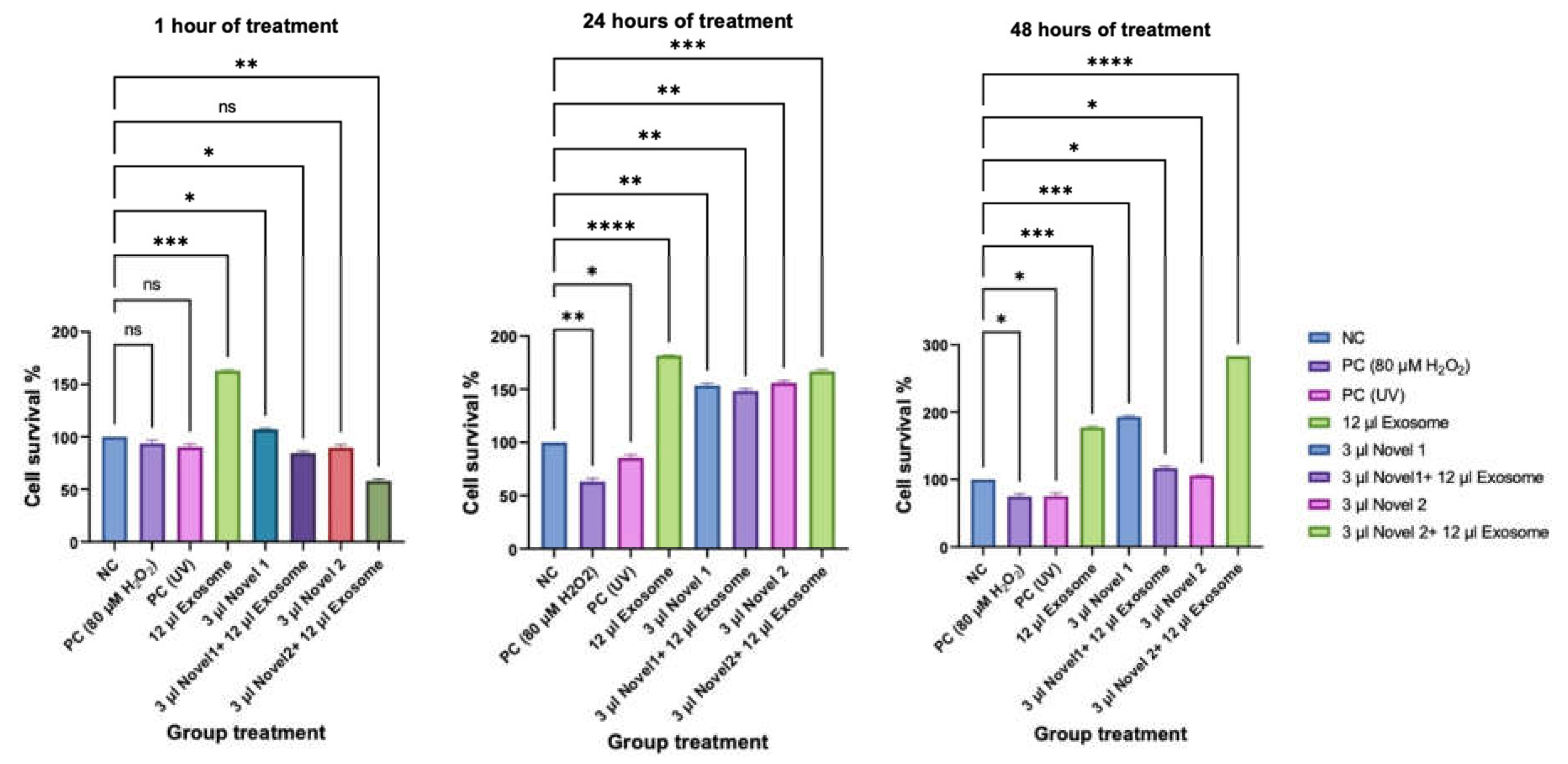
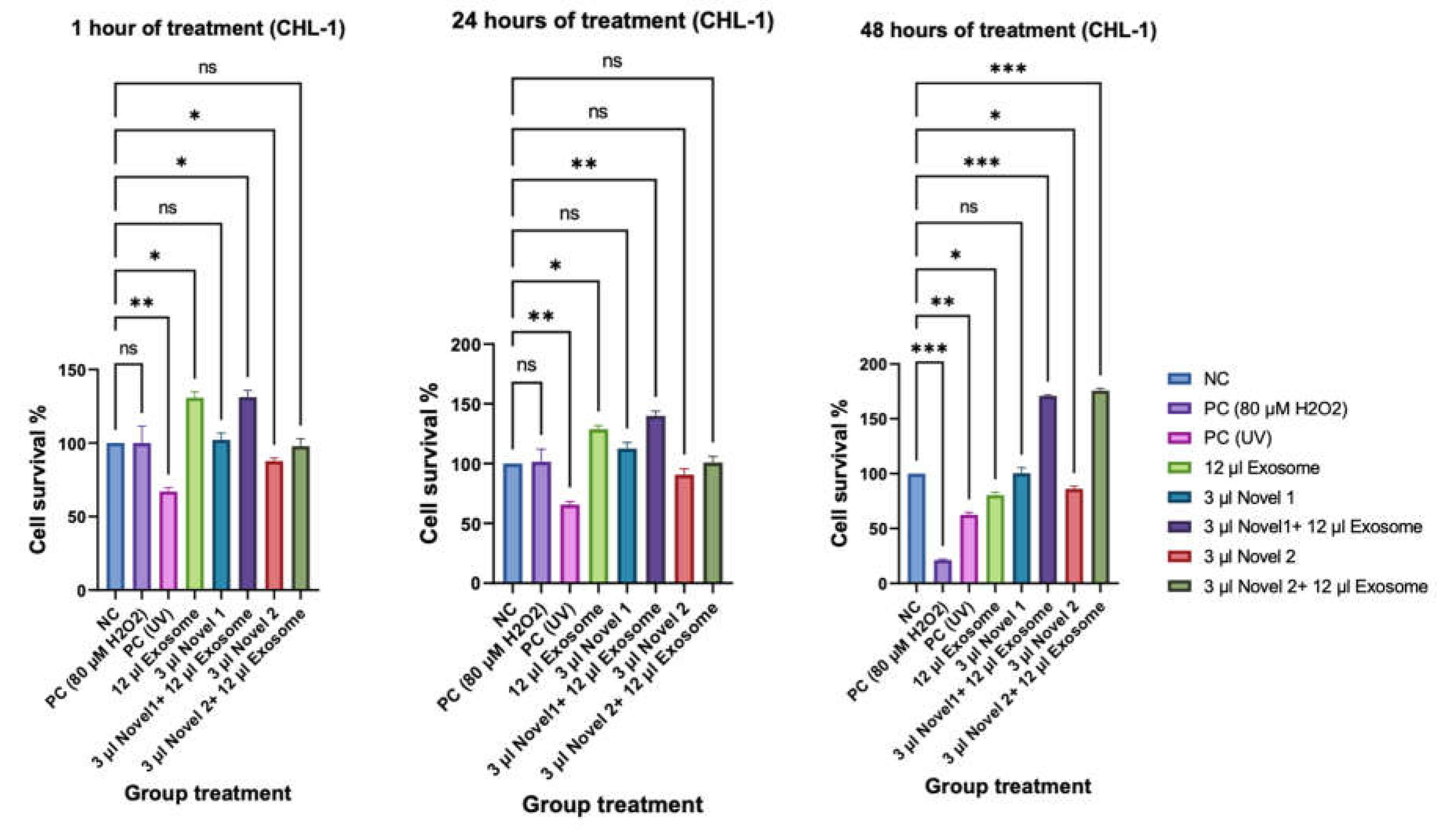
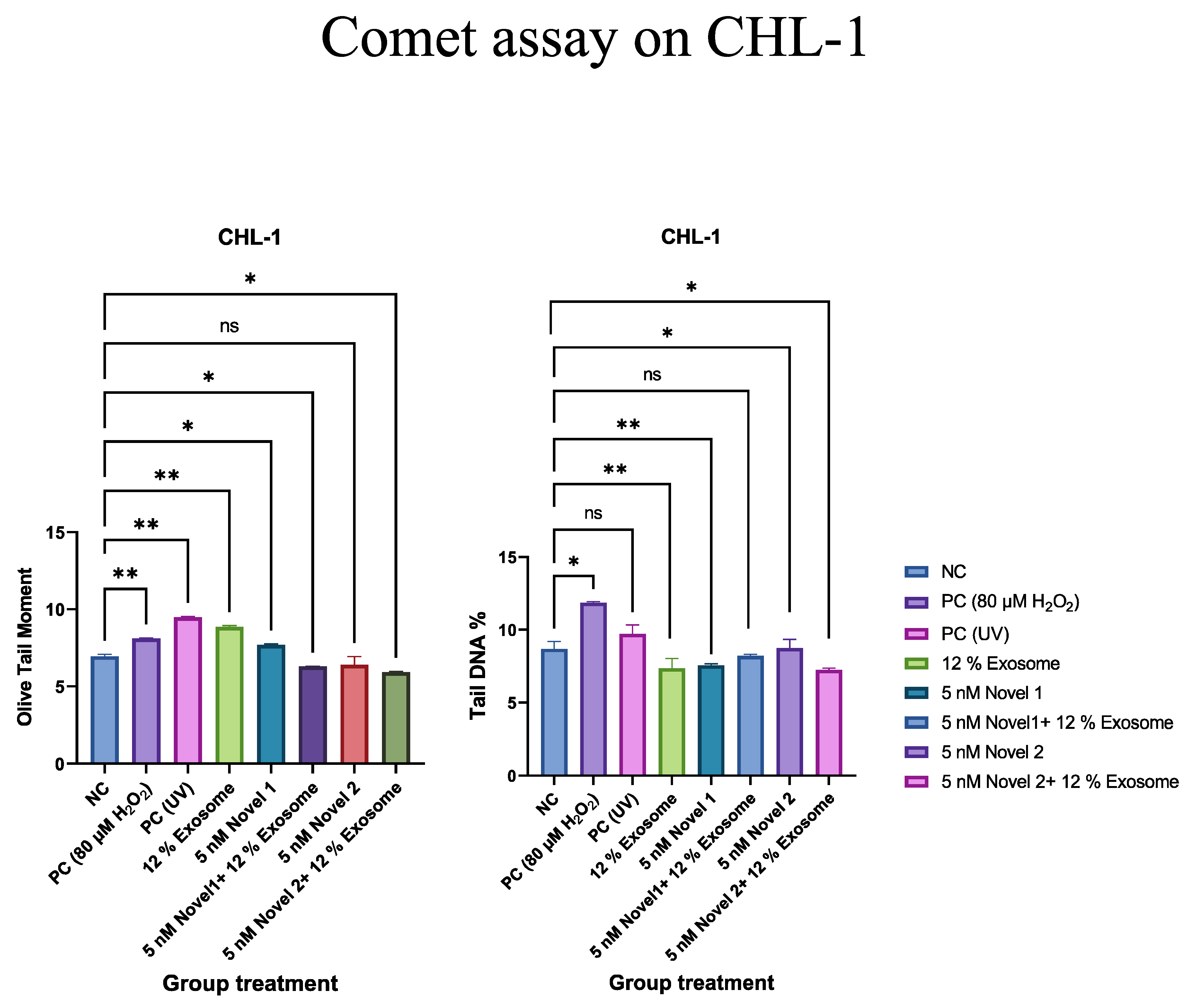
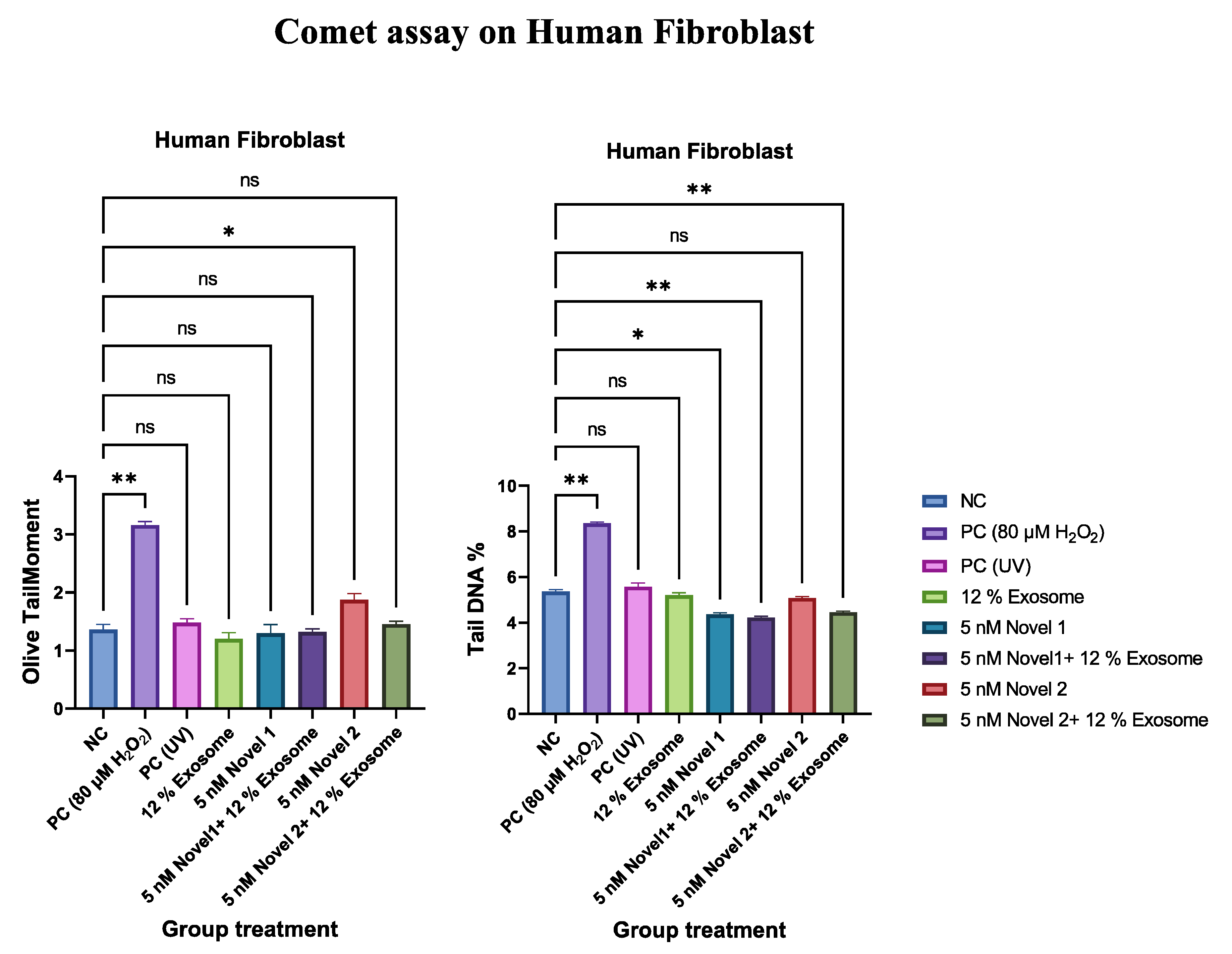
Comet Assay
Discussion
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Ethics Approval and Consent to Participate
Consent for Publication
References
- Caporali, S.; Alvino, E.; Lacal, P.M.; Levati, L.; Giurato, G.; Memoli, D.; Caprini, E.; Cappellini, G.C.A.; D’atri, S. Targeting the PI3K/AKT/mTOR pathway overcomes the stimulating effect of dabrafenib on the invasive behavior of melanoma cells with acquired resistance to the BRAF inhibitor. Int. J. Oncol. 2016, 49, 1164–1174. [Google Scholar] [CrossRef]
- Debela, D.T.; Muzazu, S.G.; Heraro, K.D.; Ndalama, M.T.; Mesele, B.W.; Haile, D.C.; Kitui, S.K.; Manyazewal, T. New approaches and procedures for cancer treatment: Current perspectives. SAGE Open Med. 2021, 9. [Google Scholar] [CrossRef] [PubMed]
- DUNKENBERGER, L. , REISS, K. & DEL VALLE, L. 2022. Comet Assay for the Detection of Single and Double-Strand DNA Breaks. Methods Mol Biol, 2422, 263-269.
- Goydel, R.S.; Rader, C. Antibody-based cancer therapy. Oncogene 2021, 40, 3655–3664. [Google Scholar] [CrossRef]
- Ha, T.-Y. The Role of MicroRNAs in Regulatory T Cells and in the Immune Response. Immune Netw. 2011, 11, 11–41. [Google Scholar] [CrossRef] [PubMed]
- HART, M. , NICKL, L., WALCH-RUECKHEIM, B., KRAMMES, L., RHEINHEIMER, S., DIENER, C., TAENZER, T., KEHL, T., SESTER, M., LENHOF, H. P., KELLER, A. & MEESE, E. 2020. Wrinkle in the plan: miR-34a-5p impacts chemokine signaling by modulating CXCL10/CXCL11/CXCR3-axis in CD4(+), CD8(+) T cells, and M1 macrophages. J Immunother Cancer, 8.
- Kalluri, R.; LeBleu, V.S. The biology, function, and biomedical applications of exosomes. Science 2020, 367, eaau6977. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.; Croce, C.M. MicroRNA: trends in clinical trials of cancer diagnosis and therapy strategies. Exp. Mol. Med. 2023, 55, 1314–1321. [Google Scholar] [CrossRef] [PubMed]
- Lykke-Andersen, S.; Brodersen, D.E.; Jensen, T.H. Origins and activities of the eukaryotic exosome. J. Cell Sci. 2009, 122, 1487–1494. [Google Scholar] [CrossRef] [PubMed]
- Mao, J.J.; Ismaila, N.; Bao, T.; Barton, D.; Ben-Arye, E.; Garland, E.L.; Greenlee, H.; Leblanc, T.; Lee, R.T.; Lopez, A.M.; et al. Integrative Medicine for Pain Management in Oncology: Society for Integrative Oncology–ASCO Guideline. J. Clin. Oncol. 2022, 40, 3998–4024. [Google Scholar] [CrossRef] [PubMed]
- Maresca, L.; Stecca, B.; Carrassa, L. Novel Therapeutic Approaches with DNA Damage Response Inhibitors for Melanoma Treatment. Cells 2022, 11, 1466. [Google Scholar] [CrossRef] [PubMed]
- Menon, A.; Abd-Aziz, N.; Khalid, K.; Poh, C.L.; Naidu, R. miRNA: A Promising Therapeutic Target in Cancer. Int. J. Mol. Sci. 2022, 23, 11502. [Google Scholar] [CrossRef] [PubMed]
- Naeem, P.; Baumgartner, A.; Ghaderi, N.; Sefat, F.; Alhawamdeh, M.; Heidari, S.; Shahzad, F.; Swaminathan, K.; Akhbari, P.; Isreb, M.; et al. Anticarcinogenic impact of extracellular vesicles (exosomes) from cord blood stem cells in malignant melanoma: A potential biological treatment. J. Cell. Mol. Med. 2022, 27, 222–231. [Google Scholar] [CrossRef] [PubMed]
- O'Brien, J.; Hayder, H.; Zayed, Y.; Peng, C. Overview of MicroRNA Biogenesis, Mechanisms of Actions, and Circulation. Front. Endocrinol. 2018, 9, 402. [Google Scholar] [CrossRef] [PubMed]
- Ratajczak, M.Z.; Kim, C.H.; Abdel-Latif, A.; Schneider, G.; Kucia, M.; Morris, A.J.; Laughlin, M.J.; Ratajczak, J. A novel perspective on stem cell homing and mobilization: review on bioactive lipids as potent chemoattractants and cationic peptides as underappreciated modulators of responsiveness to SDF-1 gradients. Leukemia 2011, 26, 63–72. [Google Scholar] [CrossRef]
- Ribas, A.; Wolchok, J.D. Cancer immunotherapy using checkpoint blockade. Science 2018, 359, 1350–1355. [Google Scholar] [CrossRef] [PubMed]
- Rupaimoole, R.; Slack, F.J. MicroRNA therapeutics: towards a new era for the management of cancer and other diseases. Nat. Rev. Drug Discov. 2017, 16, 203–222. [Google Scholar] [CrossRef] [PubMed]
- STERNER, R. C. & STERNER, R. M. 2021. CAR-T cell therapy: current limitations and potential strategies. Blood Cancer J, 11, 69.
- Zaborowski, M.P.; Balaj, L.; Breakefield, X.O.; Lai, C.P. Extracellular Vesicles: Composition, Biological Relevance, and Methods of Study. Bioscience 2015, 65, 783–797. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Deng, T.; Liu, R.; Bai, M.; Zhou, L.; Wang, X.; Li, S.; Wang, X.; Yang, H.; Li, J.; et al. Exosome-delivered EGFR regulates liver microenvironment to promote gastric cancer liver metastasis. Nat. Commun. 2017, 8, 15016. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




