Submitted:
22 April 2024
Posted:
23 April 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
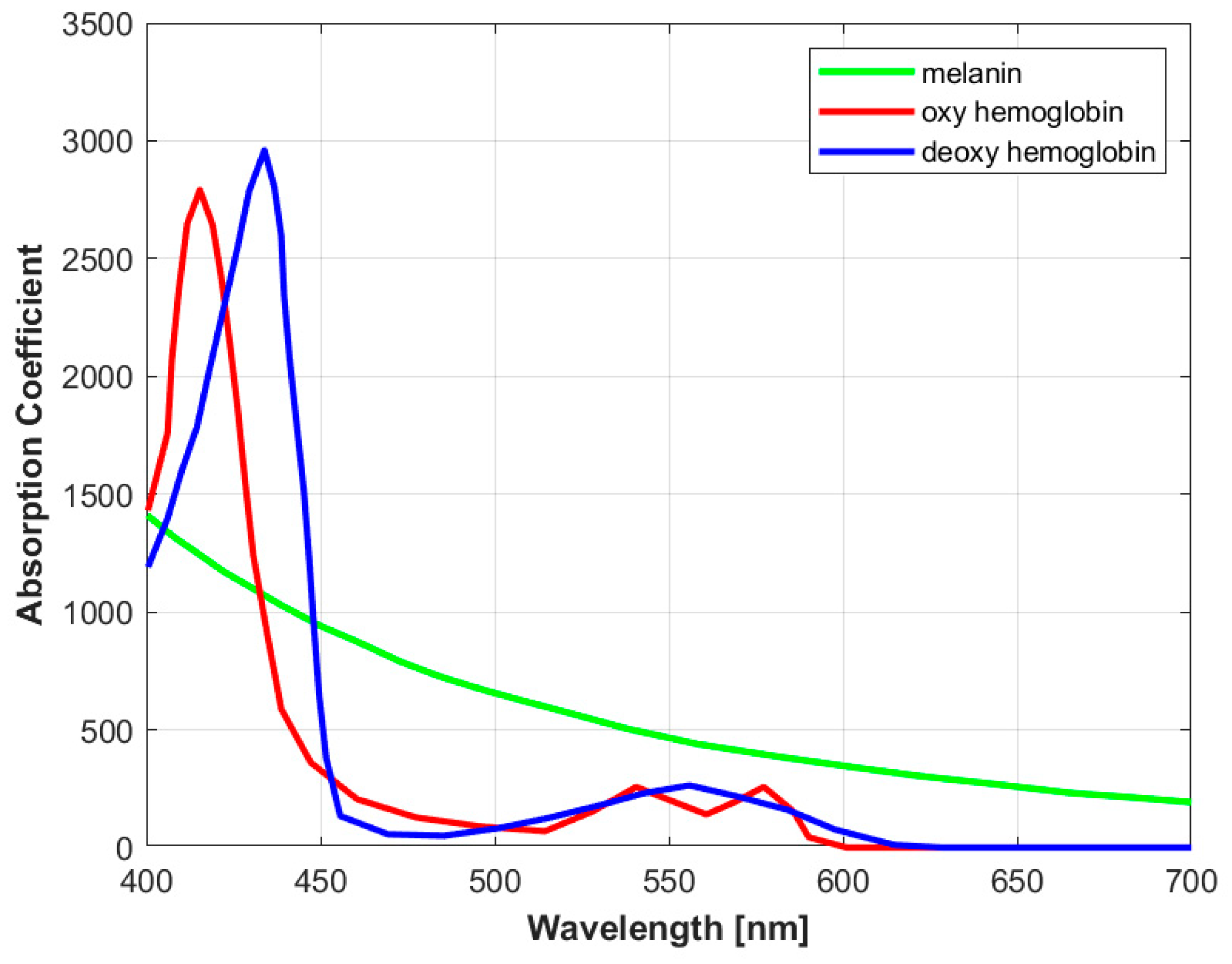
1.1. Imaging Modes: Single-Wavelength vs Multi-Spectral Imaging
1.2. Operational Classification of Optoacoustic Modalities
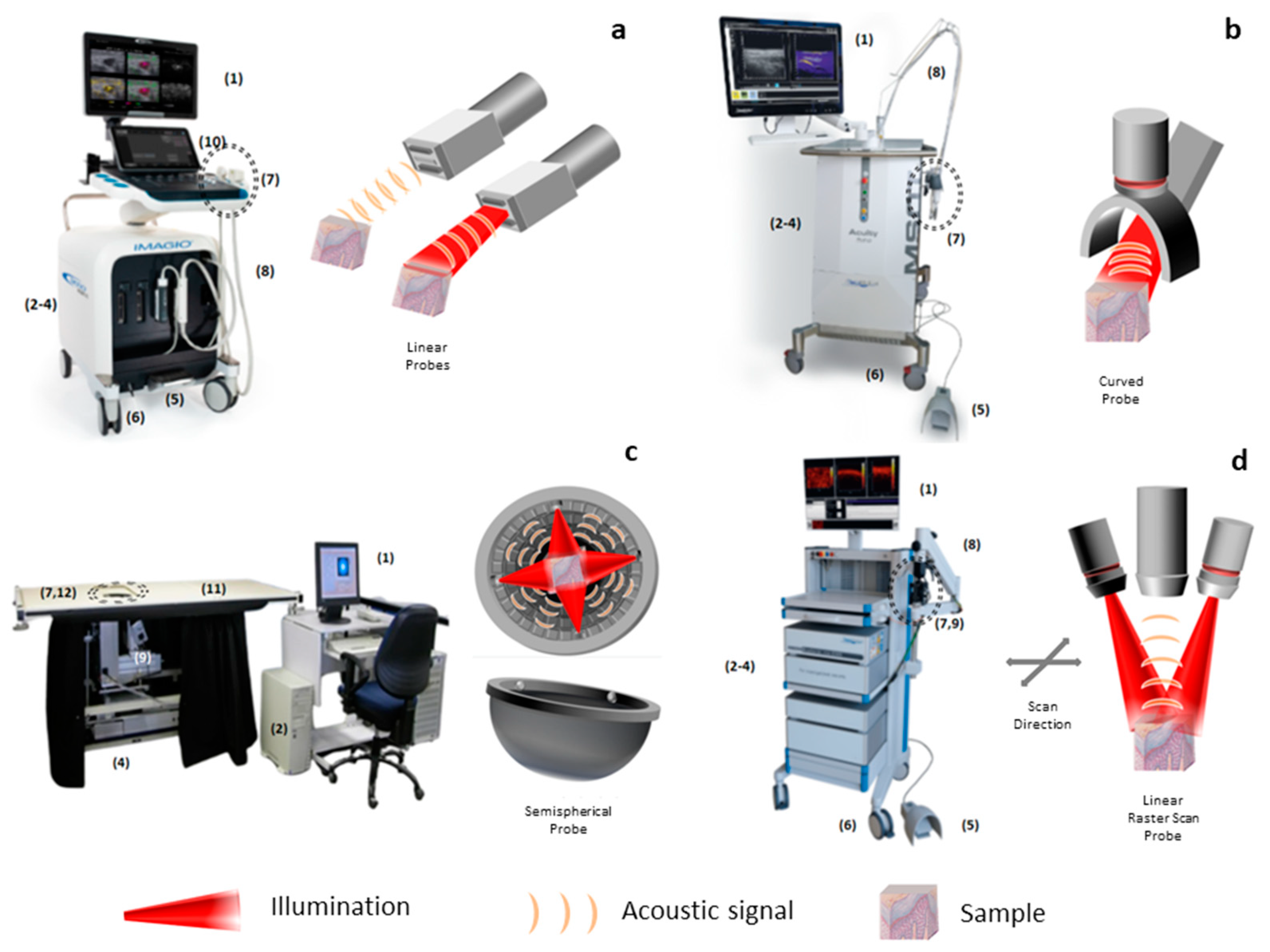
1.3. Macroscopic Systems
1.4. Mesoscopic Systems
1.5. Endoscopy
2. Review Studies
2.1. Macroscopic Systems
2.1.1. Cancer Imaging
2.1.2. Cardio-Vascular Imaging
2.1.3. Lymphatic System Imaging
2.1.4. Musculoskeletal Imaging
2.1.5. Gastrointestinal Imaging
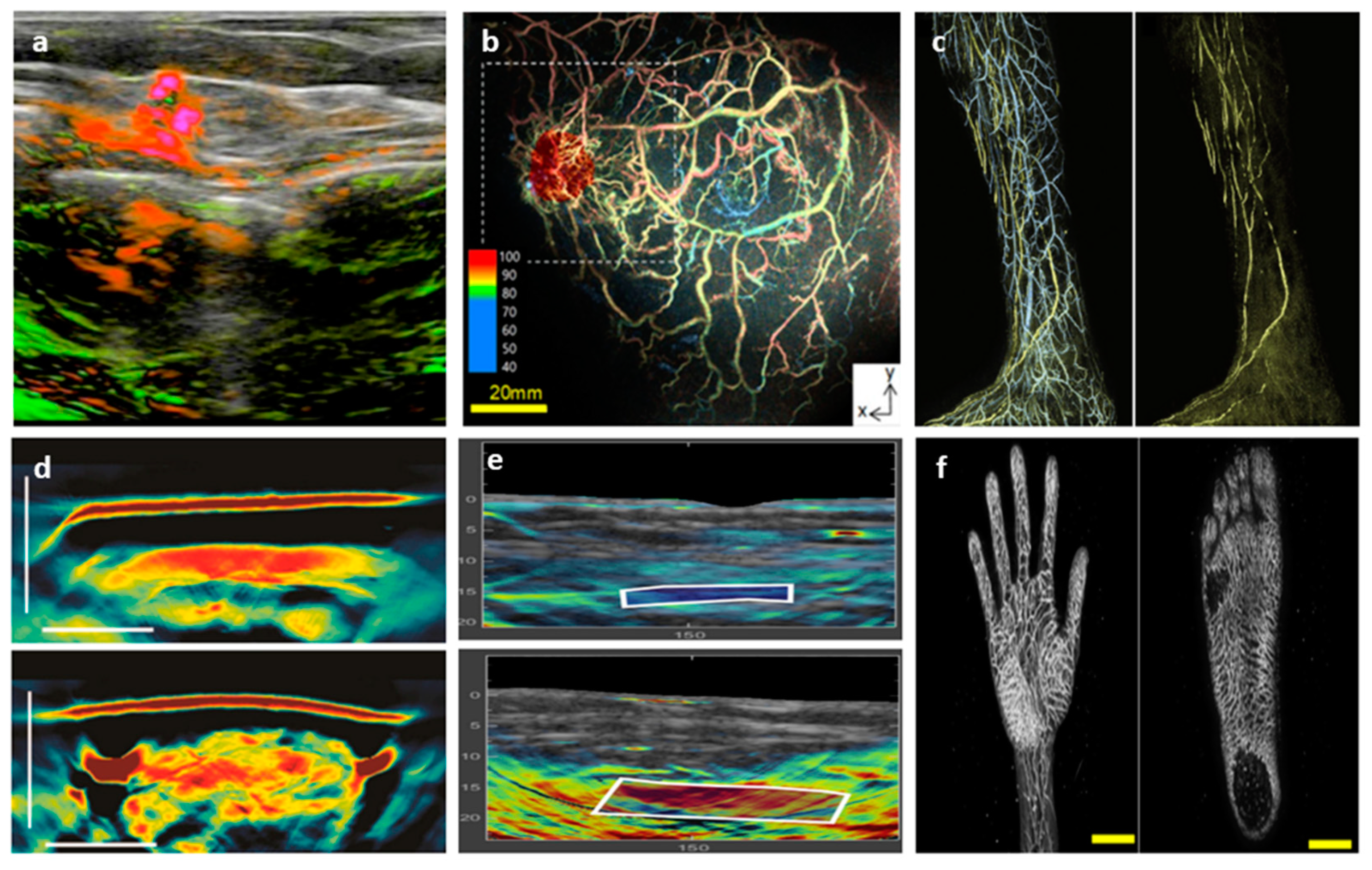
2.1.6. Miscellaneous
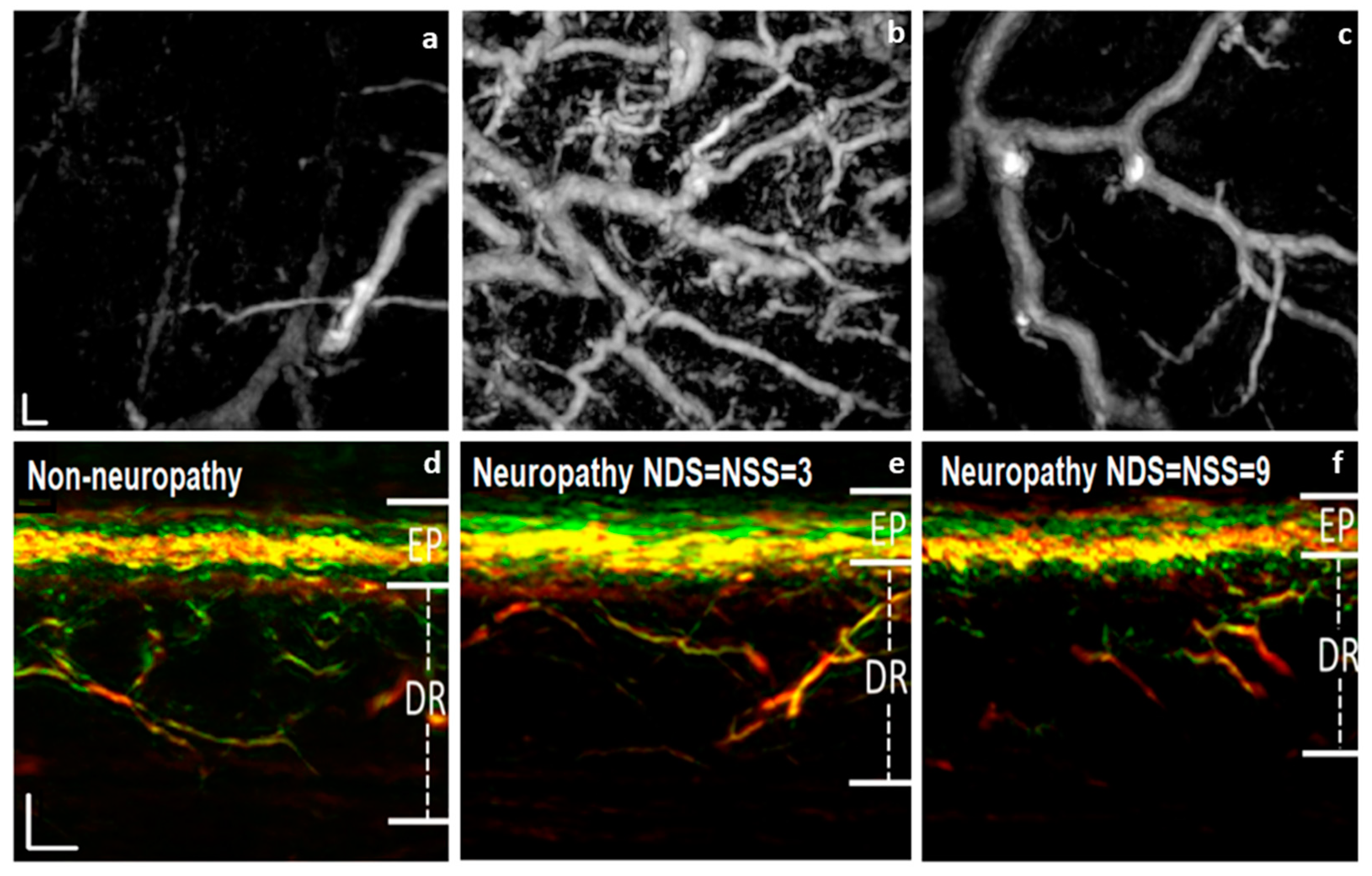
2.2. Mesoscopic Systems
3. The Path towards Clinical Translation: Are We Close to Clinical Adoption?
Author Contributions
Funding
Conflicts of Interest
Acronyms
References
- M. Omar, J. Aguirre, and V. Ntziachristos, “Optoacoustic mesoscopy for biomedicine,” Nat. Biomed. Eng., Apr. 2019. [CrossRef]
- I. Steinberg, D. M. Huland, O. Vermesh, H. E. Frostig, W. S. Tummers, and S. S. Gambhir, “Photoacoustic clinical imaging,” Photoacoustics, vol. 14, pp. 77–98, 2019. [CrossRef]
- A. Karlas, M. Pleitez, J. Aguirre, and V. Ntziachristos, “Optoacoustic Imaging in Endocrinology,” Nat. Rev. Endocrinol., 2021. [CrossRef]
- J. Aguirre, A. Giannoula, T. Minagawa, L. Funk, P. Turon, and T. Durduran, “A low memory cost model based reconstruction algorithm exploiting translational symmetry for photoacoustic microscopy,” Biomed Opt Express, vol. 4, no. 12, pp. 2813–2827, Dec. 2013. [CrossRef]
- A. Rosenthal, V. Ntziachristos, and D. Razansky, “Acoustic Inversion in Optoacoustic Tomography: A Review,” Current Medical Imaging Reviews, vol. 9, pp. 318–336, 2013.
- X. L. Dean-Ben, A. Buehler, V. Ntziachristos, and D. Razansky, “Accurate model-based reconstruction algorithm for three-dimensional optoacoustic tomography,” IEEE Trans Med Imaging, vol. 31, no. 10, pp. 1922–8, Oct. 2012. [CrossRef]
- A. Berezhnoi et al., “Optical features of human skin revealed by optoacoustic mesoscopy inthe visible and short-wave infrared regions,” Opt Lett, vol. 44, no. 17, pp. 4119–4122, Sep. 2019. [CrossRef]
- S. Tzoumas et al., “Eigenspectra optoacoustic tomography achieves quantitative blood oxygenation imaging deep in tissues,” Nat Commun, vol. 7, p. 12121, 30/online 2016. http://www.nature.com/articles/ncomms12121#supplementary-information. [CrossRef]
- B. Cox, J. G. Laufer, S. R. Arridge, and P. C. Beard, “Quantitative spectroscopic photoacoustic imaging: a review,” J Biomed Opt, vol. 17, no. 6, p. 061202, Jun. 2012. [CrossRef]
- X. L. Deán-Ben and D. Razansky, “On the link between the speckle free nature of optoacoustics and visibility of structures in limited-view tomography,” Photoacoustics, vol. 4, no. 4, pp. 133–140, 25 08/10/received 09/30/revised 10/13/accepted 2016. [CrossRef]
- A. Horiguchi et al., “Pilot study of prostate cancer angiogenesis imaging using a photoacoustic imaging system,” Urology, vol. 108, pp. 212–219, 2017. [CrossRef]
- R. Kothapalli et al., “Simultaneous transrectal ultrasound and photoacoustic human prostate imaging,” Sci. Transl. Med., vol. 11, p. 2169, Aug. 2019. [CrossRef]
- S. Nandy et al., “Evaluation of Ovarian Cancer: Initial Application of Coregistered Photoacoustic Tomography and US,” Radiology, vol. 289, no. 3, pp. 740–747, 2018. [CrossRef]
- Y. Qu et al., “In vivo characterization of connective tissue remodeling using infrared photoacoustic spectra,” J. Biomed. Opt., vol. 23, Dec. 2018. [CrossRef]
- Y. Li, G. Lu, Q. Zhou, and Z. Chen, “Advances in Endoscopic Photoacoustic Imaging,” Photonics, vol. 8, no. 7, 2021. [CrossRef]
- Y.-S. Chen, D. Yeager, and S. Y. Emelianov, “Chapter 9 - Photoacoustic Imaging for Cancer Diagnosis and Therapy Guidance,” in Cancer Theranostics, X. Chen and S. Wong, Eds., Oxford: Academic Press, 2014, pp. 139–158. [CrossRef]
- Lin, Li and Wang, Lihong V, “The emerging role of photoacoustic imaging in clinical oncology,” Nat. Rev. Clin. Oncol., vol. 19, no. 6, pp. 365–384, 2022. [CrossRef]
- A. Taruttis, A. C. Timmermans, P. C. Wouters, M. Kacprowicz, G. M. van Dam, and V. Ntziachristos, “Optoacoustic Imaging of Human Vasculature: Feasibility by Using a Handheld Probe,” Radiology, vol. 281, no. 1, pp. 256–263, 2016. [CrossRef]
- R. Akaho, M. Hirose, and N. Tsumura, “Evaluation of the robustness of estimating five components from a skin spectral image,” Opt. Rev., vol. 25, pp. 181–189, 2018. [CrossRef]
- “Premarket Approval Imagio Breas Imaging system.” Accessed: Nov. 01, 2023. [Online]. Available: https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfpma/pma.cfm?id=P200003.
- J. Zalev et al., “Clinical feasibility study of combined opto-acoustic and ultrasonic imaging modality providing coregistered functional and anatomical maps of breast tumors,” in Photons Plus Ultrasound: Imaging and Sensing 2013, A. A. Oraevsky and L. V. Wang, Eds., SPIE, 2013, p. 858103. [CrossRef]
- J. Zalev et al., “Opto-acoustic breast imaging with co-registered ultrasound,” in Medical Imaging 2014: Biomedical Applications in Molecular, Structural, and Functional Imaging, R. C. Molthen and J. B. Weaver, Eds., SPIE, 2014, p. 90381J. [CrossRef]
- A. A. Oraevsky, B. Clingman, J. Zalev, A. T. Stavros, W. T. Yang, and J. R. Parikh, “Clinical optoacoustic imaging combined with ultrasound for coregistered functional and anatomical mapping of breast tumors,” Photoacoustics, vol. 12, pp. 30–45, 2018. [CrossRef]
- G. L. G. Menezes et al., “Downgrading of Breast Masses Suspicious for Cancer by Using Optoacoustic Breast Imaging,” Radiology, vol. 288, no. 2, pp. 355–365, 2018. [CrossRef]
- E. I. Neuschler et al., “Downgrading and Upgrading Gray-Scale Ultrasound BI-RADS Categories of Benign and Malignant Masses With Optoacoustics: A Pilot Study,” Am. J. Roentgenol., vol. 211, no. 3, pp. 689–700, 2018. [CrossRef]
- E. I. Neuschler et al., “A Pivotal Study of Optoacoustic Imaging to Diagnose Benign and Malignant Breast Masses: A New Evaluation Tool for Radiologists,” Radiology, vol. 287, no. 2, pp. 398–412, 2018. [CrossRef]
- T. Kitai et al., “Photoacoustic mammography: Initial clinical results,” Breast Cancer Tokyo Jpn., vol. 21, Apr. 2012. [CrossRef]
- E. Fakhrejahani et al., “Clinical Report on the First Prototype of a Photoacoustic Tomography System with Dual Illumination for Breast Cancer Imaging,” PLOS ONE, vol. 10, no. 10, pp. 1–13, Oct. 2015. [CrossRef]
- M. Toi et al., “Visualization of tumor-related blood vessels in human breast by photoacoustic imaging system with a hemispherical detector array,” Sci. Rep., vol. 7, p. 41970, Feb. 2017. [CrossRef]
- I. Yamaga et al., “Vascular branching point counts using photoacoustic imaging in the superficial layer of the breast: A potential biomarker for breast cancer,” Photoacoustics, vol. 11, Jun. 2018. [CrossRef]
- Y. Matsumoto et al., “Visualising peripheral arterioles and venules through high-resolution and large-area photoacoustic imaging,” Sci. Rep., vol. 8, Oct. 2018. [CrossRef]
- Susanne E. Vaartjes and Johan C. G. van Hespen and Joost M. Klaase and Frank M. van den Engh and Andy K. H. The and Wiendelt Steenbergen and Ton G. van Leeuwen and Srirang Manohar, “First clinical trials of the Twente Photoacoustic Mammoscope (PAM).”.
- M. Heijblom et al., “Visualizing breast cancer using the Twente photoacoustic mammoscope: What do we learn from twelve new patient measurements?,” Opt Express, vol. 20, no. 11, pp. 11582–11597, May 2012. [CrossRef]
- M. Heijblom et al., “Appearance of breast cysts in planar geometry photoacoustic mammography using 1064-nm excitation,” J. Biomed. Opt., vol. 18, no. 12, p. 126009, 2013. [CrossRef]
- M. Heijblom et al., “Photoacoustic image patterns of breast carcinoma and comparisons with Magnetic Resonance Imaging and vascular stained histopathology,” Sci. Rep., vol. 5, p. 11778, Jul. 2015. [CrossRef]
- S. Schoustra et al., “Twente Photoacoustic Mammoscope 2: system overview and three-dimensional vascular network images in healthy breasts,” J. Biomed. Opt., vol. 24, p. 1, Oct. 2019. [CrossRef]
- R. Kruger, C. Kuzmiak, R. Lam, D. Reinecke, S. Rio, and D. Steed, “Dedicated 3D photoacoustic breast imaging,” Med. Phys., vol. 40, p. 113301, Nov. 2013. [CrossRef]
- L. Lin, P. Hu, J. Shi, C. M. Appleton, K. Maslov, and L. V. Wang, “Clinical photoacoustic computed tomography of the human breast in vivo within a single breath hold,” in Photons Plus Ultrasound: Imaging and Sensing 2018, A. A. Oraevsky and L. V. Wang, Eds., SPIE, 2018, p. 104942X. [CrossRef]
- L. Lin et al., “Single-breath-hold photoacoustic computed tomography of the breast,” Nat. Commun., vol. 9, no. 1, p. 2352, 2018. [CrossRef]
- G. Diot et al., “Multispectral Optoacoustic Tomography (MSOT) of Human Breast Cancer,” Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res., vol. 23, no. 22, p. 6912—6922, Nov. 2017. [CrossRef]
- J. Kukačka et al., “Second-generation optoacoustic imaging of breast cancer patients,” medRxiv, pp. 2021–10, 2021. [CrossRef]
- X. L. Dean-Ben, T. Fehm, M. Gostic, and D. Razansky, “Volumetric hand-held optoacoustic angiography as a tool for real-time screening of dense breast,” J. Biophotonics, vol. 9, May 2015. [CrossRef]
- N. Nyayapathi et al., “Dual Scan Mammoscope (DSM)—A New Portable Photoacoustic Breast Imaging System With Scanning in Craniocaudal Plane,” IEEE Trans. Biomed. Eng., vol. 67, no. 5, pp. 1321–1327, 2020. [CrossRef]
- S. Park, F. Brooks, U. Villa, R. Su, M. Anastasio, and A. Oraevsky, “Normalization of optical fluence distribution for three-dimensional functional optoacoustic tomography of the breast,” J. Biomed. Opt., vol. 27, Mar. 2022. [CrossRef]
- W. Roll et al., “Multispectral Optoacoustic Tomography of Benign and Malignant Thyroid Disorders: A Pilot Study,” J. Nucl. Med., vol. 60, p. jnumed.118.222174, Mar. 2019. [CrossRef]
- M. Noltes et al., “Towards in vivo characterization of thyroid nodules suspicious for malignancy using multispectral optoacoustic tomography,” Eur. J. Nucl. Med. Mol. Imaging, vol. 50, pp. 1–15, Apr. 2023. [CrossRef]
- J. Kim et al., “Multiparametric Photoacoustic Analysis of Human Thyroid Cancers In Vivo,” Cancer Res., vol. 81, no. 18, pp. 4849–4860, Sep. 2021. [CrossRef]
- M. Yang et al., “Photoacoustic/ultrasound dual imaging of human thyroid cancers: an initial clinical study,” Biomed Opt Express, vol. 8, no. 7, pp. 3449–3457, Jul. 2017. [CrossRef]
- S. Chuah et al., “Structural and functional 3D mapping of skin tumours with non-invasive multispectral optoacoustic tomography,” Skin Res. Technol., vol. 23, no. 2, pp. 221–226, 2017. [CrossRef]
- I. Stoffels et al., “Metastatic status of sentinel lymph nodes in melanoma determined noninvasively with multispectral optoacoustic imaging,” Sci. Transl. Med., vol. 7, no. 317, pp. 317ra199-317ra199, 2015. [CrossRef]
- B. Park et al., “3D Wide-field Multispectral Photoacoustic Imaging of Human Melanomas In Vivo: A Pilot Study,” J. Eur. Acad. Dermatol. Venereol. JEADV, vol. 35, Oct. 2020. [CrossRef]
- D. Jüstel et al., “Spotlight on nerves: Portable multispectral optoacoustic imaging of peripheral nerve vascularization and morphology.” arXiv, 2022. [CrossRef]
- H. Yang et al., “Soft ultrasound priors in optoacoustic reconstruction: Improving clinical vascular imaging,” Photoacoustics, vol. 19, p. 100172, 2020. [CrossRef]
- A. Karlas et al., “Multispectral optoacoustic tomography of lipid and hemoglobin contrast in human carotid atherosclerosis,” Photoacoustics, vol. 23, p. 100283, 2021. [CrossRef]
- M. Masthoff et al., “Use of Multispectral Optoacoustic Tomography to Diagnose Vascular Malformations,” JAMA Dermatol., vol. 154, Sep. 2018. [CrossRef]
- Behravesh S, Yakes W, Gupta N, Naidu S, Chong BW, Khademhosseini A, Oklu R, “Venous malformations: clinical diagnosis and treatment,” Cardiovasc Diagn Ther, vol. 6, no. 6, pp. 557–569. [CrossRef]
- D. Razansky et al., “Multispectral Optoacoustic Tomography of Matrix Metalloproteinase Activity in Vulnerable Human Carotid Plaques,” Mol. Imaging Biol. MIB Off. Publ. Acad. Mol. Imaging, vol. 14, pp. 277–85, Jul. 2011. [CrossRef]
- Van der Wal AC, Becker AE, “Atherosclerotic plaque rupture: pathologic basis of plaque stability and instability,” Cardiovasc Res, vol. 41, no. 2, pp. 334–344, 1999. [CrossRef]
- A. Dima and V. Ntziachristos, “In-vivo handheld optoacoustic tomography of the human thyroid,” Photoacoustics, vol. 4, no. 2, pp. 65–69, 2016. [CrossRef]
- M. Kroenke et al., “Multispectral Optoacoustic Tomography: A Novel Label-Free Imaging Technique for the Assessment of hyperthyroid diseases,” J. Nucl. Med., vol. 60, no. supplement 1, pp. 525–525, 2019.
- A. Karlas et al., “Flow-mediated dilatation test using optoacoustic imaging: a proof-of-concept,” Biomed Opt Express, vol. 8, no. 7, pp. 3395–3403, Jul. 2017. [CrossRef]
- Jeon BH, “Endothelial Dysfunction: From Pathophysiology to Novel Therapeutic Approaches,” Biomedicines, vol. 9, no. 11, p. 1571. [CrossRef]
- I. Tsuge et al., “Photoacoustic Tomography Shows the Branching Pattern of Anterolateral Thigh Perforators In Vivo,” Plast. Reconstr. Surg., vol. 141, no. 5, p. 1288—1292, May 2018. [CrossRef]
- J. E. Lubek and S. L. Engroff, “Chapter 71 - Anterolateral Thigh Flap,” in Current Therapy In Oral and Maxillofacial Surgery, S. C. Bagheri, R. B. Bell, and H. A. Khan, Eds., Saint Louis: W.B. Saunders, 2012, pp. 584–588. [CrossRef]
- Y. Matsumoto et al., “Label-free photoacoustic imaging of human palmar vessels: A structural morphological analysis,” Sci. Rep., vol. 8, Jan. 2018. [CrossRef]
- Y. Ishida et al., “Photoacoustic 3D imaging detects potential microvascular injuries,” medRxiv, 2020. [CrossRef]
- S. na et al., “Massively parallel functional photoacoustic computed tomography of the human brain,” Nat. Biomed. Eng., vol. 6, May 2022. [CrossRef]
- Alawneh JA, Hutchinson PAJ, Warburton E, “Stroke management: decompressive hemicraniectomy,” BMJ Clin Evid, 2015.
- W. Choi et al., “Three-dimensional Multistructural Quantitative Photoacoustic and US Imaging of Human Feet in Vivo,” Radiology, vol. 303, no. 2, pp. 467–473, 2022. [CrossRef]
- J. Kim, S. Park, Y. Jung, Y. Zhang, J. Lovell, and C. Kim, “Clinical real-time photoacoustic/ultrasound imaging system at POSTECH,” Mar. 2016, p. 970805. [CrossRef]
- J. Kim et al., “Programmable Real-time Clinical Photoacoustic and Ultrasound Imaging System,” Sci. Rep., vol. 6, p. 35137, Oct. 2016. [CrossRef]
- E. Merčep, X. L. Dean-Ben, and D. Razansky, “Combined Pulse-Echo Ultrasound and Multispectral Optoacoustic Tomography With a Multi-Segment Detector Array,” IEEE Trans. Med. Imaging, vol. PP, pp. 1–1, May 2017. [CrossRef]
- A. A. Plumb, N. T. Huynh, J. Guggenheim, E. Zhang, and P. Beard, “Rapid volumetric photoacoustic tomographic imaging with a Fabry-Perot ultrasound sensor depicts peripheral arteries and microvascular vasomotor responses to thermal stimuli,” Eur. Radiol., vol. 28, pp. 1037–1045, 2018. [CrossRef]
- C. Lee, W. Choi, J. Kim, and C. Kim, “Three-dimensional clinical handheld photoacoustic/ultrasound scanner,” Photoacoustics, vol. 18, p. 100173, 2020. [CrossRef]
- C. Özsoy et al., “LightSpeed: A Compact, High-Speed Optical-Link-Based 3D Optoacoustic Imager,” IEEE Trans. Med. Imaging, vol. PP, pp. 1–1, Apr. 2021. [CrossRef]
- Y. Skridlevskiy, I. Petrov, I. Patrikeev, R. Esenaliev, and D. Prough, “Multiwavelength optoacoustic system for noninvasive monitoring of cerebral venous oxygenation: A pilot clinical test in the internal jugular vein,” Opt. Lett., vol. 31, pp. 1827–9, Jul. 2006. [CrossRef]
- J. Yang, G. Zhang, S. Qiquan, M. Wu, L. Huang, and H. Jiang, “Detecting hemodynamic changes in the foot vessels of diabetic patients by photoacoustic tomography,” J. Biophotonics, vol. 13, May 2020. [CrossRef]
- Y. Suzuki et al., “Subcutaneous Lymphatic Vessels in the Lower Extremities: Comparison between Photoacoustic Lymphangiography and Near-Infrared Fluorescence Lymphangiography,” Radiology, vol. 295, p. 191710, Feb. 2020. [CrossRef]
- Y. Suzuki, H. Kajita, N. Imanishi, S. Aiso, and K. Kishi, “Observation of a Lymphatic Pump in a Human by Using Photoacoustic Imaging,” Plast. Reconstr. Surg. Glob. Open, vol. 8, p. e2914, Jun. 2020. [CrossRef]
- Y. Suzuki et al., “Use of photoacoustic imaging to determine the effects of aging on lower extremity lymphatic vessel function,” J. Vasc. Surg. Venous Lymphat. Disord., vol. 10, May 2021. [CrossRef]
- A. Oh et al., “Photoacoustic lymphangiography before and after lymphaticovenular anastomosis,” Arch. Plast. Surg., vol. 48, pp. 323–328, May 2021. [CrossRef]
- Verhey EM, Kandi LA, Lee YS, Morris BE, Casey WJ, Rebecca AM, Marks LA, Howard MA, Teven CM, “Outcomes of Lymphovenous Anastomosis for Lower Extremity Lymphedema: A Systematic Review,” Plast. Reconstr. Surg., vol. 10, no. 10, 2022. [CrossRef]
- Venugopal V, Pavlakis S, Duchenne Muscular Dystrophy. StatPearls, 2023. [Online]. Available: https://www.ncbi.nlm.nih.gov/books/NBK482346/.
- A. P. Regensburger et al., “Detection of collagens by multispectral optoacoustic tomography as an imaging biomarker for Duchenne muscular dystrophy,” Nat. Med., pp. 1–11, 2019. [CrossRef]
- Karlas et al., “Multispectral optoacoustic tomography of muscle perfusion and oxygenation under arterial and venous occlusion: A human pilot study,” J. Biophotonics, vol. 13, no. 6, p. e201960169, 2020. [CrossRef]
- G. Diot, A. Dima, and V. Ntziachristos, “Multispectral opto-acoustic tomography of exercised muscle oxygenation,” Opt. Lett., vol. 40, pp. 1496–9, Apr. 2015. [CrossRef]
- A. P. Regensburger et al., “Multispectral optoacoustic tomography for non-invasive disease phenotyping in pediatric spinal muscular atrophy patients,” Photoacoustics, vol. 25, p. 100315, 2022. [CrossRef]
- H. Chaytow, K. M. E. Faller, Y.-T. Huang, and T. H. Gillingwater, “Spinal muscular atrophy: From approved therapies to future therapeutic targets for personalized medicine,” Cell Rep. Med., vol. 2, no. 7, p. 100346, 2021. [CrossRef]
- J. Shubert and M. Lediju Bell, “Photoacoustic imaging of a human vertebra: Implications for guiding spinal fusion surgeries,” Phys. Med. Biol., vol. 63, Jun. 2018. [CrossRef]
- J. Yang et al., “Photoacoustic imaging of hemodynamic changes in forearm skeletal muscle during cuff occlusion,” Biomed. Opt. Express, vol. 11, Jul. 2020. [CrossRef]
- McDowell C, Farooq U, Haseeb M, Inflammatory Bowel Disease. StatPearls, 2023. [Online]. Available: https://www.ncbi.nlm.nih.gov/books/NBK470312/.
- M. J. Waldner et al., “Multispectral Optoacoustic Tomography in Crohn’s Disease: Noninvasive Imaging of Disease Activity,” Gastroenterology, vol. 151, no. 2, pp. 238–240, 2016. [CrossRef]
- F. Knieling et al., “Multispectral Optoacoustic Tomography for Assessment of Crohn’s Disease Activity,” N. Engl. J. Med., vol. 376, no. 13, pp. 1292–1294, 2017. [CrossRef]
- Ha F, Khalil H, “Crohn’s disease: a clinical update,” Ther. Adv Gastroenterol, vol. 8, no. 6, pp. 352–9, 2015. [CrossRef]
- F. Knieling et al., “Multispectral Optoacoustic Tomography in Ulcerative Colitis - A First-in-human Diagnostic Clinical Trial,” J. Nucl. Med., vol. 58, no. supplement 1, pp. 1196–1196, 2017.
- M. Gajendran et al., “A comprehensive review and update on ulcerative colitis,” Dis. Mon., vol. 65, no. 12, p. 100851, 2019. [CrossRef]
- F. Knieling et al., “Contrast-enhanced Multispectral Optoacoustic Tomography for Functional Assessment of the Gastrointestinal Tract,” Dec. 2022. [CrossRef]
- L.-P. Paulus et al., “Multispectral optoacoustic tomography of the human intestine – temporal precision and the influence of postprandial gastrointestinal blood flow,” Photoacoustics, vol. 30, p. 100457, 2023. [CrossRef]
- C.-P. Adler, “Inflammatory Joint Diseases,” in Bone Diseases: Macroscopic, Histological, and Radiological Diagnosis of Structural Changes in the Skeleton, Berlin, Heidelberg: Springer Berlin Heidelberg, 2000, pp. 441–455. [CrossRef]
- P. J. van den Berg, K. Daoudi, H. J. B. Moens, and W. Steenbergen, “Feasibility of photoacoustic/ultrasound imaging of synovitis in finger joints using a point-of-care system,” Photoacoustics, vol. 8, pp. 8–14, 2017. [CrossRef]
- O. Abeyakoon et al., “Optoacoustic imaging detects hormone-related physiological changes of breast parenchyma,” Ultraschall Med.-Eur. J. Ultrasound, vol. 40, no. 06, pp. 757–763, 2019. [CrossRef]
- Thiyagarajan DK, Basit H, Jeanmonod R, Physiology, Menstrual Cycle. StatPearls, 2023. [Online]. Available: https://www.ncbi.nlm.nih.gov/books/NBK500020/.
- L. Monteiro Rodrigues, T. F. Granja, and S. F. de Andrade, “Optoacoustic Imaging Offers New Insights into In Vivo Human Skin Vascular Physiology,” Life, vol. 12, no. 10, 2022. [CrossRef]
- Rosenberry R, Nelson MD, “Reactive hyperemia: a review of methods, mechanisms, and considerations,” Am J Physiol Regul Integr Comp Physiol, vol. 318, no. 3, pp. 605–618, 2020. [CrossRef]
- I. Tsuge et al., “Preoperative vascular mapping for anterolateral thigh flap surgeries: A clinical trial of photoacoustic tomography imaging,” Microsurgery, vol. 40, Nov. 2019. [CrossRef]
- H. Shimizu et al., “Three-dimensional visualization of thoracodorsal artery perforators using photoacoustic imaging,” J. Plast. Reconstr. Aesthet. Surg., vol. 75, no. 9, pp. 3166–3173, 2022. [CrossRef]
- A. A. Amin, M. Rifaat, A. Farahat, and T. Hashem, “The role of thoracodorsal artery perforator flap in oncoplastic breast surgery,” J. Egypt. Natl. Cancer Inst., vol. 29, no. 2, pp. 83–87, 2017. [CrossRef]
- K. Nagae et al., “Real-time 3D Photoacoustic Visualization System with a Wide Field of View for Imaging Human Limbs,” F1000Research, vol. 7, p. 1813, Feb. 2019. [CrossRef]
- N.-A. Fasoula et al., “Multicompartmental non-invasive sensing of postprandial lipemia in humans with multispectral optoacoustic tomography,” Mol. Metab., vol. 47, p. 101184, 2021. [CrossRef]
- L. Grünherz et al., “Preoperative Mapping of Lymphatic Vessels by Multispectral Optoacoustic Tomography,” Lymphat. Res. Biol., vol. 20, no. 6, pp. 659–664, 2022. [CrossRef]
- J. Aguirre et al., “Precision assessment of label-free psoriasis biomarkers with ultra-broadband optoacoustic mesoscopy,” Nat. Biomed. Eng., vol. 1, p. 0068, 10/online 2017. https://www.nature.com/articles/s41551-017-0068#supplementary-information. [CrossRef]
- W. Bost, R. Lemor, and M. Fournelle, “Optoacoustic Imaging of Subcutaneous Microvasculature With a Class one Laser,” IEEE Trans. Med. Imaging, vol. 33, May 2014. [CrossRef]
- J. Yang, G. Zhang, M. Wu, S. Qiquan, L. Huang, and H. Jiang, “Photoacoustic assessment of hemodynamic changes in foot vessels,” J. Biophotonics, vol. 12, p. e201900004, Mar. 2019. [CrossRef]
- E. Z. Zhang et al., “Multimodal photoacoustic and optical coherence tomography scanner using an all optical detection scheme for 3D morphological skin imaging,” Biomed Opt Express, vol. 2, no. 8, pp. 2202–2215, Aug. 2011. [CrossRef]
- Zabihian et al., “In vivo dual-modality photoacoustic and optical coherence tomography imaging of human dermatological pathologies.,” Biomed. Opt. Express, vol. 6 9, pp. 3163–78, 2015. [CrossRef]
- J. Aguirre, M. Schwarz, D. Soliman, A. Buehler, M. Omar, and V. Ntziachristos, “Broadband mesoscopic optoacoustic tomography reveals skin layers,” Opt Lett, vol. 39, no. 21, pp. 6297–6300, Nov. 2014. [CrossRef]
- J. Aguirre et al., “Assessing nailfold microvascular structure with ultra-wideband raster-scan optoacoustic mesoscopy,” Photoacoustics, vol. 10, pp. 31–37, 2018. [CrossRef]
- H. He et al., “Fast Optoacoustic Mesoscopy of Microvascular Endothelial Dysfunction in Cardiovascular Risk and Disease,” bioRxiv, 2021. [CrossRef]
- H. He et al., “Optoacoustic skin mesoscopy opens a window to systemic effects of diabetes,” Jun. 2020. [CrossRef]
- M. Schwarz, A. Buehler, J. Aguirre, and V. Ntziachristos, “Three-dimensional multispectral optoacoustic mesoscopy reveals melanin and blood oxygenation in human skin in vivo,” J. Biophotonics, vol. 9, Nov. 2015. [CrossRef]
- M. Schwarz, J. Aguirre, D. Soliman, A. Buehler, and V. Ntziachristos, “Unmixing chromophores in human skin with a 3D multispectral optoacoustic mesoscopy system,” in Photons Plus Ultrasound: Imaging and Sensing 2016, A. A. Oraevsky and L. V. Wang, Eds., in Society of Photo-Optical Instrumentation Engineers (SPIE) Conference Series, vol. 9708. Mar. 2016, p. 970855. [CrossRef]
- Hindelang et al., “Optoacoustic mesoscopy shows potential to increase accuracy of allergy patch testing,” Contact Dermatitis, vol. 83, no. 3, pp. 206–214, 2020. [CrossRef]
- B. Hindelang et al., “Quantification of skin sensitivity to ultraviolet radiation using ultra-wideband optoacoustic mesoscopy,” Br. J. Dermatol., vol. 184, Aug. 2020. [CrossRef]
- O. Braun-Falco, G. Plewig, H. H. Wolff, and R. K. Winkelmann, “Erythematous and Erythematosquamous Skin Diseases,” in Dermatology, Berlin, Heidelberg: Springer Berlin Heidelberg, 1991, pp. 403–466. [CrossRef]
- Berezhnoi, M. Schwarz, A. Buehler, S. V. Ovsepian, J. Aguirre, and V. Ntziachristos, “Assessing hyperthermia-induced vasodilation in human skin in vivo using optoacoustic mesoscopy,” J. Biophotonics, vol. 11, no. 11, p. e201700359, 2018. [CrossRef]
- S. Moustakidis, M. Omar, J. Aguirre, P. Mohajerani, and V. Ntziachristos, “Fully Automated Identification of Skin Morphology in Raster-Scan Optoacoustic Mesoscopy using Artificial Intelligence,” Med. Phys., vol. 46, Jul. 2019. [CrossRef]
- J. Aguirre et al., “Precision assessment of label-free psoriasis biomarkers with ultra-broadband optoacoustic mesoscopy,” Nat. Biomed. Eng., vol. 1, p. 0068, May 2017. [CrossRef]
- Hindelang et al., “Enabling precision monitoring of psoriasis treatment by optoacoustic mesoscopy,” Sci. Transl. Med., vol. 14, no. 644, p. eabm8059, 2022. [CrossRef]
- S. J. Ford et al., “Structural and functional analysis of intact hair follicles and pilosebaceous units by volumetric multispectral optoacoustic tomography,” J. Invest. Dermatol., vol. 136, no. 4, pp. 753–761, 2016. [CrossRef]
- Y. Yew et al., “Raster-scanning optoacoustic mesoscopy (RSOM) imaging as an objective disease severity tool in atopic dermatitis patients,” J. Am. Acad. Dermatol., vol. 84, Jun. 2020. [CrossRef]
- Maser, Raelene E and Nielsen, Viggo K and Bass, Eric B and Manjoo, Qurashia and Dorman, Janice S and Kelsey, Sheryl F and Becker, Dorothy J and Orchard, Trevor J, “Measuring Diabetic Neuropathy: Assessment and Comparison of Clinical Examination and Quantitative Sensory Testing,” Diabetes Care, vol. 12, no. 4, pp. 270–275, 1989. [CrossRef]
- Feldman, Eva L and Stevens, M J and Thomas, P K and Brown, M B and Canal, N and Greene, D A, “A Practical Two-Step Quantitative Clinical and Electrophysiological Assessment for the Diagnosis and Staging of Diabetic Neuropathy,” Diabetes Care, vol. 17, no. 11, pp. 1281–1289, 1994. [CrossRef]
- G. A. Van Norman, “Drugs, Devices, and the FDA: Part 2: An Overview of Approval Processes: FDA Approval of Medical Devices,” JACC Basic Transl. Sci., vol. 1, no. 4, pp. 277–287, Jun. 2016. [CrossRef]
- A. V. Kaplan et al., “Medical device development: from prototype to regulatory approval,” Circulation, vol. 109, no. 25, pp. 3068–3072, Jun. 2004. [CrossRef]
| Optoacoustic Mesoscopy | Optoacoustic Macroscopy | |
|---|---|---|
|
Bandwidth Lateral Resolution Axial Resolution Depth |
10-200 MHz <100 µm 10-100 µm 0-10 mm |
<10 MHz 100-500 µm 100-500 µm >10 mm |
| Medical Specialty | Cardiology, Dermatology, Endocrinology, Hematology, Oncology, Pathology, Plastic Surgery, Rheumatology |
Cardiology, Dermatology, Endocrinology, Gastroenterology, General Surgery, Hematology, Inmunology, Neurology, Obstetrics and Gynaecology, Oncology, Orthopaedics, Pathology, Paediatrics, Podiatry, Rheumatology, Urology, Vascular Surgery |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





