1. Introduction
In addition to protecting the head from external stimuli such as temperature, ultraviolet rays, and bruising, hair also excretes toxic substances such as heavy metals. Beautiful hair also has a significant impact on the visual effect of significantly changing one's appearance and on the psychological effect of increasing one's self-confidence. The demand for hair-care products is growing, especially with the recovery of economic activity due to the easing of behavioral restrictions caused by the Covid-19 pandemic, and the increasing number of elderly people and the growing problem of hair loss are expected to contribute significantly to the future growth of the hair-care market. Currently, two drugs, minoxidil (MXD) and finasteride, are approved by the U.S. Food and Drug Administration (FDA) for the treatment of hair loss. Although the details of MXD's mechanism of action are unknown, it is known to prolong the duration of the hair growth phase by inducing vasodilation of the scalp [1-4]. Finasteride has been demonstrated to prevent male pattern baldness by inhibiting 5α-reductase activity, which affects the metabolism of male hormones [
5]. However, side effects such as scalp problems including rashes and itching have been reported with MXD, and liver dysfunction, erectile dysfunction, and rashes have been reported with finasteride [
6,
7]. Thus, natural ingredients and organic formulations are in high demand in the hair care product market.
Studies have shown that fish oil, which is rich in n-3 polyunsaturated fatty acids such as eicosapentaenoic acid (EPA), docosapentaenoic acid (DPA), and docosahexaenoic acid (DHA), has high antioxidant capacity, reduces systemic inflammation, and is generally known to prevent atherosclerosis [
8,
9]. Intake of foods with such antioxidant capacity and circulation-promoting effects is known to contribute to various health-promoting effects, and it has been reported that intake of foods with high antioxidant capacity promotes hair growth. Human hair is mostly made of amino acids, but fatty acids are also constituents of hair, and a decrease in fatty acids is known to lead to a decrease in water retention in the hair texture, and further to a decrease in hair luster. In addition, a recent systematic review of hair growth and supplements [
10] listed about a dozen dietary supplements that promote hair growth, including omega 3 and 6, which contain antioxidants. In addition, omega-3 fatty acids have blood flow-promoting properties, which may promote blood flow to the hair matrix cells in the hair follicles, which are important for hair growth and development in particular. There have also been reports of omega-3 and its effectiveness in promoting hair growth [
11].
Recent studies have confirmed that cold-pressed whale oil (WO) has high antioxidant and anti-inflammatory effects [
12,
13]. The high stearic acid content of the WO makes it suitable as a cosmetic material, since stearic acid in WO is a component that helps improve the barrier function and is used in conditioners and treatments. Furthermore, WO contains a large amount of DPA and EPA, which could be applied to scalp care and hair growth products. Experiments were conducted to confirm the hair-growth-promoting effects of WO, aiming to make effective use of the parts that are normally discarded and to utilize WO as a functional ingredient.
2. Materials and Methods
2.1. Sample Preparation and Whale Oil (WO)
Bryde's whale (Balaenoptera brydei) skin was extracted with heated steam at 200°C for 20 minutes and frozen. The WO was provided by Kyodo Senpaku Co. Ltd (Japan).
2.2. MTT Assay
Human Follicle Dermal Papilla Cells (HFDPC) were seeded in 96-well culture plates at 3 × 104 cells/100 μL/well and pre-cultured for 24 hours at 37°C and 5% CO2. Cultures were grown in DMEM (Dulbecco's Modified Eagle's Medium "Nissui" (2), Nissui Pharmaceutical) medium with 10% FBS (fetal bovine serum) and 1% antibiotic (Sigma A5955) at a ratio of 1%. After preincubation, the medium was replaced with a new medium, and the WO, Docosapentaenoic Acid (DPA), and Eicosapentaenoic Acid (EPA) adjusted to a concentration of 0.0-5.0% with D/E solution (DMSO:Ethanol=1:1), and as a positive control Minoxidil (MXD), which is used in hair growth products, was added at 5-30 μM concentration in D/E solution. 24-120 hours of incubation at 37°C and 5 % CO2 was followed by incubation with MTT (3-[4,5-]dimethylthiazol-2-2) from the Cell Growth Kit I (Roche Life Science). After incubation at 37°C and 5% CO2 for 4 hours, 100 μL/well of solubilizing solution (0.01 M HCl, 10% SDS) was added and incubated at 37°C and 5% CO2 for 24 to 120 hours. After that, 100 μL/well of solubilizing solution (0.01 M HCl, 10% SDS) was added and incubated for 17 hours at 37°C, 5% CO2. After incubation, absorbance was measured at 570 nm using an iMark microplate reader (Bio-Rad) with a reference wavelength of 650 nm. The cell viability (%) was determined by taking the number of viable cells without the evaluation sample as 100 when the evaluation sample was added. Each measurement was repeated twice and the average (Average of values for each 12 wells) value was calculated. All results below are expressed as mean ± SE, using the Turkey method. p < 0.05 is considered a significant difference.
2.3. Confirmation of Gene Expression Involved in Hair Growth
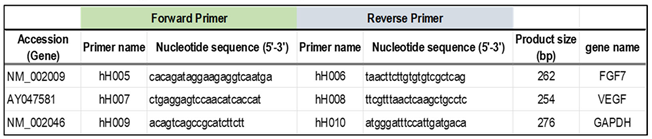
Total RNA was extracted from HFDPCs with a mixture of WO, DPA, EPA (0.0-5.0%), and Minoxidil (5-30 μM) in D/E solution (DMSO:Ethanol=1:1) and incubated for 30, 60, and 90 minutes using the RNeasy Micro Kit (QIAGEN) Total RNA was extracted according to the standard protocol. RNA concentration and 260/280 and 260/230 ratios were measured using a spectrophotometer to confirm RNA quality. 100-500 ng of RNA was used to synthesize cDNA using the Affinity Script QPCR cDNA synthesis kit (Agilent), followed by the Emerald Amp PCR Master (TAKARA) with specific primers for FGF7 and VEGF genes (
Table 1). PCR reactions (initial denaturation at 97°C for 5 min, thermal denaturation at 95°C for 45 s, annealing at 55°C for 45 s, and extension at 72°C for 1 min were performed for 27 to 45 cycles, After the PCR reactions, the PCRs were separated on a 1.5% agarose gel and visualized with ethidium bromide and UV light. The expression levels of the visualized target genes were corrected for the expression level of the GAPDH gene, a housekeeping gene, and plotted (n=3).
2.4. Evaluation of Hair Growth Using Mouse Black Skin Model
To evaluate the hair growth-promoting effects of WO, a back skin model of C57BL/6J mice was used for comparison. This is because MXD is used to treat alopecia [
13] and is commonly used as a positive control in many hair studies. To improve the penetration of the test samples, propylene glycol: ethanol: water (5:3:2) was used as the VC group, and preliminary experiments were conducted with 1, 10, and 20% concentrations of WO. After preliminary experiments the concentration of WO was determined to be 20%. C57BL/6J mice were divided into three groups (6 mice/group) and housed ad libitum at 22 ± 2°C, 6:00-18:00, 12 hr/12 hr light/dark cycle. The hair cycle was adjusted by shaving the back of the mice and then shaving again 1 week later. A 200 uL of the adjusted sample was applied to the back of the mice daily, and observations and photographs were taken at 0, 1, and 3 weeks. Photographs were analyzed using ImageJ software. The ratio of hair growth area to shaved area was calculated and the hair growth rate (%) was measured.
2.5. Histological Observations
In the hair growth evaluation experiment, the whole back skin tissue of mice was fixed with 4% paraformaldehyde solution and replaced with PBS after 3 weeks of application of the conditioned sample. Paraffin sections were dehydrated and permeated with xylene for embedding in paraffin. 5 μm paraffin sections were treated with hematoxylin/eosin stain, dehydrated, permeated, and sealed with cover glass. Section images were photographed with a BZ-X 710 brightfield system (Keyence) at ×20 magnification, and images were analyzed using ImageJ software. The total number of hair follicles in the dermis and hypodermis and the thickness of the hypodermis were measured and the mean number of hair follicles and mean thickness of the hypodermis were calculated (n=6).
2.6. Whole-Genome DNA Microarray Analysis
In hair growth evaluation experiments, whole mouse back skin tissue was sampled at 1 and 3 weeks after application of the conditioned samples and stored at -80°C. The skin tissue was pulverized to a fine powder in liquid nitrogen and stored at -80°C until RNA isolation. Skin tissue was ground to a fine powder in liquid nitrogen and stored at -80°C until RNA isolation. DeNovix) and formaldehyde-agarose gel electrophoresis. Microarray analysis was performed using the Whole Mouse Genome Oligo DNA Microarray Kit 4x44K (Agilent) and the Dye-swap method to identify genes whose expression changed after whale oil and MXD treatment (up-regulation: Up (≥1.5-fold), down-regulation: Down (≥1.5-fold)) compared to VC, Down-regulation: Down (≤0.75-fold)) were listed. The Functional_Categories (KEYWORDS) and pathway (KEGG pathway) of the list of variable genes selected by the microarray analysis were analyzed using Database for Annotation, Visualization and Integrated Discovery (DAVID) v6.8.
3. Results
3.1. Proliferative Effects of HFDPC by MTT Assay
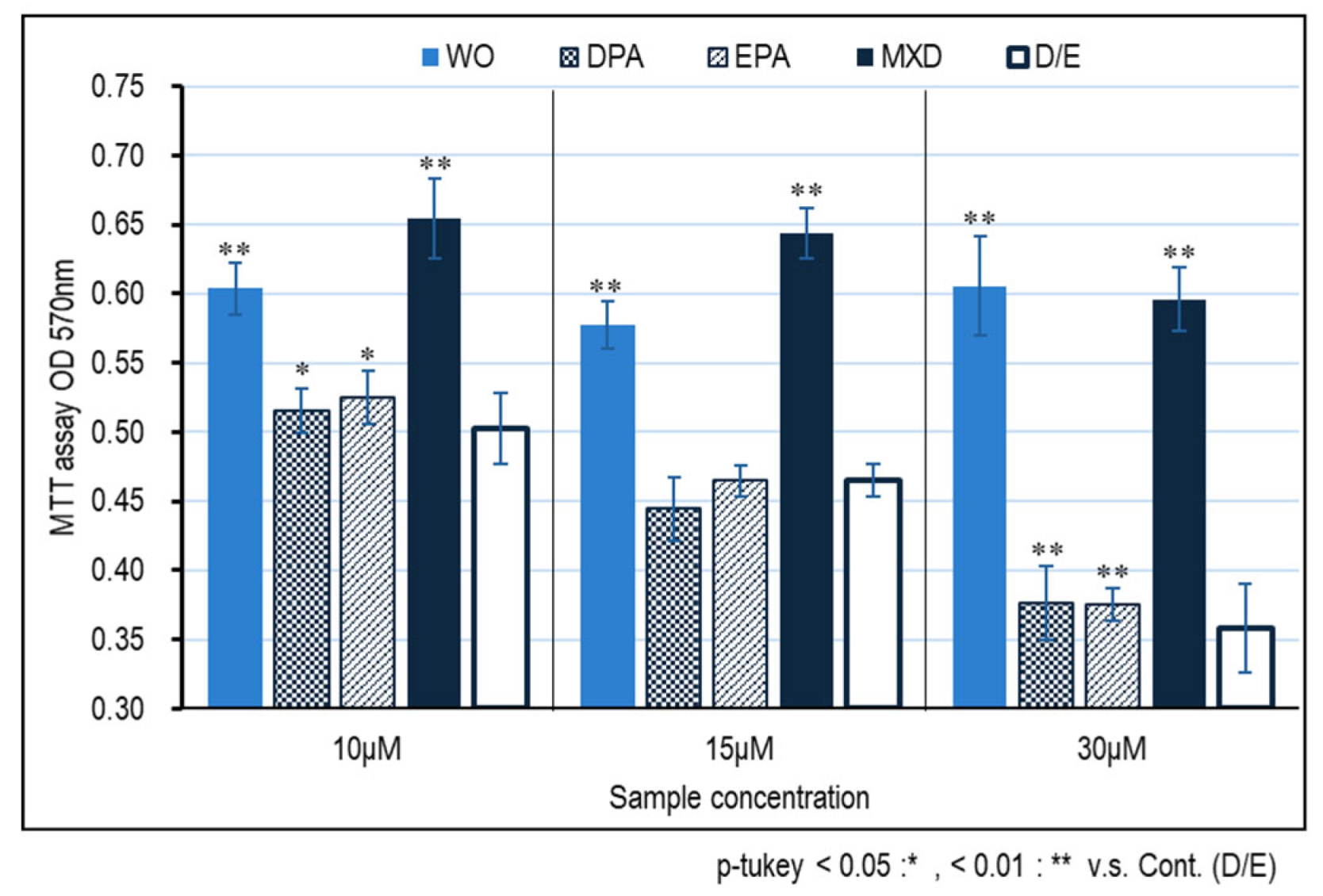
The results of cell proliferation effects using immortalized human papilla cell HFDPC by MTT assay are shown in
Figure 1. Significant cell proliferative effects of the WO and minoxidil were observed at all concentrations of 10, 15, and 30 μM compared to the control (D/E); at concentrations of 10 and 15 μM, the proliferative effect of minoxidil was higher than that of WO, but at 30 μM, whale oil was as high as that of minoxidil. The proliferative effect of WO on immortalized human papilla cells was less effective than that of WO with DPA and EPA alone, suggesting that components other than DPA and EPA were involved in the proliferative effect of WO on immortalized human papilla cells.
3.2. Hair Growth Marker Gene Expression Results
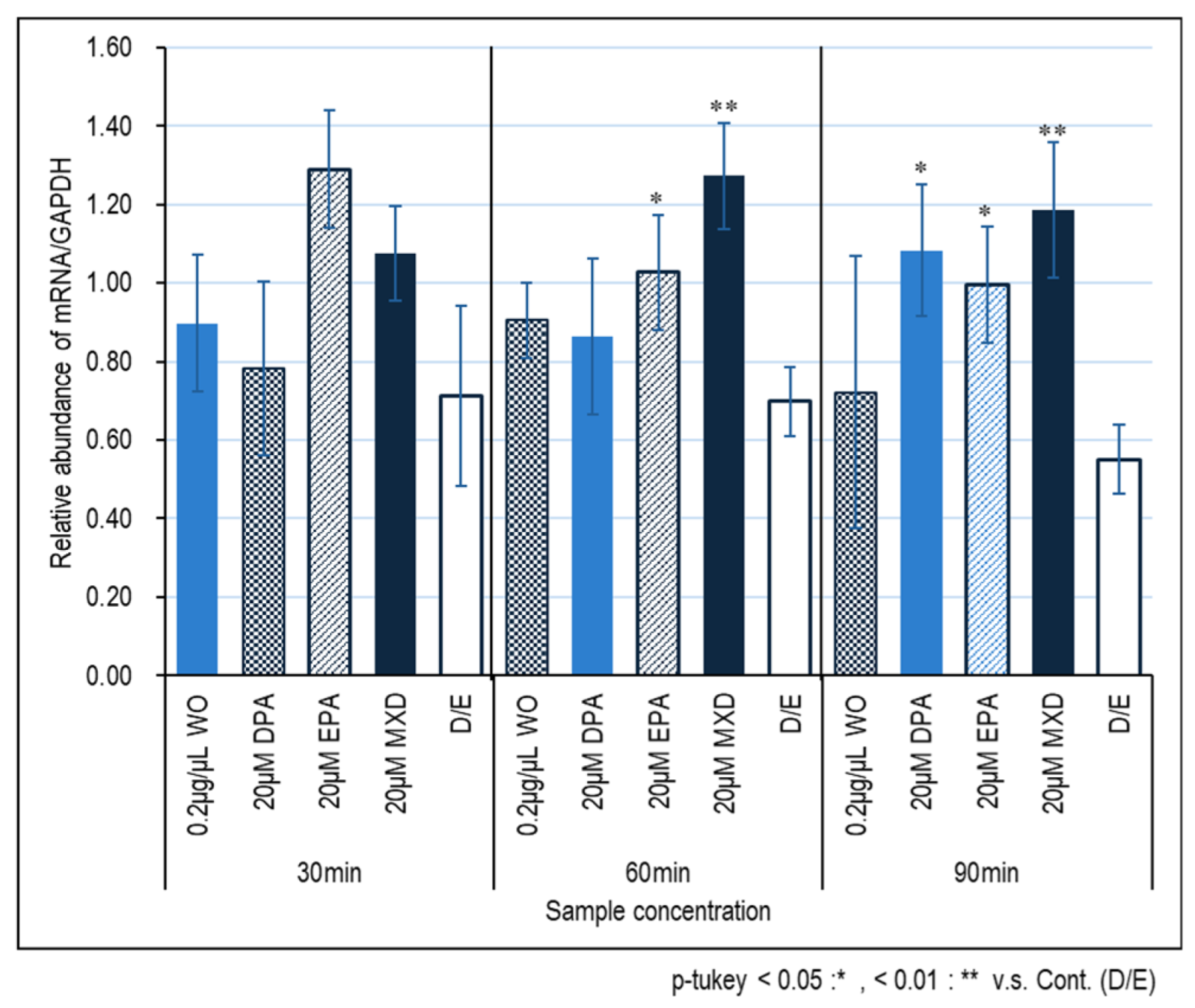
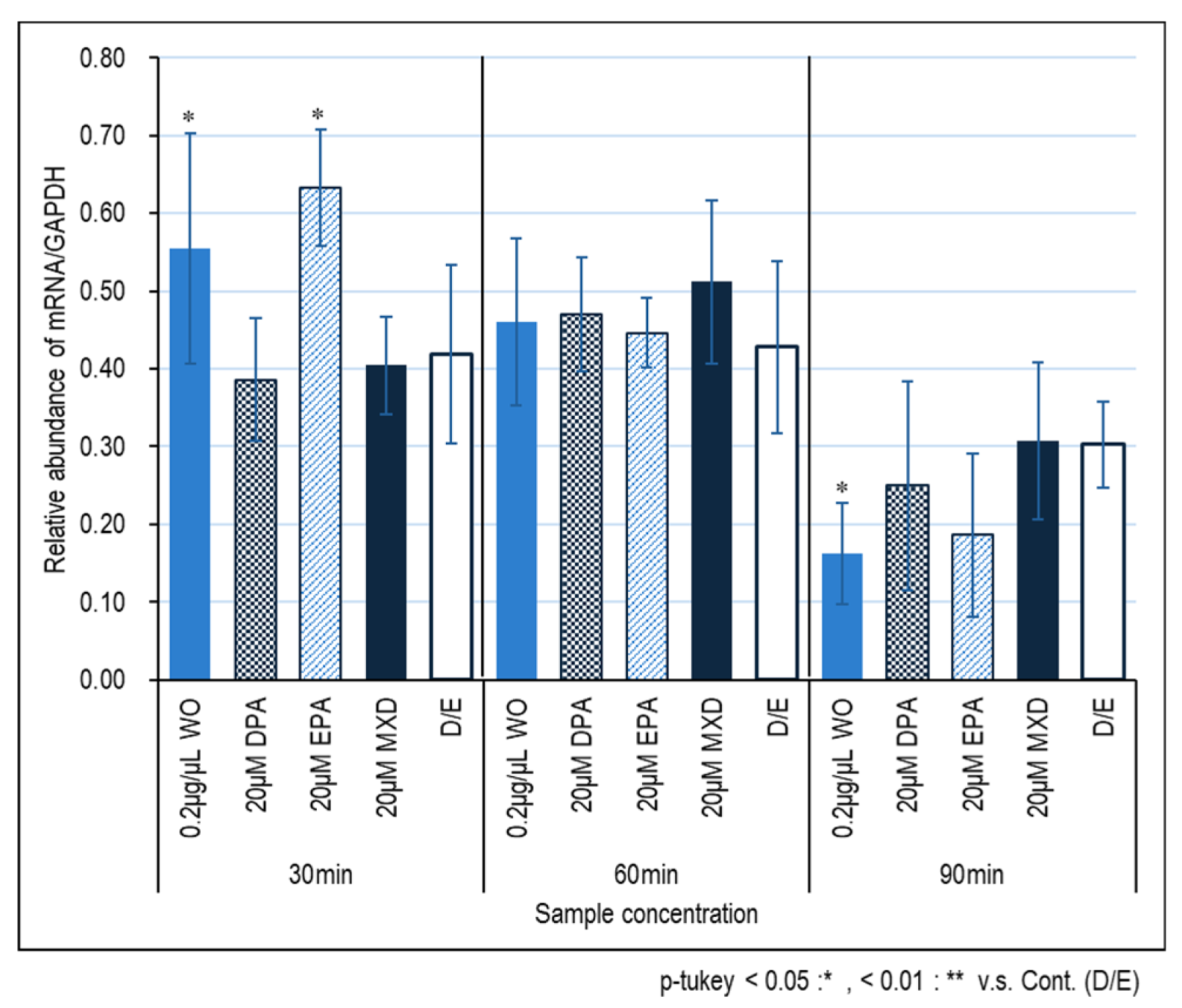
Figure 2 and
Figure 3 show the expression results of the hair growth marker genes, FGF7 and VEGF, in immortalized papilla cells induced by the WO. No significant effect of the WO on FGF7 expression was observed, but significant effects of DPA and DPA treated for 60 and 90 min and 90 min, respectively, were observed (Fig. 2). On the other hand, no significant effect of minoxidil on VEGF was observed, whereas significant effect of minoxidil on VEGF expression was observed in WO and EPA treated for 30 min.
3.3. Hair Growth Promoting Action
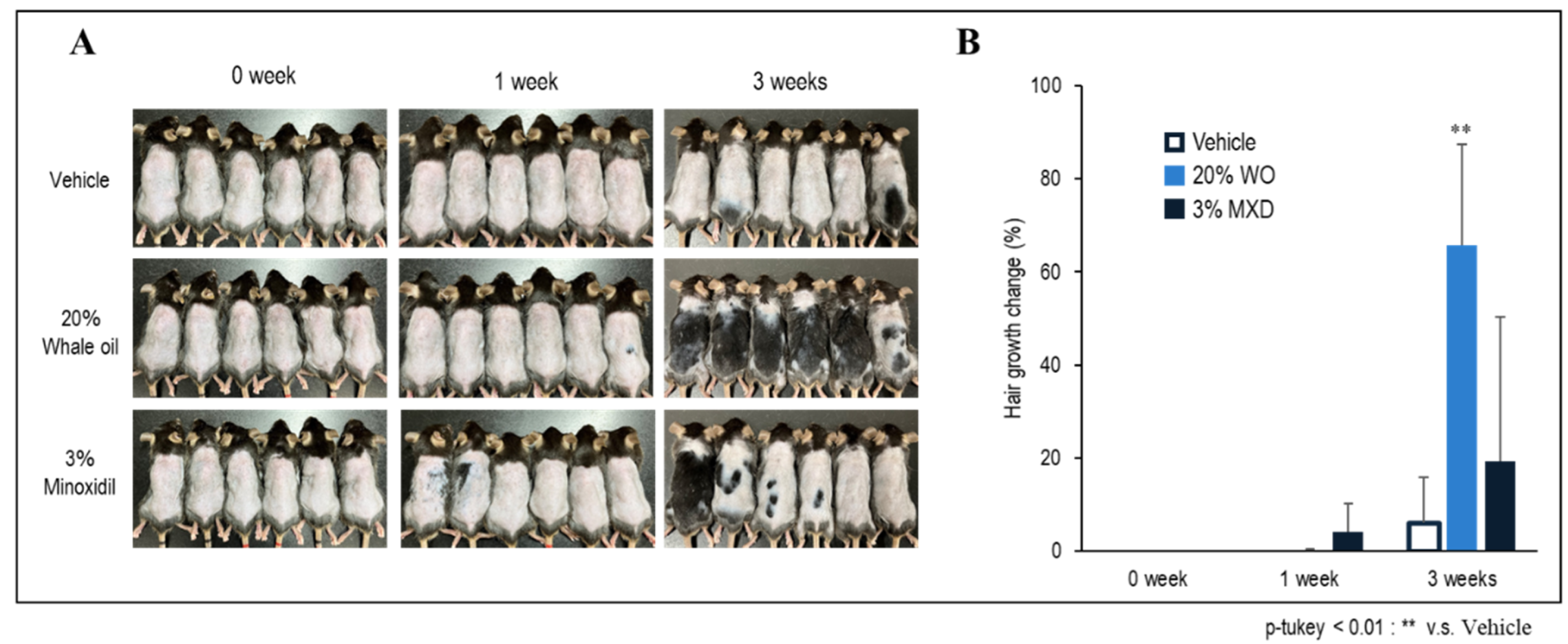
Figure 4-A and
Figure 4-B show the progress of hair growth in the back of mice at 0, 1, and 3 weeks after the application of the conditioned samples. 1 week after the application of minoxidil, the hair growth rate was high, but at 3 weeks, the WO showed a significantly higher hair growth promotion effect. While there were individual differences in minoxidil, there were almost no individual differences in WO, and a high hair-growth effect was confirmed in all individuals (
Figure 4-A, 3weeks).
3.4. Optical Microscopic Observation with H&E Staining
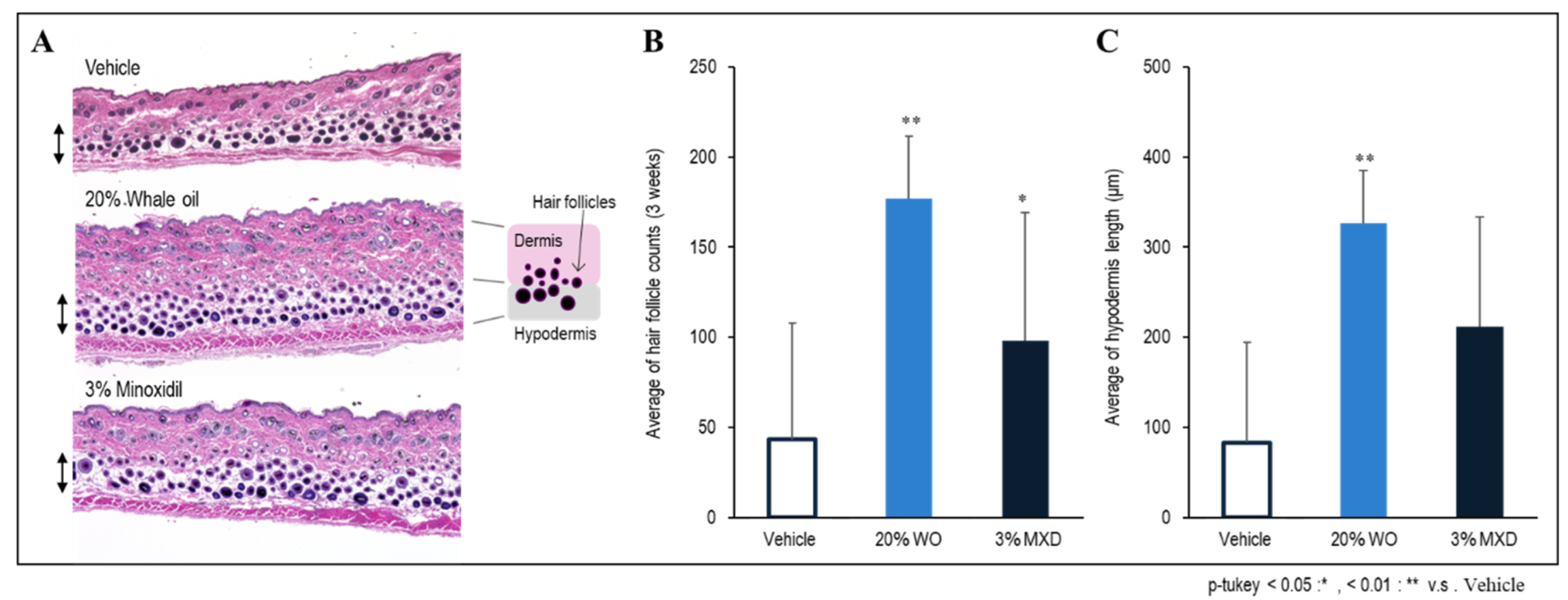
Figure 5 shows the results of measuring the total number of hair follicles in the skin tissue of the entire back layer of mice three weeks after application of the sample. As shown in
Figure 5-B, the number of hair follicles was significantly increased in both WO and minoxidil, but the number of hair follicles was particularly high in WO. In addition, the skin micrographs showed that the hair follicles were densely packed (
Figure 5-A). The thickness of the subcutaneous tissue was also measured, and a significant increase in thickness was observed in whale oil (
Figure 5-B). The thickness of subcutaneous tissue is related to: (1) protection against environmental factors and external stress, and its involvement in moisturizing function to prevent moisture loss; (2) protection of hair follicles by strengthening the formation of a layer of adipose tissue under the dermis and hair follicles, including heat insulation; and (3) indirect positive effects on hair growth by promoting the supply of necessary nutrients. (2) Thus, it is believed that an increase in the thickness of subcutaneous tissue creates an environment conducive to healthy hair growth and further improves hair growth [
14].
3.5. Transcriptomic Analysis Results
The cDNA microarray data have been submitted to NCBI's Gene Expression Omnibus under the GEO series accession number GSE263884 (
https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE263884; accessed 2024.04.13).
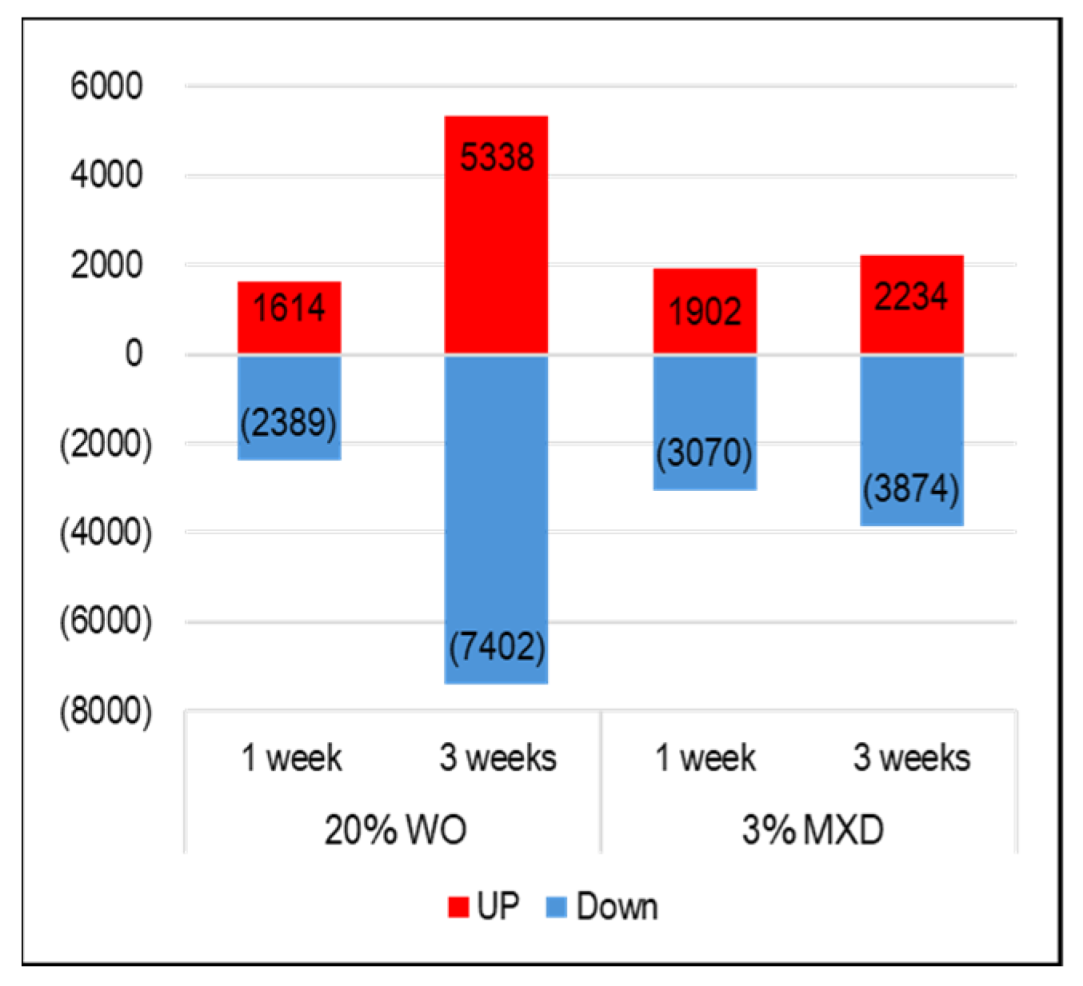
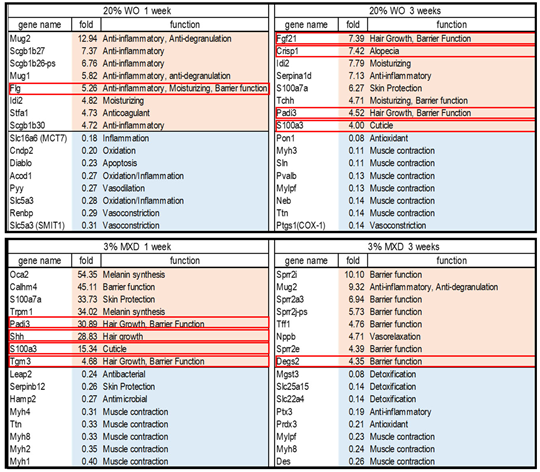
Figure 6 shows the results of the investigation of the expression variation gene data obtained from the DNA microarray analysis of mouse skin. In WO, the number of genes whose expression increased (UP: red) was 1614 and decreased (Down: blue) was 2389 in the first week, and in the third week, the number of genes whose expression increased was 5338 and decreased was 7402. Similarly, at week 1 minoxidil there was an increase of 1902 gene transcripts and a decrease of 3070 gene transcripts, and at week 3 there was an increase of 2234 gene transcripts and a decrease of 3874 transcripts. Investigating the top 30 genes among the list of genes with variable expression identified several factors associated with hair growth. The identified genes and their brief functions are listed in
Table 2. Genes circled in red are associated with hair growth and are described below.
3.5.1. Whale Oil (WO) 1 Week
Flg (Filaggrin): A decrease of filaggrin causes skin barrier function deterioration. Many patients with atopic dermatitis have been found to have genetic abnormalities of filaggrin, and filaggrin is attracting attention as a key substance in the treatment of atopic dermatitis. Filaggrin gene mutations also cause a decrease in natural moisturizing factors in the stratum corneum, leading to cutaneous xerosis and epithelial barrier abnormalities [
15]. Filaggrin normalizes skin pH and is involved in improving barrier function [
16].
3.5.2. Whale Oil (WO) 3 Weeks
Fgf21 (fibroblast growth factor 21): It is involved in the regulation of glucose metabolism and promotes blood glucose uptake by adipocytes Fgf21 (-/-) mice have been shown to have lower body weight, slower hair re-growth, less hair volume, and smaller hair follicle diameter compared to wild-type mice Fgf21 has been shown to affect hair follicle development and growth cycle in mice, suggesting that this may be related to the Pi3k/Akt and Mapk/Erk signaling pathways [
17].
Crisp1 (cysteine-rich secretory protein 1): The mouse strain C3H/HeJ, which is prone to alopecia areata, is deficient in Crisp1 protein in the hair shaft. Although clinically C3H/HeJ mice have normal hair, abnormal Crisp1 expression may predispose them to alopecia areata and affect the severity of the disease [
18].
Padi3 (peptidylarginine deiminase type III): Padi3 is essential for hair follicle formation, and mutations in the Padi3 gene are the cause of a rare hair disorder called uncombed hair syndrome, which is associated with centrifugal scarring alopecia of the scalp, a more frequent condition affecting mainly African women [
19].
S100a3 (S100 calcium-binding protein A3): S100a3 is associated with cuticle maturation and is thought to contribute to its enhancement [
20]. Furthermore, blockade of S100a3 activity inhibits hair growth in mice, suggesting that S100A3 can be used as a target for hair loss treatment [
21].
3.5.3. Minoxidil 1 Week
Shh (Sonic hedgehog): Shh is important for hair development and the hair cycle. In growing skin, Shh is expressed at high levels in the follicular matrix and functions as a mitogen to promote regeneration during the growth phase; Shh signaling is also essential for proper regulation of hair follicle morphogenesis [
22].
Tgm3 (transglutaminase 3): Tgm3 is involved in various processes related to hair follicle morphogenesis, promoting hair formation and strengthening and also supporting hair shaft growth Many Tgm3 -/- mice exhibit hair abnormalities and have been found to have distorted cuticles. In addition, Tgm3 contributes to the maintenance of skin barrier integrity [
23].
3.5.4. Minoxidil 3 Weeks
Degs2 (sphingolipid delta (4)-desaturase 2): Degs2 is a ceramide synthase, and reduced ceramide levels and altered ceramide composition are associated with atopic dermatitis and psoriasis. It has been suggested to be important for skin barrier function [
24].
3.6. Biological Functional Enrichment Analysis
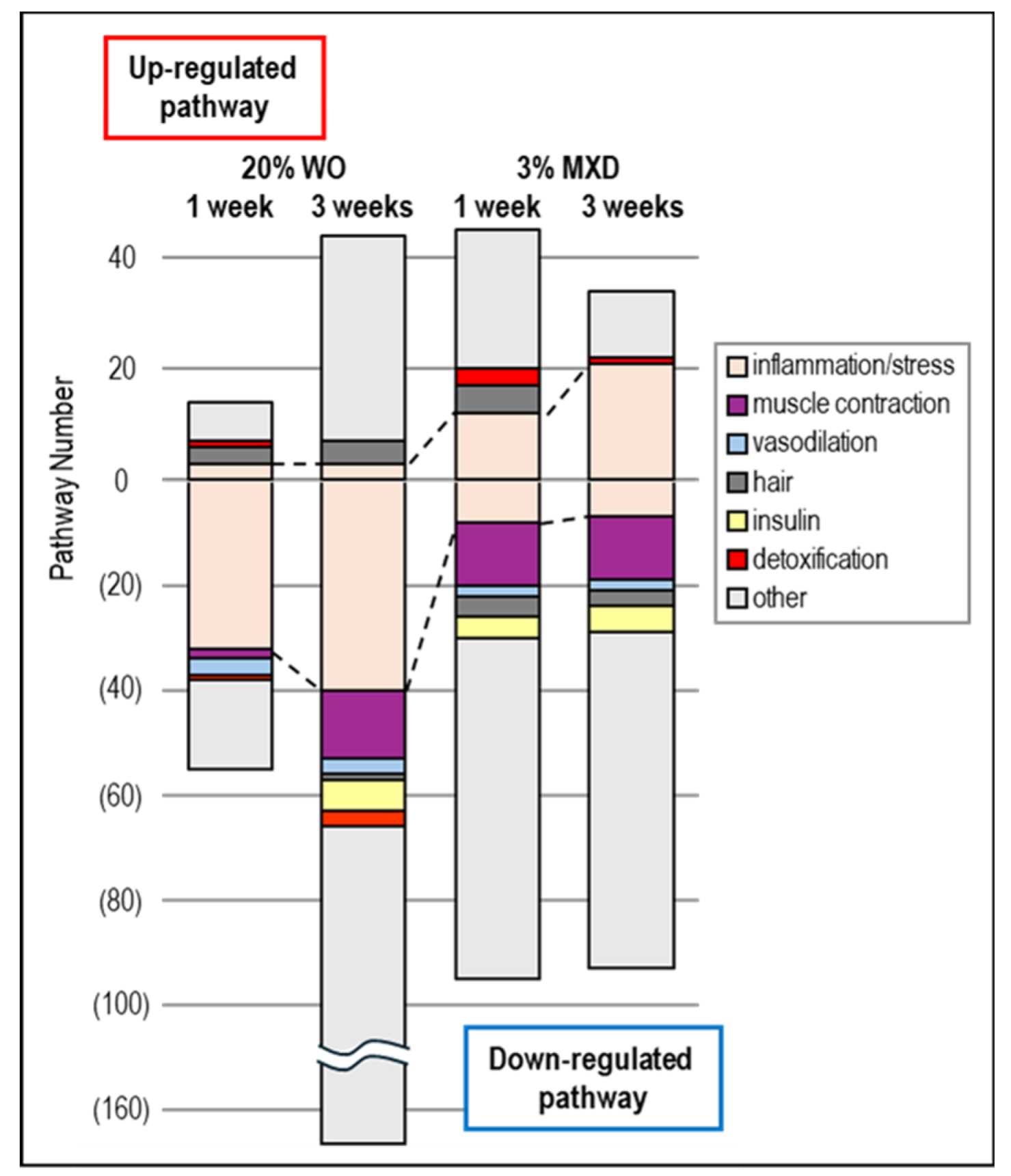
To ascertain what biological function pathways were associated with the variable expression gene clusters obtained by DNA microarray analysis of WO treatment, we performed an enrichment analysis using the DAVID tool. Each pathway in the resulting KEGG pathway was statistically significant (p-value ≤ 0.05), including detoxification (mmu00480:Glutathione metabolism, mmu00983:Drug metabolism - other enzymes), insulin (mmu04911:Insulin secretion, mmu04910:Insulin signaling pathway, etc.), hair (mmu04916:Melanogenesism, mmu04310:Wnt signaling pathway [
25], etc.), inflammation and stress response (mmu04060:Cytokine-cytokine receptor interaction, mmu04062:Chemokine signaling pathway, etc.), muscle contraction (mmu04270:Vascular smooth muscle contraction, mmu04260:Cardiac muscle contraction, mmu04924:Renin secretion, etc.), vasodilation (mmu04022:cGMP-PKG signaling pathway, mmu04926:Relaxin signaling pathway, etc.) were categorized and shown graphically in
Figure 7.
As shown in
Figure 7, vasodilation and muscle contraction pathways were common in Down category, but the percentage of muscle contraction pathway was particularly high in Down category for both WO and minoxidil. This was thought to be most likely due to the promotion of vasodilation by decreasing vasoconstriction-related gene expression. These results suggest that minoxidil has a vasodilating effect on blood flow by dilating blood vessels, and WO may have a similar vasodilating effect. In addition, inflammation-related pathways were observed in both the UP and Down categories, but minoxidil was found to have a higher proportion of inflammation pathways in the UP category. Further confirmation of this inflammatory pathway revealed that the mmu04657:IL-17 signaling pathway and mmu04668:TNF signaling pathway, which are known to play a central role as regulators of inflammatory responses, were upregulated in minoxidil. signaling pathway, which are known to be up-regulated by minoxidil, whereas they were down-regulated by WO. Furthermore, the apoptosis and epherocytosis pathways were also classified as up-regulated in minoxidil and down-regulated in WO. The results are summarized in
Figure 8. More interestingly, insulin-related pathways were found to be predominantly Down in all of WO and minoxidil (
Figure 7).
4. Discussion
Many factors related to hair growth were detected by the DNA microarray analysis. As shown in
Table 2, many of the up-regulated genes were found to be related to anti-inflammation, hair growth, and skin barrier in WO, and to hair growth, skin barrier, and melanin synthesis in minoxidil. In addition, many of the down-regulated genes were found to be related to "vasoconstriction" and "muscle contraction". Furthermore, KEGG pathway analysis (Fig. 7) confirmed that pathways classified as muscle contraction-related were down-regulated. This suggests that WO and minoxidil may cause muscle relaxation and vasodilation as a common function. Minoxidil was developed by an American pharmaceutical company in the 1960s as an antihypertensive for hypertension due to its vasodilating effect. It is believed to have a hair-growth effect from the improvement of blood flow due to this vasodilatation [26, 27] The present results suggest that WO has the same vasodilator effect as minoxidil.
Furthermore, as to the point that the insulin-related pathway was Down for both WO and minoxidil, diazoxide, the only drug approved by the U.S. Food and Drug Administration for the treatment of hyperinsulinemic hypoglycemia, has been shown to increase blood glucose by inhibiting insulin secretion through opening of ATP-sensitive potassium (KATP) channels. Hypertrichosis has been reported as a side effect [28, 29], and indeed diazoxide is also known to stimulate hair growth [
30]. Like diazoxide, minoxidil is a potent KATP channel opener, and as a result, decreased expression of many genes classified in the insulin secretion-related pathway were detected. Whale oil also showed a large decrease in the expression of genes classified in the insulin secretion-related pathway, suggesting that WO may also have a KATP channel-opening effect.
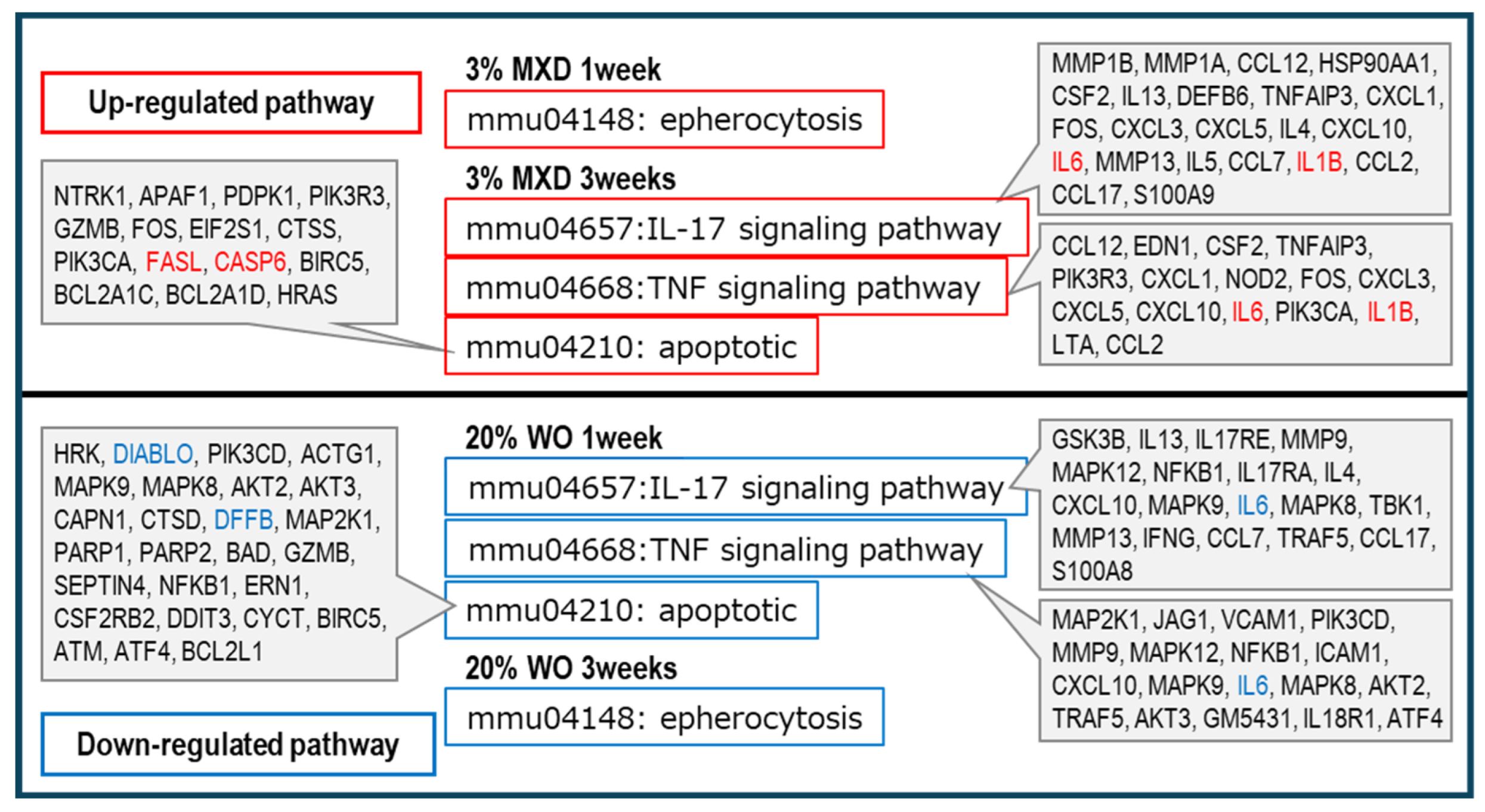
IL17 and TNF signals are known to play a central role as regulators of inflammatory responses. As shown in
Figure 8, IL17 and TNF signals, which promote inflammation, were increased by minoxidil and decreased by WO. IL-1B, which was included in the IL17 and TNF signals in minoxidil, is a potent proinflammatory cytokine that is rarely detected in normal tissues and is produced and secreted by inflammation-inducing stimuli [
31].
Furthermore, for mmu04210: apoptotic and mmu04148: epherocytosis, minoxidil was classified as an ‘Increase’, and increased expression of FASL and CASP6, which are involved in activating apoptosis, was confirmed. On the other hand, WO was classified as a ‘Decrease’, and decreased expression of DIABL and DFFB, which activates apoptosis, was observed (
Figure 8).
Apoptosis is promoted by intracellular stresses such as oxidative stress, and furthermore, epherocytosis has an inflammation-reducing effect that processes dead cells before toxic reactive oxygen species, proteases and caspases are released by a mechanism in which dead cells are removed by phagocytes after apoptosis [
32]. It has also been suggested that reducing apoptosis promotes hair growth and minimizes hair loss [
33]. Therefore, considering these results, it is possible that the WO may promote hair growth more than minoxidil by inhibiting or reducing apoptosis.
The side effects of minoxidil include dermatitis such as eczema, hives, and itching, and the probability of minoxidil-induced side effects is estimated to be about 8%. Propylene glycol contained in the solvent used to penetrate minoxidil may be considered as a cause of the high incidence of dermatitis, but propylene glycol itself is not the cause because the solvent used for whale oil in this experiment also contained propylene glycol. Therefore, the results of this current research suggest that although dermatitis symptoms were not observed, inflammation and oxidative stress may have increased with minoxidil compared to WO at the transcript level. These results suggest that WO, like minoxidil, is a vasodilator and may promote hair growth by reducing inflammation, oxidative stress, and apoptosis.
Author Contributions
Conceptualization, formal analysis, methodology, software, writing—original draft preparation, writing—review and editing, J.S., F.T., R.R., S.S.; validation, resources, F.T., S.H., S.S.; investigation, J.S, A.K., A.K., M.Y.; data curation, visualization, J.S., A.K., M.Y.; supervision, F.T., S.S.; project administration F.T., S.S. All authors have read and agreed to the submitted manuscript.
Funding
This work was supported in part by Kyodo Senpaku Inc. Ltd. (Japan).
Institutional Review Board Statement
This study was approved by the Animal Care Committee of Hoshi University School of Pharmacy and Pharmaceutical Sciences.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available in the article and submitted databases. The raw data are available upon a reasonable request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
Sample Availability
Samples of the compounds are not available from the authors.
References
- Meisheri, K.D.; Cipkus, L.A.; Taylor, C.J. Mechanism of action of minoxidil sulfate-induced vasodilation: a role for increased K+ permeability. J. Pharmacol. Exp. Ther. 1988, 245, 751–760.
- Buhl, A.E.; Waldon, D.J.; Conrad, S.J.; Mulholland, M.J.; Shull, K.L.; Kubicek, M.F., Johnson, G.A., Brunden, M.N., Stefanski, K.J., Stehle, R.G., et al. Potassium channel conductance: a mechanism affecting hair growth both in vitro and in vivo. J. Invest. Dermatol. 1992, 98, 315–319. [CrossRef]
- Lachgar, S.; Charveron, M.; Gall, Y.; Bonafe, J.L. Minoxidil upregulates the expression of vascular endothelial growth factor in human hair dermal papilla cells. Br. J. Dermatol. 1988, 138, 407–411. [CrossRef]
- Messenger, A.G.; Rundegren, J. Minoxidil: mechanisms of action on hair growth. Br. J. Dermatol. 2004, 150, 186–194.
- Mysore, V.; Shashikumar, B.M. Guidelines on the use of finasteride in androgenetic alopecia. Indian J. Dermatol. Venereol. Leprol. 2016, 82, 128–134. [CrossRef]
- Melcangi, R.C.; Caruso, D.; Abbiati, F.; Giatti, S.; Calabrese, D.; Piazza, F.; Cavaletti, G. Neuroactive steroid levels are modified in cerebrospinal fluid and plasma of post-finasteride patients showing persistent sexual side effects and anxious/depressive symptomatology. J. Sex. Med. 2013, 10, 2598–2603. [CrossRef]
- Kakali, D.; Anu, T.S.; Ashok, M.; Beena, B.; Ramesh, B.; Anand, C.B. Eclipta alba extract with potential for hair growth promoting activity. J. Ethnopharmacol. 2009, 124, 450–456.
- Madden, J.; Brunner, A.; Dastur, N.D.; Tan, R.M.; Nash, G.B.; Rainger, G.E.; Shearman, C.P.; Caldr, P.C.; Grimble, R.F. Fish oil induced increase in walking distance, but not ankle brachial pressure index, in peripheral arterial disease is dependent on both body mass index and inflammatory genotype. Prostaglandins Leukot. Essent. Fatty Acids. 2007, 76(6), 331–340. [CrossRef]
- Holy, E.W.; Forestier, M.; Richter, E.K.; Akhmedov, A.; Leiber, F.; Camici, G.G.; Mocharla, P.; Luscher, T.F.; Beer, J.H.; Tanner, F.C. Dietary alpha-linolenic acid inhibits arterial thrombus formation, tissue factor expression, and platelet activation. Arterioscler. Thromb. Vasc. Biol. 2011, 31(8), 1772–1780.
- Drake, L.; Reyes-Hadsall, S.; Martinez, J.; Heinrich, C.; Huang, K.; Mostaghimi, A. Evaluation of the Safety and Effectiveness of Nutritional Supplements for Treating Hair Loss: A Systematic Review. JAMA Dermatol. 2023, 159(1), 79-86.
- Nichols, A.J.; Hughes, O.B.; Canazza, A.; Zaiac, M.N. An Open-Label Evaluator Blinded Study of the Efficacy and Safety of a New Nutritional Supplement in Androgenetic Alopecia: A Pilot Study. J. Clin. Aesthet. Dermatol. 2017, 10(2), 52-56.
- Walquist, M.J.; Stormo, S.K.; Østerud, B.; Elvevoll, E.O.; Eilertsen, K.E. Cold-pressed minke whale oil reduces circulating LDL/VLDL-cholesterol, lipid oxidation and atherogenesis in apolipoprotein E-deficient mice fed a Western-type diet for 13 weeks. Nutr. Metab. (Lond). 2018, 15, 35. doi: 10.1186/s12986-018-0269-8. [CrossRef]
- Walquist, M.J.; Stormo, S.K.; Jensen, I.J.; Østerud, B.; Eilertsen, K.E. Antioxidant and Anti-Inflammatory Activities in Extracts from Minke Whale (Balaenoptera acutorostrata) Blubber. Mediators Inflamm. 2017, 2017, 3835851. doi: 10.1155/2017/3835851. [CrossRef]
- Pérez-Mora, S.; Ocampo-López, J.; Gómez-García, M.D.C..; Pérez-Ishiwara, D.G. BFNB Enhances Hair Growth in C57BL/6 Mice through the Induction of EGF and FGF7 Factors and the PI3K-AKT-β-Catenin Pathway. Int. J. Mol. Sci. 2023, 24(15), 12110. [CrossRef]
- Kezic, S.; O'Regan, G.M.; Yau, N.; Sandilands, A.; Chen, H.; Campbell, L.E.; Kroboth, K.; Watson, R.; Rowland, M.; McLean, W.H.; Irvine, A.D. Levels of filaggrin degradation products are influenced by both filaggrin genotype and atopic dermatitis severity. Allergy 2011, 66, 934–940. [CrossRef]
- Choi, E.H.; Kang, H. Importance of Stratum Corneum Acidification to Restore Skin Barrier Function in Eczematous Diseases. Ann Dermatol. 2024, 36(1), 1-8. doi: 10.5021/ad.23.078. [CrossRef]
- Liu, X.; Zhang, P.; Zhang, X.; Li, X.; Bai, Y.; Ao, Y.; Hexig, B.; Guo, X.; Liu, D. Fgf21 knockout mice generated using CRISPR/Cas9 reveal genetic alterations that may affect hair growth. Gene 2020, 733, 144242. doi: 10.1016/j.gene.2019.144242. [CrossRef]
- Sundberg, J.P.; Awgulewitsch, A.; Pruett, N.D.; Potter, C.S.; Silva, K.A.; Stearns, T.M.; Sundberg, B.A.; Muñoz, M.W.; Cuasnicu, P.S.; King, L.E. Jr.; Rice, R.H. Crisp1 and alopecia areata in C3H/HeJ mice. Exp. Mol. Pathol. 2014, 97(3), 525-528. doi: 10.1016/j.yexmp.2014.10.010. [CrossRef]
- Méchin, M.C.; Simon, M. Deimination in epidermal barrier and hair formation. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2023, 378(1890), 20220245. doi: 10.1098/rstb.2022.0245. [CrossRef]
- Takahashi, T.; Mamada, A.; Kizawa, K.; Suzuki, R. Age-dependent damage of hair cuticle: contribution of S100A3 protein and its citrullination. J. Cosmet. Dermatol. 2016, 15(3), 211-218. doi: 10.1111/jocd.12202. [CrossRef]
- Guan, W.; Deng, Q.; Yu, X.L.; Yuan, Y.S.; Gao, J.; Li, J.J.; Zhou, L.; Xia, P.; Han, G.Y.; Han, W.; Yu, Y. Blockade of S100A3 activity inhibits murine hair growth. Genet. Mol. Res. 2015, 14(4), 13532-13544. doi: 10.4238/2015.
- Rishikaysh, P.; Dev, K.; Diaz, D.; Qureshi, W.M.; Filip, S.; Mokry, J. Signaling involved in hair follicle morphogenesis and development. Int. J. Mol. Sci. 2014, 15(1), 1647-1670. doi: 10.3390/ijms15011647. [CrossRef]
- Chermnykh, E.S.; Alpeeva, E.V.; Vorotelyak, E.A. Transglutaminase 3: The Involvement in Epithelial Differentiation and Cancer. Cells 2020, 9(9), 1996. doi: 10.3390/cells9091996. [CrossRef]
- Ota, A.; Morita, H.; Naganuma, T.; Miyamoto, M.; Jojima, K.; Nojiri, K.; Matsuda, J.; Kihara, A. Bifunctional DEGS2 has higher hydroxylase activity toward substrates with very-long-chain fatty acids in the production of phytosphingosine ceramides. J. Biol. Chem. 2023, 299(4), 104603. doi: 10.1016/j.jbc.2023.104603. [CrossRef]
- Choi, Y.S.; Zhang, Y.; Xu, M.; Yang, Y.; Ito, M.; Peng, T.; Cui, Z.; Nagy, A.; Hadjantonakis, A.K.; Lang, R.A.; et al. Distinct functions for Wnt/beta-catenin in hair follicle stem cell proliferation and survival and interfollicular epidermal homeostasis. Cell Stem Cell. 2013, 13, 720–733. doi: 10.1016/j.stem.2013.10.003. [CrossRef]
- Suchonwanit, P.; Thammarucha, S.; Leerunyakul, K. Minoxidil and its use in hair disorders: a review. Drug Des. Devel. Ther. 2019, 13, 2777-2786. doi: 10.2147/DDDT.S214907.
- Shorter, K.; Farjo, N.P.; Picksley, S.M.; Randall, V.A. Human hair follicles contain two forms of ATP-sensitive potassium channels, only one of which is sensitive to minoxidil. Faseb J. 2008, 22(6), 1725–1736. doi: 10.1096/fj.07-099424. [CrossRef]
- Patel, P.; Nessel, T.A.; Kumar, D.D. Minoxidil. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing, 2024.
- Boskabadi, S.J.; Ramezaninejad, S.; Sohrab, M.; Farhadi, R. (2023). Diazoxide-Induced Hypertrichosis in a Neonate With Transient Hyperinsulinism. Clin. Med. Insights Case Rep. 2023, 16, 11795476231151330. doi: 10.1177/11795476231151330. [CrossRef]
- Davies, G.C.; Thornton, M.J.; Jenner, T.J.; Chen, Y.J.; Hansen, J.B.; Carr, R.D.; Randall, V.A. Novel and established potassium channel openers stimulate hair growth in vitro: implications for their modes of action in hair follicles. J. Invest. Dermatol. 2005, 124(4), 686-694. doi: 10.1111/j.0022-202X.2005.23643.x. [CrossRef]
- Jiang, H.; Guo, Z.; Zeng, K.; Tang, H.; Tan, H.; Min, R.; Huang, C. IL-1beta knockdown inhibits cigarette smoke extract-induced inflammation and apoptosis in vascular smooth muscle cells. PLoS One 2023, 18(2), e0277719. doi: 10.1371/journal.pone.0277719. [CrossRef]
- Yin, C.; Heit, B. Cellular Responses to the Efferocytosis of Apoptotic Cells. Front Immunol. 2021, 12, 631714. doi:10.3389/fimmu.2021.631714. [CrossRef]
- Wang, W.; Wang, H.; Long, Y.; Li, Z.; Li, J. Controlling Hair Loss by Regulating Apoptosis in Hair Follicles: A Comprehensive Overview. Biomolecules 2023, 14(1), 20. doi: 10.3390/biom14010020. [CrossRef]
Figure 1.
HFDPC proliferation effect of WO by MTT assay. The graph shows the results on the third day after addition of the test sample. Results were presented as mean ± SE ** p < 0.01, * p < 0.05 when compared to the respective D/E values by Student's t-test.
Figure 1.
HFDPC proliferation effect of WO by MTT assay. The graph shows the results on the third day after addition of the test sample. Results were presented as mean ± SE ** p < 0.01, * p < 0.05 when compared to the respective D/E values by Student's t-test.
Figure 2.
FGF7 gene expression in HFDPC by RT-PCR. The graph shows the expression of the FGF7 gene 30, 60, and 90 minutes after addition of the test sample. Results were presented as mean ± SE ** p < 0.01, * p < 0.05 when compared to the respective D/E values by Student's t-test (n =3).
Figure 2.
FGF7 gene expression in HFDPC by RT-PCR. The graph shows the expression of the FGF7 gene 30, 60, and 90 minutes after addition of the test sample. Results were presented as mean ± SE ** p < 0.01, * p < 0.05 when compared to the respective D/E values by Student's t-test (n =3).
Figure 3.
VEGF gene expression in HFDPC by RT-PCR. The graph shows the expression of the VEGF gene 30, 60, and 90 minutes after addition of the test sample. Results were presented as mean ± SE ** p < 0.01, * p < 0.05 when compared to the respective D/E values by Student's t-test (n =3).
Figure 3.
VEGF gene expression in HFDPC by RT-PCR. The graph shows the expression of the VEGF gene 30, 60, and 90 minutes after addition of the test sample. Results were presented as mean ± SE ** p < 0.01, * p < 0.05 when compared to the respective D/E values by Student's t-test (n =3).
Figure 4.
Effect of whale oil (WO) application on hair growth on the back of mice. A: Progressive growth of hairs on the back of mice; B: Percentage of hair growth area on the back of mice.
Figure 4.
Effect of whale oil (WO) application on hair growth on the back of mice. A: Progressive growth of hairs on the back of mice; B: Percentage of hair growth area on the back of mice.
Figure 5.
Experimental measurement of the number of hair follicles and thickness of subcutaneous tissue in the back skin tissue of mice. A: H&E-stained photo of mouse back skin tissue; B: Number of hair follicles; C: Thickness measurement of subcutaneous tissue (Hypodermis).
Figure 5.
Experimental measurement of the number of hair follicles and thickness of subcutaneous tissue in the back skin tissue of mice. A: H&E-stained photo of mouse back skin tissue; B: Number of hair follicles; C: Thickness measurement of subcutaneous tissue (Hypodermis).
Figure 6.
Number of genes whose expression changes were observed due to whale oil (WO) and minoxidil. The graph shows genes whose expression increased in red, and the number of genes whose expression decreased in blue.
Figure 6.
Number of genes whose expression changes were observed due to whale oil (WO) and minoxidil. The graph shows genes whose expression increased in red, and the number of genes whose expression decreased in blue.
Figure 7.
Pathway analysis of genes with altered expression in mouse back skin. Data obtained from KEGG pathway analysis using the DAVID tool were categorized into inflammation, muscle contraction, vasodilation, hair, insulin, detoxification, and others, and the number of pathways were counted and graphed.
Figure 7.
Pathway analysis of genes with altered expression in mouse back skin. Data obtained from KEGG pathway analysis using the DAVID tool were categorized into inflammation, muscle contraction, vasodilation, hair, insulin, detoxification, and others, and the number of pathways were counted and graphed.
Figure 8.
Discussion of inflammation-related and apoptosis pathway analysis results. The pathway terms selected to contrast minoxidil and whale oil in the KEGG pathway analysis classified as inflammation were mmu046657: IL-17 signaling pathway, mmu04668: TNF signaling pathway, and mmu04210 : apoptotic, and mmu04148: epherocytosis.
Figure 8.
Discussion of inflammation-related and apoptosis pathway analysis results. The pathway terms selected to contrast minoxidil and whale oil in the KEGG pathway analysis classified as inflammation were mmu046657: IL-17 signaling pathway, mmu04668: TNF signaling pathway, and mmu04210 : apoptotic, and mmu04148: epherocytosis.
Table 1.
Primer designs used for RT-PCR analysis.
Table 1.
Primer designs used for RT-PCR analysis.
Table 2.
Factors related to hair growth in the top genes of expression variation. (Red letters: increased expression, blue letters: decreased expression).
Table 2.
Factors related to hair growth in the top genes of expression variation. (Red letters: increased expression, blue letters: decreased expression).
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).