Submitted:
17 April 2024
Posted:
18 April 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
Cell Culture
Mice
Transfection of siRNA
LPS-induced acute lung injury (ALI)
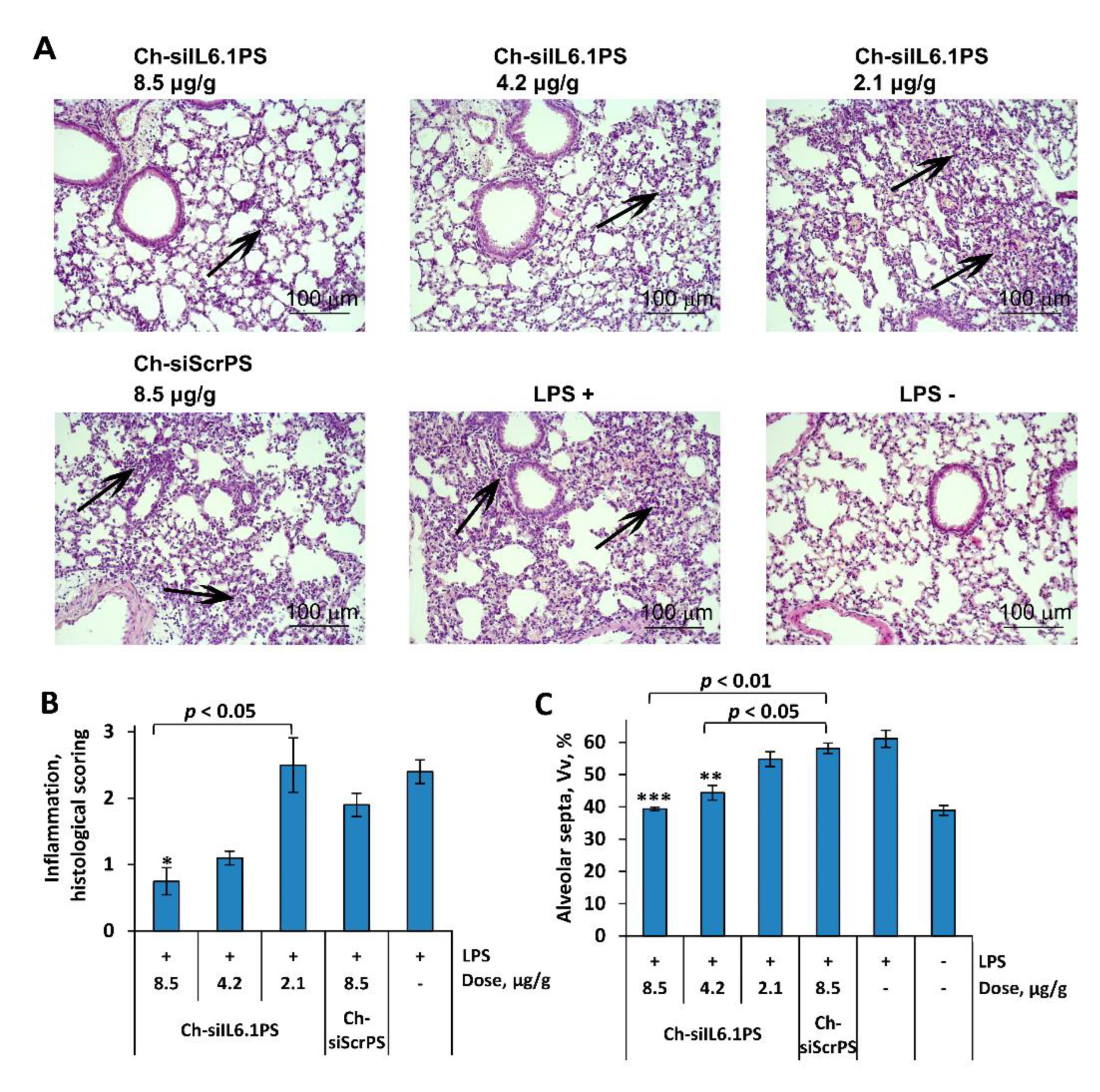
Histology
Statistical analysis
3. Results
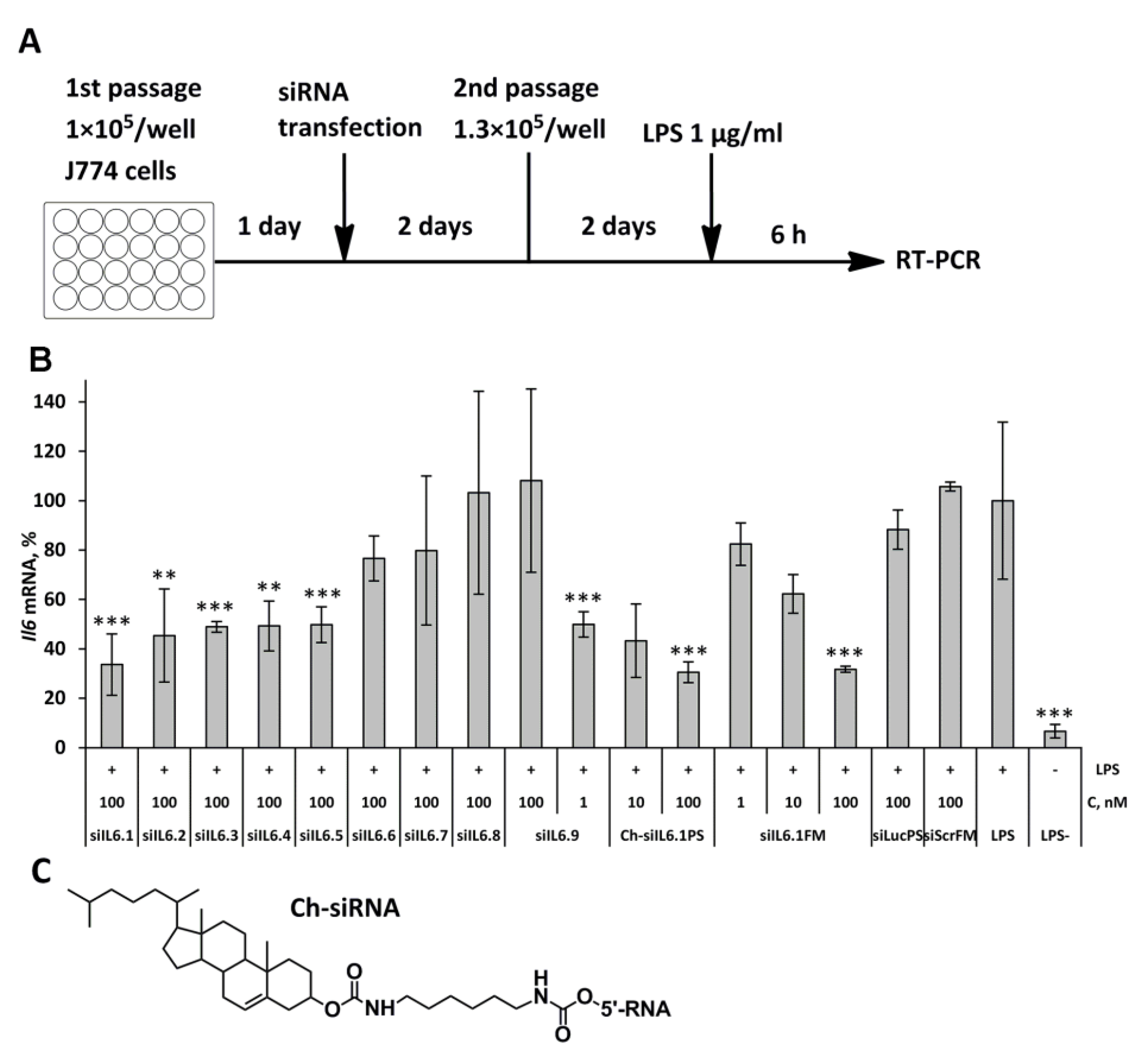
3.1. Silencing activities of anti-Il6 siRNAs in vitro
| Designation | Sequence1 |
|---|---|
| IL6.1.AS | GUUmAUmGCCUmAAGCmAUmAUCmAGUUU |
| IL6.1.S | ACUmGAUmAUmGCUUmAGGCmAUmAACGC |
| IL6.2.AS | UmAAGGACCmAAGACCmAUCCmA |
| IL6.2.S | UmGGAUmGGUCUUmGGUCCUUmAGC |
| IL6.3.AS | GUCmACUUmGAAAUmGUUmAUmAUmGU |
| IL6.3.S | AUmAUmAACmAUUUCmAAGUmGACmAC |
| IL6.4.AS | UUmGGGACmACUmAUUUUmAAUUmAU |
| IL6.4.S | AAUUmAAAAUmAGUmGUCCCmAACmA |
| IL6.5.AS | UmGCCUmAAGCmAUmAUCmAGUUUmGU |
| IL6.5_S | AAACUmGAUmAUmGCUUmAGGCmAUmA |
| IL6.6.AS | UmGCUmAAUUUmAAAUmAUmGUUUUU |
| IL6.6.S | AAACmAUmAUUUmAAAUUmAGCmAAU |
| IL6.7.AS | AGUCGGAGGCUUmAAUmUCmACmA |
| IL6.7.S | UmGUmAAUUmAAGCCUCCGACUUmG |
| IL6.8.AS | CUmACCmAAACUmGGAUmAUmAAUCmA |
| IL6.8.S | AUUmAUmAUCCmAGUUUmGGUmAGCmA |
| IL6.9.AS | CmAGGAAAUUUmGCCUmAUUmGAAA |
| IL6.9.S | UCmAAUmAGGCmAAAUUUCCUmGAU |
| IL6.1FM.AS | GmUmUmAmUmGmCfCmUfAfAfGmCmAmUmAmUmCmAmGmUmUmUm |
| IL6.1FM.S | AmCfUmGmAmUfAmUmGmCmUmUmAmGfGmCfAmUmAmAmCmGmCm |
| IL6.1PS.AS | Gm*Um*UmAmUmGmCfCmUfAfAfGmCmAmUmAmUmCmAmGmUm*Um*Um |
| IL6.1PS.S | Am*Cf*UmGmAmUfAmUmGmCmUmUmAmGfGmCfAmUmAmAmCm*Gm*Cm |
| Ch-IL6.1PS.AS | Ch-Gm*Um*UmAmUmGmCfCmUfAfAfGmCmAmUmAmUmCmAmGmUm*Um*Um |
| LucPS.AS | Cm*Gm*UmUmAmUmUfUmAfUfCfGmGmAmGmUmUmGmCm*Am*Gm |
| LucPS.S | Gm*Cf*AmAmCmUfCmCmGmAmUmAmAmAfUmAfAmCmGm*Cm*Gm |
| ScrFM.AS | AmAfUmAmUmCfUmGmCmUmCmUmUmCfAmUfGmCmGmGmGm |
| ScrFM.S | CmGmCmAmUmGmAfAmGfAfGfCmAmGmAmUmAmUmUmCmGm |
| ScrPS.AS | Am*Af*UmAmUmCfUmGmCmUmCmUmUmCfAmUfGmCmGm*Gm*Gm |
| Ch-ScrPS.S | Ch-Cm*Gm*CmAmUmGmAfAmGfAfGfCmAmGmAmUmAmUmUm*Cm*Gm |
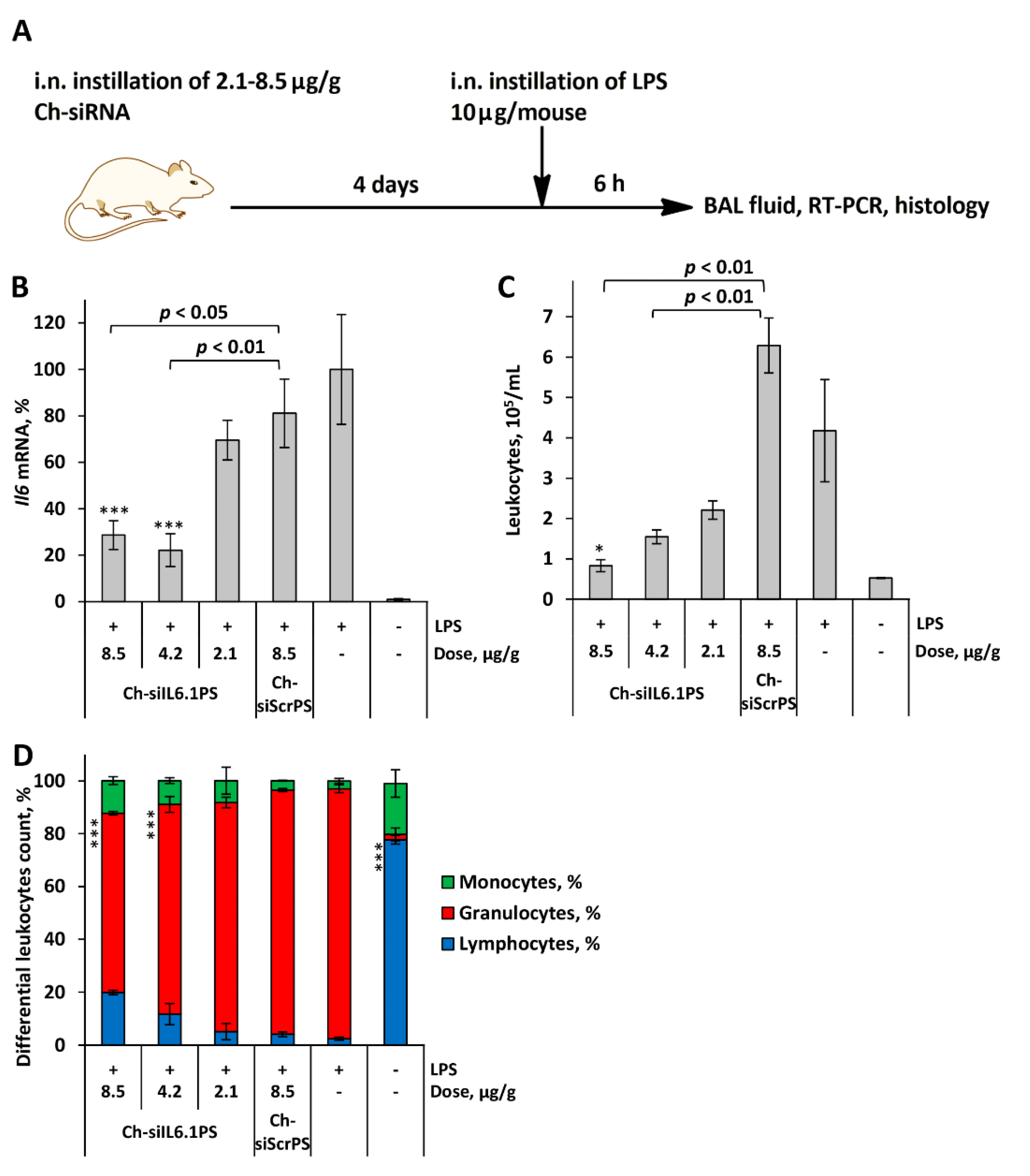
3.2. Ch-siIL6.1PS silences Il6 mRNA level and reduces the severity of acute lung injury in mice
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dana, H.; Mahmoodi Chalbatani, G.; Mahmoodzadeh, H.; Karimloo, R.; Rezaiean, O.; Moradzadeh, A.; Mehmandoost, N.; Moazzen, F.; Mazraeh, A.; Marmari, V.; et al. Molecular Mechanisms and Biological Functions of SiRNA. Int. J. Biomed. Sci. 2017, 13, 48–57. [Google Scholar] [CrossRef] [PubMed]
- Hu, B.; Zhong, L.; Weng, Y.; Peng, L.; Huang, Y.; Zhao, Y.; Liang, X.J. Therapeutic SiRNA: State of the Art. Signal Transduct. Target. Ther. 2020, 5, 101. [Google Scholar] [CrossRef] [PubMed]
- Brown, K.M.; Nair, J.K.; Janas, M.M.; Anglero-Rodriguez, Y.I.; Dang, L.T.H.; Peng, H.; Theile, C.S.; Castellanos-Rizaldos, E.; Brown, C.; Foster, D.; et al. Expanding RNAi Therapeutics to Extrahepatic Tissues with Lipophilic Conjugates. Nat. Biotechnol. 2022, 40, 1500–1508. [Google Scholar] [CrossRef] [PubMed]
- Alterman, J.F.; Godinho, B.M.D.C.; Hassler, M.R.; Ferguson, C.M.; Echeverria, D.; Sapp, E.; Haraszti, R.A.; Coles, A.H.; Conroy, F.; Miller, R.; et al. A Divalent SiRNA Chemical Scaffold for Potent and Sustained Modulation of Gene Expression throughout the Central Nervous System. Nat. Biotechnol. 2019, 37, 884–894. [Google Scholar] [CrossRef] [PubMed]
- Zuckerman, J.E.; Gale, A.; Wu, P.; Ma, R.; Davis, M.E. SiRNA Delivery to the Glomerular Mesangium Using Polycationic Cyclodextrin Nanoparticles Containing SiRNA. Nucleic Acid Ther. 2015, 25, 53–64. [Google Scholar] [CrossRef] [PubMed]
- Thai, H.B.D.; Kim, K.R.; Hong, K.T.; Voitsitskyi, T.; Lee, J.S.; Mao, C.; Ahn, D.R. Kidney-Targeted Cytosolic Delivery of SiRNA Using a Small-Sized Mirror DNA Tetrahedron for Enhanced Potency. ACS Cent. Sci. 2020, 6, 2250–2258. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Hardie, J.; Liu, Y.; Ray, M.; Luo, X.; Das, R.; Landis, R.F.; Farkas, M.E.; Rotello, V.M. Nanocapsule-Mediated Cytosolic SiRNA Delivery for Anti-Inflammatory Treatment. J. Control. Release 2018, 283, 235–240. [Google Scholar] [CrossRef] [PubMed]
- Miller, J.B.; Kos, P.; Tieu, V.; Zhou, K.; Siegwart, D.J. Development of Cationic Quaternary Ammonium Sulfonamide Amino Lipids for Nucleic Acid Delivery. ACS Appl. Mater. Interfaces 2018, 10, 2302–2311. [Google Scholar] [CrossRef] [PubMed]
- Chernikov, I. V.; Vlassov, V. V.; Chernolovskaya, E.L. Current Development of SiRNA Bioconjugates: From Research to the Clinic. Front. Pharmacol. 2019, 10, 444. [Google Scholar] [CrossRef]
- Eberle, F.; Giessler, K.; Deck, C.; Heeg, K.; Peter, M.; Richert, C.; Dalpke, A.H. Modifications in Small Interfering RNA That Separate Immunostimulation from RNA Interference. J. Immunol. 2008, 180(5), 3229–3237. [Google Scholar] [CrossRef]
- Sioud, M.; Furset, G.; Cekaite, L. Suppression of Immunostimulatory SiRNA-Driven Innate Immune Activation by 2′-Modified RNAs. Biochem. Biophys. Res. Commun. 2007, 361, 122–126. [Google Scholar] [CrossRef] [PubMed]
- Manoharan, M.; Akinc, A.; Pandey, R.K.; Qin, J.; Hadwiger, P.; John, M.; Mills, K.; Charisse, K.; Maier, M.A.; Nechev, L.; et al. Unique Gene-Silencing and Structural Properties of 2′-Fluoro-Modified SiRNAs. Angew. Chemie Int. Ed. 2011, 50, 2284–2288. [Google Scholar] [CrossRef]
- Judge, A.D.; Bola, G.; Lee, A.C.H.; MacLachlan, I. Design of Noninflammatory Synthetic SiRNA Mediating Potent Gene Silencing in Vivo. Mol. Ther. 2006, 13, 494–505. [Google Scholar] [CrossRef] [PubMed]
- Robbins, M.; Judge, A.; Liang, L.; McClintock, K.; Yaworski, E.; MacLachlan, I. 2′-O-Methyl-Modified RNAs Act as TLR7 Antagonists. Mol. Ther. 2007, 15, 1663–1669. [Google Scholar] [CrossRef]
- Chernikov, I. V.; Gladkikh, D. V.; Meschaninova, M.I.; Ven’yaminova, A.G.; Zenkova, M.A.; Vlassov, V. V.; Chernolovskaya, E.L. Cholesterol-Containing Nuclease-Resistant SiRNA Accumulates in Tumors in a Carrier-Free Mode and Silences MDR1 Gene. Mol. Ther. - Nucleic Acids 2017, 6, 209–220. [Google Scholar] [CrossRef] [PubMed]
- Biscans, A.; Coles, A.; Haraszti, R.; Echeverria, Di.; Hassler, M.; Osborn, M.; Khvorova, A. Diverse Lipid Conjugates for Functional Extra-Hepatic SiRNA Delivery in Vivo. Nucleic Acids Res. 2019, 47, 1082–1096. [Google Scholar] [CrossRef] [PubMed]
- Chernikov, I. V.; Meschaninova, M.I.; Gladkikh, D. V.; Ven’yaminova, A.G.; Zenkova, M.A.; Vlassov, V. V.; Chernolovskaya, E.L. Interaction of Lipophilic Conjugates of Modified SiRNAs with Hematopoietic Cells In Vitro and In Vivo. Russ. J. Bioorganic Chem. 2021, 47, 399–410. [Google Scholar] [CrossRef]
- Cooper, G.S.; Bynum, M.L.K.; Somers, E.C. Recent Insights in the Epidemiology of Autoimmune Diseases: Improved Prevalence Estimates and Understanding of Clustering of Diseases. J. Autoimmun. 2009, 33, 197–207. [Google Scholar] [CrossRef] [PubMed]
- El-Gabalawy, H.; Guenther, L.C.; Bernstein, C.N. Epidemiology of Immune-Mediated Inflammatory Diseases: Incidence, Prevalence, Natural History, and Comorbidities. J. Rheumatol. 2010, 37, 2–10. [Google Scholar] [CrossRef]
- Lerner, A.; Jeremias, P.; Matthias, T. The World Incidence and Prevalence of Autoimmune Diseases Is Increasing. Int. J. Celiac Dis. 2015, 3, 151–155. [Google Scholar] [CrossRef]
- Yasunaga, M. Antibody Therapeutics and Immunoregulation in Cancer and Autoimmune Disease. Semin. Cancer Biol. 2020, 64, 1–12. [Google Scholar] [CrossRef]
- Li, P.; Zheng, Y.; Chen, X. Drugs for Autoimmune Inflammatory Diseases: From Small Molecule Compounds to Anti-TNF Biologics. Front. Pharmacol. 2017, 8, 460. [Google Scholar] [CrossRef]
- Connor, V. Anti-TNF Therapies: A Comprehensive Analysis of Adverse Effects Associated with Immunosuppression. Rheumatol. Int. 2011, 31, 327–337. [Google Scholar] [CrossRef]
- Yu, M.B.; Firek, A.; Langridge, W.H.R. Predicting Methotrexate Resistance in Rheumatoid Arthritis Patients. Inflammopharmacology 2018, 26, 699–708. [Google Scholar] [CrossRef]
- Ragaller, M.; Richter, T. Acute Lung Injury and Acute Respiratory Distress Syndrome. J. Emergencies, Trauma Shock 2010, 3, 43–51. [Google Scholar] [CrossRef] [PubMed]
- Gotzev, R.; Kenarov, P. Acute Respiratory Distress Syndrome (ARDS). Anaesthesiol. Intensive Care 2013, 42, 43–49. [Google Scholar]
- Zoulikha, M.; Xiao, Q.; Boafo, G.F.; Sallam, M.A.; Chen, Z.; He, W. Pulmonary Delivery of SiRNA against Acute Lung Injury/Acute Respiratory Distress Syndrome. Acta Pharm. Sin. B 2022, 12, 600–620. [Google Scholar] [CrossRef]
- Trovato, M.; Sciacchitano, S.; Facciolà, A.; Valenti, A.; Visalli, G.; Di Pietro, A. Interleukin-6 Signalling as a Valuable Cornerstone for Molecular Medicine (Review). Int. J. Mol. Med. 2021, 47, 107. [Google Scholar] [CrossRef] [PubMed]
- Florentin, J.; Zhao, J.; Tai, Y.Y.; Vasamsetti, S.B.; O’Neil, S.P.; Kumar, R.; Arunkumar, A.; Watson, A.; Sembrat, J.; Bullock, G.C.; et al. Interleukin-6 Mediates Neutrophil Mobilization from Bone Marrow in Pulmonary Hypertension. Cell. Mol. Immunol. 2021, 18, 374–384. [Google Scholar] [CrossRef]
- Chen, I.C.; Wang, S.C.; Chen, Y.T.; Tseng, H.H.; Liu, P.L.; Lin, T.C.; Wu, H.E.; Chen, Y.R.; Tseng, Y.H.; Hsu, J.H.; et al. Corylin Ameliorates Lps-Induced Acute Lung Injury via Suppressing the Mapks and Il-6/Stat3 Signaling Pathways. Pharmaceuticals 2021, 14, 1046. [Google Scholar] [CrossRef]
- Meschaninova, M.I.; Novopashina, D.S.; Semikolenova, O.A.; Silnikov, V.N.; Venyaminova, A.G. Novel Convenient Approach to the Solid-Phase Synthesis of Oligonucleotide Conjugates. Molecules 2019, 24, 4266. [Google Scholar] [CrossRef] [PubMed]
- Foster, D.J.; Brown, C.R.; Shaikh, S.; Trapp, C.; Schlegel, M.K.; Qian, K.; Sehgal, A.; Rajeev, K.G.; Jadhav, V.; Manoharan, M.; et al. Advanced SiRNA Designs Further Improve In Vivo Performance of GalNAc-SiRNA Conjugates. Mol. Ther. 2018, 26, 708–717. [Google Scholar] [CrossRef] [PubMed]
- Chernikov, I. V.; Ponomareva, U.A.; Meschaninova, M.I.; Bachkova, I.K.; Teterina, A.A.; Gladkikh, D. V.; Savin, I.A.; Vlassov, V. V.; Zenkova, M.A.; Chernolovskaya, E.L. Cholesterol-Conjugated Supramolecular Multimeric SiRNAs: Effect of SiRNA Length on Accumulation and Silencing In Vitro and In Vivo. Nucleic Acid Ther. 2023, 33, 361–373. [Google Scholar] [CrossRef] [PubMed]
- Hassler, M.R.; Turanov, A.A.; Alterman, J.F.; Haraszti, R.A.; Coles, A.H.; Osborn, M.F.; Echeverria, D.; Nikan, M.; Salomon, W.E.; Roux, L.; et al. Comparison of Partially and Fully Chemically-Modified SiRNA in Conjugate-Mediated Delivery in Vivo. Nucleic Acids Res. 2018, 46, 2185–2196. [Google Scholar] [CrossRef] [PubMed]
- Chernikov, I. V.; Staroseletz, Y.Y.; Tatarnikova, I.S.; Sen’kova, A. V.; Savin, I.A.; Markov, A. V.; Logashenko, E.B.; Chernolovskaya, E.L.; Zenkova, M.A.; Vlassov, V. V. SiRNA-Mediated Timp1 Silencing Inhibited the Inflammatory Phenotype during Acute Lung Injury. Int. J. Mol. Sci. 2023, 24, 1641. [Google Scholar] [CrossRef] [PubMed]
- Liao, W.; Dong, J.; Peh, H.Y.; Tan, L.H.; Lim, K.S.; Li, L.; Wong, W.S.F. Oligonucleotide Therapy for Obstructive and Restrictive Respiratory Diseases. Molecules 2017, 22, 139. [Google Scholar] [CrossRef]
- Gupta, R.; Fonacier, L.S. Adverse Effects of Nonsystemic Steroids (Inhaled, Intranasal, and Cutaneous): A Review of the Literature and Suggested Monitoring Tool. Curr. Allergy Asthma Rep. 2016, 16, 44. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Chandley, P.; Rohatgi, S. Recent Advances in the Development of Monoclonal Antibodies and Next-Generation Antibodies. ImmunoHorizons 2023, 7, 886–897. [Google Scholar] [CrossRef] [PubMed]
- van Schouwenburg, P.A.; Bartelds, G.M.; Hart, M.H.; Aarden, L.; Wolbink, G.J.; Wouters, D. A Novel Method for the Detection of Antibodies to Adalimumab in the Presence of Drug Reveals “Hidden” Immunogenicity in Rheumatoid Arthritis Patients. J. Immunol. Methods 2010, 362, 82–88. [Google Scholar] [CrossRef] [PubMed]
- Willoughby, J.L.S.; Chan, A.; Sehgal, A.; Butler, J.S.; Nair, J.K.; Racie, T.; Shulga-Morskaya, S.; Nguyen, T.; Qian, K.; Yucius, K.; et al. Evaluation of GalNAc-SiRNA Conjugate Activity in Pre-Clinical Animal Models with Reduced Asialoglycoprotein Receptor Expression. Mol. Ther. 2018, 26, 105–114. [Google Scholar] [CrossRef]
- Corydon, I.J.; Fabian-Jessing, B.K.; Jakobsen, T.S.; Jørgensen, A.C.; Jensen, E.G.; Askou, A.L.; Aagaard, L.; Corydon, T.J. 25 Years of Maturation: A Systematic Review of RNAi in the Clinic. Mol. Ther. - Nucleic Acids 2023, 33, 469–482. [Google Scholar] [CrossRef]
- An, G. Pharmacokinetics and Pharmacodynamics of GalNAc-Conjugated SiRNAs. J. Clin. Pharmacol. 2024, 64, 45–57. [Google Scholar] [CrossRef]
- Ene, C.V.; Nicolae, I.; Geavlete, B.; Geavlete, P.; Ene, C.D. IL-6 Signaling Link between Inflammatory Tumor Microenvironment and Prostatic Tumorigenesis. Anal. Cell. Pathol. 2022, 2022, 5980387. [Google Scholar] [CrossRef]
- Roda, G.; Chien Ng, S.; Kotze, P.G.; Argollo, M.; Panaccione, R.; Spinelli, A.; Kaser, A.; Peyrin-Biroulet, L.; Danese, S. Crohn’s Disease. Nat. Rev. Dis. Prim. 2020, 6, 23. [Google Scholar] [CrossRef]
- Petrova, N.S.; Chernikov, I. V.; Meschaninova, M.I.; Dovydenko, I.S.; Venyaminova, A.G.; Zenkova, M.A.; Vlassov, V. V.; Chernolovskaya, E.L. Carrier-Free Cellular Uptake and the Gene-Silencing Activity of the Lipophilic SiRNAs Is Strongly Affected by the Length of the Linker between SiRNA and Lipophilic Group. Nucleic Acids Res. 2012, 40, 2330–2344. [Google Scholar] [CrossRef]
- Gilleron, J.; Querbes, W.; Zeigerer, A.; Borodovsky, A.; Marsico, G.; Schubert, U.; Manygoats, K.; Seifert, S.; Andree, C.; Stöter, M.; et al. Image-Based Analysis of Lipid Nanoparticle-Mediated SiRNA Delivery, Intracellular Trafficking and Endosomal Escape. Nat. Biotechnol. 2013, 31, 638–646. [Google Scholar] [CrossRef]
- Nair, J.K.; Attarwala, H.; Sehgal, A.; Wang, Q.; Aluri, K.; Zhang, X.; Gao, M.; Liu, J.; Indrakanti, R.; Schofield, S.; et al. Impact of Enhanced Metabolic Stability on Pharmacokinetics and Pharmacodynamics of GalNAc-SiRNA Conjugates. Nucleic Acids Res. 2017, 45, 10969–10977. [Google Scholar] [CrossRef]
- van oud Alblas, A.B.; van Furth, R. Origin, Kinetics, and Characteristics in the Normal Macrophages Steady State. J Exp Med. 1979, 149, 1504–1518. [Google Scholar] [CrossRef]
- Veiga, N.; Goldsmith, M.; Diesendruck, Y.; Ramishetti, S.; Rosenblum, D.; Elinav, E.; Behlke, M.A.; Benhar, I.; Peer, D. Leukocyte-Specific SiRNA Delivery Revealing IRF8 as a Potential Anti-Inflammatory Target. J. Control. Release 2019, 313, 33–41. [Google Scholar] [CrossRef]
- Zhang, J.; Shen, H.; Xu, J.; Liu, L.; Tan, J.; Li, M.; Xu, N.; Luo, S.; Wang, J.; Yang, F.; et al. Liver-Targeted SiRNA Lipid Nanoparticles Treat Hepatic Cirrhosis by Dual Antifibrotic and Anti-Inflammatory Activities. ACS Nano 2020, 14, 6305–6322. [Google Scholar] [CrossRef]
- Lee, J.; Son, W.; Hong, J.; Song, Y.; Yang, C.S.; Kim, Y.H. Down-Regulation of TNF-α via Macrophage-Targeted RNAi System for the Treatment of Acute Inflammatory Sepsis. J. Control. Release 2021, 336, 344–353. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Guo, R.; Huo, S.; Chen, H.; Song, Q.; Jiang, G.; Yu, Y.; Huang, J.; Xie, S.; Gao, X.; et al. CaP-Based Anti-Inflammatory HIF-1α SiRNA-Encapsulating Nanoparticle for Rheumatoid Arthritis Therapy. J. Control. Release 2022, 343, 314–325. [Google Scholar] [CrossRef] [PubMed]
- Mandal, A.; Kumbhojkar, N.; Reilly, C.; Dharamdasani, V.; Ukidve, A.; Ingber, D.E.; Mitragotri, S. Treatment of Psoriasis with NFKBIZ SiRNA Using Topical Ionic Liquid Formulations. Sci. Adv. 2020, 6, eabb6049. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





