Submitted:
16 April 2024
Posted:
17 April 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Material
2.2. SLM Process
2.3. Methods of Sample Characterisation
2.3.1. Density
2.3.2. Differential Scanning Calorimetry, DSC
2.3.3. Phase Analysis
2.3.4. Sample Morphology
2.3.5. Hardness and Young’s Modulus
3. Results
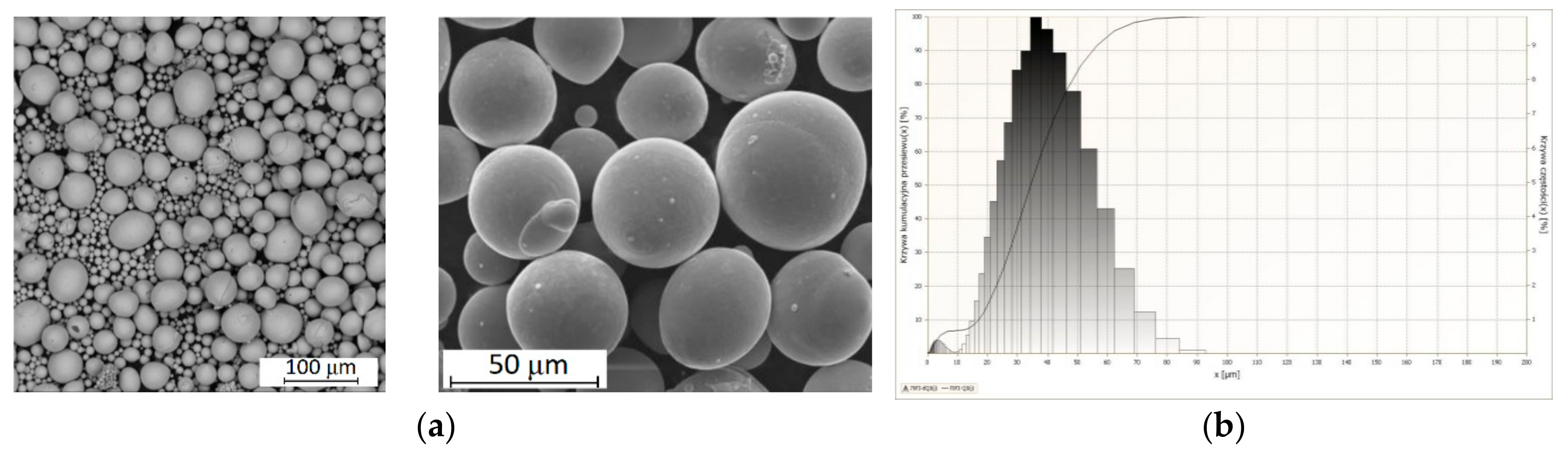
3.1. Powder Characteristics
3.1.1. Morphology
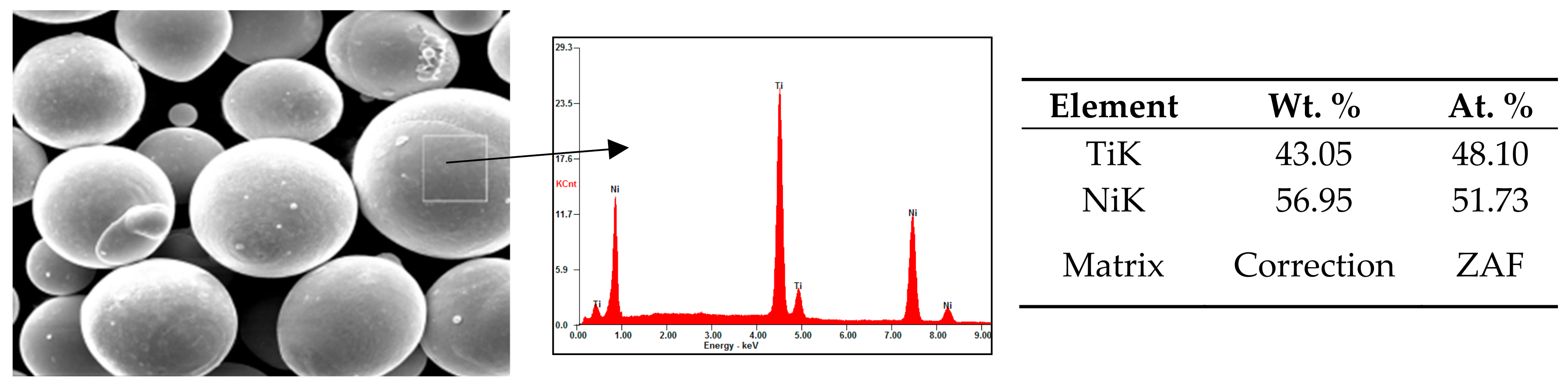
3.1.2. Elemental Composition
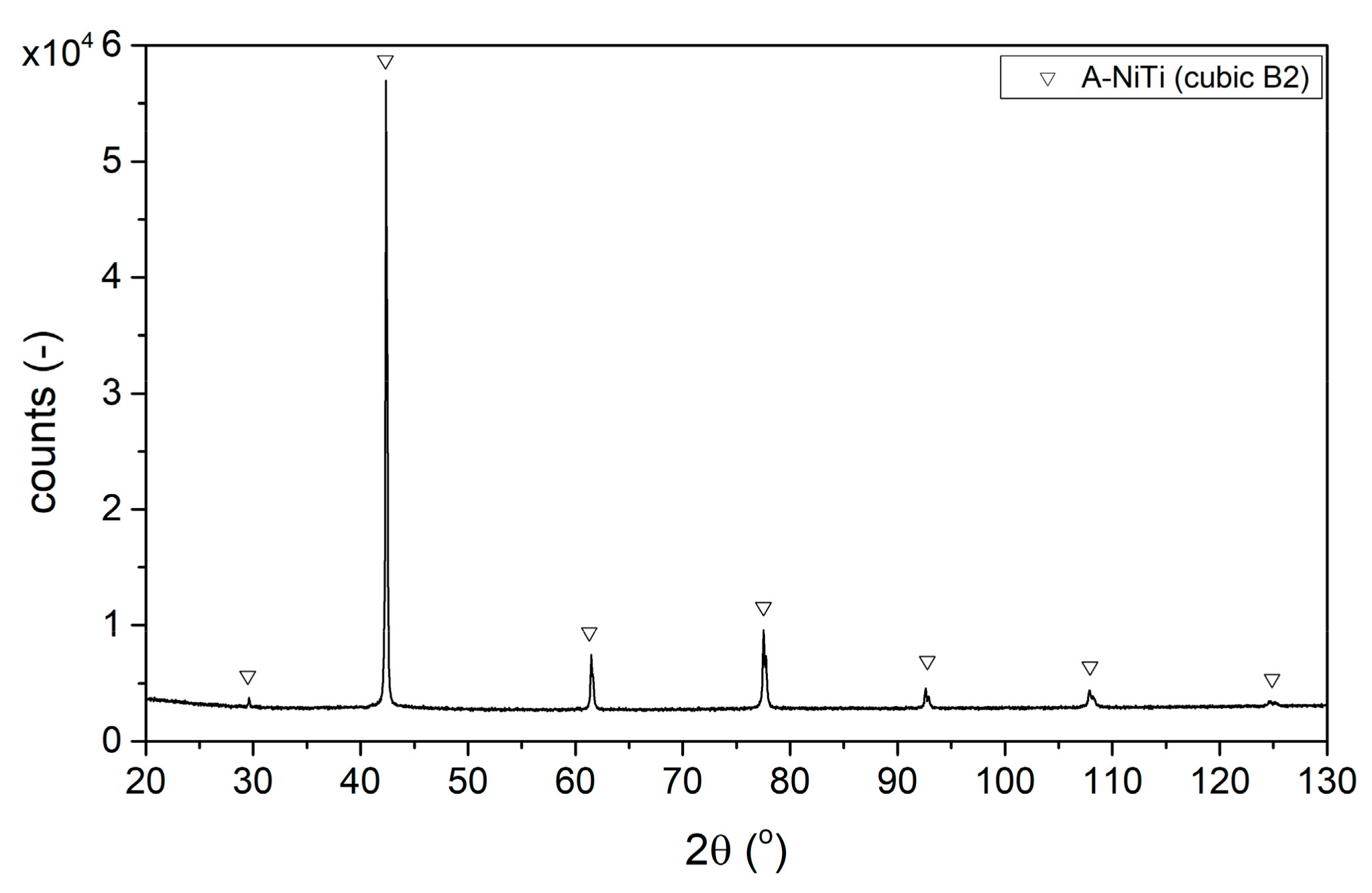
3.1.3. Phase Structure
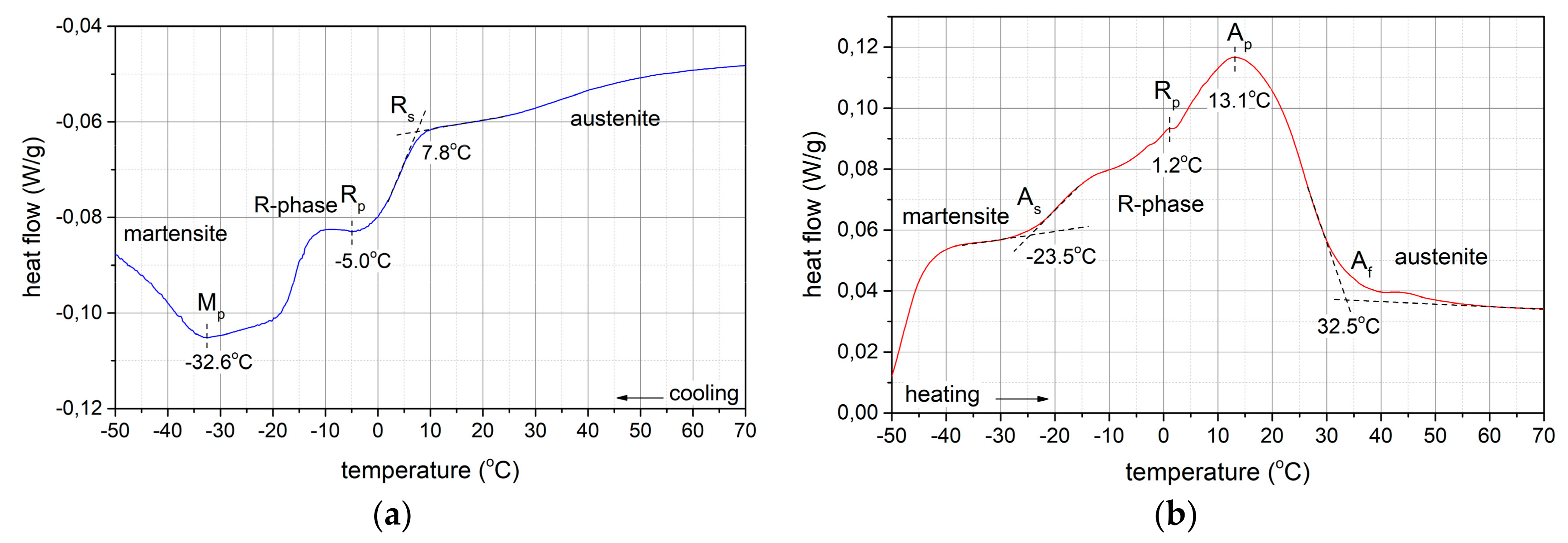
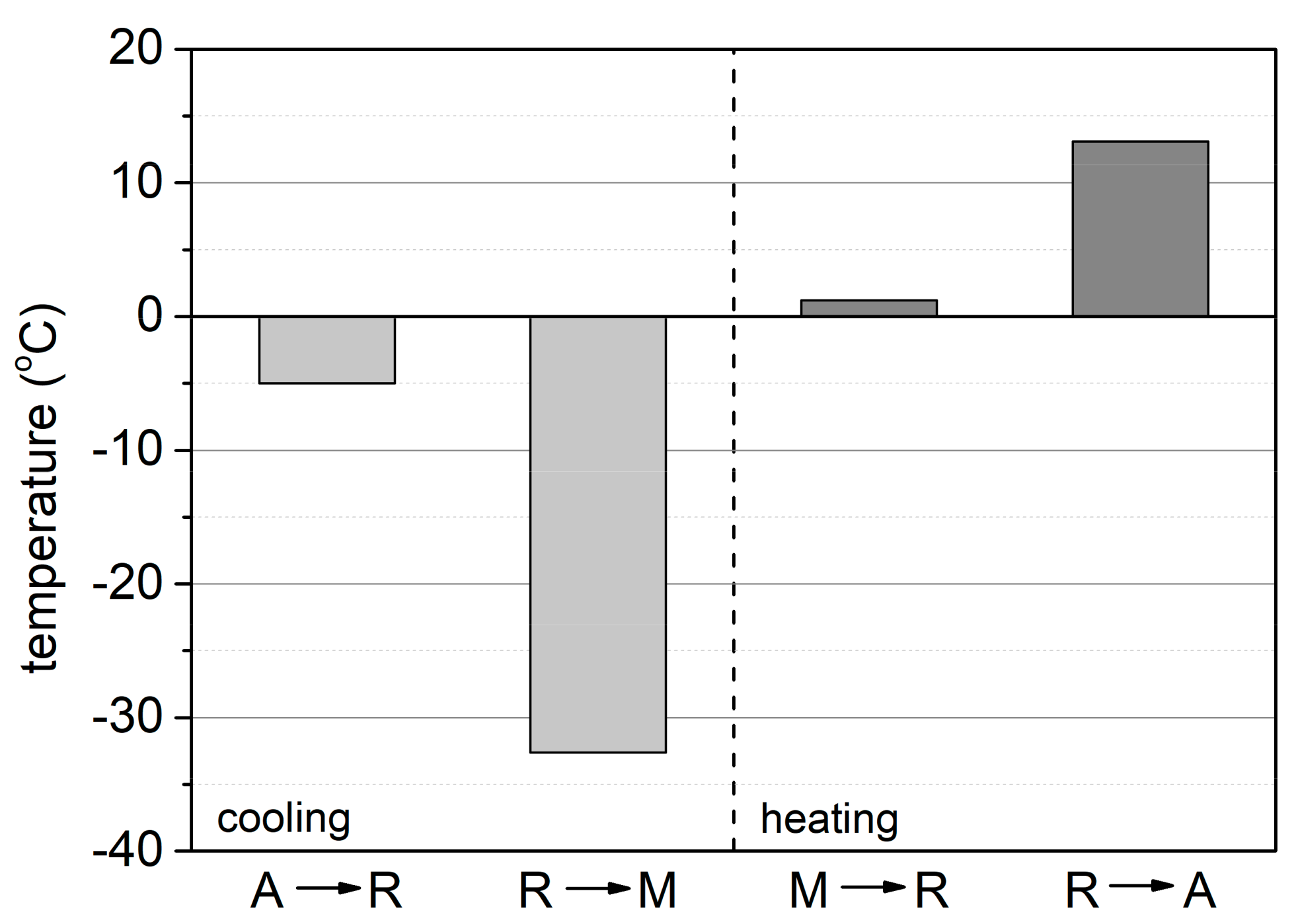
3.1.4. Phase Transformations
- TRmax = -5 °C, A→R transformation during cooling
- TMmax = -32,6 °C, R → M transformation during cooling
- TRmax = 1,2 °C, M → R transformation during heating
- TAmax = 13,10 °C, R→A transformation during heating
3.2. Input-Output Correlation
3.2.1. Input Parameters
3.2.2. Sample Characteristics
Density
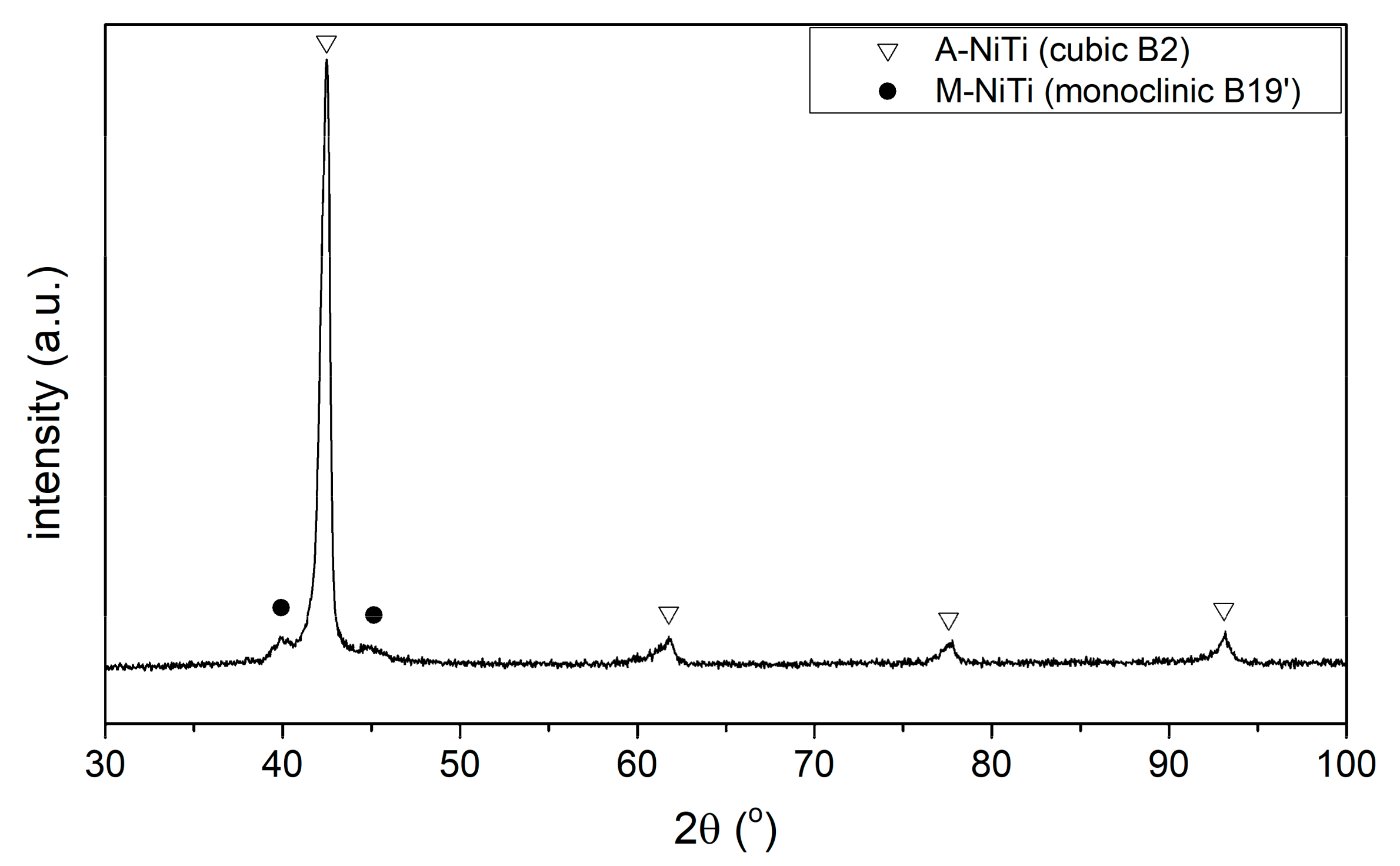
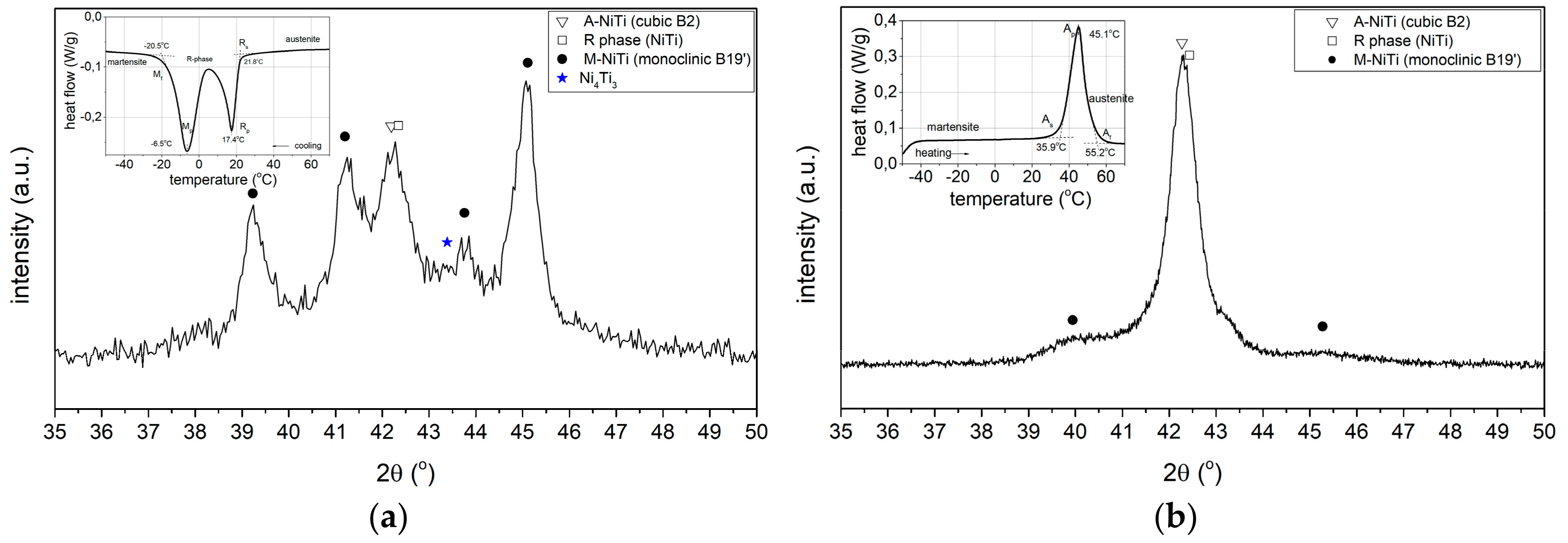
XRD Phase Analysis
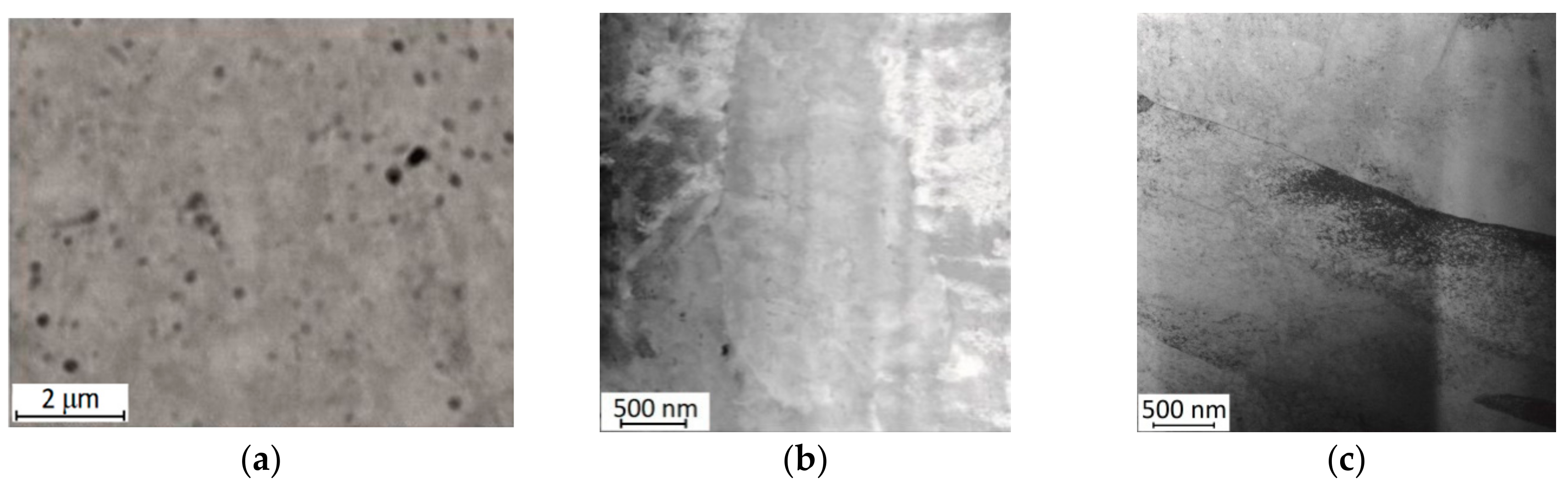
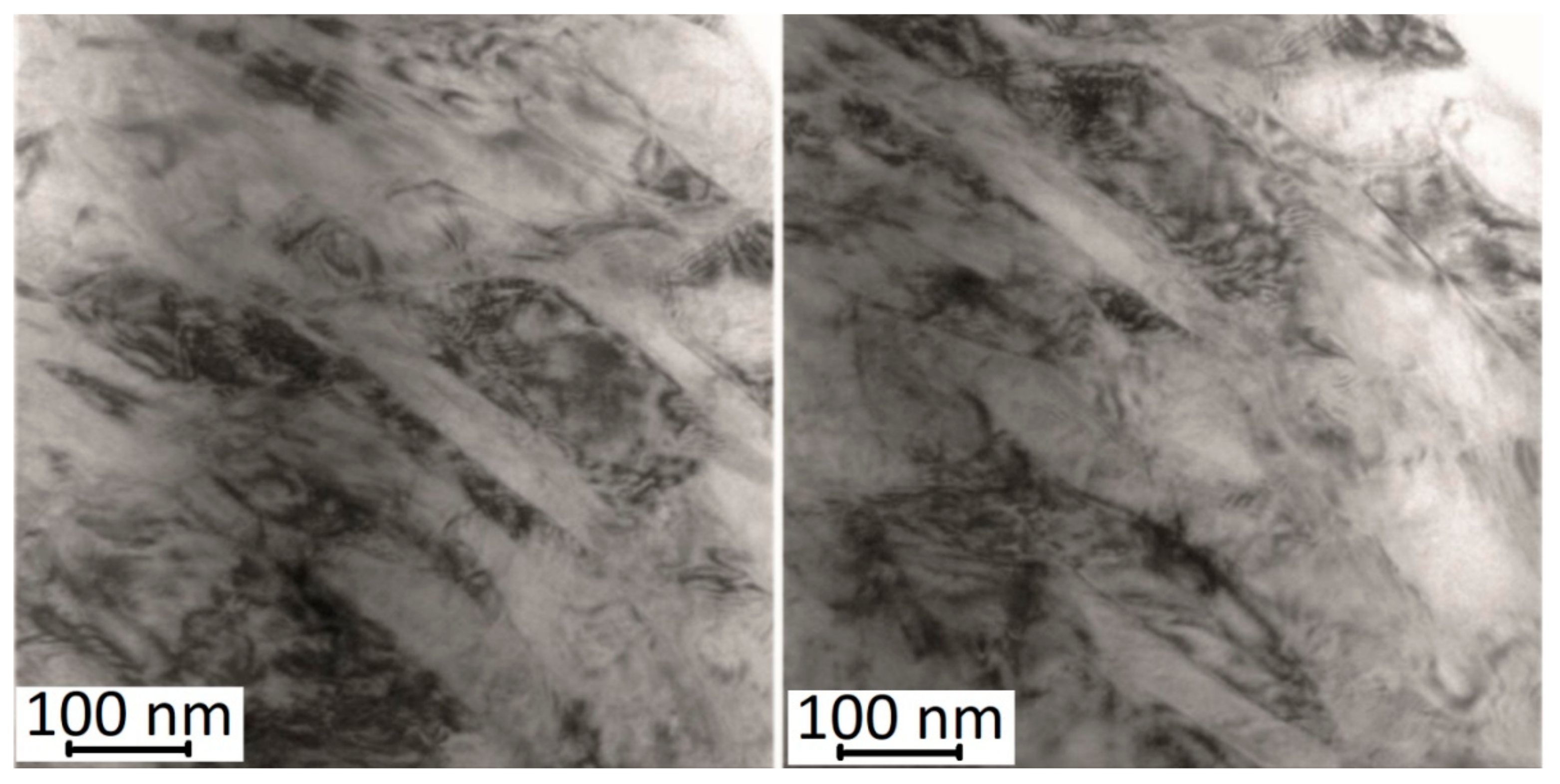
Microstructure
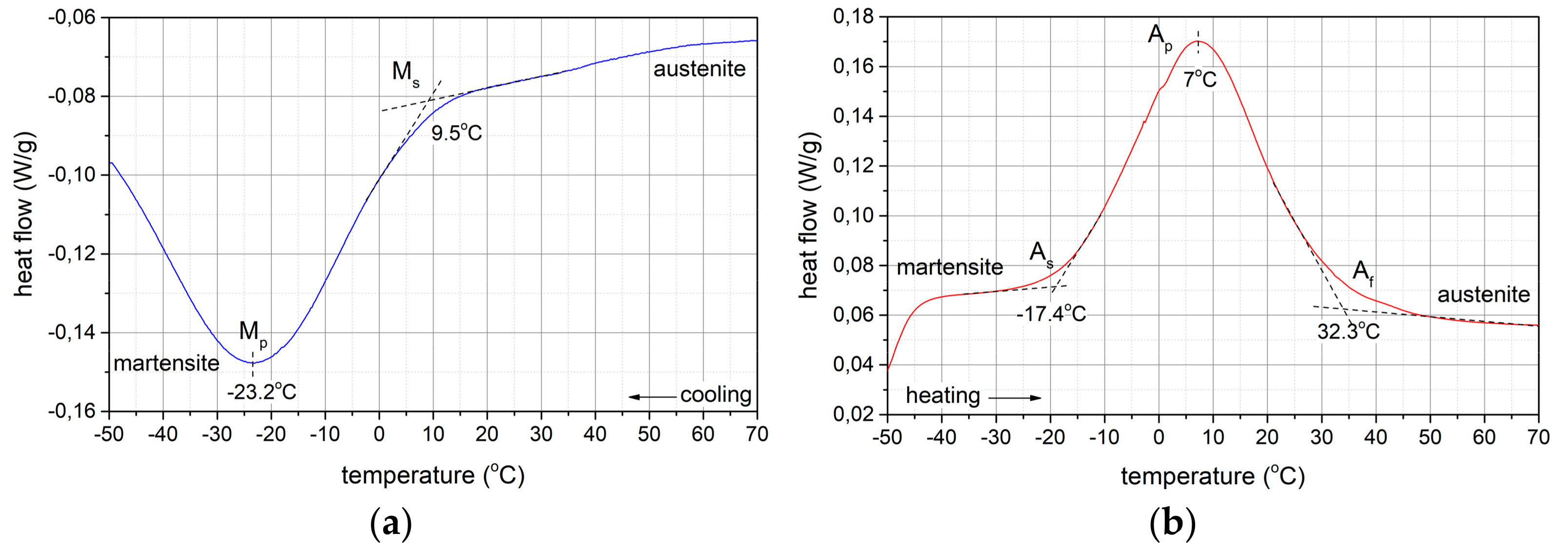
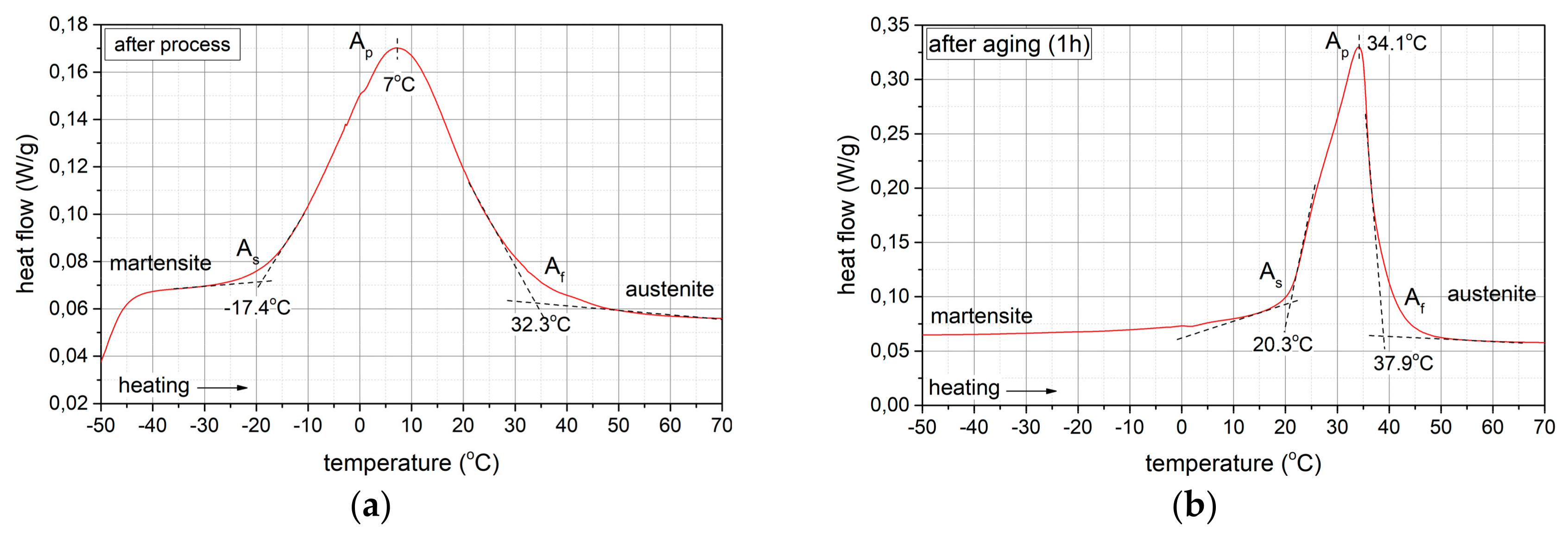
Phase Transformations
- TMmax = -23.3 °C:A → M transformation during cooling,
- TAmax = 7 °C:M→A transformation during heating.
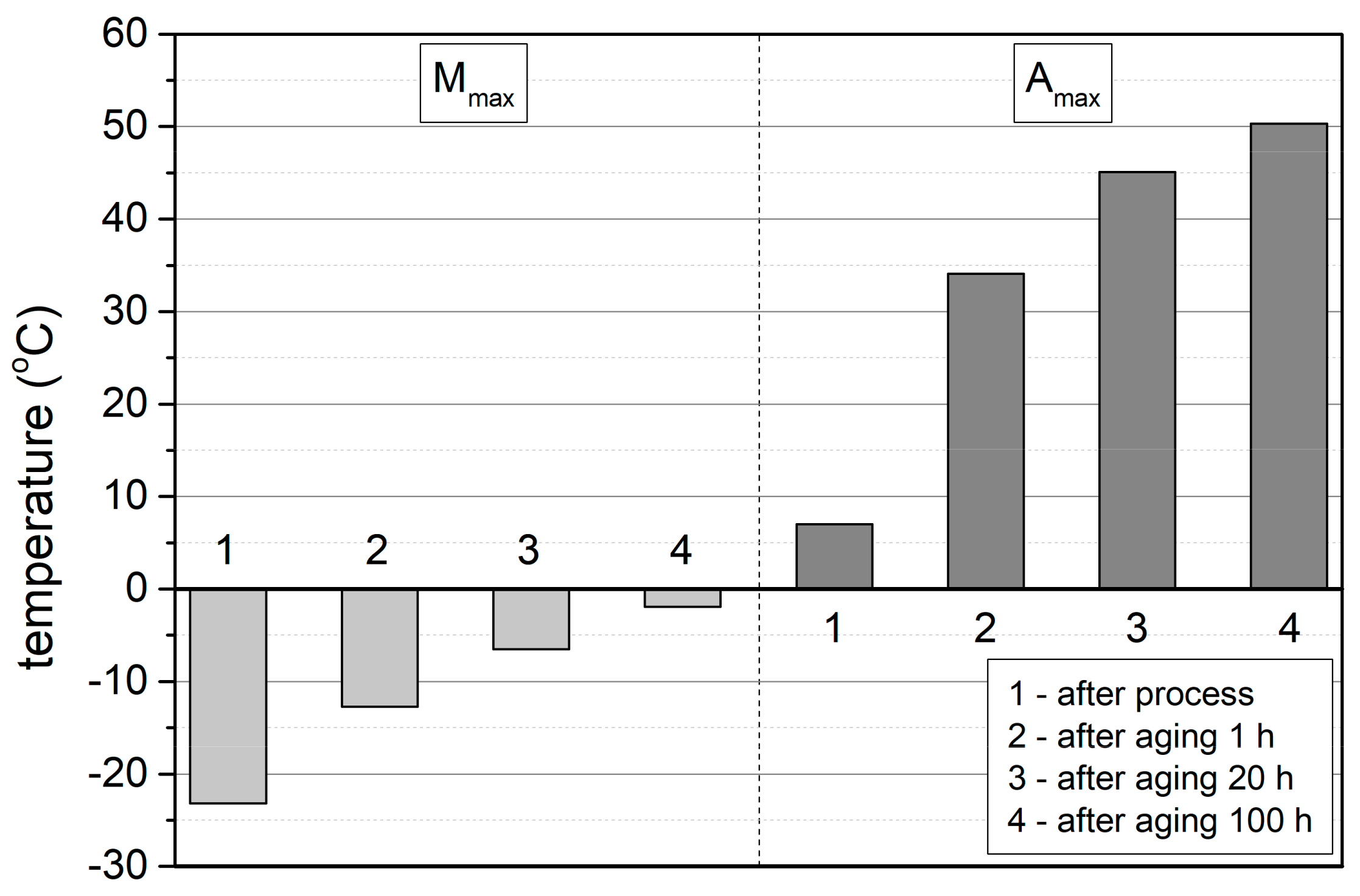
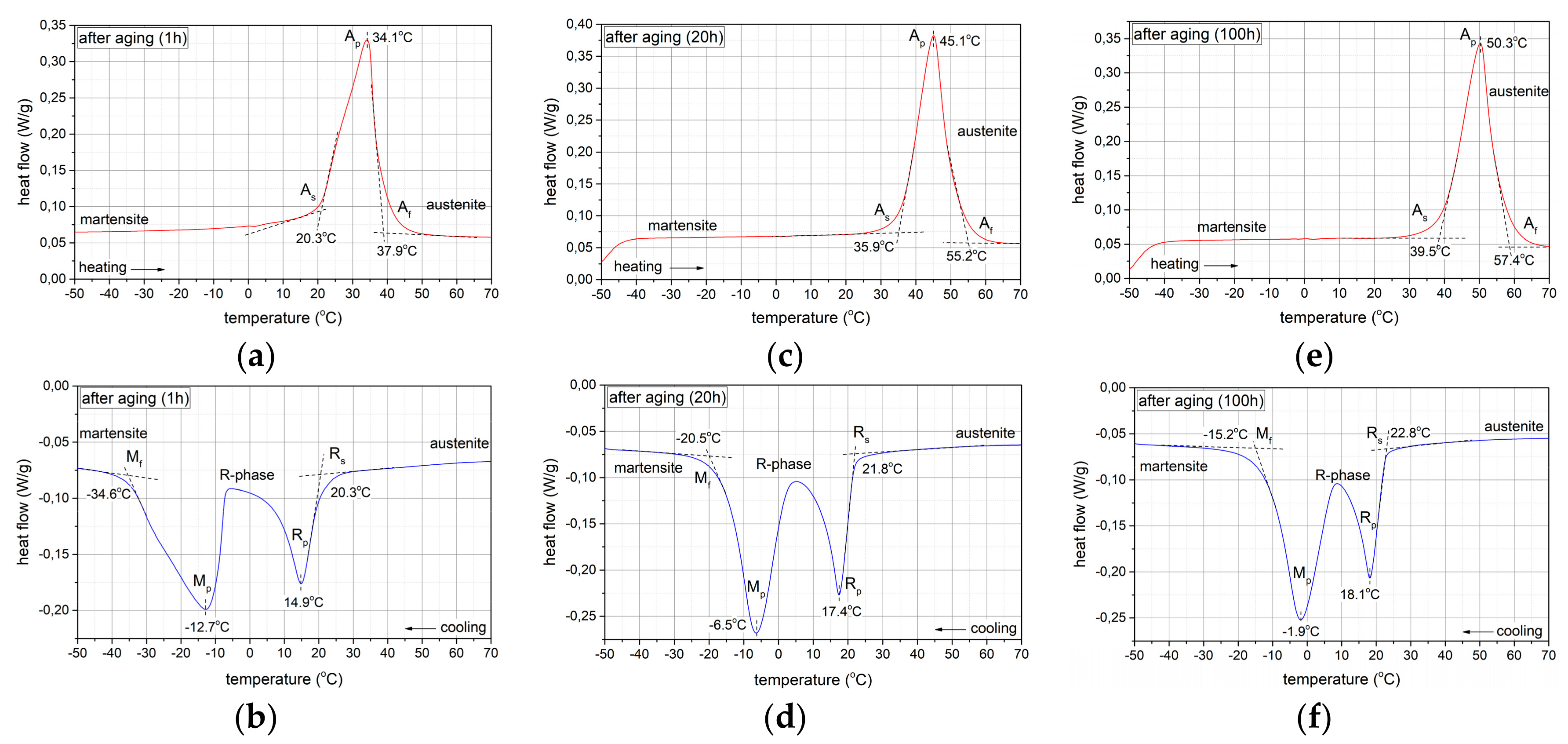
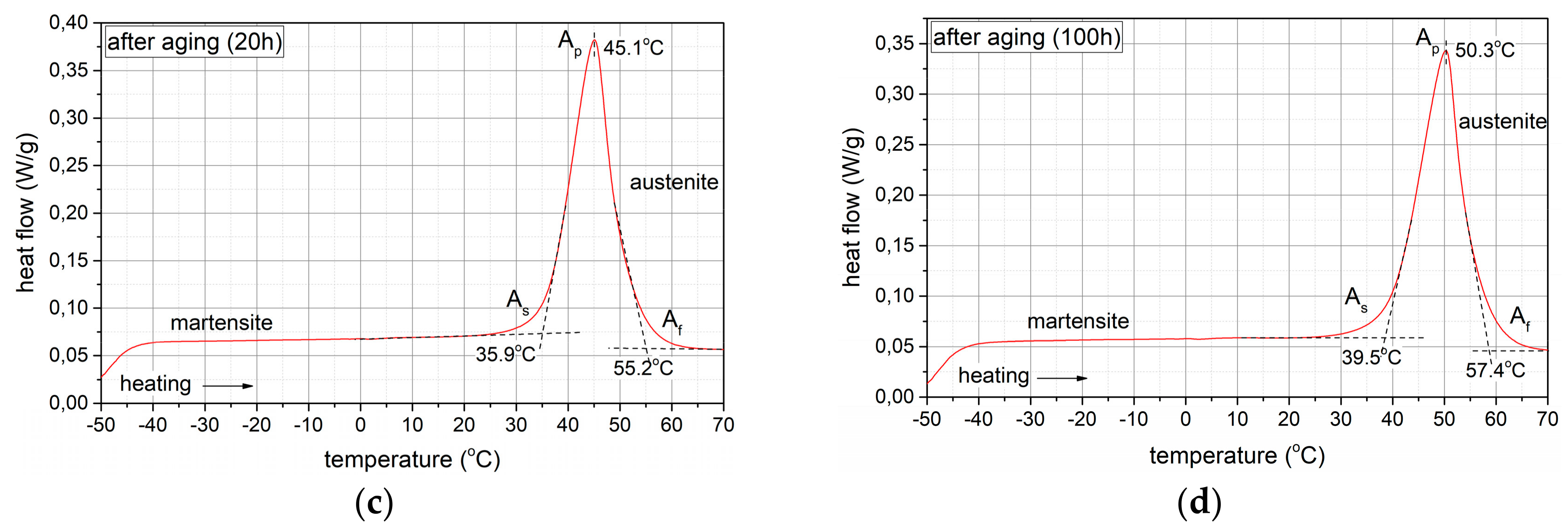
3.3. Isothermal Ageing
3.3.1. Phase Composition Analysis
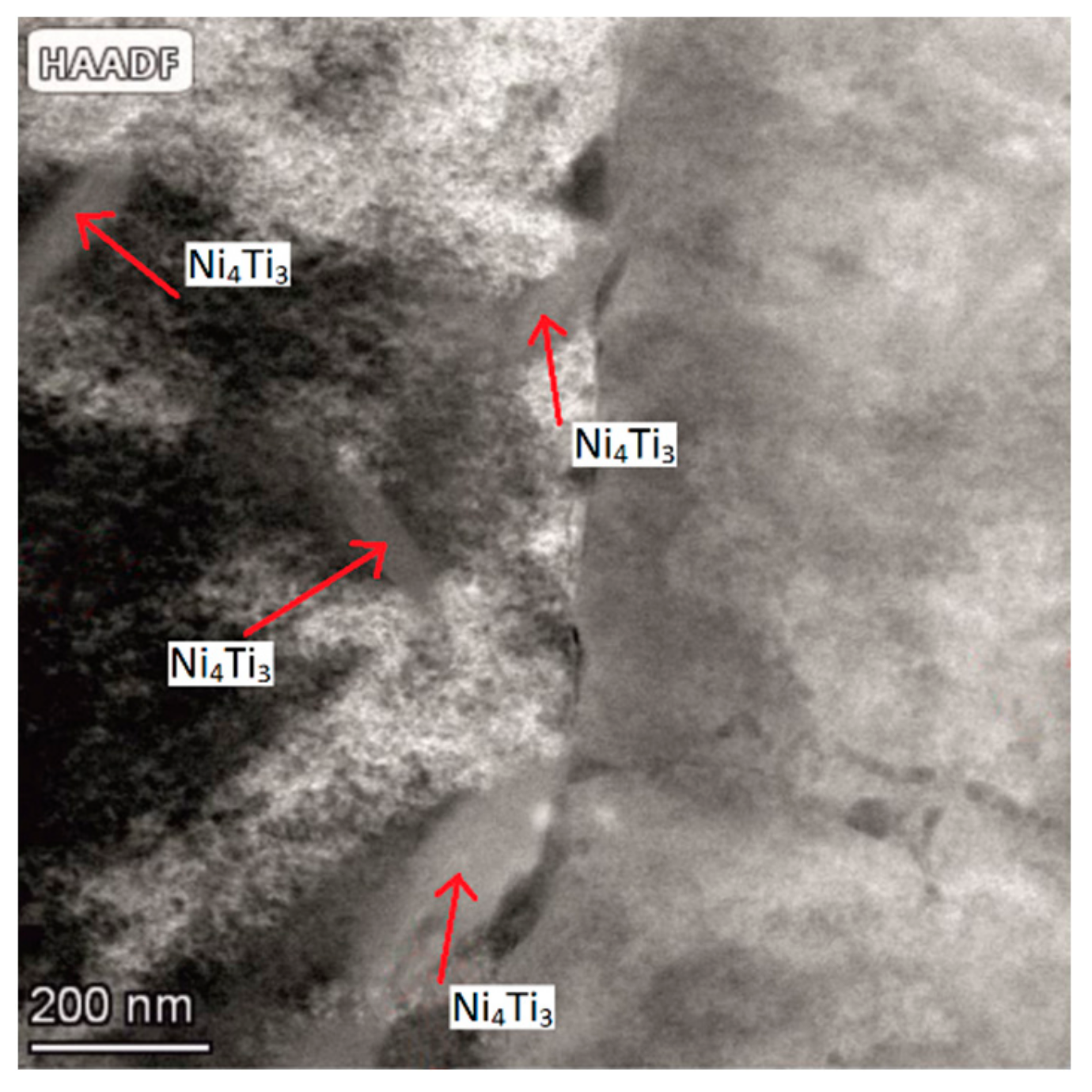
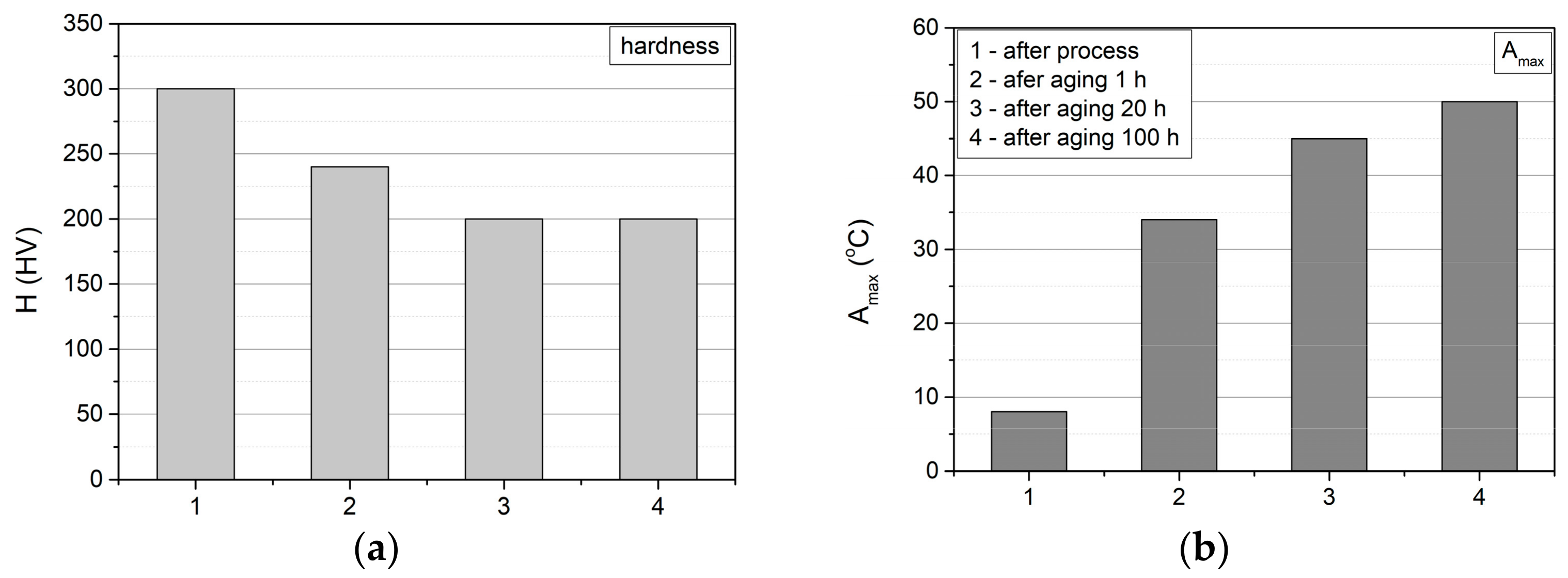
3.3.2. Hardness and Young’s Modulus
4. Conclusions
- During cooling, the type of transformation changes from one-step after solution annealing to two-step after ageing for 1, 20 and 100 h.
- During heating of the samples from low temperatures, for all the ageing times, only a one-step transformation from M(B19′) to A(B2) is observed in the DSC thermograms.
- The transformation temperature M(B19′) → A(B2) increases with the ageing time.
- The width of the total transformation temperature range M(B19′) → A(B2) during heating varies from large (ΔT=49.70 °C) after solution annealing to narrow (ΔT=19.30 °C) after 20 h of ageing.
- The change in the hardness values as a result of the ageing process corresponds to the change in temperature, at which the maximum peak reflecting the M(B19′) → A(B2), transformation is observed, i.e., the changes in hardness minimise after 20 h of ageing.
- Comparison with literature data proves that, irrespective of the NiTi alloy composition and sample manufacturing technology, a sufficiently long ageing process time (in the cases analysed, at 500 °C) leads to the occurrence of the martensite → austenite transformation in the same temperature range.
Author Contributions
Funding
Conflicts of Interest
References
- Aimar, A.; Palermo, A.; Innocenti, B. The role of 3D printing in medical applications: A state of the art. J. Healthc. Eng. 2019, 2019, 5340616. [Google Scholar] [CrossRef] [PubMed]
- Velasco-Hogan, A.; Xu, J.; Meyers, M.A. Additive manufacturing as a method to design and optimize bioinspired structures. Adv. Mater. 2018, 30, 1800940. [Google Scholar] [CrossRef] [PubMed]
- Sabahi, N.; Chen, W.; Wang, C.H.; Kruzic, J.J.; Li, X. A review on additive manufacturing of shape-memory materials for biomedical applications. JOM 2020, 72, 1229–1253. [Google Scholar] [CrossRef]
- Yan, Q.; Dong, H.; Su, J.; Han, J.; Song, B.; Wei, Q.; Shi, Y. A review of 3D printing technology for medical applications. Engineering 2018, 4, 729–742. [Google Scholar] [CrossRef]
- Ye, J.; Wilson, D.A.; Tu, Y.; Peng, F. 3D-Printed Micromotors for Biomedical Applications. Adv. Mater. Technol. 2020, 5, 2000435. [Google Scholar] [CrossRef]
- Ahangar, P.; Cooke, M.E.; Weber, M.H.; Rosenzweig, D.H. Current biomedical applications of 3D printing and additive manufacturing. Appl. Sci. 2019, 9, 1713. [Google Scholar] [CrossRef]
- Yuan, L.; Ding, S.; Wen, C. Additive manufacturing technology for porous metal implant applications and triple minimal surface structures: A review. Bioact. Mater. 2018, 4, 56–70. [Google Scholar] [CrossRef] [PubMed]
- Sacco, E.; Moon, S.K. Additive manufacturing for space: Status and promises. Int. J. Adv. Man. Technol. 2019, 105, 4123–4146. [Google Scholar] [CrossRef]
- Ishfaq, K.; Asad, M.; Mahmood, M.A.; Abdullah, M.; Pruncu, C. Opportunities and challenges in additive manufacturing used in space sector: A comprehensive review. RapidPrototyping J. 2022, 28, 2027–2042. [Google Scholar] [CrossRef]
- Lu, H.Z.; Ma, H.W.; Luo, X.; Wang, Y.; Wang, J.; Lupoi, R.; Yin, S.; Yang, C. Microstructure, shape memory properties, and in vitro biocompatibility of porous NiTi scaffolds fabricated via selective laser melting. J. Mater. Res. Technol. 2021, 15, 6797–6812. [Google Scholar] [CrossRef]
- Zhang, Y.; Attarilar, S.; Wang, L.; Lu, W.; Yang, J.; Fu, Y. A review on design and mechanical properties of additively manufactured NiTi implants for orthopedic applications. Int. J. Bioprinting 2021, 7, 340. [Google Scholar] [CrossRef] [PubMed]
- Chmielewska, A.; Dobkowska, A.; Kijeńska-Gawrońska, E.; Jakubczak, M.; Krawczyńska, A.; Choińska, E.; Jastrzębska, A.; Dean, D.; Wysocki, B.; Święszkowski, W. Biological and corrosionevaluation of in situ alloyedNiTifabricatedthrough laser powderbedfusion (LPBF). Int. J. Mol. Sci. 2021, 22, 13209. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Guo, Y.; Liu, Y.; Jia, B.; Sha, P.; Li, L.; Yu, Z.; Zhang, Z.; Ren, L. An extremely efficiency method to achieve stable superhydrophobicity on the surface of additive manufactured NiTi Alloys: “Ultrasonic Fluorination”. Appl. Surf. Sci. 2023, 612, 155947. [Google Scholar] [CrossRef]
- Habijan, T.; Haberland, C.; Meier, H.; Frenzel, J.; Wittsiepe, J.; Wuwer, C.; Greulich, C.; Schildhauer, T.A.; Köller, M. The biocompatibility of dense and porous nickel–titanium produced by selective laser melting. Mater. Sci. Eng. C 2013, 33, 419–426. [Google Scholar] [CrossRef] [PubMed]
- Chekotu, J.C.; Goodall, R.; Kinahan, D.; Brabazon, D. Control of Ni-Ti phase structure, solid-state transition temperatures and enthalpies via control of L-PBF process parameters. Mater. Design 2022, 218, 110715. [Google Scholar] [CrossRef]
- Ye, D.; Li, S.F.; Misra, R.D.K.; Zheng, R.; Yang, Y.F. Ni-loss compensation and thermomechanical property recovery of 3D printed NiTi alloys by pre-coating Ni on NiTi powder. Addit. Manuf. 2021, 47, 102344. [Google Scholar] [CrossRef]
- Saedi, S.; Turabi, A.S.; Andani, M.T.; Haberland, C.; Elahinia, M.; Karaca, H. Thermomechanical characterization of Ni-rich NiTi fabricated by selective laser melting. Smart Mater. Struct. 2016, 25, 035005. [Google Scholar] [CrossRef]
- Saedi, S.; Turabi, A.S.; Andani, M.T.; Haberland, C.; Karaca, H.; Elahinia, M. The influence of heat treatment on the thermomechanical response of Ni-rich NiTi alloys manufactured by selective laser melting. J. Alloys Compd. 2016, 677, 204–210. [Google Scholar] [CrossRef]
- Feng, B.; Wang, C.; Zhang, Q.; Ren, Y.; Cui, L.; Yang, Q.; Hao, S. Effect of laser hatch spacing on the pore defects, phase transition and properties of selective laser melting fabricated NiTi shape memory alloys. Mater. Sci. Eng. A 2022, 840, 142965. [Google Scholar] [CrossRef]
- Khoo, Z.X.; Liu, Y.; An, J.; Chua, C.K.; Shen, Y.F.; Kuo, C.N. A review of selective laser melted NiTi shape memory alloy. Materials 2018, 11, 519. [Google Scholar] [CrossRef] [PubMed]
- Allafi, J.K.; Ren, X.; Eggeler, G. The mechanism of multistage martensitic transitions in aged Ni-rich NiTi shape memory alloys. Acta Mater. 2002, 50, 793–803. [Google Scholar] [CrossRef]
- Zhou, N.; Shen, C.; Wagner, M.X.; Eggeler, G.; Mills, M.J.; Wang, Y. Effect of Ni4Ti3 precipitation on martensitic transition in Ti–Ni. Acta Mater. 2010, 58, 6685–6694. [Google Scholar] [CrossRef]
- Yao, X.; Amin-Ahmadi, B.; Li, Y.; Cao, S.; Ma, X.; Zhang, X.P.; Schryvers, D. Optimization of Automated Crystal Orientation Mapping in a TEM for Ni4Ti3 Precipitation in All-Round SMA. ShapeMem. Superelast. 2016, 2, 286–297. [Google Scholar] [CrossRef]
- Ma, C.; Gu, D.; Dai, D.; Xia, M.; Chen, H. Selective growth of Ni4Ti3 precipitate variants induced by complicated cyclic stress during laser additive manufacturing of NiTi-based composites. Mater. Charact. 2018, 143, 191–196. [Google Scholar] [CrossRef]
- Novák, P.; Pokorný, P.; Vojtěch, V.; Knaislová, A.; Školáková, A.; Čapek, J.; Kopeček, J. Formation of Ni–Ti intermetallics during reactive sintering at 500–650 °C. Mater. Chem. Phys. 2015, 155, 113–121. [Google Scholar] [CrossRef]
- Ma, J.; Franco, B.; Tapia, G.; Karayagiz, K.; Johnson, L.; Liu, J.; Arroyave, R.; Karaman, I.; Elwany, A. Spatial control of functional response in 4D-printed active metallic structures. Sci. Rep. 2017, 7, 46707. [Google Scholar] [CrossRef] [PubMed]
- Elahinia, M.; Moghaddam, N.S.; Amerinatanzi, A.; Saedi, S.; Toker, G.P.; Karaca, H.; Bigelow, G.S.; Benafan, O. Additive manufacturing of NiTiHf high temperature shape memory alloy. Scripta Materialia 2018, 145, 90–94. [Google Scholar] [CrossRef]
- Bormann, T.; de Wild, M.; Beckmann, F.; Müller, B. Assessing the morphology of selective laser melted NiTi-scaffolds for a three-dimensional quantification of the one-way shape memory effect. Proc. SPIE 2013, 8689, 281–288. [Google Scholar] [CrossRef]
- Bormann, T.; Schumacher, R.; Müller, B.; Mertmann, M.; De Wild, M. Tailoring selective laser melting process parameters for NiTi implants. J. Mater. Eng. Perform. 2012, 21, 2519–2524. [Google Scholar] [CrossRef]
- Bose, S.; Ke, D.; Sahasrabudhe, H.; Bandyopadhyay, A. Additive manufacturing of biomaterials. Prog. Mater. Sci. 2018, 93, 45–111. [Google Scholar] [CrossRef] [PubMed]
- DebRoy, T.; Wei, H.L.; Zuback, J.S.; Mukherjee, T.; Elmer, J.W.; Milewski, J.O.; Beese, A.M.; Wilson-Heid, A.; De, A.; Zhang, W. Additive manufacturing of metallic components—Process, structure and properties. Prog. Mater. Sci. 2018, 92, 112–224. [Google Scholar] [CrossRef]
- Horvay, K.M.; Schade, C.T. Development of nitinol alloys for additive manufacturing. Mater. Sci. Technol. 2018, 2018, 63–70. [Google Scholar]
- Uchil, J.; Kumara, K.G.; Mahesh, K.K. Effect of thermal cycling on R-phase stability in a NiTi shape memory alloy. Mater. Sci. Eng. A 2002, 332, 25–28. [Google Scholar] [CrossRef]
- Khalil-Allafi, J.; Schmahl, W.W.; Toebbens, D.M. Space group and crystal structure of the R-phase in binary NiTi shape memory alloys. Acta Mater. 2006, 54, 3171–3175. [Google Scholar] [CrossRef]
- Wang, X.; Kustov, S.; Verlinden, B.; Van Humbeeck, J. Fundamental development on utilizing the R-phase transition in NiTi shape memory alloys. Shape Mem. Superelast. 2015, 1, 231–239. [Google Scholar] [CrossRef]
- Honarvar, M.; Konh, B.; Podder, T.K.; Dicker, A.P.; Yu, Y.; Hutapea, P. X-ray diffraction investigations of shape memory NiTi wire. J. Mater. Eng. Perform. 2015, 24, 3038–3048. [Google Scholar] [CrossRef]
- Feng, B.; Kong, X.; Hao, S.; Liu, Y.; Yang, Y.; Yang, H.; Cui, L. In-situ synchrotron high energy X-ray diffraction study of micro-mechanical behaviour of R phase reorientation in nanocrystalline NiTi alloy. Acta Mater. 2020, 194, 565–576. [Google Scholar] [CrossRef]
- Duerig, T.W.; Bhattacharya, K. The influence of the R-phase on the superelastic behavior of NiTi. ShapeMem. Superelast. 2015, 1, 153–161. [Google Scholar] [CrossRef]
- Šittner, P.; Landa, M.; Lukáš, P.; Novák, V. R-phase transition phenomena in thermomechanically loaded NiTi polycrystals. Mech. Mater. 2006, 38, 475–492. [Google Scholar] [CrossRef]
- Chekotu, J.C.; Groarke, R.; O’Toole, K.; Brabazon, D. Advances in selective laser melting of nitinol shape memory alloy part production. Materials 2019, 12, 809. [Google Scholar] [CrossRef] [PubMed]
- Michutta, J.; Somsen, C.; Yawny, A.; Dlouhy, A.; Eggeler, G. Elementary martensitic transition processes in Ni-rich NiTi single crystals with Ni4Ti3 precipitates. Acta Mater. 2006, 54, 3525–3542. [Google Scholar] [CrossRef]
- Khalil-Allafi, J.; Dlouhy, A.; Eggeler, G. Ni4Ti3-precipitation during aging of NiTi shape memory alloys and its influence on martensitic phase transitions. Acta Mater. 2002, 50, 4255–4274. [Google Scholar] [CrossRef]
- Barras, C.D.J.; Myers, K.A. Nitinol–its use in vascular surgery and other applications. EJVES 2000, 19, 564–569. [Google Scholar] [CrossRef] [PubMed]
- Laurentis, K.J.D.; Mavroidis, C. Mechanical design of a shape memory alloy actuated prosthetic hand. Technol. Health Care 2002, 10, 91–106. [Google Scholar] [CrossRef] [PubMed]
- Bataillard, L.; Bidaux, J.E.; Gotthardt, R. Interaction between microstructure and multiple-step transition in binary NiTi alloys using in-situ transmission electron microscopy observations. Philos. Mag. A 1998, 78, 327–344. [Google Scholar] [CrossRef]
- Waitz, T.; Kazykhanov, V.; Karnthaler, H.P. Martensitic phase transitions in nanocrystalline NiTi studied by TEM. Acta Materialia 2004, 52, 137–147. [Google Scholar] [CrossRef]
- Gall, K.; Juntunen, K.; Maier, H.J.; Sehitoglu, H.; Chumlyakov, Y.I. Instrumented micro-indentation of NiTi shape-memory alloys. Acta Materialia 2001, 49, 3205–3217. [Google Scholar] [CrossRef]
- Waitz, T.; Karnthaler, H.P. Martensitic transition of NiTi nanocrystals embedded in an amorphous matrix. Acta Materialia 2004, 52, 5461–5469. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




