1. Introduction
According to the World Health Organisation (WHO) ischemic stroke is the second leading cause (11% among all) of death in the world [
1]. As stated by the Russian registry annual stroke incidence is 3.28 (4.15; 2.74) per 1000 while mortality is 0.96 (1.18; 0.81) per 1000 population. Ischemic stroke is five times more frequent than intracranial bleeding and in 15-20% of cases it is caused by brachiocephalic arteries (BCA) atherosclerosis which sometimes additionally increases the risks due to its asymptomatic [
2,
3,
4]. Ultrasound examination, which is usually administered either by neurologist in case of neurological symptoms or cardiologist to verify arterial hypertension target organs lesions or to rule out the diagnosis of multifocal atherosclerosis when other arteries are damaged (coronary arteries, peripheral arteries of lower limbs), is the most wide-spread diagnostic method of brachiocephalic arteries atherosclerosis. Contrast enhanced computed tomography (CT) of brachiocephalic arteries is less common. Detecting vulnerable plaques as the risk factor of stroke is one of the most sufficient advantages of computed tomography, however, CT is an expensive, time-consuming procedure which also requires high-cost equipment and qualified medical staff. Moreover, during CT a patient undergoes a certain radiation and contrast drugs exposure.
Thus, nowadays there is no safe and effective screening method which would not only help to detect the brachiocephalic arteries atherosclerosis but to identify the groups of patients of high risk who have unstable atherosclerotic lesions.
The aim of this research is to study the features of blood plasma lipidomic profile in unstable brachiocephalic arteries.
2. Materials and Methods
The material was collected on the basis of the Cardiology Clinic of the University Clinical Hospital № 1, I.M. Sechenov First Moscow State Medical University (Sechenov University) from August 2022 to October 2023.
Ninety blood plasma samples were collected. The first group contained data from 52 patients with severe atherosclerotic lesions, the second group – data from 38 patients with no BCA atherosclerosis. Age medians of the 1st and 2nd groups were 68.0 [63.0;72.3] and 49.5 [39.5;58.0] respectively. Men predominated in both groups. Smoking, arterial hypertension, diabetes mellitus, ischemic vascular complications and chronic kidney disease were met in a group of patients with BCA atherosclerosis more frequently (
Table 1).
Sample Description
Fifty eight atherosclerosis plaques and blood plasma samples from patients with sufficient brachiocephalic atherosclerosis and 38 blood plasma samples from patients with no atherosclerosis were included. After histological and morphological examinations the plaques were divided into 2 groups: ‘stable’ and ‘unstable’ which means presenting destabilization features such as hemorrhage, large lipid core, etc.
Blood Samples Preparation for Target Lipidomic Analysis
Blood samples preparation for the lipidomic analysis involves the steps described below. The samples are safely stored at the temperature of -80C before the analysis and later undergo careful defrosting on ice. Two hundred and fifty μl of plasma is collected from each sample and transferred into eppendorfs. Five μl of internal standard is added to that aliquots. Next step is adding 200 μl of methanol and 750 μl of heptane to the samples and then they are placed into the orbital shaker for 10 minutes. As soon as the incubation of the sample ends it is subjected to centrifugation at maximum speed for 10 minutes. Six hundred and fifty μl of supernatant is collected and transferred into eppendorfs. The obtained samples are lyophilized until dry and then reconstructed into 50 μl of B phase. After that the samples undergo the centrifugation again and 40 μl of supernatant is transferred into marked vials with screw caps and submitted for analysis.
Blood samples preparation for metabolites detection was held that way: 100 μl of aliquot of each blood plasma sample (calibrator or quality control sample) is mixed with 50 μl of isotope-labeled internal standards (ISTD) (D7-Arg, 1,55 μm ) and 40 μl of methanol in microtiter plate for protein precipitation. After 10 minutes long incubation microtiter plate is centrifuged for 5 minutes at 13000 xg. Thereafter 40 μl of supernatant is transferred into a falcon and mixed with 40 μl of water, then 1 μl of the obtained solution is entered into the LC/MS/MS system.
Reagent for Target Lipidomic Analysis
The fatty acid methyl ester standards were purchased from Larodan (Sweden) and contain the following saturated fatty acids: С12:0, С14:0, C16:0, C17:0 (IS), C18:0, C20:0, C22:0, C24:0; omega-7 unsaturated fatty acids: C16:1 cis; omega-9 unsaturated fatty acids: C18:1 cis, C20:1 cis, C24:1 cis; trans fatty acid: C16:1 trans, С18:1 trans, С18:2 trans; omega-6 unsaturated fatty acids: С18:2 n-6, С18:3 n-6, С20:2 n-6, С20:3 n-6, С20:4 n-6, С22:4 n-6, C22:5 n-6; omega-3 unsaturated fatty acids: EPA C20:5 n-3, DHA C22:6 n-3.
The solvents used were methanol (Merk, LiChrosolv, hypergrade for LC-MS, USA), acetonitrile, and isopropanol (for HPLC gradient, AppliChem GmbH, Darmstadt, Germany). Deionized water for HPLC was obtained using Merck Milli-Q Advantage A10 apparatus (Merck KGaA, Germany or France).
Methodic HPLC-MS/MS
The procedure for plasma samples preparation and analysis is described in Eroshchenko N. N. et al., 2023 [
5]. In brief, 40 μl of blood plasma was placed in a 2 ml Eppendorf safe-lock tube. Then, 50 μl of an internal standard solution of methyl heptadecanoate (C17:0) with a concentration of 500 μg ml-1 in methanol was added. After that, 910 µl of methanol with BHT 200 μg ml-1 was added, and the solution was mixed on a shaker for 30 minutes at 2000 rpm. The sample was centrifuged for 10 minutes at 19000 g. Then, 500 μl of the supernatant was transferred into a 1.5 ml glass crimp vial and 500 μl of a 15% solution of boron trifluoride (BF3) in methanol was added. The vial was crimp-sealed, mixed, and placed in a thermostat at 100°C for 90 minutes. After incubation, the samples were transferred to a refrigerator at +4°C for 30 minutes. The supernatant was transferred to a 1.5 ml glass vial with a screw cap. Deionized water (500 µl) was added, and the resulting samples were analyzed using the HPLC-MS/MS method.
The LC-20AD Prominence (Shimazdu, Japan) was used for chromatographic separation. Eluent A was prepared by adding 0.1% aqueous formic acid to deionized water and a 5 mM ammonium formate solution. Eluent B was a mixture of acetonitrile and isopropanol (1:1) with the addition of 1% eluent A. Detection was performed on a Sciex 4500 QTRAP mass spectrometer (Sciex, Canada) using electrospray ionization in positive mode. The results of the sample analysis were processed using Analyst 1.6.3 and MultiQuant 3.0 software (Sciex, Canada). Ionization source parameters: Curtain Gas (CUR) – 35; Collision Gas (CAD) - Medium; IonSpray Voltage (IS) - 5500; Temperature (TEM) - 0; Ion Source Gas 1 (GS1) - 50; Ion Source Gas 2 (GS2) - 50.
Data was obtained using multiple reaction monitoring (MRM) in positive mode with [M+NH4]+ ions. List of MRM (Q1/Q3): C12:0 (232.3/215.3); C14:0 (260.3/243.3), C16:0 (288.3/271.3), C18:0 (316.2/299.2), C20:0 (344.3/327.3), C22:0 (372.3/355.3), C24:0 (400.3/383.3), C17:0 IS (302.3/285.3), C16:1 cis (286.3/269.3), C18:1 cis (314.3/297.3), C20:1 cis (342.3/325.3), C24:1 cis (398.3/381.3), C16:1 trans (286.3/269.3), C18:1 trans (314.3/297.3), C18:2 trans (312.3/295.3), C18:2 n-6 (312.3/295.3), C18:3 n-6 (310.3/293.3), C20:2 n-6 (340.3/323.3), C20:3 n-6 (338.3/321.3), C20:4 n-6 (336.3/319.3), C22:4 n-6 (364.3/347.3), C22:5 n-6 (362.3/345.3), EPA C20:5 n-3 (334.3/317.3), DHA C22:6 n-3 (360.3/343.3).
The Protocol of Panoramic Lipid Plasma Analysis
Sample Preparation
Prior to the start of sample preparation, the samples were stored at -80 °C. The samples were thawed on ice. After defrosting, the samples were placed on the vortex for 10 seconds. Aliquots of 100 µl were taken from the samples and transferred to separate eppendorphs. Next, 400 µl of 1-butanol containing an internal standard was added to the samples. The samples were placed on a shaker for 10 minutes at 1200 rpm at room temperature. The samples were then centrifuged at 13,000 rpm for 10 minutes at 5C. After the end of centrifugation, 200 microliters of the organic layer were taken from the samples and transferred for analysis.
Twenty-five percent of the samples studied were prepared in two bio-repeats.
Analysis Conditions
A Sciex 6600QTOF time-of-flight mass spectrometer with a calibrant delivery system (CDS) with an Agilent 1290 Infinity II liquid chromatograph was used for the analysis. Ion source settings: TEM = 300°C; GS1 = 55; 2 = 55; CUR = 30; IS = 5500. Ion detection was carried out in the positive ionization mode of the sample in the TOFMS mode in the range 350-1700 m/z.
Chromatographic separation of the test sample components was carried out in the RPLC chromatography mode using the Waters ACQUITY C8 chromatographic column (2.1x100 mm 1.7um ): phase A (water:acetonitrile (4:6); 10 mM ammonium formate); phase B (acetonitrile:isopropanol (1:9); 10 mM ammonium formate); sample input volume 2 µl. Chromatographic gradient: 0 min 10% B; 0.15 min 10% B; 2 min 30% B; 2.5 min 48% B; 11 min 65% B; 12 min 99% B; 14 min 99% B; 14.1 min 10%B; 16 min 10% B; flow rate was 0.25 ml/min, thermostat temperature was 55°C.
The samples in the analytical series were randomized and examined in two technical repeats per sample.
The initial solution for the quality control samples was obtained by combining aliquots from a common sample pool. Next, the initial solution for the quality control samples was divided into aliquots and frozen. Before starting the analysis, the required number of quality control samples was thawed and analyzed as part of the analytical series; at least 10% of the analytical series accounted for quality control samples.
Processing the Results
To process the results, the SCIEX MasterView software was used; Skyline, MSDIAL. MSDIAL software with a generated lipid library and the MS-DIAL LipidBlast library (ver 68) were used for lipids annotation.
Statistics
Statistical analysis was carried out using the programming language R v4.2 and Python v.3.10 [^R].
Normality (using Chapiro-Wilk test), mean, standard deviation, median, interquartile range, 95% confidence interval, minimum and maximum values are calculated for quantitative indicators. Proportion and absolute number of values were calculated for categorial and qualitative characteristics.
Comparative analysis for normally distributed quantitative characteristics was carried out based on Welch's t-test (2 groups) while for non-normally distributed quantitative characteristic — using the Mann-Whitney U test (2 groups).
Comparative analysis for categorial and qualitative characteristics was carried out using Pearson’s square method; if inapplicable, Fisher’s exact test is used.
Mathematical modeling pipeline is to evaluate lipidome affecting on endpoints such as being either in study or control group and atherosclerotic plaque stability or instability.
3. Results
There is a statistically significant difference in the content target lipidomic profile (
Table 2).
There is a statistically significant difference in the values of the indices SFA (more for the control group), UFA (more for the main one), MUFA (more for the control group), omega-3 index.
Due to the unknown role of each quantitative indicator and all of them together in relation to such endpoints as group membership and stability/instability of atherosclerotic plaque in the main group, cluster analysis was performed only on lipidome data.
The quality of clustering was assessed using ROC analysis, where membership in the main or control group was used as a reference.
We tried different clustering algorithms and assigned BCA, stable/unstable atherosclerotic plaque to groups with/without atherosclerosis.
The best separation into clusters corresponding to the outcome was achieved using the Ward algorithm. The clustering - belonging to a group.
Results of dividing all indicators of the targeted and panoramic lipidome into 2 clusters using the Ward algorithm. Division into clusters correlated well with group membership: AUC 0.82 [0.65;0.94], Sens 0.80 [0.50;1.00], Spec 0.85 [0.75;0.93], NPV 0.50 [0.23;0.75], PPV 0.96 [0.89; 1.00].
The characteristics of both classes according to clinical, anamnestic and others are presented in Supplementary Materials (Table S1)
In one of the clusters, it is, in fact, completely represented by patients without atherosclerosis. Separation is provided by both types of lipidome (targeted and panoramic). We conducted a similar analysis in the group with BCA atherosclerosis and, when divided into 2 clusters, a weak relationship between clustering and plaque stability/instability was noticed. This can be interpreted as the inability to use these signs to characterize the atherosclerotic process as stable or unstable: AUC 0.55 [0.46;0.63], Sens 0.08 [0.0;0.21], Spec 0.82 [0.68;0.94], NPV 0.29 [0.0;0.63], PPV 0.51 [0.36;0.65].
Due to the small number included in the study and the significant number of potential predictors, non-standard approaches were used to select predictors most strongly associated with the outcome (group membership, stability/instability of atherosclerotic plaque). For each LASSO regression model created in the leave-one-out cross validation process, we selected 10 predictors with the highest absolute coefficients. Then we averaged the coefficients for each model and formed a list of predictors with the highest averaged coefficients.
Ten predictors with the highest averaged absolute coefficients: TG_54.7_O_TG_18.3_18.3_18.1_O_M..NH4_7.62, PC_40.8_PC_20.4_20.4_M..H_4.86, TG_42.0_TG_14.0_14.0_14.0_M..Na_10.0, TG_42.1_TG_12.0_12.0_18.1_M..Na_9.39, TG_54.1_TG_18.0_18.0_18.1_OA1_M..NH4_12.81, DG_13.0_M..Na_2.95, TG_48.2_TG_16.0_16.1_16.1_M..Na_10.94, G_48.3_TG_16.1_16.1_16.1_M..Na_10.29, TG_O_55.1_TG_O_19.1_18.0_18.0_M..NH4_12.81, PC_O_36.3_PC_O_18.1_18.2_M..H_5.7. These predictors have the largest absolute coefficients from the regression model. The quality of the resulting model was very high. AUC 0,86 [0,71;097], Sens 0.92 [0,85;0,98], Spec 0,8 [0,5;1], NPV 0,67 [0,36;0,92], PPV 0,96 [0,9;1].
When using target lipidome data, 10 predictors were also identified that had the highest coefficient (C16.0_1_percmol', DHA_C22.6n3_2_percmol, C20.2_n_6_1_percmol, C18.2_trans_1_percmol, UFA_relat, SFA_relat, C12.0_1_percmol, C22.5_n_6_ 1_percmol, C18.1_cis_1_percmol, MUFA_relat) . AUC 0.95 [0.83;1], Sens 1 [1;1], Spec 0.9 [0.67;1], NPV 1 [1;1], PPV 0.98 [0.94;1 ].
Panoramic lipidomic analysis identified the following 10 predictors ('G_52.4_O_TG_18.2_18.2_16.0_O_M..NH4_8.61, LPC_O_18.1_M..H_2.44, PI_34.2_M..NH4_4.55, TG_54.6_TG_16.0_18.2_20 .4_M..NH4_10.85, DG_28.3_OA1_M..Na_0.96, DG_41.3_M..Na_10.88, SM_36.2_O3_M..H_5.93, PI_36.4_M..NH4_4.53, TG_54.7_O_TG_18.3_18. 3_18.1_O_M..NH4_7.62, SM_41.3_O2_M..H_6.06). The quality of the model is also good. AUC 0.98 [0.94;1], Sens 0.96 [0.87;1], Spec 1.0 [1;1], NPV 0.97 [0.89;1], PPV 1 [1 ;1].
For stable/unstable groups we have next ten predictors with the highest averaged absolute coefficients: TG_54.7_O_TG_18.3_18.3_18.1_O_M..NH4_7.62, NAE_22.6_M..H_2.88, PC_O_38.3_PC_O_18.0_20.3_M..H_6.35, C18.2_trans_1_percmol, DG_41.3_M..Na_10.88, LPC_22.5_M..H_1.91, LPC_O_18.1_M..H_2.44, PI_34.2_M..NH4_4.55, LPC_18.3_0.0_M..H_1.51, PC_37.3_M..H_5.63. Resulting modell is high quality: AUC 0.58 [0.44 0.715], sens 0.625 [0.421 0.81 ], spec 0.536 [0.345 0.724], npv 0.625 [0.417 0.812], ppv 0.536 [0.345 0.72 ].
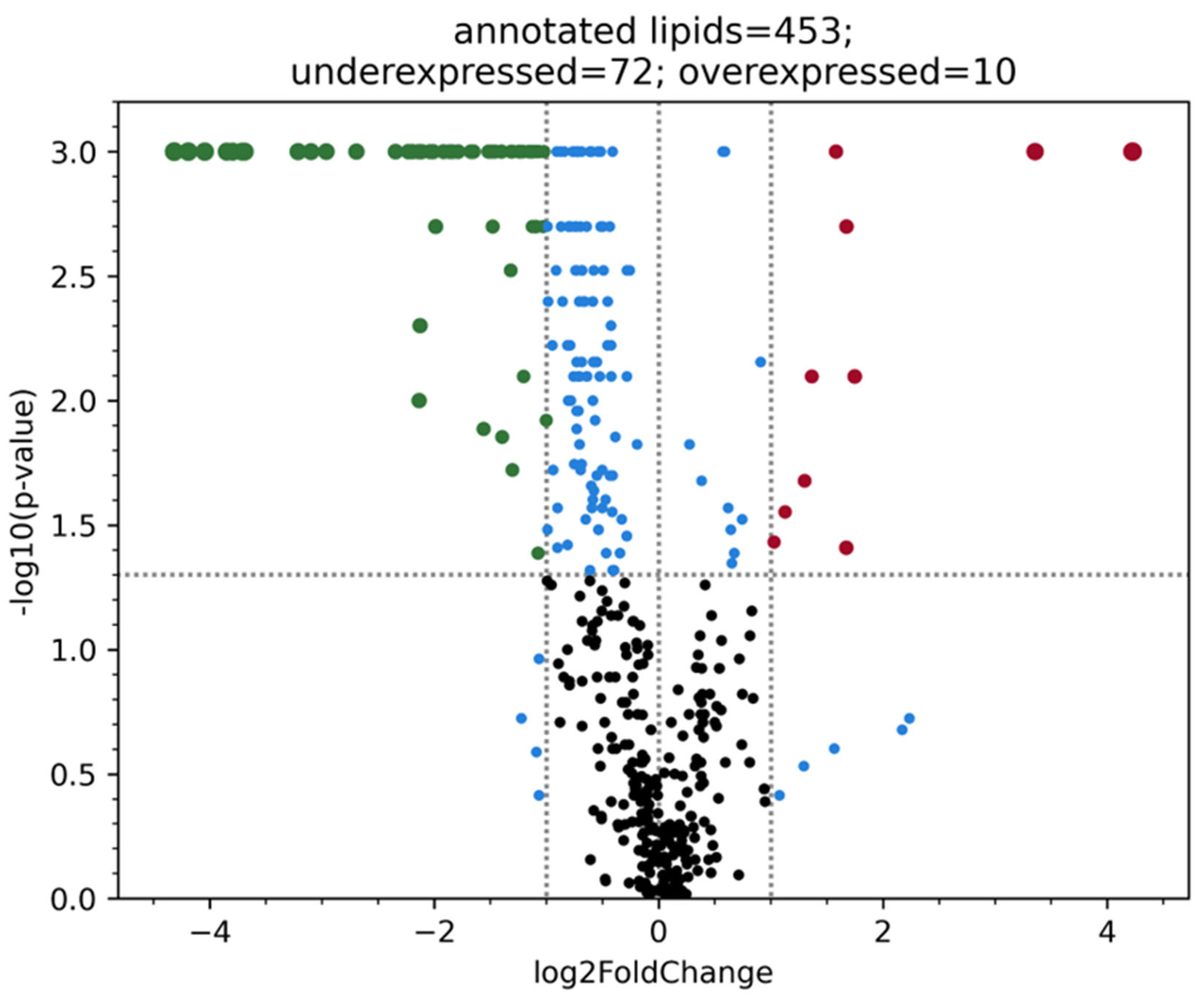
When analyzing the overview lipidome - 423 lipids in patients with or without atherosclerosis BCA (
Figure 1).
The batch effect which is possibly associated with the samples storage duration was noticed during overview lipidome analysis. Thus, ten blood plasma samples with the same time of storage from the second group were included in the analysis. When conducting target lipidome analysis no batch effect was noticed, that is why, all samples were used for the research. Most likely this effect was caused by ceramides, dimetlarginines and other lipids oxidation in course of time, so comparison of samples with different storage duration cannot provide objectivity. It is worth mentioning that fatty acids are stable enough and are not subject to rapid oxidation when stored at -80 degrees.
In groups divided by stability and instability of atherosclerotic plaques, a significant difference was also considered: p-value < 0.05 and abs(fold change) > 2. However, even in this case, we did not observe any significant differences in terms of the established criteria for the blood plasma lipidome between patients with stable and unstable plaques.
4. Discussion
Nowadays lipidome clinical application is limitedly used in cardiological practice because of a proven decrease of adverse outcomes due to the therapy aimed to reduce the levels of atherogenic lipids. Nevertheless, recent decades have shown a remarkably increased interest of the scientific community in studying the lipidome within a wide range of pathologies and for various fluids of the body [
6]. Australian scientists have already identified some changes in the lipidome which help to differentiate Alzheimer’s disease with a high level of accuracy [
7]. Other researches have shown a negative effect of lipid metabolism changes in diabetes mellitus patients on blodstream [
8]. Fifteen lipids associated with high risk of type 2 diabetes mellitus developing in patients with coronary heart disease in 5 years in CARDIOPREV research, moreover, a new classifier showing high efficacy in glucose metabolism disorders prognosis was created based on this information [
9]. Furthermore, 21 lipids associated with diabetes mellitus were also found in research with a 16-year-long observation period made by Miao G. Several scientific works demonstrate lipidomic features specific for chronic kidney disease, elevated risk of atrial fibrillation, systemic lupus erythematosus [
10].
This study is dedicated to studying lipidome characteristics in brachycephalic atherosclerosis including the cases of unstable plaques. There is a great variety of works aimed at studying the characteristics of lipid metabolism in diverse manifestations of atherosclerosis. According to our data, in patients with significant atherosclerosis of the BCA, when comparing groups with stable or unstable atherosclerotic plaque, no significant differences in lipidomic profiling of blood plasma were detected. Most likely, this is due to the presence in these same patients of other atherosclerotic plaques that contribute to the lipidome.
In a study by Sojo, L. et al. the association of subclinical atherosclerosis in patients with type 1 diabetes mellitus with the spectrum of lipids was studied; the most significant positive associations for plaques localized in the brachiocephalic arteries were obtained for sphingomyelin [
14]. Sufficient association between plasma lipids and absence of carotid plaques in patients of Chinese population was found by Liu Y., it was discovered for HDL-C, Non-HDL-C, TC/HDL-C, LDL-C/HDL-C; HDL-C, LDL-C, Non-HDL-C, TC/HDL-C, LDL-C/HDL-C levels correlated with carotid plaques absence [
15].
In a research by You Q. et al. the characteristics of lipidome were studied among people with atherosclerotic lesions of main (20 people) and small (20 people) brachycephalic vessels, in comparison with healthy volunteers (14 people). An increase of the ceramides Cer (d36:3), Cer (d34:2), Cer (d38:6), Cer (d36:4) and Cer (d16:0/18:1) characterized damage to the main vessels; sphingomyelin SM (d34:1) and ceramides Cer (d34:2), Cer (d36:4), Cer (d16:0/18:1); Cer (d38:6), Cer (d36:3) and Cer (d32:0) levels increase is typical for internal vessels lesion; when comparing the two groups presented above, a rise in Cer (d36:4) and SM (d34:1) was detected [
16].
A study be Nieddu G. et al. has shown that lipids belonging to the group of phosphatidylethanolamine (PE), sphingomyelin (SM) and diacylglycerol (DG) can be used to differentiate plaque types: the analysis revealed significant changes for LDL PE (38:6), SM (32:1) and SM (32:2) when comparing vulnerable and stable atherosclerotic lesions [
17].
Slijkhuis N. B. et al. compared blood plasma lipidome and carotid plaques lipidome after endarterectomy. Both lipidomes were rich in ceramides (37% for plasma and 63% for plaques), cholesterolyl oleate and cholesterolyl linoleate were most common and cholesterolyl oleate was mostly present in plaques. Free fatty acids FFA (16:0) and FFA (18:1) were detected with equal frequency in both plasma and plaques, although FFA (16:0) was more common in the latter. FFA (18:1), FFA(18:2), FFA(20:4) and FFA(22:4) were identified in areas of plaques rich in macrophages, which, according to the authors, indirectly indicates their pro-inflammatory role. Phosphatidylcholines were more common in plasma, but two of them, PC(32:0) and PC(34:1), predominated in plaques [
18].
5. Conclusions
The blood lipidome parameters revealed in our study can be used in the detection of atherosclerotic lesions of the brachiocephalic arteries. Triacylglycerides had the highest representation 14:0, 18:0, 18:3, 54:7, 55:1; diacylglycerides 13:0, 41:3, phosphatidylcholine 18:1, 18:2, 36:3, 37.3, 40:8. However, at the moment, there are no indicators of targeted or panoramic plasma lipidome with a sufficient level of evidence that would help characterize the unstable course of atherosclerosis. The resulting models have a good diagnostic ability.
The study of lipidomics makes a great contribution to understanding the development of the disease, expands the possibilities of diagnosis, allows you to use the principles of personalized medicine, but requires further research.
Author Contributions
Conceptualization Anastasiia Lomonosova, Daria Gognieva, Dmitry Shchekochikhin and Philipp Kopylov; methodology Anastasiia Lomonosova, Daria Gognieva, Artemy Silantiev, Magomed Abdullaev, Anna Nartova, Nikolay Eroshchenko, Roman Komarov, Andrey Dzyundzya and Philipp Kopylov; Software Aleksandr Suvorov and Artemy Silantiev; validation Aleksandr Suvorov, Artemy Silantiev, Nikolay Eroshchenko, Roman Komarov, Andrey Dzyundzya, Dmitry Shchekochikhin and Philipp Kopylov; formal analysis Anastasiia Lomonosova, Daria Gognieva, Aleksandr Suvorov, Artemy Silantiev, Alina Abasheva, Yana Vasina , Magomed Abdullaev, Anna Nartova and Nikolay Eroshchenko; study Anastasiia Lomonosova, Daria Gognieva, Magomed Abdullaev, Anna Nartova, Nikolay Eroshchenko, Roman Komarov, Andrey Dzyundzya and Philipp Kopylov; resources Anastasiia Lomonosova, Daria Gognieva, Aleksandr Suvorov, Artemy Silantiev, Magomed Abdullaev, Anna Nartova, Nikolay Eroshchenko, Roman Komarov, Andrey Dzyundzya and Philipp Kopylov; data curation Anastasiia Lomonosova, Daria Gognieva, Artemy Silantiev, Magomed Abdullaev, Anna Nartova, Nikolay Eroshchenko and Philipp Kopylov; preparing a draft Anastasiia Lomonosova, Daria Gognieva, Yana Vasina , Magomed Abdullaev, Anna Nartova, Nikolay Eroshchenko and Andrey Dzyundzya; review and Editing Anastasiia Lomonosova, Daria Gognieva, Aleksandr Suvorov, Artemy Silantiev, Yana Vasina, Nikolay Eroshchenko, Roman Komarov and Philipp Kopylov; visualization Anastasiia Lomonosova, Aleksandr Suvorov, Artemy Silantiev, Alina Abasheva and Yana Vasina; supervision Anastasiia Lomonosova, Daria Gognieva, Aleksandr Suvorov, Artemy Silantiev, Alina Abasheva, Yana Vasina, Magomed Abdullaev, Anna Nartova, Nikolay Eroshchenko, Roman Komarov, Andrey Dzyundzya, Dmitry Shchekochikhin and Philipp Kopylov; project administration Anastasiia Lomonosova, Daria Gognieva and Philipp Kopylov; attracting funding Anastasiia Lomonosova, Daria Gognieva, Dmitry Shchekochikhin and Philipp Kopylov.
Funding
The work of Daria Gognieva, Anna Nartova and Magomed Abdullaev was funded by RSF grant № 23-75-01134 “Identification of new biomarkers of unstable atherosclerotic lesions based on lipidomic and metabolomic analysis of plaques and peripheral blood of patients with brachiocephalic atherosclerosis”.The work of Anastasiia Lomonosova, Artemy Silantiev, Aleksandr Suvorov, Philipp Kopylov was financed by the Ministry of Science and Higher Education of the Russian Federation within the framework of state support for the creation and development of World-Class Research Centers “Digital biodesign and personalized healthcare” № 075-15-2022-304.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by and approved by the Local ethics committee of I.M. Sechenov First Moscow State Medical University (Sechenov University), Protocol Code №16-22, 01.09.2022.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study. Written informed consent has been obtained from the patients to publish this paper.
Data Availability Statement
The data can be provided at the official request of the Principal Investigator, due to the fact that our local ethics committee does not allow them to be provided openly.
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- The top 10 causes of death: https://www.who.int/news-room/fact-sheets/detail/the-top-10-causes-of-death (accessed on 27/03/2024).
- Stakhovskaya LV, Klochikhina OA, Bogatyreva MD, Kovalenko VV. Epidemiology of stroke in the Russian Federation: results of territory's population registry, 2009-2010. S.S. Korsakov Journal of Neurology and Psychiatry. 2013;113(5):4-10. (In Russ.).
- Lemenev V.L., Luk'ianchikov V.A., Beliaev A.A. Cerebrovascular disease and stenotic lesion of the brachiocephalic arteries: epidemiology, clinical manifestations, treatment. Consilium Medicum. 2019; 21 (9): 29–32. [CrossRef]
- Kopylov FIu, Bykova AA, Shchekochikhin DIu, Elmanaa KhE, Dziundzia AN, Vasilevsky YuV, Simakov SS. Asymptomatic atherosclerosis of the brachiocephalic arteries: Current approaches to diagnosis and treatment. Therapeutic Archive, 2017,89(4):95 100. (In Russ.). [CrossRef]
- N.N. Eroshchenko, V.V. Veselov, A.V. Pirogov, E.Y. Danilova, A.N. Kirushin, A.L. Paravyan, G. Cravotto. Development and validation of a HPLC-MS/MS method for the analysis of fatty acids - in the form of FAME ammonium adducts - in human whole blood and erythrocytes to determine omega-3 index. Journal of Chromatography B, 2023, Vol.1227. [CrossRef]
- Agatonovic-Kustrin S, Morton DW, Smirnov V, Petukhov A, Gegechkori V, Kuzina V, Gorpinchenko N, Ramenskaya G. Analytical Strategies in Lipidomics for Discovery of Functional Biomarkers from Human Saliva. Dis Markers, 2019 Dec 4;2019:6741518. [CrossRef]
- Liu, Y., Thalamuthu, A., Mather, K.A. et al. Plasma lipidome is dysregulated in Alzheimer’s disease and is associated with disease risk genes. Transl Psychiatry 2021, 11, 344. [CrossRef]
- Toma, L.; Stancu, C.S.; Sima, A.V. Endothelial Dysfunction in Diabetes Is Aggravated by Glycated Lipoproteins; Novel Molecular Therapies. Biomedicines, 2021, 9, 18. [CrossRef]
- Villasanta-Gonzalez, A., Mora-Ortiz, M., Alcala-Diaz, J.F. et al. Plasma lipidic fingerprint associated with type 2 diabetes in patients with coronary heart disease: CORDIOPREV study. Cardiovasc Diabetol, 2023, 22, 199 . [CrossRef]
- Guanhong Miao, Ying Zhang, Zhiguang Huo, Wenjie Zeng, Jianhui Zhu, Jason G. Umans, Gert Wohlgemuth, Diego Pedrosa, Brian DeFelice, Shelley A. Cole, Amanda M. Fretts, Elisa T. Lee, Barbara V. Howard, Oliver Fiehn, Jinying Zhao; Longitudinal Plasma Lipidome and Risk of Type 2 Diabetes in a Large Sample of American Indians With Normal Fasting Glucose: The Strong Heart Family Study. Diabetes Care 1 December 2021, 44 (12): 2664–2672. [CrossRef]
- Marczak, L.; Idkowiak, J.; Tracz, J.; Stobiecki, M.; Perek, B.; Kostka-Jeziorny, K.; Tykarski, A.; Wanic-Kossowska, M.; Borowski, M.; Osuch, M.; et al. Mass Spectrometry-Based Lipidomics Reveals Differential Changes in the Accumulated Lipid Classes in Chronic Kidney Disease. Metabolites 2021, 11, 275. [CrossRef]
- Toledo, E., Wittenbecher, C., Razquin, C. et al. Plasma lipidome and risk of atrial fibrillation: results from the PREDIMED trial. J Physiol Biochem, 2023, 79, 355–364. [CrossRef]
- Marczak, L.; Idkowiak, J.; Tracz, J.; Stobiecki, M.; Perek, B.; Kostka-Jeziorny, K.; Tykarski, A.; Wanic-Kossowska, M.; Borowski, M.; Osuch, M.; et al. Mass Spectrometry-Based Lipidomics Reveals Differential Changes in the Accumulated Lipid Classes in Chronic Kidney Disease. Metabolites 2021, 11, 275. [CrossRef]
- Sojo, L.; Santos-González, E.; Riera, L.; Aguilera, A.; Barahona, R.; Pellicer, P.; Buxó, M.; Mayneris-Perxachs, J.; Fernandez-Balsells, M.; Fernández-Real, J.-M. Plasma Lipidomics Profiles Highlight the Associations of the Dual Antioxidant/Pro-oxidant Molecules Sphingomyelin and Phosphatidylcholine with Subclinical Atherosclerosis in Patients with Type 1 Diabetes. Antioxidants 2023, 12, 1132. [CrossRef]
- Liu Y, Zhu Y, Jia W, Sun D, Zhao L, Zhang C, Wang C, Chen G, Fu S, Bo Y, Xing Y. Association between lipid profiles and presence of carotid plaque. Sci Rep, 2019 Nov 29;9(1):18011. [CrossRef]
- You Q, Peng Q, Yu Z, Jin H, Zhang J, Sun W, Huang Y. Plasma lipidomic analysis of sphingolipids in patients with large artery atherosclerosis cerebrovascular disease and cerebral small vessel disease. Biosci Rep, 2020 Sep 30;40(9):BSR20201519. [CrossRef]
- Nieddu, G.; Michelucci, E.; Formato, M.; Ciampelli, C.; Obino, G.; Signore, G.; Di Giorgi, N.; Rocchiccioli, S.; Lepedda, A.J. Molecular Characterization of Plasma HDL, LDL, and VLDL Lipids Cargos from Atherosclerotic Patients with Advanced Carotid Lesions: A Preliminary Report. Int. J. Mol. Sci. 2022, 23, 12449. Academic Editor: Gerhard. [CrossRef]
- Slijkhuis N, Towers M, Mirzaian M, Korteland SA, Heijs B, van Gaalen K, Nieuwenhuizen I, Nigg A, van der Heiden K, de Rijke YB, van der Lugt A, Sijbrands EJG, Claude E, van Soest G. Identifying lipid traces of atherogenic mechanisms in human carotid plaque. Atherosclerosis, 2023 Nov;385:117340. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).